Floodplain Forest Soil Nematode Communities as Influenced by Non-Native Acer negundo L. Invasion
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling, Nematodes Extraction, and Processing
2.3. Nematode Community Analysis
2.4. Soil Properties Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
3.2. Nematode Community Indices and Faunal Profile Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kimmins, J.P. Forest Ecology: A Foundation for Sustainable Forest Management and Environmental Ethics in Forestry, 3rd ed.; Prentice Hall: Upper Saddle, NJ, USA, 2004. [Google Scholar]
- Burton, P.J.C.; Messier, D.W.S.; Adamowicz, W.L. Towards Sustainable Management of the Boreal Forest; NRC Research Press: Ottawa, ON, Canada, 2003. [Google Scholar]
- Drescher, A.; Prots, B. Fraxinus pennsylvanica-an invasive tree species in Middle Europe: Case studies from the Danube basin. Contrib. Bot. 2016, 51, 55–69. [Google Scholar]
- Danielewicz, W. Ekologiczne Uwarunkowania Zasięgow Drzew i Krzewow na Aluwialnych Obszarach Doliny Odry; Wydawnictwo Uniwersytetu Przyrodniczego w Poznaniu: Poznań, Poland, 2008; 268p. [Google Scholar]
- Saccone, P.; Pagès, J.P.; Girel, J.; Brun, J.J.; Michalet, R. Acer negundo invasion along a successional gradient: Early direct facilitation by native pioneers and late indirect facilitation by conspecifics. New Phytol. 2010, 187, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Tokarska-Guzik, B.; Zając, M. The role of rivers and streams in the migration of alien plants into the Polish Carpathians. Biodivers. Res. Conserv. 2011, 23, 43–56. [Google Scholar] [CrossRef]
- Straigyte, L.; Cekstere, G.; Laivins, M.; Marozas, V. The spread, intensity and invasiveness of the Acer negundo in Riga and Kaunas. Dendrobiology 2015, 74, 157–168. [Google Scholar] [CrossRef]
- Maděra, P.; Řepka, R.; Koutecký, T.; Šebesta, J. Vascular plant biodiversity of floodplain forest in Morava and Dyje rivers confluence (Forest District Soutok), Czech Republic. J. Landsc. Ecol. 2018, 11, 64–97. [Google Scholar] [CrossRef]
- Hübl, E. Vegetation and Flora Near the Danube in Austria. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Cham, Switzerland, 2020; pp. 65–86. [Google Scholar]
- Tabacchi, E.; Planty-Tabacchi, A.M. Recent changes in riparian vegetation: Possible consequences on dead wood processing along rivers. River Res. Appl. 2003, 19, 251–263. [Google Scholar] [CrossRef]
- Vykhor, B.; Prots, B. Ash-leaved maple (Acer negundo L.) in the Transcarpathia: Ecology, distribution and impact on environment. Stud. Biol. 2013, 7, 119–130. [Google Scholar] [CrossRef]
- Emelyanov, A.V.; Frolova, S.V. Ash-leaf maple (Acer negundo L.) in coastal phytocenoses of the Vorona River. Russ. J. Biol. Invasions 2011, 2, 161. [Google Scholar] [CrossRef]
- Kostina, M.V.; Yasinskaya, O.I.; Barabanshchikova, N.S.; Orlyuk, F.A. Toward a issue of box elder invasion into the forests around Moscow. Russ. J. Biol. Invasions 2016, 7, 47–51. [Google Scholar] [CrossRef]
- Veselkin, D.V.; Dubrovin, D.I. Diversity of the grass layer of urbanized communities dominated by invasive Acer negundo. Russ. J. Ecol. 2019, 50, 413–421. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Scheu, S.; Ramirez, K.S.; Lemanceau, P.; Eggleton, P.; Jones, A.; Moreira, F.M.S.; Barrios, E.; De Deyn, G.B.; Briones, M.J.I.; et al. Global Soil Biodiversity Atlas; European Commission: Luxembourg, 2016; 167p. [Google Scholar]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Viketoft, M.; Palmborg, C.; Sohlenius, B.; Huss-Danell, K.; Bengtsson, J. Plant species effects on soil nematode communities in experimental grasslands. Appl. Soil Ecol. 2005, 30, 90–103. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Migunova, V.D.; Ackermann, M.; Ruess, L.; Scheu, S. Changes in plant species richness induce functional shifts in soil nematode communities in experimental grassland. PLoS ONE 2011, 6, e24087. [Google Scholar] [CrossRef]
- Dietrich, P.; Cesarz, S.; Liu, T.; Roscher, C.; Eisenhauer, N. Effects of plant species diversity on nematode community composition and diversity in a long-term biodiversity experiment. Oecologia 2021, 197, 297–311. [Google Scholar] [CrossRef]
- Griffiths, B.; Neilson, R.; Bengough, A.G. Soil factors determined nematode community composition in a two year pot experiment. Nematology 2003, 5, 889–897. [Google Scholar] [CrossRef]
- Briar, S.S.; Grewal, P.S.; Somasekhar, N.; Stinner, D.; Miller, S.A. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Appl. Soil Ecol. 2007, 37, 256–266. [Google Scholar] [CrossRef]
- Nisa, R.U.; Tantray, A.Y.; Kouser, N.; Allie, K.A.; Wani, S.M.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L.; Shah, A.A. Influence of ecological and edaphic factors on biodiversity of soil nematodes. Saudi J. Biol. Sci. 2021, 28, 3049–3059. [Google Scholar] [CrossRef]
- Jurová, J.; Renčo, M.; Gömöryová, E.; Čerevková, A. Effects of the invasive common milkweed (Asclepias syriaca) on nematode communities in natural grasslands. Nematology 2020, 22, 423–438. [Google Scholar] [CrossRef]
- Čerevková, A.; Ivashchenko, K.; Miklisová, D.; Ananyeva, N.; Renčo, M. Influence of invasion by Sosnowsky’s hogweed on nematode communities and microbial activity in forest and grassland ecosystems. Glob. Ecol. Conserv. 2020, 21, e00851. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Homolová, Z. Nematode communities indicate the negative impact of Reynoutria japonica invasion on soil fauna in ruderal habitats of Tatra national park in Slovakia. Glob. Ecol. Conserv. 2021, 26, e01470. [Google Scholar] [CrossRef]
- Lazzaro, L.; Mazza, G.; d’Errico, G.; Fabiani, A.; Giuliani, C.; Inghilesi, A.F.; Lagomarsino, A.; Landi, S.; Lastrucci, L.; Pastorelli, R.; et al. How ecosystems change following invasion by Robinia pseudoacacia: Insights from soil chemical properties and soil microbial, nematode, microarthropod and plant communities. Sci. Total Environ. 2018, 622, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Neher, D.A.; Wu, J.; Barbercheck, M.E.; Anas, O. Ecosystem type affects interpretation of soil nematode community measures. Appl. Soil Ecol. 2005, 30, 47–64. [Google Scholar] [CrossRef]
- Mekonen, S.; Petros, I.; Hailemariam, M. The role of nematodes in the processes of soil ecology and their use as bioindicators. Agric. Biol. J. N. Am. 2017, 8, 132–140. [Google Scholar]
- Renčo, M.; Čerevková, A.; Hlava, J. Life in a contaminated environment: How soil nematodes can indicate long-term heavy-metal pollution. J. Nematol. 2022, 54, 20220053. [Google Scholar] [CrossRef]
- Renčo, M.; Gömöryová, E.; Čerevková, A. Close-to-nature forest management effects on soil nematodes and microbial activity in pine plantations on aeolian sands. Community Ecol. 2024, 25, 337–348. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Ružičková, H.; Banásová, V.; Kalivoda, H. Morava River alluvial meadows on the Slovak–Austrian border (Slovak part): Plant community dynamics, floristic and butterfly diversity–threats and management. J. Nat. Conserv. 2004, 12, 157–169. [Google Scholar] [CrossRef]
