Forestry Plans as the Source of Environmental Data for the Analysis of Bird Community Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Bird Data
2.3. Environmental Data
| AbiAlb | Abies alba Mill. | Silver Fir |
|---|---|---|
| AbiGra | Abies grandis (Douglas) Lindl. | Grand Fir |
| AcePla | Acer platanoides L. | Norway Maple |
| AcePse | Acer pseudoplatanus L. | Sycamore Maple |
| AesHip | Aesculus hippocastanum L. | Horse Chestnut |
| AlnGlu | Alnus glutinosa (L.) Gaertner | Common Alder |
| AlnInc | Alnus incana (L.) Moench | Speckled Alder |
| BetPen | Betula pendula Roth | Silver Birch |
| BetPub | Betula pubescens Ehrh. | Pubescent Birch |
| CarBet | Carpinus betulus L. | Hornbeam |
| CerAvi | Cerasus avium (L.) Moench | Wild Cherry |
| DusAln | Duschekia alnobetula (Ehr.) Pouzar | Green Alder |
| FagSyl | Fagus sylvatica L. | European Beech |
| FraExc | Fraxinus excelsior L. | Ash |
| LarDec | Larix decidua Mill. | Larch |
| PicAbi | Picea abies (L.) Karsten | Norway Spruce |
| PicPun | Picea pungens Engelm. | Colorado Spruce |
| PinMug | Pinus mugo Turra | Dwarf Mountain Pine |
| PinSyl | Pinus sylvestris L. | Scots Pine |
| Pinus | Pinus sp. | Pine |
| PopTre | Populus tremula L. | Aspen |
| PseMen | Pseudotsuga menziesii (Mirbel) Franco | Douglas Fir |
| QuePet | Quercus petraea (Mattyschka) Liebl. | Sessile Oak |
| QueRob | Quercus robur (L.) | Pedunculate Oak |
| SalCap | Salix caprea L. | Goat Willow |
| Salix | Salix alba L. | White Willow |
| Shr | Shrubs | |
| SorAuc | Sorbus aucuparia L. | Rowan Tree |
| SorTor | Sorbus torminalis (L.) Crantz | Wild Services Tree |
| TilCor | Tilia cordata Mill. | Small-leaved Lime |
| UlmMin | Ulmus minor Mill. | Elm Tree |
2.4. Data Analysis
2.4.1. Structure of Forest Stands and Their Bird Communities
2.4.2. Species Richness of Bird Communities
3. Results
3.1. Bird Species Richness
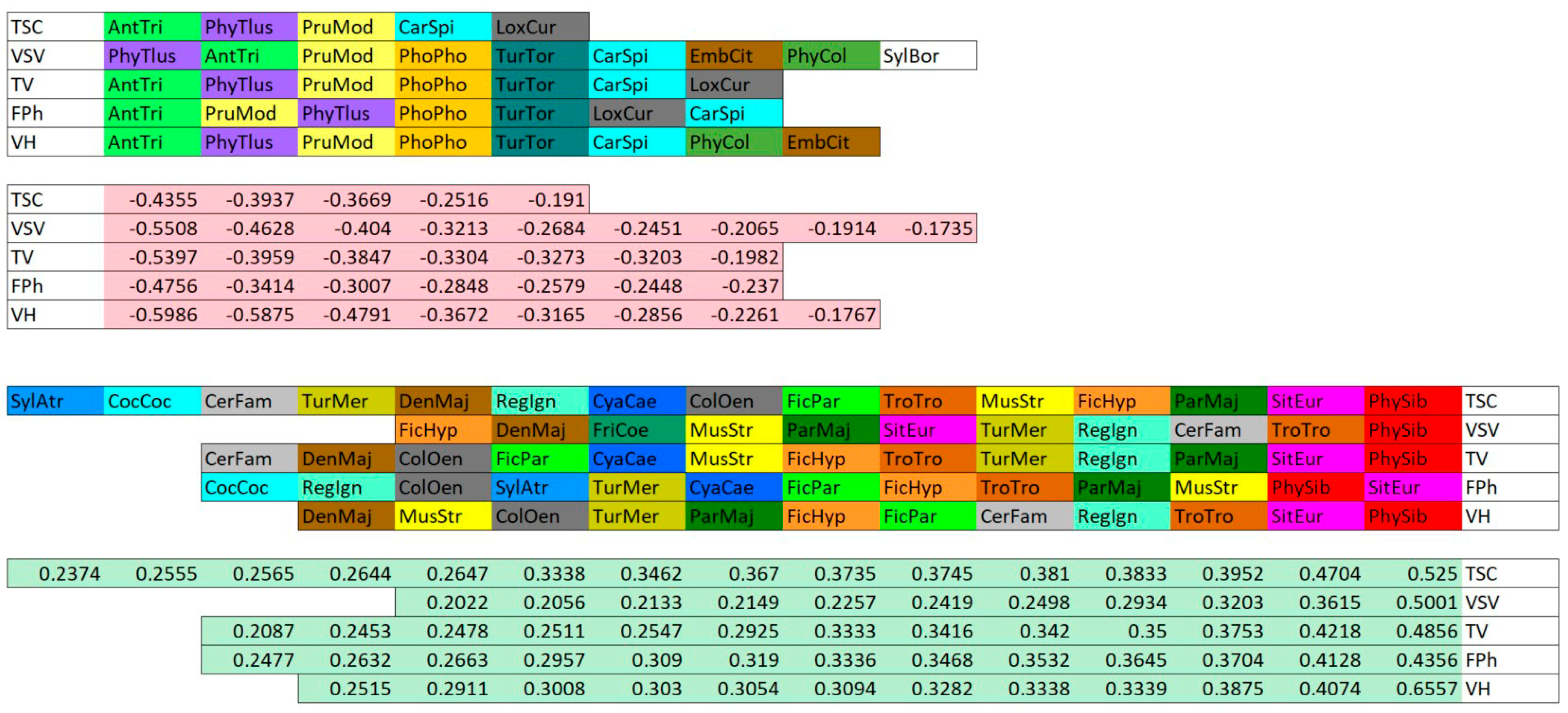
3.2. Bird Communities Composition
| Selected | n | f | Non-Selected | n | f |
|---|---|---|---|---|---|
| Eurasian Chaffinch | 285 | 100.0 | European Robin | 272 | 95.4 |
| Blackcap | 282 | 98.9 | Song Thrush | 261 | 91.6 |
| Common Chiffchaff | 238 | 83.5 | Coal Tit | 260 | 91.2 |
| Winter Wren | 217 | 76.1 | Goldcrest | 224 | 78.6 |
