The Diversity and Phylogenetic Relationships of a Chaetopterus Symbiont Community in Djibouti, with Redescription of Chaetopterus djiboutiensis Gravier, 1906 Stat. Nov. (Annelida: Chaetopteridae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Analyses
2.3. Molecular Analyses
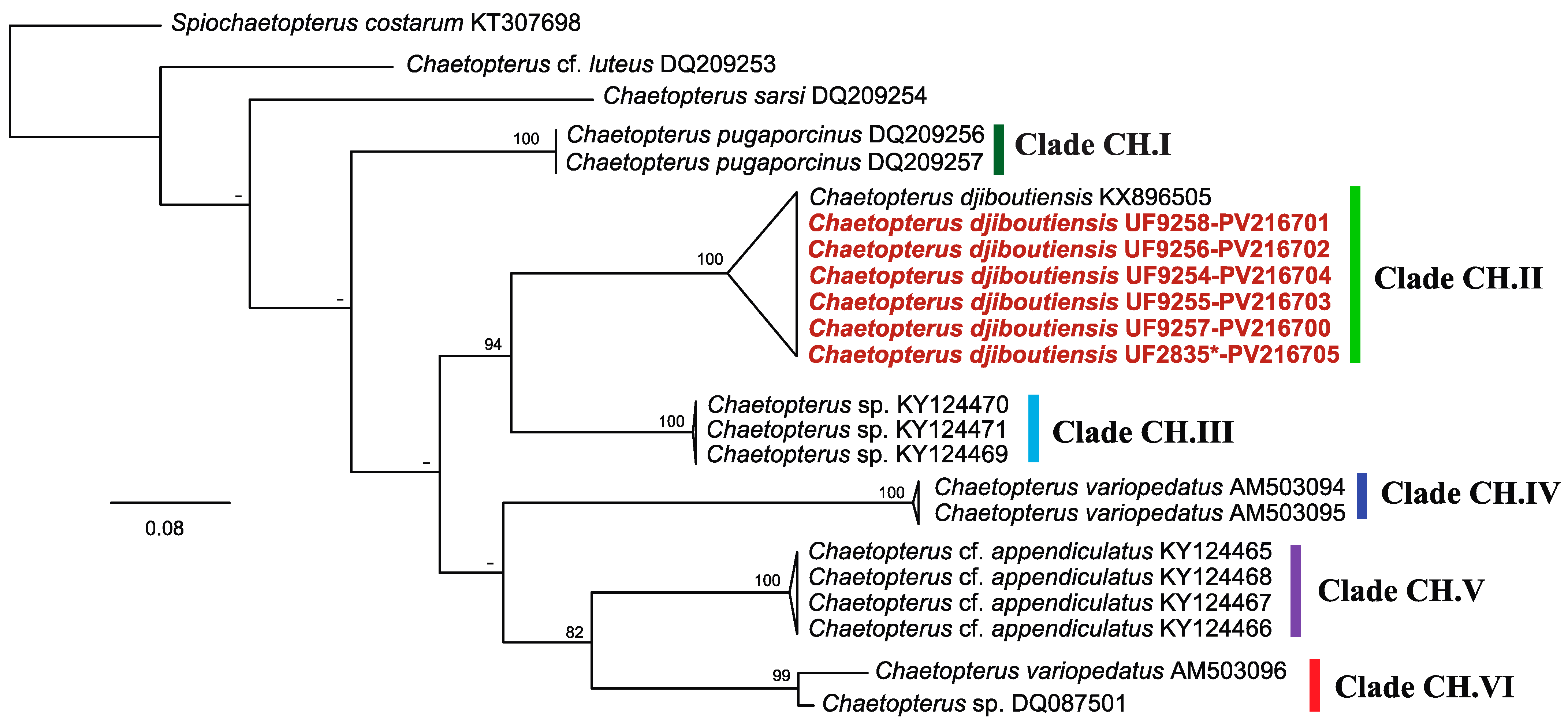
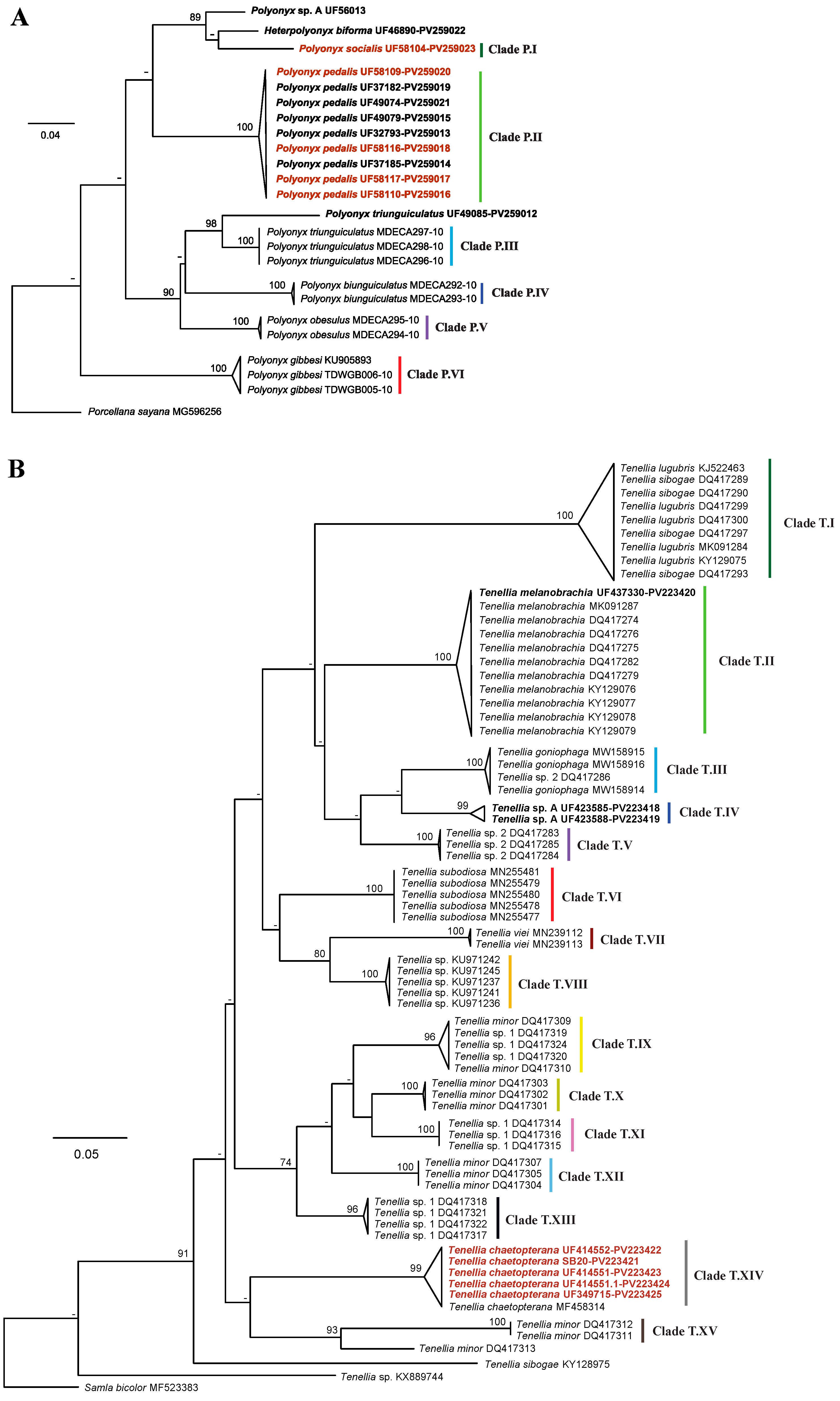
2.4. Phylogenetic Analyses
3. Results
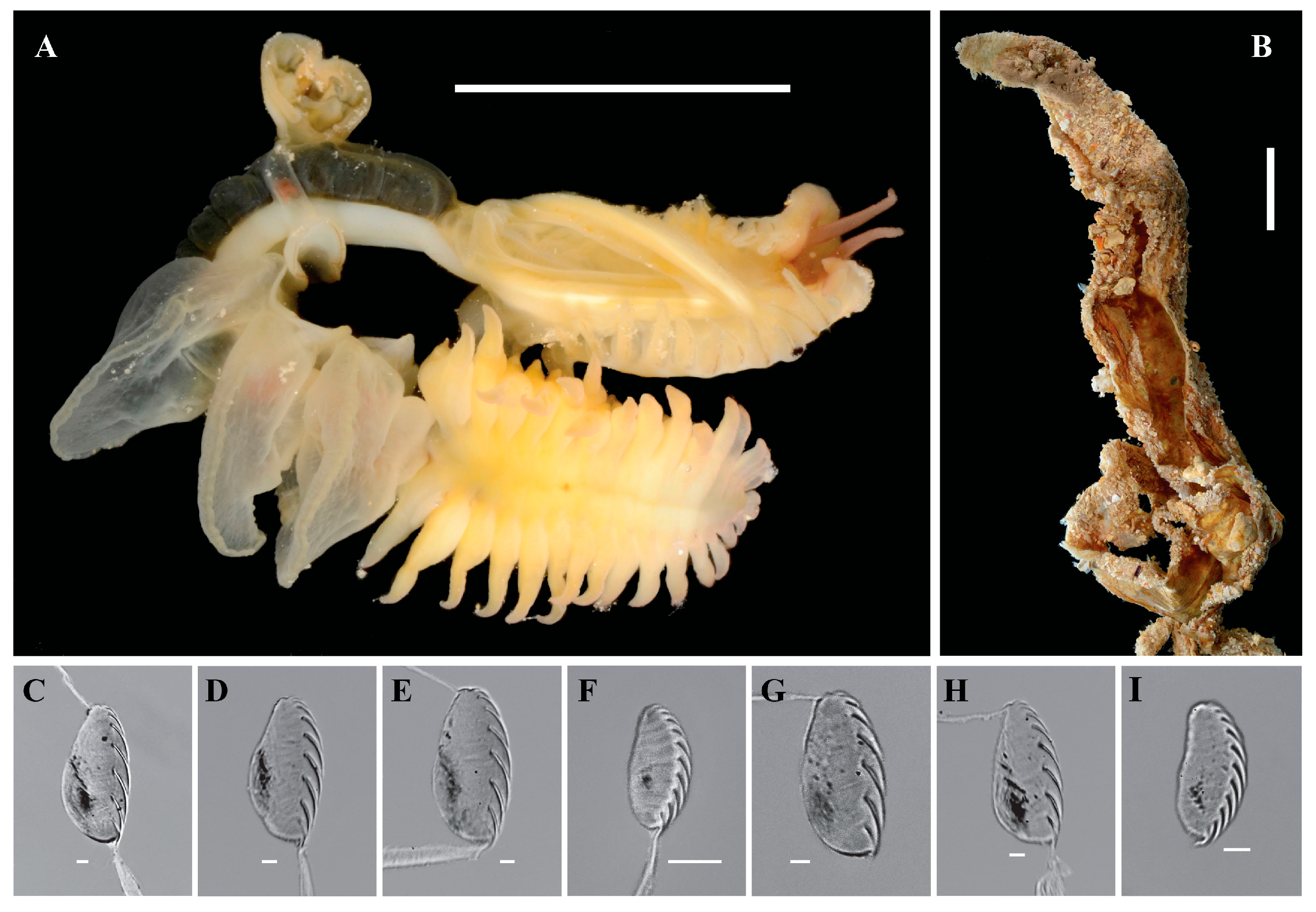
3.1. Taxonomic Account
3.2. Morpho-Molecular Results
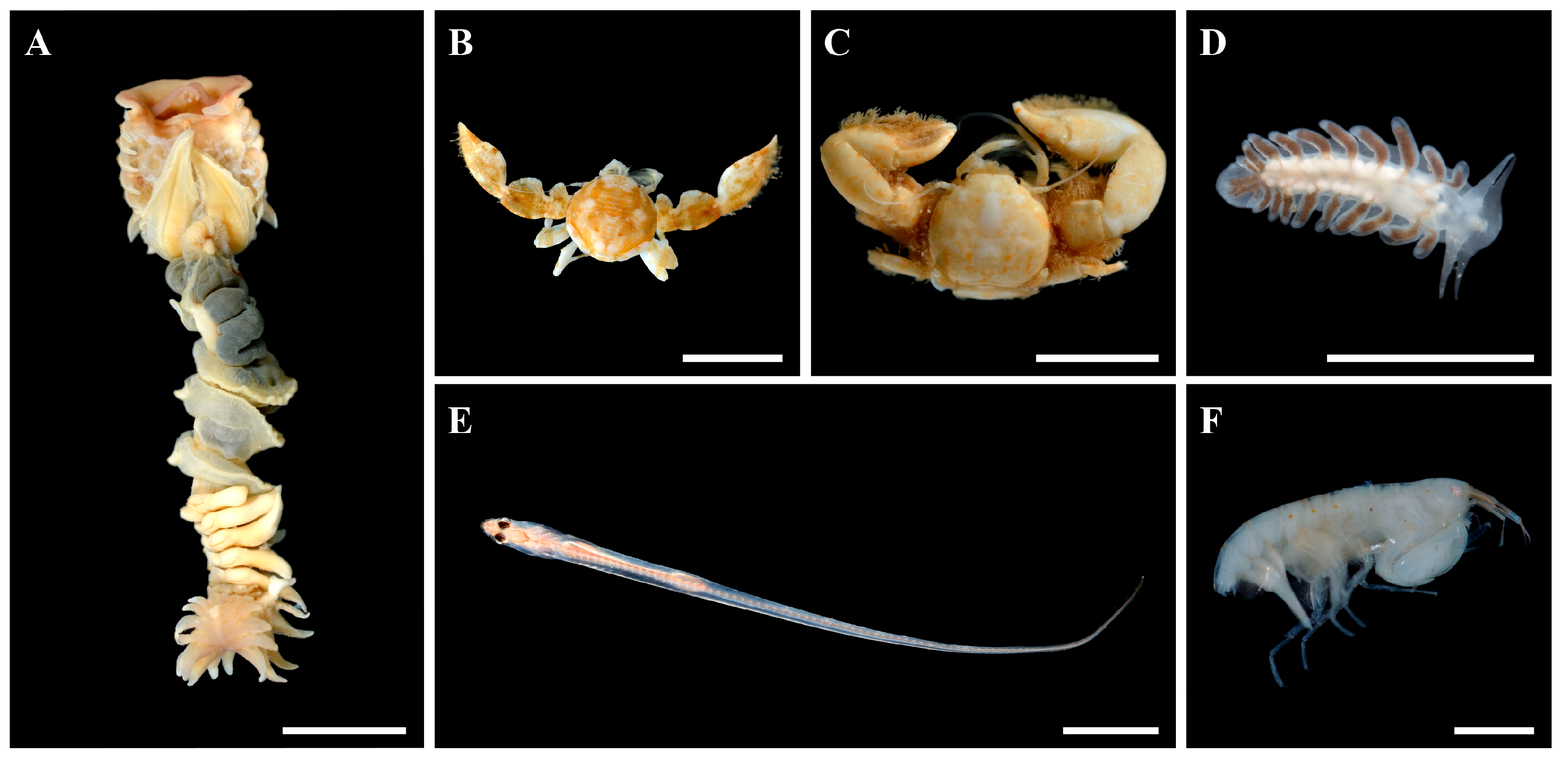
3.2.1. Host: Chaetopterus djiboutiensis
3.2.2. Symbionts: Crabs
3.2.3. Symbionts: Nudibranch
3.2.4. Symbionts: Fish
3.2.5. Symbionts: Amphipods
3.3. Symbiont Community Structure
4. Discussion
4.1. Host and Symbionts Identification
4.2. Symbiont Distribution and Association Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reaka, M. Biodiversity of Caribbean Coral Reefs. In Caribbean Marine Biodiversity; Miloslavich, P., Klein, E., Eds.; Des Tech Publishers: Lancaster, PA, USA, 2005; pp. 259–276. [Google Scholar]
- Hoegh-Guldberg, O. Climate Change, Coral Bleaching and the Future of the World’s Coral Reefs. Mar. Freshw. Res. 1999, 50, 259–276. [Google Scholar] [CrossRef]
- Bouchet, P.; Lozouet, P.; Maestrati, P.; Heros, V. Assessing the Magnitude of Species Richness in Tropical Marine Environments: Exceptionally High Numbers of Molluscs at a New Caledonia Site. Biol. J. Linn. Soc. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Williams, J.D.; McDermott, J.J. Hermit Crab Biocoenoses: A Worldwide Review of the Diversity and Natural History of Hermit Crab Associates. J. Exp. Mar. Biol. Ecol. 2004, 305, 1–128. [Google Scholar] [CrossRef]