- Kubíček, F.; Šimonovič, V.; Kollár, J.; Kanka, R. Herb layer biomass of the Morava River floodplain forests. Ekológia 2008, 27, 23–30. [Google Scholar]
- Petrášová-Šibíková, M.; Bacigál, T.; Jarolímek, I. Fragmentation of hardwood floodplain forests–how does it affect species composition? Community Ecol. 2017, 18, 97–108. [Google Scholar] [CrossRef]
- Brown, A.G.; Harper, D.; Peterken, G.F. European floodplain forests: Structure, functioning and management. Glob. Ecol. Biogeogr. Lett. 1997, 6, 169–178. [Google Scholar] [CrossRef]
- Frymark-Szymkowiak, A.; Kieliszewska-Rokicka, B. The Fine Root Distribution and Morphology of Mature White Poplar in Natural Temperate Riverside Forests under Periodically Flooded or Dry Hydrological Conditions. Forests 2023, 14, 223. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.D.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–325. [Google Scholar] [PubMed]
- Wasilewska, L. Soil invertebrates as bioindicators, with special reference to soil-inhabiting nematodes. Russ. J. Nematol. 1997, 5, 113–126. [Google Scholar]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Wasilewska, L. The effect of age of meadows on succession and diversity in soil nematode communities. Pedobiologia 1994, 38, 1–11. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Heip, C.H.; Herman, P.M.; Soetaert, K. Indices of diversity and evenness. Oceanis 1988, 24, 61–88. [Google Scholar]
- Hrivňakova, K.; Makovnikova, J.; Barančikova, G.; Bezak, P.; Bezakova, Z.; Dodok, R.; Grečo, V.; Chlpik, J.; Kobza, J.; Lištjak, M.; et al. Jednotné Pracovné Postupy Rozborov Pôd; Soil Science and Conservation Research Institute: Bratislava, Slovakia, 2011. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Roversi, P.F.; Nanelli, P. Arthropods and Nematodes: Functional Biodiversity in Forest Ecosystems. In Forest Ecosystems—More than Just Trees; Blanco, J.A., Lo, J.H., Eds.; InTech: Rijeka, Croatia, 2012; pp. 29–52. [Google Scholar]
- Merganič, J.; Merganičová, K.; Marušák, R.; Audolenská, V. Plant diversity of forests. In Forest Ecosystems—More than Just Trees; Blanco, J.A., Lo, J.H., Eds.; InTech: Rijeka, Croatia, 2012; pp. 3–28. [Google Scholar]
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.A.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Veselkin, D.V.; Dubrovin, D.I.; Pustovalova, L.A. High canopy cover of invasive Acer negundo L. affects ground vegetation taxonomic richness. Sci. Rep. 2021, 11, 20758. [Google Scholar] [CrossRef] [PubMed]
- Tsandekova, O.L.; Sheremetova, S.A.; Ufimtsev, V.I.; Khrustaleva, I.A. Changes in Flora Species Composition and Soil Enzyme Activity in Communities of Acer negundo in Kemerovo Region. Russ. J. Biol. Invasions 2023, 14, 658–665. [Google Scholar] [CrossRef]
- Lazarova, S.; Coyne, D.; Rodríguez, M.G.; Peteira, B.; Ciancio, A. Functional diversity of soil nematodes in relation to the impact of agriculture—A review. Diversity 2021, 13, 64. [Google Scholar] [CrossRef]
- Yeates, G.W. Abundance, diversity, and resilience of nematode assemblages in forest soils. Can. J. For. Res. 2007, 37, 216–225. [Google Scholar] [CrossRef]
- Pietsch, K.A.; Ogle, K.; Cornelissen, J.H.C.; Cornwell, W.K.; Banisch, G.; Craine, J.M.; Jackson, B.G.; Kattge, J.; Peltzer, D.A.; Penuelas, J.; et al. Global relationship of wood and leaf litter decomposability: The role of functional traits within and across plant organs. Glob. Ecol. Biogeogr. 2014, 23, 1046–1057. [Google Scholar] [CrossRef]
- Heděnec, P.; Nilsson, L.O.; Zheng, H.; Gundersen, P.; Schmidt, I.K.; Rousk, J.; Vesterdal, L. Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Soil Biol. Biochem. 2020, 149, 107933. [Google Scholar] [CrossRef]
- Cesarz, S.; Ruess, L.; Jacob, M.; Jacob, A.; Schaefer, M.; Scheu, S. Tree species diversity versus tree species identity: Driving forces in structuring forest food webs as indicated by soil nematodes. Soil Biol. Biochem. 2013, 62, 36–45. [Google Scholar] [CrossRef]
- Renčo, M.; Jurová, J.; Čerevková, A. Invasiveness of Impatiens parviflora in Carpathian beech forests: Insights from soil nematode communities. Diversity 2024, 16, 393. [Google Scholar] [CrossRef]
- Čerevková, A.; Miklisová, D.; Bobuľská, L.; Renčo, M. Impact of the invasive plant Solidago gigantea on soil nematodes in a semi-natural grassland and a temperate broadleaved mixed forest. J. Helminthol. 2020, 94, e51. [Google Scholar] [CrossRef]
- Poiras, L. Nematodes in the deciduous forests of Moldova. Bul. Ştiinţific. Rev. Etnogr. Ştiinţele Nat. Şi Muzeol. (Ser. Nouă) 2006, 17, 87–96. [Google Scholar]
- Salamon, J.A.; Wolters, V. Nematoda response to forest conversion. Eur. J. Soil Biol. 2009, 45, 184–191. [Google Scholar] [CrossRef]
- Háněl, L.; Čerevková, A. Species and genera of soil nematodes in forest ecosystems of the Vihorlat Protected Landscape Area, Slovakia. Helminthologia 2010, 47, 123–135. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Gömöryová, E. Soil nematode fauna and microbial characteristics in an early-successional forest ecosystem. Forests 2019, 10, 888. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; Van Ruijven, J.; Berendse, F.; Van Der Putten, W.H. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 2004, 106, 576–586. [Google Scholar] [CrossRef]
- Wohlgemuth, T.; Gossner, M.M.; Campagnaro, T.; Marchante, H.; van Loo, M.; Vacchiano, G.; Castro-Díez, P.; Dobrowolska, D.; Gazda, A.; Keren, S.; et al. Impact of non-native tree species in Europe on soil properties and biodiversity: A review. NeoBiota 2022, 78, 45–69. [Google Scholar] [CrossRef]
- Bakonyi, G.; Nagy, P.; Kovacs-Lang, E.; Kovacs, E.; Barabas, S.; Répasi, V.; Seres, A. Soil nematode community structure as affected by temperature and moisture in a temperate semiarid shrubland. Appl. Soil Ecol. 2007, 37, 31–40. [Google Scholar] [CrossRef]
- Olatunji, O.A.; Gong, S.; Tariq, A.; Pan, K.; Sun, X.; Chen, W.; Zhang, L.; Dakhil, M.A.; Huang, D.; Tan, X. The effect of phosphorus addition, soil moisture, and plant type on soil nematode abundance and community composition. J. Soils Sediments 2019, 19, 1139–1150. [Google Scholar] [CrossRef]
- Lišková, M.; Čerevková, A. Nematode communities of river banks and adjacent meadows in the Slovak Republic. Helminthologia 2005, 42, 223–232. [Google Scholar]
- Liang, W.; Steinberger, Y. Temporal changes in nematode community structure in a desert ecosystem. J. Arid Environ. 2001, 48, 267–280. [Google Scholar] [CrossRef]
- Pen-Mouratov, S.; Rakhimbaev, M.; Steinberger, Y. Seasonal and spatial variation in nematode communities in a Negev Desert ecosystem. J. Nematol. 2003, 35, 157–166. [Google Scholar] [PubMed]
- De Jager, N.R.; Swanson, W.; Strauss, E.A.; Thomsen, M.; Yin, Y. Flood pulse effects on nitrification in a floodplain forest impacted by herbivory, invasion, and restoration. Wetl. Ecol. Manag. 2015, 23, 1067–1081. [Google Scholar] [CrossRef]
- Matthews, J.W.; McIntyre, S.; Peralta, A.L.; Rodgers, C. Long-term assessment of alternative strategies for the restoration of floodplain forest in the presence of an invasive grass. Phalaris arundinacea. Wetlands 2020, 40, 655–665. [Google Scholar] [CrossRef]
 study area in the protected area Záhorie on the Slovak side of the Morava River.