| Eurasian Blackbird | 207 | 72.6 | Common Woodpigeon | 192 | 67.4 |
| Hedge Accentor | 188 | 66.0 | Mistle Thrush | 191 | 67.0 |
| Firecrest | 163 | 57.2 | Crested Tit | 113 | 39.6 |
| Eurasian Siskin | 158 | 55.4 | Eurasian Bullfinch | 103 | 36.1 |
| Eurasian Treecreeper | 150 | 52.6 | Eurasian Jay | 79 | 27.7 |
| Willow Warbler | 141 | 49.5 | Black Woodpecker | 66 | 23.2 |
| Wood Warbler | 116 | 40.7 | Common Whitethroat | 32 | 11.2 |
| Tree Pipit | 98 | 34.4 | Common Raven | 26 | 9.1 |
| Great Spotted Woodpecker | 95 | 33.3 | Grey Wagtail | 24 | 8.4 |
| Red Crossbill | 91 | 31.9 | Marsh Tit | 17 | 6.0 |
| Wood Nuthatch | 78 | 27.4 | Eurasian Nutcracker | 15 | 5.3 |
| Great Tit | 68 | 23.9 | Lesser Redpoll | 12 | 4.2 |
| Blue Tit | 51 | 17.9 | Common Starling | 11 | 3.9 |
| Redstart | 34 | 11.9 | Hooded Crow | 11 | 3.9 |
| Pied Flycatcher | 28 | 9.8 | Magpie | 8 | 2.8 |
| Stock Dove | 27 | 9.5 | White Wagtail | 6 | 2.1 |
| Ring Ouzel | 24 | 8.4 | Lesser Whitethroat | 6 | 2.1 |
| Garden Warbler | 22 | 7.7 | Dipper | 6 | 2.1 |
| Spotted Flycatcher | 17 | 6.0 | Willow Tit | 6 | 2.1 |
| Yellowhammer | 16 | 5.6 | Turtle Dove | 6 | 2.1 |
| Hawfinch | 14 | 4.9 | Fieldfare | 5 | 1.8 |
| Red-breasted Flycatcher | 11 | 3.9 | Common Rosefinch | 4 | 1.4 |
| Meadow Pipit | 4 | 1.4 | |||
| Greenish Warbler | 3 | 1.1 | |||
| Linnet | 3 | 1.1 | |||
| Long-tailed Tit | 3 | 1.1 | |||
| Grey-headed Woodpecker | 3 | 1.1 |
| AegCau | Aegithalos caudatus | Long-Tailed Tit |
|---|---|---|
| AntPra | Anthus pratensis | Meadow Pipit |
| AntTri | Anthus trivialis | Tree Pipit |
| CarCab | Carduelis cabaret | Lesser Redpoll |
| CarCan | Carduelis cannabina | Linnet |
| CarEry | Carpodacus erythrinus | Common Rosefinch |
| CarSpi | Carduelis spinus | Eurasian Siskin |
| CerFam | Certhia familiaris | Eurasian Treecreeper |
| CinCin | Cinclus cinclus | Dipper |
| CocCoc | Coccothraustes coccothraustes | Hawfinch |
| ColOen | Columba oenas | Stock Dove |
| ColPal | Columba palumbus | Common Woodpigeon |
| CorCor | Corvus corax | Common Raven |
| CorCor | Corvus cornix | Hooded Crow |
| CyaCae | Cyanistes caeruleus | Blue Tit |
| DenMaj | Dendrocopos major | Great Spotted Woodpecker |