- Anker, A.; Murina, G.; Lira, C.; Caripe, J.A.V.; Palmer, A.R.; Jeng, M. Macrofauna Associated with Echiuran Burrows: A Review with New Observations of the Innkeeper Worm, Ochetostoma Erythrogrammon Leuckart and Rüppel, in Venezuela. Zool. Stud. 2005, 44, 157–190. [Google Scholar]
- Macdonald, K.S.; Ríos, R.; Duffy, J.E. Biodiversity, Host Specificity, and Dominance by Eusocial Species among Sponge-Dwelling Alpheid Shrimp on the Belize Barrier Reef. Divers. Distrib. 2006, 12, 165–178. [Google Scholar] [CrossRef]
- Britayev, T.A.; Mekhova, E.S. Assessment of Hidden Diversity of Crinoids and Their Symbionts in the Bay of Nhatrang, Vietnam. Org. Divers. Evol. 2011, 11, 275–285. [Google Scholar] [CrossRef]
- Hoeksema, B.W. The Hidden Biodiversity of Tropical Coral Reefs. Biodiversity 2017, 18, 8–12. [Google Scholar] [CrossRef]
- Knowlton, N.; Jackson, J.B.C. Shifting Baselines, Local Impacts, and Global Change on Coral Reefs. PLoS Biol. 2008, 6, e54. [Google Scholar] [CrossRef]
- Caley, M.J.; Buckley, K.A.; Jones, G.P. Separating Ecological Effects of Habitat Fragmentation, Degradation, and Loss on Coral Commensals. Ecology 2001, 82, 3435. [Google Scholar] [CrossRef]
- Kiers, T.E.; Palmer, T.M.; Ives, A.R.; Bruno, J.F.; Bronstein, J.L. Mutualisms in a Changing World: An Evolutionary Perspective. Ecol. Lett. 2010, 13, 1459–1474. [Google Scholar] [CrossRef]
- Rouse, G.W.; Pleijel, F. Chaetopteridae Audouin and Milne Edwards, 1833. In Polychaetes; Oxford University Press: Oxford, UK, 2001; pp. 256–260. [Google Scholar]
- Osborn, K.J.; Rouse, G.W.; Goffredi, S.K.; Robison, B.H. Description and Relationships of Chaetopterus pugaporcinus, an Unusual Pelagic Polychaete (Annelida, Chaetopteridae). Biol. Bull. 2007, 212, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Nishi, E.; Rouse, G.W. Phylogenetic Analyses of Chaetopteridae (Annelida). Zool. Scr. 2017, 46, 596–610. [Google Scholar] [CrossRef]