study area in the protected area Záhorie on the Slovak side of the Morava River.
 study area in the protected area Záhorie on the Slovak side of the Morava River.
study area in the protected area Záhorie on the Slovak side of the Morava River.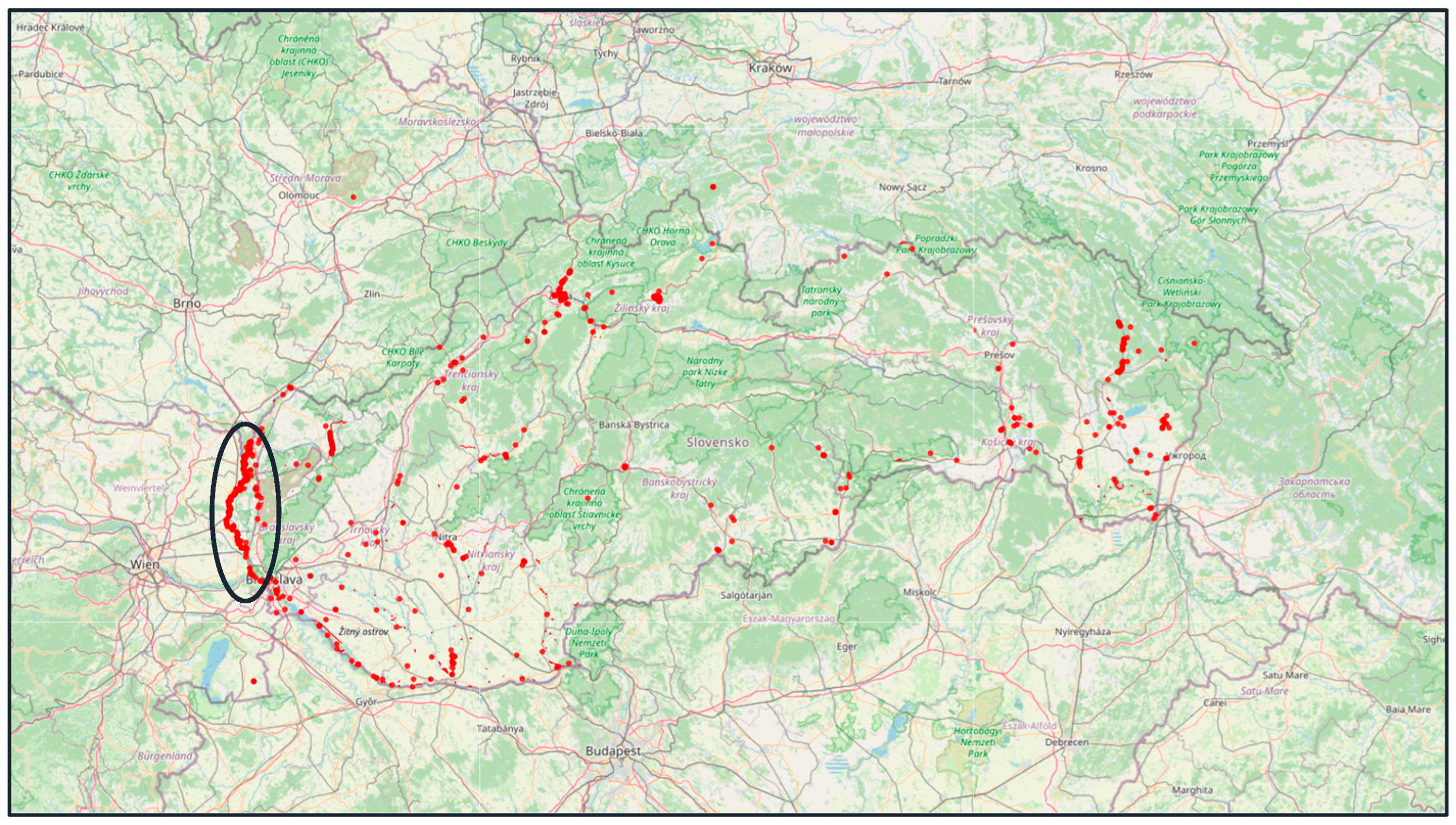
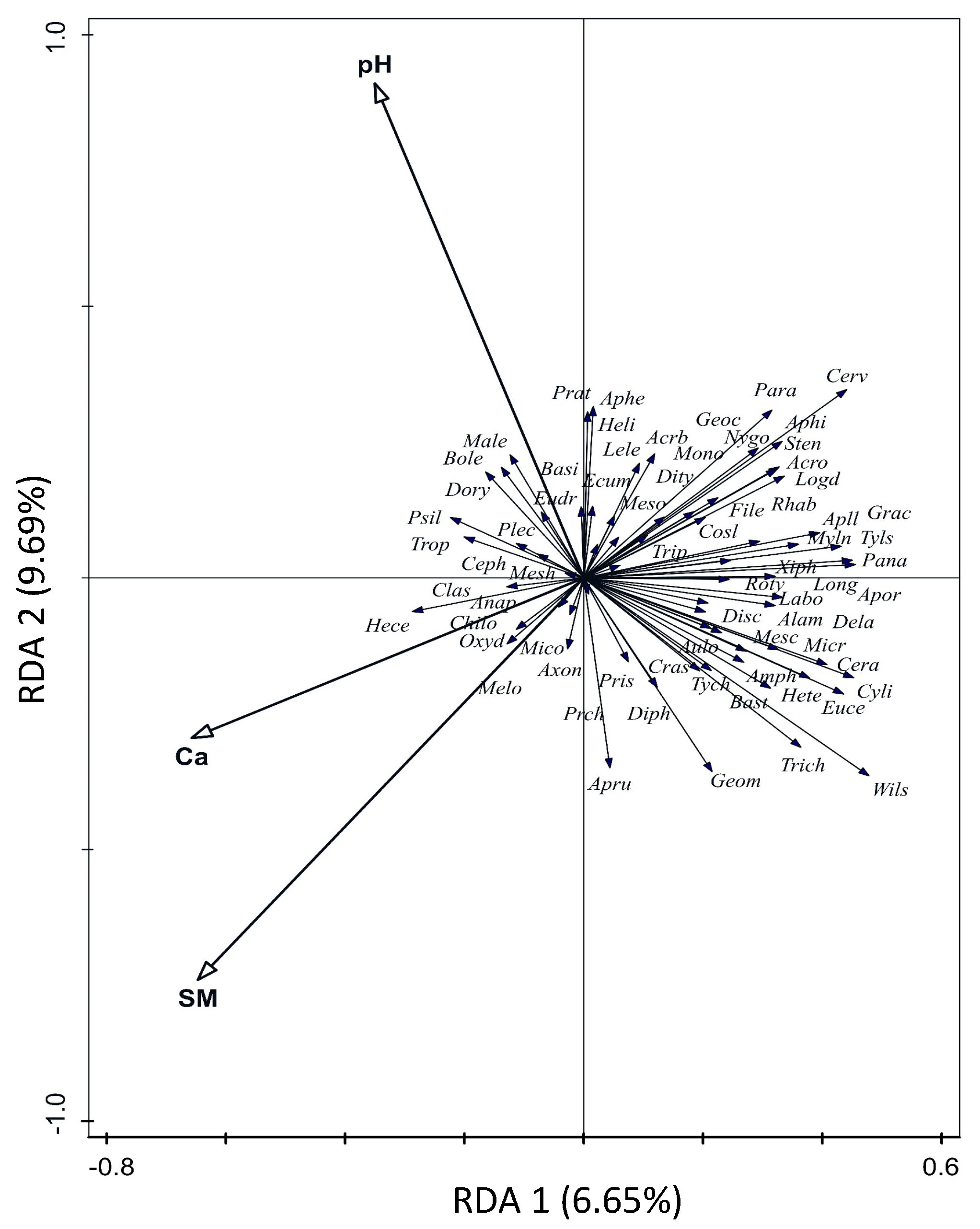


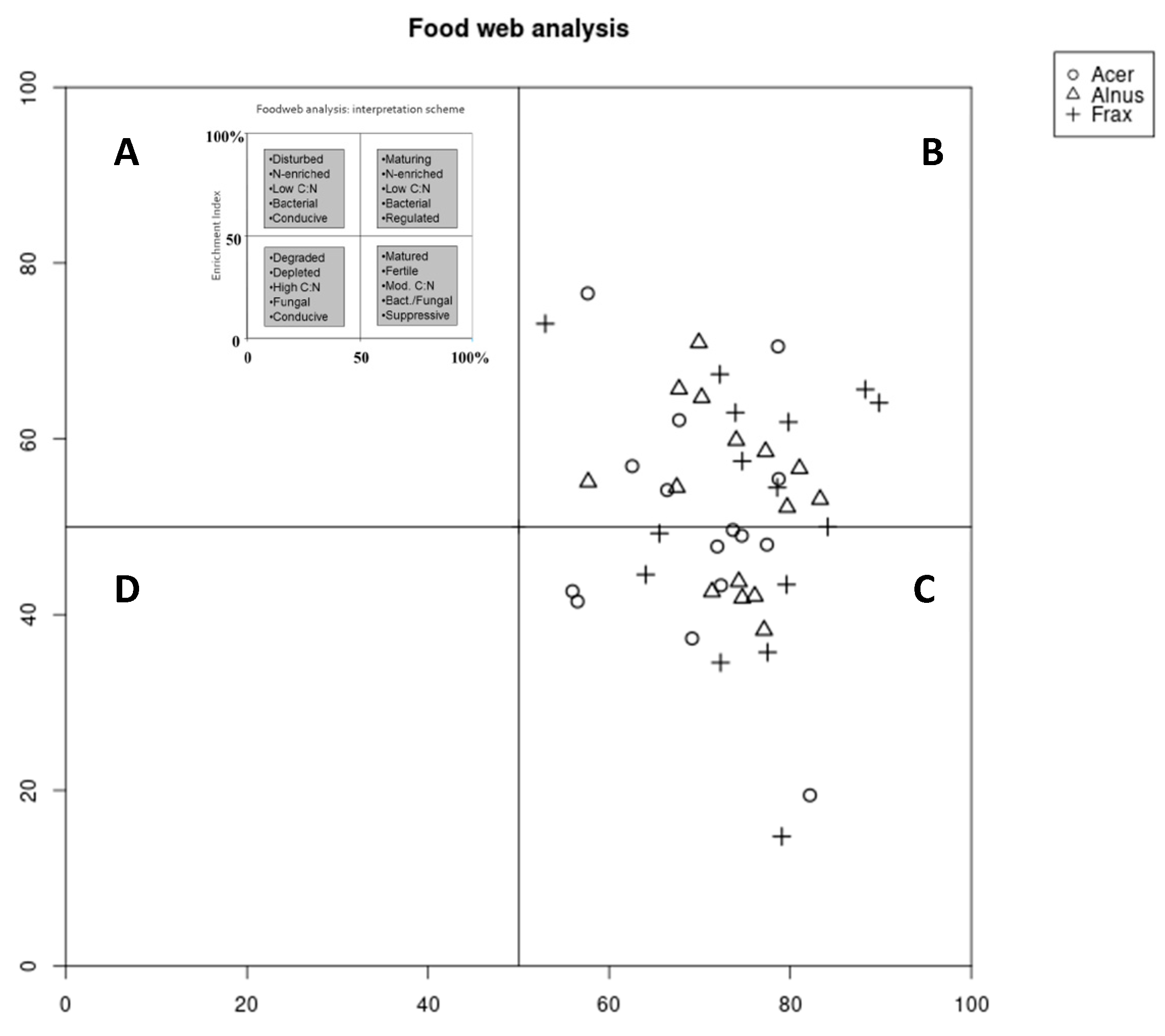
| Parameter | Acer negundo (AN) | Alnus glutinosa (AG) | Fraxinus excelsior (FE) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.D. | min | max | Mean ± S.D. | min | max | Mean ± S.D. | min | max | ||
| pH (H2O) | 7.37 ± 0.42 | 6.41 | 7.81 | 7.11 ± 0.46 | 6.00 | 7.56 | 7.09 ± 0.43 | 5.97 | 7.53 | 0.658 * |
| C (g/kg) | 29.72 ± 9.99 | 9.07 | 43.5 | 35.27 ± 14.84 | 10.40 | 65.35 | 30.36 ± 13.57 | 14.51 | 45.29 | 0.268 |
| N (g/kg) | 2.307 ± 0.96 | 0.68 | 3.85 | 2.822 ± 1.19 | 0.73 | 4.03 | 2.353 ± 0.90 | 1.24 | 3.86 | 0.324 |
| CO32− (g/kg) | 25.952 ± 38.83 | 1.92 | 37.51 | 9.515 ± 8.78 | 0.50 | 22.62 | 16.293 ± 41.43 | 1.50 | 20.47 | 0.097 |
| Corg (g/kg) | 26.608 ± 10.42 | 8.64 | 46.66 | 34.209 ± 15.10 | 10.21 | 65.24 | 28.527 ± 11.15 | 14.59 | 45.01 | 0.287 |
| Mg (mg/kg) | 421.53 ± 155.2 | 128.8 | 681.2 | 430.67 ± 177.5 | 147.0 | 817.5 | 441.80 ± 193.7 | 192.1 | 742.5 | 0.168 |
| Ca (mg/kg) | 3497.93 ± 1074.9 | 1696.1 | 5449.2 | 3093.53 ± 1180.9 | 1330.1 | 4984.2 | 3339.73 ± 1240.9 | 1902.1 | 5401.2 | 0.133 |
| K (mg/kg) | 187.93 ± 44.8 | 130.8 | 273.3 | 199.59 ± 90.8 | 111.1 | 341.8 | 228.01 ± 171.2 | 71.7 | 742.6 | 0.207 |
| P (mg/kg) | 41.283 ± 19.6 | 6.1 | 84.6 | 39.544 ± 25.5 | 11.2 | 99.6 | 40.442 ± 27.6 | 5.83 | 93.7 | 0.387 |
| SM (%) | 26.738 ± 4.5 | 15.7 | 32.4 | 23.192 ± 3.4 | 21.3 | 30.9 | 22.585 ± 3.8 | 16.9 | 26.3 | 0.294 |
| Indices | AN | AG | FE | p-Value |
|---|---|---|---|---|
| Nematode abundance | 1646.1 ± 1027.9 a | 1354.9 ± 1127.6 a | 1608.1 ± 997.2 a | 0.127 |
| Genera richness (S) | 33.5 ± 7.7 a | 36.7 ± 8.1 a | 36.6 ± 7.3 a | 0.369 |
| Maturity index (MI) | 2.68 ± 0.34 a | 2.72 ± 0.23 a | 2.82 ± 0.28 a | 0.087 |
| Shannon–Weaver diversity index (H′) | 3.03 ± 0.29 a | 3.31 ± 0.25 a | 3.19 ± 0.15 a | 0.455 |
| Pielou evenness index (J′) | 0.82 ± 0.02 a | 0.92 ± 0.05 a | 0.89 ± 0.03 a | 0.241 |
| Margalef index (SR) | 4.51 ± 0.73 a | 5.22 ± 0.82 a | 5.05 ± 0.84 a | 0.493 |
| Trophic diversity index (TD) | 17.69 ± 5.99 a | 19.53 ± 5.15 a | 18.92 ± 4.41 a | 0.697 |
| Nematode channel ratio (NCR) | 0.80 ± 0.13 a | 0.79 ± 0.12 a | 0.81 ± 0.07 a | 0.875 |
| Wasilewska index (WI) | 1.25 ± 0.52 a | 3.52 ± 0.27 b | 1.12 ± 0.61 a | 0.038 |
| Family | Genera | AN (n = 15) | AG (n = 15) | FA (n = 15) |
|---|---|---|---|---|
| Alaimidae | Alaimus, Apmhidelus | 56.7 ± 46.40 | 43.9 ± 45.3 | 62.9 ± 52.5 |
| Anatonchidae | Anatonchus, Miconchus | 6.0 ± 11.3 | 4.6 ± 8.4 | 8.4 ± 11.6 |
| Anguinidae | Ditylenchus, Nothotylenchus | 27.6 ± 42.1 | 21.8 ± 30.1 | 27.4 ± 57.7 |
| Aphanolaimidae | Aphanolaimus | 1.1 ± 3.5 | 1.3 ± 3.9 | - |
| Aphelenchidae | Aphelenchus | 70.1 ± 26.4 | 19.6 ± 24.4 | 35.5 ± 42.7 |
| Aphelenchoididae | Aphelenchoides | 25.8 ± 42.2 | 17.7 ± 41.2 | 23.3 ± 22.6 |