| DryMar | Dryocopus martius | Black Woodpecker |
| EmbCit | Emberiza citrinella | Yellowhammer |
| EriRub | Erithacus rubecula | European Robin |
| FicHyp | Ficedula hypoleuca | Pied Flycatcher |
| FicPar | Ficedula parva | Red-breasted Flycatcher |
| FriCoe | Fringilla coelebs | Eurasian Chaffinch |
| GarGla | Garrulus glandarius | Eurasian Jay |
| LopCri | Lophophanes cristatus | Crested Tit |
| LoxCur | Loxia curvirostra | Red Crossbill |
| MotAlb | Motacilla alba | White Wagtail |
| MotCin | Motacilla cinerea | Grey Wagtail |
| MusStr | Muscicapa striata | Spotted Flycatcher |
| NucCar | Nucifraga caryocatactes | Eurasian Nutcracker |
| ParMaj | Parus major | Great Tit |
| PerAte | Periparus ater | Coal Tit |
| PhoPho | Phoenicurus phoenicurus | Redstart |
| PhyCol | Phylloscopus collybita | Common Chiffchaff |
| PhySib | Phylloscopus sibilatrix | Wood Warbler |
| PhyTro | Phylloscopus trochilus | Willow Warbler |
| PhyTro | Phylloscopus trochiloides | Greenish Warbler |
| PicCan | Picus canus | Grey-headed Woodpecker |
| PicPic | Pica pica | Magpie |
| PoeMon | Poecile montanus | Willow Tit |
| PoePal | Poecile palustris | Marsh Tit |
| PruMod | Prunella modularis | Dunnock |
| PyrPyr | Pyrrhula pyrrhula | Eurasian Bullfinch |
| RegIgn | Regulus ignicapillus | Firecrest |
| RegReg | Regulus regulus | Goldcrest |
| SitEur | Sitta europaea | Eurasian Nuthatch |
| StrTur | Streptopelia turtur | Turtle Dove |
| StuVul | Sturnus vulgaris | Common Starling |
| SylAtr | Sylvia atricapilla | Blackcap |
| SylBor | Sylvia borin | Garden Warbler |
| SylCom | Sylvia communis | Whitethroat |
| SylCur | Sylvia curruca | Lesser Whitethroat |
| TroTro | Troglodytes troglodytes | Winter Wren |
| TurMer | Turdus merula | Eurasian Blackbird |
| TurPhi | Turdus philomelos | Song Thrush |
| TurPil | Turdus pilaris | Fieldfare |
| TurTor | Turdus torquatus | Ring Ouzel |
| TurVis | Turdus viscivorus | Mistle Thrush |
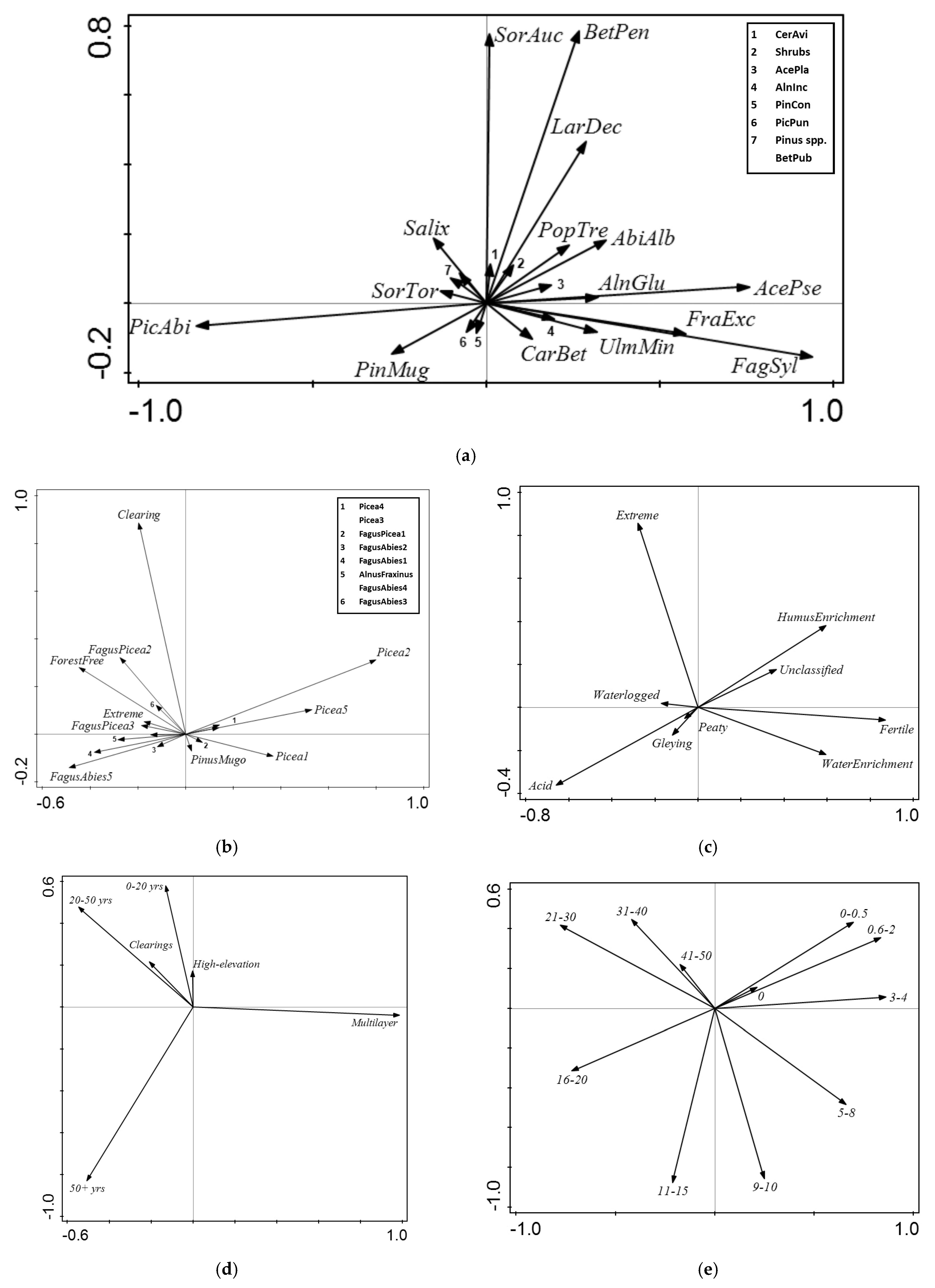
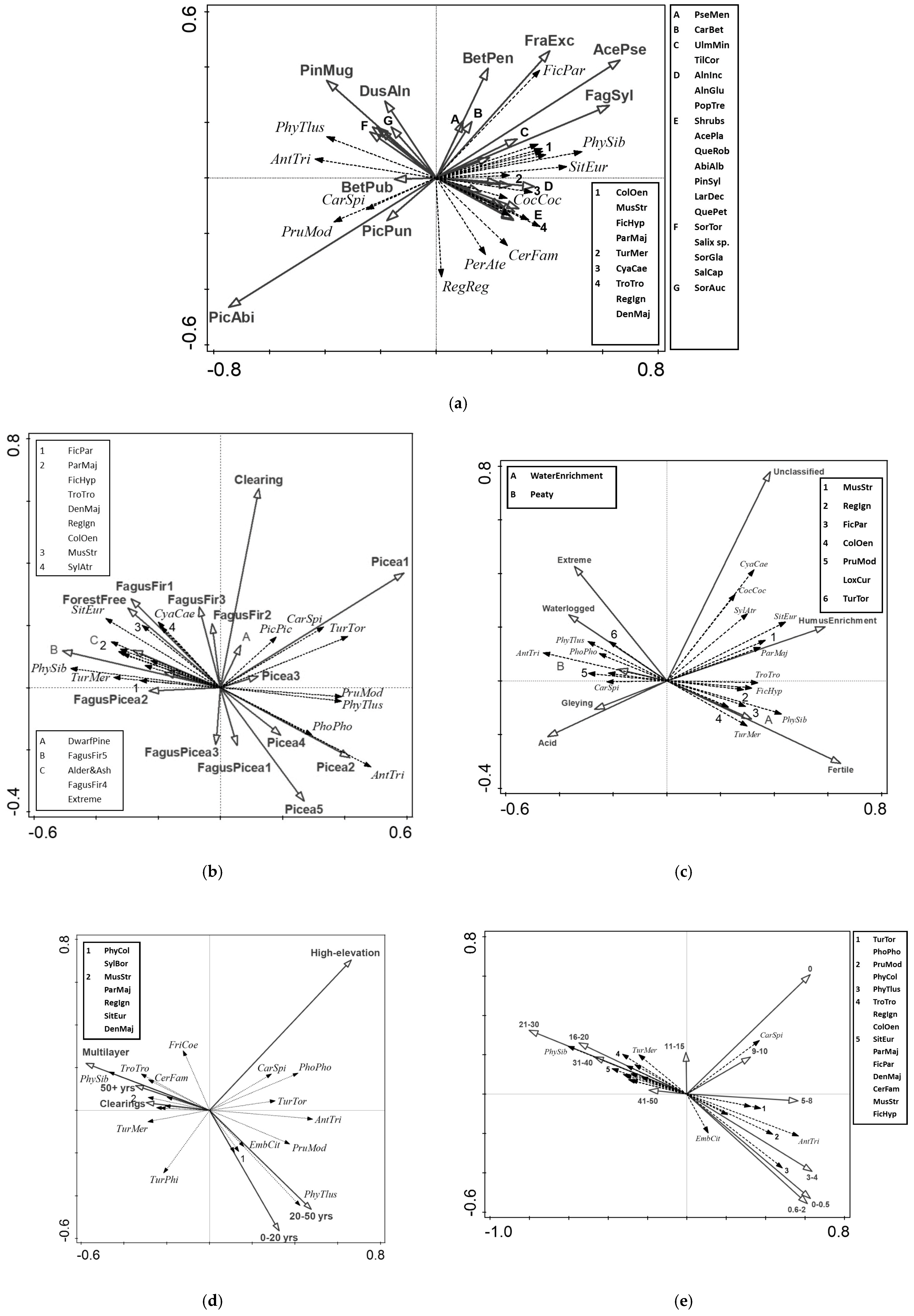
3.3. RDA—Tree Species Composition
3.4. RDA—Target Forest Vegetation
3.5. RDA—Forest Phytosociology
3.6. RDA—Vertical Structure of Forest Vegetation
3.7. RDA—Vegetation Height
4. Discussion
4.1. Use of Forest Management Plans in the Research and Management of Forest Bird Communities