- Grove, M.W.; Finelli, C.M.; Wethey, D.S.; Woodin, S.A. The Effects of Symbiotic Crabs on the Pumping Activity and Growth Rates of Chaetopterus variopedatus. J. Exp. Mar. Biol. Ecol. 2000, 246, 31–52. [Google Scholar] [CrossRef]
- Britayev, T.A.; Mekhova, E.; Deart, Y.; Martin, D. Do Syntopic Host Species Harbour Similar Symbiotic Communities? The Case of Chaetopterus spp. (Annelida: Chaetopteridae). PeerJ 2017, 5, e2930. [Google Scholar] [CrossRef]
- Britayev, T.A.; Martin, D. Chaetopteridae. In Handbook of Zoology—Annelida: Basal Groups and Pleisto-Annelidae, Sedentaria I.; Purschke, G., Böggemann, M., Westheide, W., Eds.; Walter de Gruyter: Berlin, Germany, 2019; Volume 1, pp. 156–175. [Google Scholar]
- Renier, S.A. Prospetto Della Classe Dei Vermi Nominati e Ordinati Secondo Il Sistema Di Bosc. Padua 1804, 38, 15–27. [Google Scholar]
- Fauvel, P. Polychetes Sedentaires. Addenda Aux Errantes, Archiannelides, Myzostomaires. In Faune de France; Paul Lechevalier: Paris, France, 1927; Volume 5, pp. 1–488. [Google Scholar]
- Petersen, M.E. Chaetopterus variopedatus (Renier) (Annelida: Polychaeta: Chaetopteridae) a Species Complex. What Species Are Being Used at MBL? Biol. Bull. 1984, 167, 513. [Google Scholar]
- Petersen, M.E.; Britayev, T.A. A New Genus and Species of Polynoid: Scaleworm Commensal with Chaetopterus appendiculatus Grube from the Banda Sea (Annelida: Polychaeta), with a Review of Commensals of Chaetopteridae. Bull. Mar. Sci. 1997, 60, 261–276. [Google Scholar]
- Nishi, E. Partial Revision of Japanese Chaetopterus (Chaetopteridae, Polychaeta), Including Description of Three New Species from Southern Pacific Side of Central Japan. Actinia 2001, 14, 1–26. [Google Scholar]