| Aporcelaimidae | Aporcelaimellus, Aporcelaimus, Paraxonchium | 43.1 ± 30.8 | 61.2 ± 64.1 | 53.5 ± 24.8 |
| Aulolaimidae | Aulolaimus | 11.5 ± 8.6 | 12.8 ± 2.3 | 22.7 ± 6.9 |
| Bastianiidae | Bastiania | 13.0 ± 7.5 | 2.6 ± 5.5 | 15.8 ± 3.7 |
| Belondiridae | Axonchium, Oxydirus | 54.1 ± 107.2 | 19.3 ± 26.4 | 21.9 ± 44.3 |
| Boleodoridae | Basiria, Boleodorus | 16.9 ± 25.5 | 8.8 ± 19.6 | 8.8 ± 12.8 |
| Cephalobidae | Acrobeles, Acrobeloides, Cephalobus, Cervidellus, Eucephalobus, Heterocephalobus, Chiloplacus | 262.1 ± 169.8 | 304.8 ± 187.5 | 215.9 ± 95.8 |
| Criconematidae | Criconema, Mesocriconema, Ogma | 16.1 ± 5.7 | 13.4 ± 22.3 | 26.3 ± 8.2 |
| Cylindrolaimidae | Cylindrolaimus | 5.1 ± 1.2 | 9.2 ± 7.7 | 6.7 ± 3.9 |
| Diphtherophoridae | Diphtherophora | 27.7 ± 34.6 | 5.3 ± 2.8 | 14.1 ± 10.8 |
| Diplogasteridae | Allodiplogaster | - | 1.4 ± 0.7 | 1.3 ± 1.0 |
| Dorylaimidae | Labronema, Mesodorylaimus, Prodorylaimus | 33.9 ± 45.9 | 13.6 ± 18.8 | 20.2 ± 11.7 |
| Ecphyadophoridae | Ecphyadophora, Lelenchus | 2.0 ± 4.5 | 1.4 ± 3.0 | 2.0 ± 2.5 |
| Hemicycliophoridae | Hemicycliophora | - | 4.9 ± 10.2 | 9.5 ± 4.3 |
| Heteroderidae | Heterodera | 3.3 ± 5.6 | 4.5 ± 5.8 | 19.9 ± 6.3 |
| Hoplolaimidae | Helicotylenchus, Pratylenchoides, Rotylenchus | 126.1 ± 110.1 | 113.1 ± 58.8 | 110.1 ± 69.4 |
| Leptonchidae | Tylencholaimellus | 1.3 ± 0.5 | 3.2 ± 5.2 | 7.7 ± 10.2 |
| Longidoridae | Longidorus, Paralongidorus | 25.5 ± 40.1 | 31.2 ± 25.4 | 32.5 ± 15.6 |
| Meloidogynidae | Meloidogyne | 0.5 ± 1.8 | 1.7 ± 2.3 | 4.3 ± 9.8 |
| Mesorhabditidae | Mesorhabditis | 13.2 ± 10.2 | 16.8 ± 24.1 | 12.7 ± 20.9 |
| Metateratocephalidae | Euteratocephalus | - | - | 1.0 ± 1.5 |
| Microlaimidae | Prodesmodora | 2.5 ± 7.6 | - | 2.8 ± 2.0 |
| Monhysteridae | Geomonhystera, Monhystrella | 5.9 ± 17.7 | 7.7 ± 12.4 | 9.9 ± 1.3 |
| Mononchidae | Clarkus, Mononchus | 17.7 ± 10.8 | 13.7 ± 24.4 | 16.3 ± 15.1 |
| Mydonomidae | Dorylaimoides | 17.6 ± 21.5 | 3.7 ± 6.2 | 13.3 ± 19.9 |
| Mylonchulidae | Mylonchulus | 26.6 ± 11.2 | 32.2 ± 21.4 | 30.1 ± 30.5 |
| Neotylenchidae | Deladenus, Hexatylus | 2.3 ± 8.9 | 2.1 ± 4.2 | 1.6 ± 5.7 |
| Nordiidae | Longidorella | 12.8 ± 9.2 | 18.0 ± 23.4 | 9.6 ± 15.4 |
| Nygolaimidae | Nygolaimus | 3.3 ± 6.7 | 4.5 ± 9.3 | 7.5 ± 14.7 |
| Odontolaimidae | Odontolaimus | - | 2.5 ± 5.3 | 0.6 ± 2.1 |
| Panagrolaimidae | Panagrolaimus | 4.0 ± 8.9 | 17.9 ± 10.5 | 2.3 ± 6.2 |
| Paraphelenchidae | Paraphelenchus | 3.3 ± 2.5 | 3.1 ± 5.8 | 2.0 ± 6.7 |
| Paratylenchidae | Gracilacus, Paratylenchus | 44.7 ± 72.5 | 54.4 ± 35.5 | 63.5 ± 77.7 |
| Plectidae | Anaplectus, Ceratoplectus, Ereptonema, Plectus, Wilsonema | 21.7 ± 45.6 | 12.9 ± 25.3 | 14.2 ± 23.4 |
| Pratylenchidae | Pratylenchus | 58.8 ± 61.2 | 21.9 ± 15.2 | 20.6 ± 30.7 |
| Prismatolaimidae | Prismatolaimus | 16.0 ± 15.2 | 5.6 ± 10.6 | 18.7 ± 10.9 |
| Qudsianematidae | Crassolabium, Discolaimus, Ecumenicus, Epidorylaimus, Eudorylaimus, Microdorylaimus | 10.4 ± 22.1 | 41.4 ± 26.1 | 62.6 ± 23.5 |
| Rhabiditdae | Rhabditis | 45.5 ± 26.9 | 57.9 ± 50.1 | 71.2 ± 56.3 |
| Rhabdolaimidae | Rhabdolaimus | - | - | 3.3 ± 2.1 |
| Seinuridae | Aprutides, Seinura | 2.1 ± 3.5 | 2.5 ± 5.4 | 3.4 ± 11.4 |