4.2. Bird Community Diversity and Vegetation Characteristics from Forest Management Plans
4.3. Species Composition of Bird Communities and Vegetation Characteristics from Forest Management Plans
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stratfod, J.A.; Sekercioglu, C.H. Birds in forests ecosystems. In Handbook of Forest Ecology, 1st ed.; Peh, K.S.H., Corlett, R.T., Bergeron, Y., Eds.; Routledge: London, UK, 2015. [Google Scholar] [CrossRef]
- Block, W.M.; Brennan, L.A. The habitat concept in ornithology. In Current Ornithology; Power, D.M., Ed.; Plenum Press: London, UK, 1993; pp. 35–91. [Google Scholar]
- Muiruri, E.W.; Rainio, K.; Koricheva, J. Do birds see the forest for the trees? Scale-dependent effects of tree diversity on avian predation of artificial larvae. Oecologia 2016, 180, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.K.; Holmes, R.T. Foraging behavior of forest birds: The relationship among search tactics, diet and habitat structure. Ecology 1982, 63, 1918–1931. [Google Scholar] [CrossRef]
- Whelan, C.J. Foliage structure influences foraging of insectivorous forest birds: An experimental study. Ecology 2001, 82, 219–231. [Google Scholar] [CrossRef]
- Quine, C.P.; Fuller, R.J.; Smith, K.W.; Grice, P.V. Stand management: A threat or opportunity for birds in British woodland? Ibis 2007, 149, 161–174. [Google Scholar] [CrossRef]
- Wood, E.M.; Pidgeon, A.M.; Liu, F.; Mladenoff, D.J. Birds see the trees inside the forest: The potential impacts of changes in forest composition on songbirds during spring migration. For. Ecol. Manag. 2012, 280, 176–186. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W. On bird species diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W.; Preer, J. On bird species diversity II. Prediction of bird censuses from habitat measurements. Am. Nat. 1962, 96, 167–174. [Google Scholar] [CrossRef]
- Jones, J. Habitat selection studies in avian ecology: A critical review. Auk 2001, 118, 557–562. [Google Scholar] [CrossRef]
- James, F.C.; Wamer, N.O. Relationships between temperate forest bird communities and vegetation structure. Ecology 1982, 63, 159–171. [Google Scholar] [CrossRef]