- Martin, D.; Gil, J.; Carreras-Carbonell, J.; Bhaud, M. Description of a New Species of Mesochaetopterus (Annelida, Polychaeta, Chaetopteridae), with Redescription of Mesochaetopterus xerecus and an Approach to the Phylogeny of the Family. Zool. J. Linn. Soc. 2008, 152, 201–225. [Google Scholar] [CrossRef]
- Tilic, E.; Rouse, G.W. Hidden in Plain Sight, Chaetopterus dewysee sp. nov. (Chaetopteridae, Annelida)—A New Species from Southern California. Eur. J. Taxon. 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Read, G.B.; Fauchald, K. World Polychaeta Database. Chaetopterus Cuvier. 1830. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=129229 (accessed on 1 March 2025).
- Ng, P.K.L.; Nakasone, Y. Taxonomy and Ecology of the Porcellanid Crab Polyonyx cometes Walker, 1887 (Crustacea: Decapoda), with Description of a New Genus. J. Nat. Hist. 1993, 27, 1103–1117. [Google Scholar] [CrossRef]
- Johnson, D.S. On Some Commensal Decapod Crustaceans from Singapore (Palaemonidae and Porcellanidae). J. Zool. 1967, 153, 499–526. [Google Scholar] [CrossRef]
- Britayev, T.A. Pilargis Berkeleyae (Polychaeta, Pilargidae) as a Commensal of a Sedentary Polychaete Chaetopterus cautus (Chaetopteridae). Zool. Zh. 1993, 72, 147–151. [Google Scholar]
- Osawa, M. Heteropolyonyx biforma, New Genus and Species, from Japan, and Redescription of Polyonyx utinomii (Decapoda: Porcellanidae). J. Crustac. Biol. 2001, 21, 506–520. [Google Scholar] [CrossRef]
- Paulay, G. (Florida Museum of Natural History, Gainesville, FL, USA); Uyeno, D. (Kagoshima University, Kagoshima, Japan); Moroz, L. (University of Florida, Gainesville, FL, USA). Personal communication, 2017. Polyonyx biforma Specimen Notes in FLMNH IZ Specify Web Portal.