| Steinernematidae | Steinernema | 10.9 ± 5.3 | 9.8 ± 18.6 | 12.7 ± 19.1 |
| Telotylenchidae | Amplimerlinius, Bitylenchus, Geocenamus, Trophurus | 10.8 ± 21.5 | 5.3 ± 15.2 | 8.3 ± 9.9 |
| Trichodoridae | Paratrichodorus, Trichodorus | 7.8 ± 21.5 | 4.9 ± 14.5 | 9.1 ± 11.9 |
| Tripylidae | Tripyla | 2.2 ± 5.9 | 1.2 ± 3.2 | 5.5 ± 9.3 |
| Tylenchidae | Coslenchus, Filenchus, Malenchus, Psilenchus, Tylenchus | 45.5 ± 24.8 | 34.2 ± 59.9 | 51.7 ± 79.1 |
| Tylencholaimidae | Tylencholaimus | 8.8 ± 15.9 | 16.1 ± 25.1 | 2.4 ± 6.1 |
| Xiphinematidae | Xiphinema | 7.6 ± 18.1 | 6.7 ± 10.2 | 20.2 ± 39.9 |
| Trophic Group/Genus | Acer negundo (AN) | Alnus glutinosa (AG) | Fraxinus excelsior (FE) | ||||
|---|---|---|---|---|---|---|---|
| Bacterivores | c-p | A | D% | A | D% | A | D% |
| Acrobeles | 2 | 920.3 (61.2) | 3.42 | 617.4 (41.2) | 2.34 | 744.8 (49.7) | 3.21 |
| Acrobeloides | 2 | 1009.2 (67.4) | 4.19 | 722.9 (48.2) | 3.71 | 978.3 (65.2) | 3.99 |
| Alaimus | 4 | 754.0 (50.3) | 3.28 | 597.9 (39.9) | 2.53 | 833.9 (55.6) | 3.77 |
| Allodiplogaster | 3 | - | - | 22.2 (1.5) | 0.04 | 19.1 (1.3) | 0.05 |
| Amphidelus | 4 | 96.9 (6.5) | 0.53 | 61.6 (4.1) | 0.36 | 109.5 (7.3) | 0.49 |
| Anaplectus | 2 | 248.0 (16.5) | 0.85 | 150.5 (10.1) | 1.01 | 223.3 (14.9) | 0.78 |
| Aphanolaimus | 3 | 17.0 (1.1) | 0.11 | 19.6 (1.3) | 0.12 | - | - |
| Aulolaimus | 3 | 173.1 (11.5) | 0.55 | 193.0 (12.9) | 0.50 | 340.0 (22.7) | 1.33 |
| Bastiania | 3 | 194.5 (12.8) | 0.61 | 39.8 (2.7) | 0.16 | 236.8 (15.8) | 0.87 |
| Cephalobus | 2 | 643.9 (42.9) | 1.93 | 527.9 (35.2) | 2.62 | 356.2 (23.8) | 1.99 |
| Ceratoplectus | 2 | 53.9 (3.4) | 0.12 | 93.9 (6.3) | 0.38 | 100.6 (6.7) | 0.40 |
| Cervidellus | 2 | 305.8 (20.9) | 1.14 | 538.4 (35.9) | 1.65 | 154.1 (10.3) | 0.70 |
| Cylindrolaimus | 3 | 75.9 (5.0) | 0.18 | 137.6 (9.2) | 0.36 | 77.5 (5.2) | 0.29 |
| Ereptonema | 3 | 34.4 (2.3) | 0.07 | 39.7 (2.6) | 0.11 | 25.1 (1.7) | 0.04 |
| Eucephalobus | 2 | 809.9 (53.9) | 4.70 | 1279.9 (85.1) | 6.28 | 906.1 (60.4) | 3.88 |
| Euteratocephalus | 2 | - | - | - | - | 15.1 (1.0) | 0.11 |
| Geomonhystera | 2 | 77.8 (5.2) | 0.17 | 113.8 (7.7) | 0.72 | 149.2 (10.0) | 0.53 |
| Heterocephalobus | 2 | 177.4 (11.8) | 0.88 | 79.3 (5.3) | 0.63 | 54.3 (3.6) | 0.41 |
| Chiloplacus | 2 | 65.1 (4.4) | 0.28 | 200.9 (13.4) | 1.12 | 34.2 (2.3) | 0.15 |
| Mesorhabditis | 1 | 198.2 (13.2) | 0.84 | 252.2 (16.8) | 1.09 | 185.4 (12.4) | 0.67 |
| Monhystrella | 2 | 11.4 (0.8) | 0.04 | - | - | - | - |
| Odontolaimus | 3 | - | - | 35.5 (2.4) | 0.16 | 8.6 (0.6) | 0.05 |
| Panagrolaimus | 1 | 56.0 (3.7) | 0.16 | 251.2 (16.8) | 1.19 | 32.8 (2.2) | 0.10 |
| Plectus | 2 | 1212.5 (80.8) | 5.89 | 561.5 (34.7) | 3.28 | 553.2 (36.9) | 2.52 |
| Prismatolaimus | 3 | 223.6 (14.9) | 0.71 | 78.5 (78.5) | 0.55 | 282.6 (18.8) | 1.05 |
| Prodesmodora | 3 | 37.9 (2.5) | 0.14 | - | - | 42.2 (2.8) | 0.09 |
| Rhabditis | 1 | 744.0 (49.9) | 5.18 | 887.2 (59.2) | 4.89 | 1021.8 (68.2) | 4.66 |
| Rhabdolaimus | 3 | - | - | 2.4 (0.2) | 0.04 | 46.8 (3.1) | 0.13 |
| Wilsonema | 2 | 78.0 (5.2) | 0.29 | 120.5 (8.1) | 0.64 | 163.6 (10.9) | 0.68 |