- Canterbury, G.E.; Martin, T.E.; Petit, D.R.; Petit, L.J.; Bradford, D.F. Bird communities and habitat as ecological indicators of forest condition in regional monitoring. Conserv. Biol. 2000, 14, 544–558. [Google Scholar] [CrossRef]
- Lemaitre, J.; Darveau, M.; Zhao, Q.; Fortin, D. Multiscale assessment of the influence of habitat structure and composition on bird assemblages in boreal forest. Biodivers. Conserv. 2012, 21, 3355–3368. [Google Scholar] [CrossRef]
- Moudrý, V.; Moudrá, L.; Barták, V.; Bejček, V.; Gdulová, K.; Hendrychová, M.; Moravec, D.; Musil, P.; Rocchini, D.; Šťastný, K.; et al. The role of the vegetation structure, primary productivity and senescence derived from airborne LiDAR and hyperspectral data for birds diversity and rarity on a restored site. Landsc. Urban Plan. 2021, 210, 104064. [Google Scholar] [CrossRef]
- Laiolo, P. Effects of habitat structure, floral composition and diversity on a forest bird community in north-western Italy. Folia Zool. 2002, 51, 121–128. [Google Scholar]
- Moning, C.; Müller, J. Critical forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests. Ecol. Indic. 2009, 9, 922–932. [Google Scholar] [CrossRef]
- Honkanen, M.; Roberge, J.M.; Rajasärkkä, A.; Mönkkönen, M. Disentangling the effects of area, energy and habitat heterogeneity on boreal forest bird species richness in protected areas. Glob. Ecol. Biogeogr. 2010, 19, 61–71. [Google Scholar] [CrossRef]
- Elo, M.; Roberge, J.M.; Rajasarkka, A.; Mönkkonen, M. Energy density and its variation in space limit species richness of boreal forest birds. J. Biogeogr. 2012, 39, 1462–1472. [Google Scholar] [CrossRef]
- Wiens, J.A. Ecology of Bird Communities. Vol I; Cambridge University Press: Cambridge, UK, 1989; 539p. [Google Scholar]
- Easton, W.E.; Martin, K. The effect of vegetation management on breeding bird communities in British Columbia. Ecol. Appl. 1998, 8, 1092–1103. [Google Scholar] [CrossRef]
- Díaz, L. Influences of forest type and forest structure on bird communities in oak and pine woodlands in Spain. For. Ecol. Manag. 2006, 223, 54–65. [Google Scholar] [CrossRef]