- Martin, D.; Britayev, T. Symbiotic Polychaetes: Review of Known Species. Oceanogr. Mar. Biol. 1998, 36, 217–340. [Google Scholar]
- Gibbs, P.E. Aspects of Polychaete Ecology with Particular Reference to Commensalism. Philos. Trans. R Soc. Lond. B Biol. Sci. 1969, 255, 443–458. [Google Scholar] [CrossRef]
- White, K.N.; Krapp-Schickel, T. Red Sea Leucothoidae (Crustacea: Amphipoda) Including New and Re-Described Species. Eur. J. Taxon. 2017, 324, 1–40. [Google Scholar] [CrossRef]
- Britayev, T.A.; Martin, D. Scale-Worms (Polychaeta, Polynoidae) Associated with Chaetopterid Worms (Polychaeta, Chaetopteridae), with Description of a New Genus and Species. J. Nat. Hist. 2005, 39, 4081–4099. [Google Scholar] [CrossRef]
- Komai, T.; Nishi, E.; Taru, M. A New Species of Pinnixa (Crustacea: Decapoda: Brachyura: Pinnotheridae) Associated with a Tube Worm, Chaetopterus cautus (Annelida: Polychaeta), from Tokyo Bay, Japan. Zootaxa 2014, 3793, 119–132. [Google Scholar] [CrossRef]
- Marin, I.N. The Species Composition and Ecological Features of Pea Crabs of the Genus Pinnixa White, 1846 (Brachyura: Pinnotheridae) in Peter the Great Bay, the Sea of Japan. Russ. J. Mar. Biol. 2016, 42, 139–145. [Google Scholar] [CrossRef]
- Kobjakova, Z.I. Biocoenoses of the Possjet Bay of the Sea of Japan. In Explorations of the Fauna of the Seas V (XIII); Zoological Institute, Academy of Sciences of the U.S.S.R.: St. Petersburg, Russia, 1967; pp. 230–247. [Google Scholar]
- Vassilenko, S. On Taxonomy and Ecology of Commensal Crabs of Family Pinnotheridae (Crustacea, Decapoda, Brachyura) of the Sea Okhotsk and Northern Part of the Japan Sea. Proc. Zool. Soc. Lenin. 1990, 218, 75–95. [Google Scholar]
- Kornienki, E.S.; Korn, O. Illustrated Key for the Identification of Brachyuran Larvae in the Northwestern Sea of Japan; Dalnauka: Vladivostok, Russia, 2010. [Google Scholar]
- Hsueh, P.W. Intertidal Distribution, Symbiotic Association and Reproduction of Pinnotheres bidentatus (Brachyura: Pinnotheridae) from Taiwan. J. Nat. Hist. 2001, 35, 1681–1692. [Google Scholar] [CrossRef]
- Hsueh, P.W. Responses of the Pea Crab Pinnotheres taichungae to the Life History Patterns of Its Primary Bivalve Host Laternula marilina. J. Nat. Hist. 2003, 37, 1453–1462. [Google Scholar] [CrossRef]
- Osawa, M.; Fujita, Y. Polyonyx haigae McNeil, 1968 (Crustacea: Decapoda: Anomura: Porcellanidae) from the Ryukyu Islands, Southwestern Japan. Fauna Ryukyuana 2022, 65, 41–65. [Google Scholar]
- Osawa, M.; Ng, P.K.L. Revision of Polyonyx pedalis Nobili, 1906 (Crustacea: Decapoda: Anomura: Porcellanidae), with Descriptions of Three New Species. Raffles Bull. Zool. 2016, 2016, 499–518. [Google Scholar]
- Amer, M.A.; Naruse, T.; Osawa, M. First Record of Polyonyx loimicola Sankolli, 1965 (Crustacea, Decapoda, Anomura, Porcellanidae) from the Red Sea, Egypt. Mar. Biodivers. Rec. 2019, 12, 1–5. [Google Scholar] [CrossRef]
- Hsueh, P.-W.; Huang, J.-F. Polyonyx bella, New Species (Decapoda: Anomura: Porcellanidae), from Taiwan, with Notes on Its Reproduction and Swimming Behavior. J. Crustac. Biol. 1998, 18, 332–336. [Google Scholar] [CrossRef]
- Shen, C.J. Notes on the Genus Polyonyx (Porcellanidae) with Description of a New Species. Bull. Fan. Mem. Inst. Biol. 1936, 6, 275–287. [Google Scholar]
- Werding, B.; Hiller, A. A New Species of Polyonyx (Crustacea, Anomura, Porcellanidae) Inhabiting Polychaete-Worm Tubes (Annelida, Chaetopteridae) in the Indo-West Pacific. Zookeys 2019, 2019, 25–34. [Google Scholar] [CrossRef]
- Miyake, S. Studies on the Crab-Shaped Anomura of Nippon and Adjacent Waters. J. Dep. Agric. Kyushu Imp. Univ. 1943, 7, 49–158. [Google Scholar] [CrossRef]
- Eager, S.; Read, G. A New Species of Pontocypria (Ostracoda) Living on Chaetopterus sp. (Annelida: Polychaeta) from Hauraki Gulf, New Zealand. N. Z. Nat. Sci. 2004, 29, 49–54. [Google Scholar]
- Tu, T.J. Über Einige in Polychaetenr Öhren Und Muschcln Gefundne Dekapoden an Der Kuste von Schantung. Zool. Anz. 1938, 122, 177–186. [Google Scholar]
- Gray, I.E. Changes in Abundance of the Commensal Crabs of Chaetopterus. Biol. Bull. 1961, 120, 353–359. [Google Scholar] [CrossRef]
- Cuvier, G. Le Règne Animal Distingué D’après Son Organisation, Pour Servir de Base à L’histoire Naturelle des Animaux et D’introduction à L’anatomie Comparée, 2nd ed.; Déterville & Crochard: Paris, France, 1830. [Google Scholar]
- Marenzeller, E. Südjapanische Anneliden, I. Denkschriften der Kaiserlichen Akademie der Wissenschaften. Mathematisch Naturwissenschaftliche Classe 1879, 41, 109–154. [Google Scholar]