| Fungivores | |||||||
| Aphelenchoides | 2 | 587.0 (25.8) | 1.13 | 265.3 (17.7) | 1.19 | 349.1 (23.4) | 1.36 |
| Aphelenchus | 2 | 1053.5 (70.2) | 4.16 | 290.4 (19.4) | 2.23 | 532.5 (35.5) | 2.38 |
| Aprutides | 2 | - | - | 57.6 (3.8) | 0.33 | 101.7 (6.8) | 0.48 |
| Deladenus | 2 | 34.4 (2.3) | 0.07 | 9.2 (0.6) | 0.06 | 23.6 (1.6) | 0.09 |
| Diphtherophora | 3 | 415.0 (27.7) | 1.54 | 375.5 (25.1) | 1.97 | 211.1 (14.1) | 1.21 |
| Ditylenchus | 2 | 414.1 (27.6) | 1.38 | 258.1 (17.2) | 2.03 | 399.4 (26.6) | 1.35 |
| Ecphyadophora | 2 | - | - | - | - | 10.2 (0.7) | 0.05 |
| Hexatylus | 2 | - | - | 20.9 (1.4) | 0.10 | - | - |
| Nothotylenchus | 2 | - | - | 69.0 (4.6) | 0.22 | 11.8 (0.8) | 0.05 |
| Paraphelenchus | 2 | 47.2 (3.1) | 0.14 | 44.3 (3.0) | 0.09 | 27.3 (1.8) | 0.09 |
| Tylencholaimellus | 4 | 19.5 (1.3) | 0.05 | 45.6 (3.1) | 0.27 | 115.3 (7.6) | 0.61 |
| Tylencholaimus | 4 | 123.4 (8.2) | 0.34 | 256.2 (17.1) | 1.22 | 34.0 (2.7) | 0.32 |
| Plant parasites | |||||||
| Amplimerlinius | 3 | 37.2 (2.5) | 0.10 | - | - | - | - |
| Basiria | 2 | 13.0 (0.9) | 0.05 | 44.6 (3.0) | 0.33 | 74.4 (4.5) | 0.42 |
| Bitylenchus | 3 | 21.5 (1.5) | 0.11 | 22.2 (1.5) | 0.04 | - | - |
| Boleodorus (f) | 2 | 240.6 (16.1) | 1.26 | 87.9 (5.9) | 0.24 | 58.4 (3.9) | 0.26 |
| Coslenchus (f) | 2 | 317.5 (21.2) | 1.20 | 362.1 (24.1) | 1.41 | 256.1 (17.1) | 1.32 |
| Criconema | 3 | 14.5 (1.0) | 0.05 | 7.8 (0.5) | 0.05 | 41.3 (2.8) | 0.21 |
| Filenchus (f) | 2 | 1515.4 (101.1) | 5.62 | 997.8 (65.5) | 4.56 | 1394.3 (92.8) | 5.46 |
| Geocenamus | 3 | 405.1 (27.1) | 3.08 | 283.6 (18.9) | 1.78 | 402.0 (26.8) | 1.48 |
| Gracilacus | 2 | 239.9 (16.2) | 0.60 | 444.9 (29.7) | 2.62 | 1240.8 (82.7) | 4.30 |
| Helicotylenchus | 3 | 1546.1 (103.8) | 6.56 | 1280.5 (85.4) | 6.89 | 1200.4 (80.1) | 4.83 |
| Hemicycliophora | 3 | - | - | 72.8 (4.8) | 0.31 | 136.2 (9.1) | 0.38 |
| Heterodera | 3 | 49.4 (3.3) | 0.36 | 67.2 (4.7) | 0.26 | 298.6 (19.9) | 1.02 |
| Lelenchus (f) | 2 | 29.8 (2.0) | 0.30 | 21.1 (1.4) | 0.14 | 19.5 (1.3) | 0.10 |
| Longidorella | 4 | 190.3 (12.9) | 0.59 | 343.4 (22.9) | 1.26 | 153.8 (10.3) | 0.94 |
| Longidorus | 5 | 378.7 (25.5) | 1.19 | 470.7 (31.4) | 2.20 | 473.7 (31.6) | 1.42 |
| Malenchus (f) | 2 | 1287.8 (85.9) | 6.23 | 1067.3 (71.5) | 6.35 | 1778.6 (118.5) | 8.32 |
| Meloidogyne | 3 | 7.1 (0.4) | 0.05 | 28.1 (1.9) | 0.28 | 64.2 (4.3) | 0.69 |
| Mesocriconema | 3 | 228.0 (15.2) | 0.79 | 200.5 (13.4) | 0.82 | 340.6 (22.7) | 1.13 |
| Ogma | 3 | - | - | - | - | 13.1 (0.9) | 0.05 |
| Paralongidorus | 5 | - | - | - | - | 18.7 (1.3) | 0.10 |
| Paratrichodorus | 4 | - | - | 22.2 (1.5) | 0.04 | 88.7 (5.9) | 0.39 |
| Paratylenchus | 2 | 1100.1 (73.3) | 4.58 | 288.2 (19.2) | 0.96 | 655.7 (43.7) | 2.86 |
| Pratylenchoides | 3 | 118.3 (7.9) | 0.34 | 85.3 (5.7) | 0.42 | 266.0 (17.7) | 0.79 |
| Pratylenchus | 3 | 824.4 (54.9) | 2.17 | 399.3 (26.6) | 1.66 | 289.0 (19.3) | 0.95 |
| Psilenchus (f) | 2 | 130.1 (8.8) | 0.41 | 56.7 (3.7) | 0.44 | 185.4 (12.4) | 0.83 |
| Rotylenchus | 3 | 226.0 (15.7) | 0.67 | 338.9 (22.6) | 1.35 | 85.4 (24.5) | 0.50 |
| Trichodorus | 4 | 235.2 (11.8) | 1.18 | 124.9 (8.3) | 0.37 | 104.6 (15.6) | 0.55 |