- Donald, P.F.; Fuller, R.J.; Evans, A.D.; Gough, S.J. Effects of forest management and grazing on breeding bird communities in plantations of broadleaved and coniferous trees in western England. Biol. Conserv. 1998, 85, 183–197. [Google Scholar] [CrossRef]
- Reif, J.; Storch, D.; Voříšek, P.; Šťastný, K.; Bejček, V. Bird-habitat associations predict population trends in central European forest and farmland birds. Biodivers. Conserv. 2008, 17, 3307–3319. [Google Scholar] [CrossRef]
- Willson, M.F.; Comet, T.A. Bird Communities of Northern Forests: Ecological Correlates of Diversity and Abundance in the Understory. Condor 1996, 98, 350–362. [Google Scholar] [CrossRef]
- Adams, B.T.; Matthews, S.N. Diverse temperate forest bird assemblages demonstrate closer correspondence to plant species composition than vegetation structure. Ecography 2019, 42, 1752–1764. [Google Scholar] [CrossRef]
- MacArthur, R.H. Population Ecology of Some Warblers of Northeastern Coniferous Forests. Ecology 1958, 39, 599–619. [Google Scholar] [CrossRef]
- MacNally, R.; Parkinson, A.; Horrocks, G.; Young, M. Current Loads of Coarse Woody Debris on Southeastern Australian Floodplains: Evaluation of Change and Implications for Restoration. Restor. Ecol. 2002, 10, 627–635. [Google Scholar] [CrossRef]
- Holmes, R.T.; Robinson, S.K. Tree species preferences of foraging insectivorous birds in a northem hardwoods forest. Oecologia 1981, 48, 31–35. [Google Scholar] [CrossRef]
- Gabbe, A.P.; Robinson, S.K.; Brawn, J.D. Tree-Species Preferences of Foraging Insectivorous Birds: Implications for Floodplain Forest Restoration. Conserv. Biol. 2002, 16, 462–470. [Google Scholar] [CrossRef]
- Hartung, S.C.; Brawn, J.D. Effects of Savanna Restoration on the Foraging Ecology of Insectivorous Songbirds. Condor 2005, 107, 879–888. [Google Scholar] [CrossRef]
- Holmes, R.T.; Schultz, J.C. Food availability for forest birds: Effects of prey distribution and abundance on bird foraging. Can. J. Zool. 1988, 66, 720–728. [Google Scholar] [CrossRef]
- Shutt, J.D.; Bolton, M.; Benedicto Cabello, I.; Burgess, M.D.; Phillimore, A.B. The effects of woodland habitat and biogeography on blue tit Cyanistes caeruleus territory occupancy and productivity along a 220 km transect. Ecography 2018, 41, 1967–1978. [Google Scholar] [CrossRef]