- Walker, A.O. Notes on a Collection of Crustacea from Singapore. J. Linn. Soc. 1887, 20, 107–117. [Google Scholar] [CrossRef]
- Ekimova, I.; Deart, Y.; Schepetov, D. Living with a Giant Parchment Tube Worm: A Description of a New Nudibranch Species (Gastropoda: Heterobranchia) Associated with the Annelid Chaetopterus. Mar. Biodivers. 2017, 49, 289–300. [Google Scholar] [CrossRef]
- Smith, J.L.B. The Fishes of the Family Carapidae in the Western Indian Ocean. Ann. Mag. Nat. 1955, 8, 401–416. [Google Scholar] [CrossRef]
- Cowburn, B.; Samoilys, M.A.; Osuka, K.; Klaus, R.; Newman, C.; Gudka, M.; Obura, D. Healthy and Diverse Coral Reefs in Djibouti—A Resilient Reef System or Few Anthropogenic Threats? Mar. Pollut. Bull. 2019, 148, 182–193. [Google Scholar] [CrossRef]
- Gravier, C. Sur Les Annélides Polychètes de La Mer Rouge (Sabellides). Bull. Muséum D’histoire Nat. Paris 1906, 12, 33–43. [Google Scholar]
- Nobili, G. Diagnoses Préliminaires de 34 Espèces et Variétés Nouvelles, et de 2 Genres Nouveaux de Décapodes de La Mer Rouge. Bull. Mus. D’histoire Naturelle 1906, 11, 393–411. [Google Scholar]
- Hartman, O. Catalogue of the Polychaeta Annelids of the World. Allan Hancock Found. Publ. 1959, 23, 1–628. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Crossland, C. The Polychaeta of the Maldive Archipelago from the Collections Made by J. Stanley Gnrdiner in 1899. Proc. Zool. Soc. Lond. 1904, 74, 270–286. [Google Scholar] [CrossRef]
- Lessona, M. Sopra Due Nuove Specie Di Animali Invertebrati Raccolte Nel Golfo Di Genova. Atti. Soc. Ital. Sci. 1865, 8, 423–428. [Google Scholar]
- Savigny, J.C. Memoires Sur Les Animaux sans Vertebres. Paris 1816, 2, 1–239. [Google Scholar]
- White, K.N. A Taxonomic Review of the Leucothoidae (Crustacea: Amphipoda). Zootaxa 2011, 3078, 1–113. [Google Scholar] [CrossRef]
- Wang, A.; Conti-Jerpe, I.E.; Richards, J.L.; Baker, D.M. Phestilla subodiosus sp. nov. (Nudibranchia, Trinchesiidae), a Corallivorous Pest Species in the Aquarium Trade. Zookeys 2020, 909, 1–24. [Google Scholar] [CrossRef]
- Sinniger, F.; Prasetia, R.; Yorifuji, M.; Bongaerts, P.; Harii, S. Seriatopora Diversity Preserved in Upper Mesophotic Coral Ecosystems in Southern Japan. Front. Mar. Sci. 2017, 4, 155. [Google Scholar] [CrossRef]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR Primers for Mitochondrial Cytochrome c Oxidase Subunit I for Marine Invertebrates and Application in All-Taxa Biotic Surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef]
- Palumbi, S.R. Nucleic Acids II: The Polymerase Chain Reaction. In Molecular Systematics; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer Associates: Sunderland, MA, USA, 1996; pp. 205–247. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Colgan, D.J.; McLauchlan, A.; Wilson, G.D.F.; Livingston, S.P.; Edgecombe, G.D.; Macaranas, J.; Cassis, G.; Gray, M.R. Histone H3 and U2 SnRNA DNA Sequences and Arthropod Molecular Evolution. Aust. J. Zool. 1998, 46, 419. [Google Scholar] [CrossRef]
- Puslednik, L.; Serb, J.M. Molecular Phylogenetics of the Pectinidae (Mollusca: Bivalvia) and Effect of Increased Taxon Sampling and Outgroup Selection on Tree Topology. Mol. Phylogenet. Evol. 2008, 48, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Handy, S.M.; Deeds, J.R.; Ivanova, N.V.; Hebert, P.D.N.; Hanner, R.H.; Ormos, A.; Weigt, L.A.; Moore, M.M.; Yancy, H.F. A Single-Laboratory Validated Method for the Generation of DNA Barcodes for the Identification of Fish for Regulatory Compliance. J. AOAC Int. 2011, 94, 201–210. [Google Scholar] [CrossRef]
- Spears, T.; DeBry, R.W.; Abele, L.G.; Chodyla, K. Peracarid Monophyly and Interordinal Phylogeny Inferred from Nuclear Small-Subunit Ribosomal DNA Sequences (Crustacea: Malacostraca: Peracarida). Proc. Biol. Sc. Wash. 2005, 118, 117–157. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rodríguez, I.T.; Hernández, G.; Felder, D.L. Phylogenetic Relationships among Western Atlantic Porcellanidae (Decapoda: Anomura), Based on Partial Sequences of the Mitochondrial 16S RRNA Gene, with Comments on Morphology. J. Crust Biol. 2006, 6, 151–166. [Google Scholar] [CrossRef]
- Parmentier, E.; Lanterbecq, D.; Eeckhaut, I. From Commensalism to Parasitism in Carapidae (Ophidiiformes): Heterochronic Modes of Development? PeerJ 2016, 4, e1786. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Nylander, J.A.A. MrModeltest V2. Program Distributed by the Author 2004; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Rambaut, A. FigTree v1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, Scotland, 2018. [Google Scholar]