| Trophurus | 3 | 186.4 (12.4) | 0.41 | 2.2 (0.2) | 0.13 | 95.7 (6.4) | 0.44 |
| Tylenchus (f) | 2 | 126.1 (8.4) | 0.39 | 75.0 (5.0) | 0.49 | 242.6 (16.2) | 0.67 |
| Xiphinema | 5 | 106.6 (7.1) | 0.35 | 124.5 (8.3) | 0.60 | 292.7 (19.1) | 1.18 |
| Omnivores | |||||||
| Aporcelaimellus | 5 | 492.9 (32.9) | 1.94 | 616.1 (41.7) | 2.74 | 611.2 (25.5) | 2.14 |
| Aporcelaimus | 5 | 146.0 (9.7) | 0.56 | 199.9 (13.3) | 0.88 | 187.9 (10.2) | 0.87 |
| Axonchium | 5 | 355.2 (23.6) | 0.87 | 74.1 (4.5) | 0.44 | 67.4 (9.9) | 0.25 |
| Crassolabium | 4 | 365.6 (24.7) | 1.39 | 379.7 (25.3) | 1.63 | 354.9 (20.4) | 1.70 |
| Dorylaimoides | 4 | 264.0 (17.6) | 1.16 | 49.1 (3.3) | 0.32 | 196.5 (21.7) | 0.79 |
| Ecumenicus | 5 | 67.3 (4.5) | 0.30 | 44.8 (3.0) | 0.50 | 50.9 (5.5) | 0.38 |
| Epidorylaimus | 4 | 15.3 (1.1) | 0.04 | 29.9 (2.0) | 0.09 | 25.1 (6.9) | 0.04 |
| Eudorylaimus | 4 | 301.5 (20.1) | 1.01 | 210.8 (14.1) | 1.07 | 247.6 (15.1) | 1.42 |
| Labronema | 4 | 17.5 (1.2) | 0.18 | 65.7 (4.4) | 0.26 | 30.4 (2.5) | 0.15 |
| Mesodorylaimus | 4 | 455.9 (30.4) | 1.23 | 206.4 (13.7) | 0.99 | 255.9 (26.3) | 1.06 |
| Microdorylaimus | 4 | 352.1 (23.5) | 1.56 | 429.1 (128.5) | 1.68 | 543.5 (15.8) | 2.18 |
| Oxydirus | 5 | 455.6 (15.4) | 1.29 | 216.0 (14.4) | 1.40 | 261.5 (30.1) | 0.92 |
| Paraxonchium | 5 | 6.9 (0.5) | 0.08 | 101.9 (6.7) | 0.51 | 3.3 (0.4) | 0.07 |
| Prodorylaimus | 4 | 47.7 (3.2) | 0.16 | - | - | ||
| Predators | |||||||
| Anatonchus | 4 | 62.8 (4.2) | 0.16 | 13.2 (0.9) | 0.05 | - | - |
| Clarkus | 4 | 213.5 (14.5) | 0.82 | 151.5 (10.1) | 0.73 | 116.9 (10.5) | 0.49 |
| Discolaimus | 5 | 7.1 (0.5) | 0.05 | 59.4 (4.0) | 0.25 | 89.8 (11.1) | 0.37 |
| Miconchus | 4 | 27.6 (1.8) | 0.29 | 49.0 (3.3) | 0.28 | 27.0 (6.9) | 0.26 |
| Mononchus | 4 | 56.2 (3.8) | 0.19 | 54.3 (3.6) | 0.28 | 77.1 (5.6) | 0.43 |
| Mylonchulus | 4 | 398.4 (26.5) | 1.48 | 485.2 (32.3) | 2.48 | 462.1 (25.3) | 2.03 |
| Nygolaimus | 5 | 46.5 (3.1) | 0.14 | 78.5 (5.2) | 0.37 | 105.5 (2.3) | 0.32 |
| Seinura | 2 | 62.7 (4.2) | 0.13 | 18.5 (1.2) | 0.11 | - | - |
| Tripyla | 3 | 31.3 (2.1) | 0.37 | 17.6 (1.9) | 0.09 | 76.4 (7.8) | 0.29 |
| Insect parasite | |||||||
| Steinernema | 152.7 (10.2) | 0.89 | 136.6 (15.5) | 0.60 | 171.8 (6.3) | 0.6 | |
| AN | AG | FE | p-Value | |
|---|---|---|---|---|
| Bacterivores | 547.1 ± 324.1 a | 508.5 ± 425.1 a | 513.1 ± 328.5 a | 0.236 |
| Ba1 | 22.2 ± 28.9 a | 30.9 ± 42.2 a | 27.6 ± 44.8 a | 0.125 |
| Ba2 | 26.7 ± 46.1 a | 23.8 ± 30.4 a | 21.1 ± 43.6 a | 0.340 |
| Ba3 | 5.0 ± 13.2 a | 3.8 ± 10.6 a | 7.2 ± 19.4 a | 0.154 |
| Ba4 | 28.4 ± 38.5 a | 22.0 ± 35.7 a | 31.5 ± 42.7 a | 0.369 |
| Predators | 60.40 ± 49.9 a | 61.5 ± 62.7 a | 73.6 ± 65.2 a | 0.247 |
| P2 | 4.2 ± 6.7 a | 1.2 ± 11.8 a | - | 0.239 |
| P3 | 2.1 ± 5.5 a | 1.7 ± 3.1 a | 8.1 ± 1.2 b | 0.041 |
| P4 | 10.1 ± 4.0 a | 12.0 ± 5.4 a | 11.1 ± 2.6 a | 0.687 |
| P5 | 1.8 ± 4.7 a | 3.3 ± 4.2 a | 7.0 ± 12.9 a | 0.183 |
| Fungivores | 166.2 ± 145.6 a | 112.8 ± 98.7 a | 121.1 ± 127.4 a | 0.237 |
| Fu2 | 14.3 ± 37.8 a | 7.5 ± 19.9 a | 10.8 ± 28.9 a | 0.269 |
| Fu3 | 27.7 ± 34.6 a | 25.0 ± 32.8 a | 14.0 ± 22.8 a | 0.188 |