- Tomppo, E.O.; Schadauer, K. Harmonization of National Forest Inventories in Europe: Advances under COSTAction E43. For. Sci. 2012, 56, 191–200. [Google Scholar] [CrossRef]
- Kučera, M.; Adolt, R. (Eds.) Národní Inventarizace Lesů v České Republice—Výsledky Druhého Cyklu 2011–2015, 1st ed.; Ústav pro Hospodářskou Úpravu Lesů Brandýs nad Labem: Brandýs nad Labem-Stará Boleslav, Czech Republic, 2019. [Google Scholar]
- Flousek, J.; Gramsz, B.; Telenský, T. Ptáci Krkonoš—Atlas Hnízdního Rozšíření 2012–2014; Správa KRNAP: Vrchlabí, Czech Republic, 2015; 480p. [Google Scholar]
- Branton, M.; Richardson, J.S. Assessing the value of the umbrella-species concept for conservation planning with meta-analysis. Conserv. Biol. 2011, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Roberge, J.M.; Angelstam, P.E.R. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Suter, W.; Graf, R.F.; Hess, R. Capercaillie (Tetrao urogallus) and avian biodiversity: Testing the umbrella-species concept. Conserv. Biol. 2002, 16, 778–788. [Google Scholar] [CrossRef]
- Flousek, J.; Hartmanová, O.; Štursa, J.; Potocki, J. (Eds.) Krkonoše: Příroda, Historie, Život; Baset: Praha, Czech Republic, 2007; 863p. [Google Scholar]
- Lokvenc, T. Toulky Krkonošskou Minulostí; Kruh: Hradec Králové, Czech Republic, 1978; 264p. [Google Scholar]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques; Elsevier Science: Amsterdam, The Netherlands, 2000; 302p. [Google Scholar]
- Ter Braak, C.J.; Šmilauer, P.; Dray, S. Algorithmus and biplots for double constrained correspondence analysis. Environ. Ecol. Stat. 2018, 25, 171–197. [Google Scholar] [CrossRef]
- Černý, M.; Zahradníček, J.; Pařez, J.; Moravčík, P. Hospodářská úprava lesů na bázi statistické provozní inventarizace. Lesn. Práce 2000, 79, 60–62. [Google Scholar]
- Černý, M.; Zahradníček, J.; Pařez, J. Metoda integrované hospodářské úpravy lesů v lesích s bohatou strukturou. Lesn. Práce 2001, 80, 24–27. [Google Scholar]
- Plíva, K. Typologický Systém ÚHÚL; Brandýs nad Labem, ÚHÚL: Brandýs nad Labem-Stará Boleslav, Czech Republic, 1971. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 16 April 2025).
- Barbe, L.; Morel, R.; Rantier, Y.; Lebas, J.F.; Butet, A. Bird communities of a temperate forest: Spatio-temporal partitioning between resident and migratory species. J. Ornithol. 2018, 159, 457–469. [Google Scholar] [CrossRef]