- Wehe, T.; Fiege, D. Annotated checklist of the polychaete species of the seas surrounding the Arabian Peninsula: Red Sea, Gulf of Aden, Arabian Sea, Gulf of Oman, Arabian Gulf. Fauna Arab. 2002, 19, 7–238. [Google Scholar]
- Stimpson, W. Descriptions of Some New Marine Invertebrata. Proc. Acad. Nat. Sci. USA 1855, 7, 385–394. [Google Scholar] [CrossRef]
- Sars, M. Uddrag Af En Beskrivelse over Chaetopterus sarsii n.sp., Og Chaetopterus norvegicus Sars. Forh. Vidensk-Selsk. 1860, 720, 85–88. [Google Scholar]
- Zehntner, L. Voyage de MM. M. Bedot et C. Pictet Dans l’Archipel Malais. Crustacés de l’Archipel Malais. Rev. Suisse Zool. 1894, 2, 135–214. [Google Scholar] [CrossRef]
- Miers, E.J. Crustacea. Part I. The Collections from Melanesia. Crustacea. In Report on the Zoological Collections Made in the Indo-Pacific Ocean During the Voyage of H.M.S. “Alert” 1881–82; British Museum of Natural History: London, UK, 1884; pp. 178–322. [Google Scholar]
- Markle, D.F.; Olney, J.E. Systematics of the Pearlfishes (Pisces: Carapidae). Bull. Mar. Sci. 1990, 47, 269–410. [Google Scholar]
- Koeda, K. A New Pearlfish, Onuxodon albometeori sp. nov. (Ophidiiformes: Carapidae), from Taiwan. Zootaxa 2019, 4702, 6–9. [Google Scholar] [CrossRef]
- Fowler, H.W. Fishes of the Tropical Central Pacific. Bernice P. Bishop Mus. Bull. 1927, 38, 1–32. [Google Scholar]
- Rendahl, H. Results of Dr. E. Mjöbergs Swedish Scientific Expeditions to Australia, 1910–1913. XXVIII. Fische. K. Sven. Vetenskapsakad. Handl. 1921, 61, 1–24. [Google Scholar]
- Dana, J.D. United States Exploring Expedition. During the Years 1838, 1839, 1840, 1841, 1842; Under the Command of Charles Wilkes, U.S.N. XIII. Crustacea, Part I; C. Sherman: Philadelphia, PA, USA, 1852. [Google Scholar]
- Paulson, O.M. Izsliedovaniia Rakoobraznykh Krasnago Moria, s Zamietkami Otnositelno Rakoobraznych Drugikh Morei. Chast I.: Podophthalmata i Edriophthalmata (Cumacea.). In S Dvadtsatiu Odnoiu Tablitseiu Risunkov; Tipografiia S.V. Kulzhenko: Kiev, Ukraine, 1875. [Google Scholar]
- Stimpson, W. Prodromus Descriptionis Animalium Evertebratum, Quae in Expeditione Ad Oceanum Pacificum Septentrionalem, a Republica Federata Missa, Cadwaladaro Ringgold et Johanne Rodgers Ducibus, Observavit et Descripsit. Pars VII. Crustacea Anomoura. Proc. Acad. Nat. Sci. USA 1858, 10, 225–252. [Google Scholar]
- Johnson, D. The Indo-West Pacific Species of the Genus Polyonyx (Crustacea, Decapoda, Porcellanidae). Ann. Zool. 1958, 2, 95–118. [Google Scholar]
- Rudman, W.B. The Ecology and Anatomy of a New Species of Aeolid Opisthobranch Mollusc; a Predator of the Scleractinian Coral Porites. Zool. J. Linn. Soc. 1979, 65, 339–350. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Shjegstad, S.; Paul, V. Host Specificity of Four Corallivorous Phestilla Nudibranchs (Gastropoda: Opisthobranchia). Mar. Ecol. Prog. Ser. 2003, 255, 207–218. [Google Scholar] [CrossRef]
- Faucci, A.; Toonen, R.J.; Hadfield, M.G. Host Shift and Speciation in a Coral-Feeding Nudibranch. Proc. Royal Soc. B 2007, 274, 111–119. [Google Scholar] [CrossRef]
- Mehrotra, R.; Arnold, S.; Wang, A.; Chavanich, S.; Hoeksema, B.W.; Caballer, M. A New Species of Coral-Feeding Nudibranch (Mollusca: Gastropoda) from the Gulf of Thailand. Mar. Biodivers. 2020, 50, 36. [Google Scholar] [CrossRef]
- Fritts-Penniman, A.L.; Gosliner, T.M.; Mahardika, G.N.; Barber, P.H. Cryptic Ecological and Geographic Diversification in Coral-Associated Nudibranchs. Mol. Phylogenet Evol. 2020, 144, 106698. [Google Scholar] [CrossRef]
- Williams, J.T. Synopsis and Phylogenetic Analysis of the Pearlfish Subfamily Carapinae (Pisces: Carapidae). Bull. Mar. Sci. 1984, 34, 386–397. [Google Scholar]
- Krapp-Schickel, G. Revision of Mediterranean Leucothoe Species (Crustacea, Amphipoda). Boll. Mus. Civ. Stor. Nat. Verona 1975, 2, 91–118. [Google Scholar]
- Ledoyer, M. Les Gammariens de La Pente Externe Du Grand Récif de Tuléar (Madagascar). Mem. Mus. Civico Storia Nat. Verona 1979, 2, 1–150. [Google Scholar]
- Stier, A.C.; Gil, M.A.; Mckeon, C.S.; Lemer, S.; Leray, M. Housekeeping Mutualisms: Do More Symbionts Facilitate Host Performance? PLoS ONE 2012, 7, 32079. [Google Scholar] [CrossRef]
- Ory, N.C.; Dudgeon; Thiel, M. Host-Use Patterns and Factors Influencing the Choice between Anemone and Urchin Hosts by a Caridean Shrimp. J. Exp. Mar. Biol. Ecol. 2013, 449, 85–92. [Google Scholar] [CrossRef]