| Fu4 | 5.1 ± 11.7 a | 9.7 ± 19.3 a | 5.3 ± 11.1 a | 0.214 |
| Omnivores | 222.8 ± 112.1 a | 174.2 ± 157.1 a | 192.3 ± 129.1 a | 0.255 |
| Om4 | 15.2 ± 27.1 a | 11.4 ± 25.9 a | 14.2 ± 23.8 a | 0.547 |
| Om5 | 16.9 ± 38.3 a | 13.2 ± 26.4 a | 13.1 ± 27.6 a | 0.473 |
| Plant parasites | 638.3 ± 423.1 a | 487.4 ± 258.3 a | 696.7 ± 464.9 a | 0.164 |
| Pp2 | 33.3 ± 59.6 a | 23.0 ± 45.5 a | 39.4 ± 69.9 a | 0.234 |
| Pp3 | 17.5 ± 45.5 a | 13.3 ± 41.2 a | 15.9 ± 42.2 a | 0.277 |
| Pp4 | 9.5 ± 23.5 a | 10.9 ± 25.7 a | 9.5 ± 23.2 a | 0.754 |
| Pp5 | 10.8 ± 26.9 a | 13.2 ± 32.9 a | 17.5 ± 44.4 a | 0.269 |
| Insect parasites | 10.2 ± 5.2 a | 9.1 ± 10.8 a | 5.5 ± 6.6 a |
| Explained Variability | pseudoF | p | ||
|---|---|---|---|---|
| Nematode genera composition | ||||
| Acer negundo | 2.8 | 1.2 | 0.184 | + |
| Alnus glutinosa | 2.2 | 1.0 | 0.345 | + |
| Fraxinus excelsior | 1.8 | 1.5 | 0.445 | + |
| Phosphorus | 1.9 | 0.9 | 0.646 | + |
| Soil moisture | 3.6 | 1.6 | 0.034 | + |
| Potassium | 1.5 | 0.7 | 0.886 | + |
| Calcium | 3.6 | 1.7 | 0.022 | + |
| Magnesium | 2.6 | 1.2 | 0.569 | + |
| Corg | 2.2 | 1.0 | 0.382 | + |
| pH | 4.5 | 2.0 | 0.006 | + |
| Nitrogen | 1.9 | 0.9 | 0.648 | + |
| Carbon | 0.7 | 0.3 | 0.338 | + |
| Nematode trophic structure | ||||
| Acer negundo | 1.2 | 0.7 | 0.957 | + |
| Alnus glutinosa | 1.0 | 0.5 | 0.746 | + |
| Fraxinus excelsior | 0.7 | 0.3 | 0.908 | + |
| Phosphorus | 3.92 | 1.9 | 0.132 | + |
| Soil moisture | 9.15 | 4.3 | 0.020 | + |
| Potassium | 2.30 | 1.1 | 0.785 | + |
| Calcium | 1.41 | 0.7 | 0.506 | + |
| Magnesium | 0.84 | 0.4 | 0.758 | + |
| Corg | 0.28 | 0.4 | 0.966 | + |
| pH | 1.03 | 0.5 | 0.684 | + |
| Nitrogen | 0.98 | 0.4 | 0.715 | + |
| Carbon | 0.51 | 0.2 | 0.866 | + |
| Nematode functional guilds | ||||
| Acer negundo | 0.9 | 0.4 | 0.962 | + |
| Alnus glutinosa | 1.8 | 0.8 | 0.546 | + |
| Fraxinus excelsior | 1.5 | 0.8 | 0.642 | + |
| Phosphorus | 6.9 | 3.2 | 0.006 | + |
| Soil moisture | 4.9 | 2.4 | 0.039 | + |
| Potassium | 2.8 | 1.4 | 0.182 | + |
| Calcium | 3.1 | 1.5 | 0.144 | + |
| Magnesium | 2.0 | 1.0 | 0.396 | + |
| Corg | 1.1 | 0.5 | 0.864 | + |
| pH | 1.2 | 0.6 | 0.792 | + |
| Nitrogen | 1.1 | 0.5 | 0.890 | + |
| Carbon | 0.8 | 0.4 | 0.964 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renčo, M.; Čerevková, A.; Gömöryová, E. Floodplain Forest Soil Nematode Communities as Influenced by Non-Native Acer negundo L. Invasion. Diversity 2025, 17, 376. https://doi.org/10.3390/d17060376
Renčo M, Čerevková A, Gömöryová E. Floodplain Forest Soil Nematode Communities as Influenced by Non-Native Acer negundo L. Invasion. Diversity. 2025; 17(6):376. https://doi.org/10.3390/d17060376
Chicago/Turabian StyleRenčo, Marek, Andrea Čerevková, and Erika Gömöryová. 2025. "Floodplain Forest Soil Nematode Communities as Influenced by Non-Native Acer negundo L. Invasion" Diversity 17, no. 6: 376. https://doi.org/10.3390/d17060376
APA StyleRenčo, M., Čerevková, A., & Gömöryová, E. (2025). Floodplain Forest Soil Nematode Communities as Influenced by Non-Native Acer negundo L. Invasion. Diversity, 17(6), 376. https://doi.org/10.3390/d17060376