- Thiel, M.; Baeza, J.A. Factors Affecting the Social Behaviour of Crustaceans Living Symbiotically with Other Marine Invertebrates: A Modelling Approach. Symbiosis 2001, 30, 163–190. [Google Scholar]
- Lyskin, S.A.; Britaev, T.A. Symbionts of Holothurians from South Vietnam: Intra- and Interspecific Interactions. Dokl. Biol. Sci. 2005, 401, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Trott, L.B.; Trott, E.E. Pearlfishes (Carapidae: Gadiformes) Collected from Puerto Galera, Mindoro, Philippines. Copeia 1972, 1972, 839. [Google Scholar] [CrossRef]
- De Beaufort, L.F.; Chapman, W.M. Fishes of Indo-Australian Archipelago; E.J. Brill: Leiden, The Netherlands, 1951; Volume 9. [Google Scholar]
- Nazar, A.K.A.; Dharani, G.; Rao, D.V.; Santhanakumar, J.; Saravanane, N. A New Record of Pearl Fish Onuxodon margaritiferae (Rendahl, 1921) from Andaman Islands. Indian. J. Fish. 2011, 58, 141–143. [Google Scholar]
- White, K.N. Caribbean Leucothoidae (Crustacea: Amphipoda) of Panama. Gulf Caribb. Res. 2011, 23, 23–35. [Google Scholar] [CrossRef]
- Kodama, M.; White, K.N.; Hosoki, T.K.; Yoshida, R. Leucothoid Amphipod and Terebellid Polychaete Symbiosis with Description of a New Species of the Genus Leucothoe Leach, 1814 (Crustacea: Amphipoda: Leucothoidae). Syst. Biodivers. 2022, 20, 1–20. [Google Scholar] [CrossRef]
- Abele, L.G.; Patton, W.K. The Size of Coral Heads and the Community Biology of Associated Decapod Crustaceans. J. Biogeogr. 1976, 3, 35–47. [Google Scholar] [CrossRef]
- Baeza, J.A. The Origins of Symbiosis as a Lifestyle in Marine Crabs (Genus Petrolisthes) from the Eastern Pacific: Does Interspecific Competition Play a Role? Rev. Biol. Mar. Oceanogr. 2007, 42, 7–21. [Google Scholar] [CrossRef]
- Tun, K.; Chou, L.M.; Cabanban, A.; Tuan, V.; Philreefs; Yeemin, T.; Suharsono; Sour, K.; Lane, D. Status of Coral Reefs, Coral Reef Monitoring and Management in Southeast Asia. In Status of Coral Reefs of the World; Wilkinson, C., Ed.; Australian Institute of Marine Science: Townsville, Australia, 2004; pp. 235–276. [Google Scholar]
- Spalding, M. World Atlas of Coral Reefs; University of California Press: Berkeley, CA, USA, 2001. [Google Scholar]


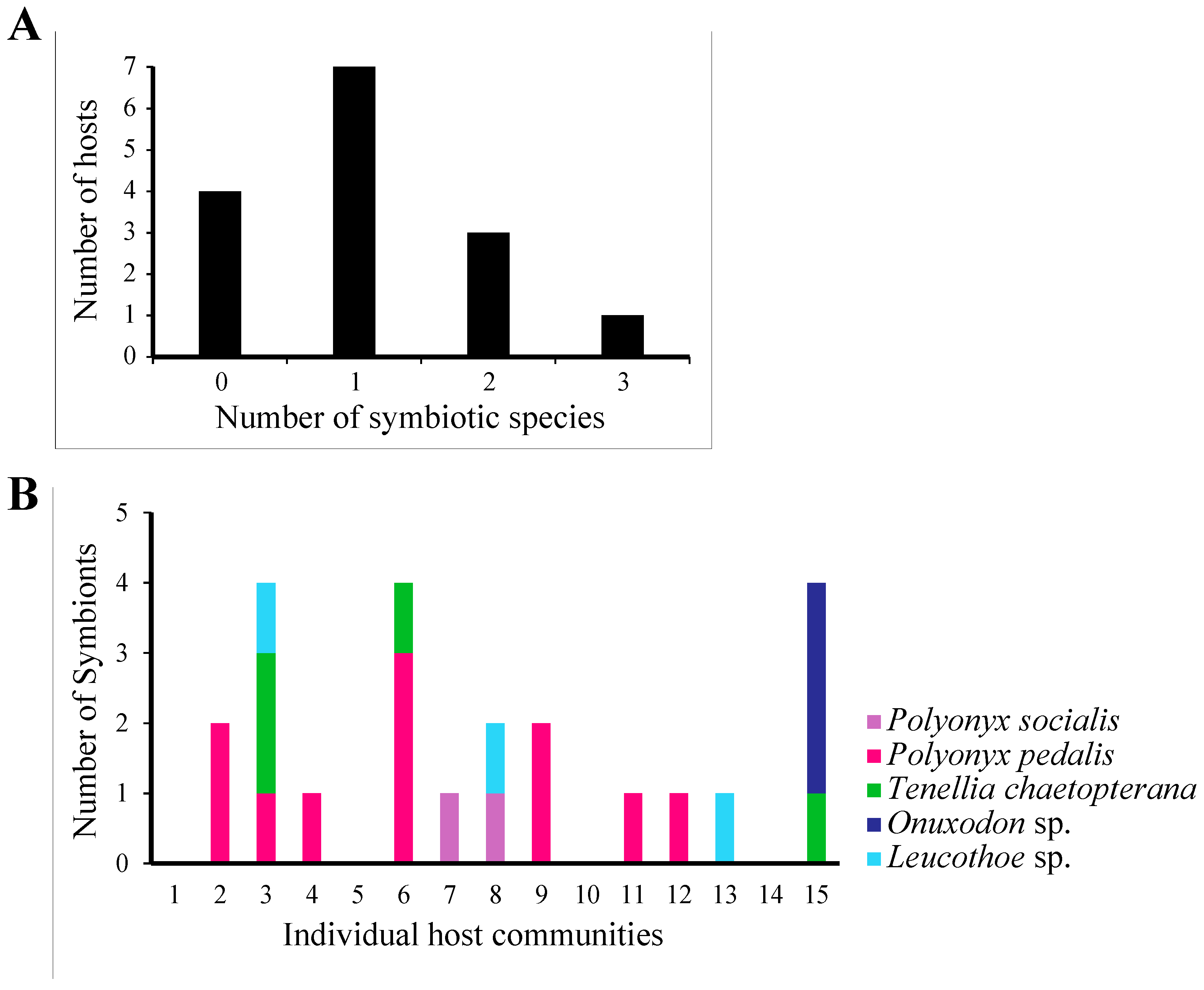
| Sample Site | Host ID # | Symbionts |
|---|---|---|
| Obock | DJI-CH-01 | None |
| Obock | DJI-CH-02 | Polyonyx pedalis (2) |
| Obock | DJI-CH-03 | Polyonyx pedalis, Tenellia chaetopterana (2), Leucothoe sp. A |
| Obock | DJI-CH-04 | Polyonyx pedalis |
| Obock | DJI-CH-05 | None |
| Obock | DJI-CH-06 | Polyonyx pedalis (3) and Tenellia chaetopterana |
| Sable Blanc East | DJI-CH-07 | Polyonyx socialis |
| Sable Blanc West | DJI-CH-08 | Polyonyx socialis and Leucothoe sp. A |
| Ghoubet East | DJI-CH-09 | Polyonyx pedalis (2) |
| Ghoubet | DJI-CH-10 | None |
| Devil Island | DJI-CH-11 | Polyonyx pedalis |
| Ghoubet East | DJI-CH-12 | Polyonyx pedalis |
| Ras Eiro | DJI-CH-13 | Leucothoe sp. A |
| The Crack | DJI-CH-14 | None |
| Obock | DJI-CH-15 | Onuxodon sp. (3) and Tenellia chaetopterana (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, S.D.; Terraneo, T.I.; Moore, J.M.; Paulay, G.; White, K.N.; Berumen, M.L.; Benzoni, F. The Diversity and Phylogenetic Relationships of a Chaetopterus Symbiont Community in Djibouti, with Redescription of Chaetopterus djiboutiensis Gravier, 1906 Stat. Nov. (Annelida: Chaetopteridae). Diversity 2025, 17, 366. https://doi.org/10.3390/d17050366
Brown SD, Terraneo TI, Moore JM, Paulay G, White KN, Berumen ML, Benzoni F. The Diversity and Phylogenetic Relationships of a Chaetopterus Symbiont Community in Djibouti, with Redescription of Chaetopterus djiboutiensis Gravier, 1906 Stat. Nov. (Annelida: Chaetopteridae). Diversity. 2025; 17(5):366. https://doi.org/10.3390/d17050366
Chicago/Turabian StyleBrown, Shannon D., Tullia I. Terraneo, Jenna M. Moore, Gustav Paulay, Kristine N. White, Michael L. Berumen, and Francesca Benzoni. 2025. "The Diversity and Phylogenetic Relationships of a Chaetopterus Symbiont Community in Djibouti, with Redescription of Chaetopterus djiboutiensis Gravier, 1906 Stat. Nov. (Annelida: Chaetopteridae)" Diversity 17, no. 5: 366. https://doi.org/10.3390/d17050366
APA StyleBrown, S. D., Terraneo, T. I., Moore, J. M., Paulay, G., White, K. N., Berumen, M. L., & Benzoni, F. (2025). The Diversity and Phylogenetic Relationships of a Chaetopterus Symbiont Community in Djibouti, with Redescription of Chaetopterus djiboutiensis Gravier, 1906 Stat. Nov. (Annelida: Chaetopteridae). Diversity, 17(5), 366. https://doi.org/10.3390/d17050366