- Reise, J.; Kukulka, F.; Flade, M.; Winter, S. Characterising the richness and diversity of forest bird species using National Forest Inventory data in Germany. For. Ecol. Manag. 2019, 432, 799–811. [Google Scholar] [CrossRef]
- Leitão, P.J.; Toraño Caicoya, A.; Dahlkamp, A.; Guderjan, L.; Griesser, M.; Haverkamp, P.J.; Norden, J.; Snäll, T.; Schröder, B. Impacts of forest management on forest bird occurrence patterns—A case study in Central Europe. Front. For. Glob. Change 2022, 5, 786556. [Google Scholar] [CrossRef]
- Fearer, T.M.; Prisley, S.P.; Stauffer, D.F.; Keyser, P.D. A method for integrating the Breeding Bird Survey and Forest Inventory and Analysis databases to evaluate forest bird–habitat relationships at multiple spatial scales. For. Ecol. Manag. 2007, 243, 128–143. [Google Scholar] [CrossRef]
- Birčák, T.; Reif, J. The effects of tree age and tree species composition on bird species richness in a Central European montane forest. Biologia 2015, 70, 1528–1536. [Google Scholar] [CrossRef]
- du Bus de Warnaffe, G.; Deconchat, M. Impact of four silvicultural systems on birds in the Belgian Ardenne: Implications for biodiversity in plantation forests. Biodivers. Conserv. 2008, 17, 1041–1055. [Google Scholar] [CrossRef]
- Hewson, C.M.; Austin, G.E.; Gough, S.J.; Fuller, R.J. Species-specific responses of woodland birds to stand-level habitat characteristics: The dual importance of forest structure and floristics. For. Ecol. Manag. 2011, 261, 1224–1240. [Google Scholar] [CrossRef]
- Bergner, A.; Avcı, M.; Eryiğit, H.; Jansson, N.; Niklasson, M.; Westerberg, L.; Milberg, P. Influences of forest type and habitat structure on bird assemblages of oak (Quercus spp.) and pine (Pinus spp.) stands in southwestern Turkey. For. Ecol. Manag. 2015, 336, 137–147. [Google Scholar] [CrossRef]
- Jobbagy, E.J.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Pokorný, P. Neklidné Časy. Kapitoly ze Společných Dějin Přírody a Lidí; Dokořán: Praha, Czech Republic, 2011; 370p. [Google Scholar]
- Šebková, B.; Šamonil, P.; Janík, D.; Adam, D.; Král, K.; Vrška, T.; Hort, L.; Unar, P. Spatial and volume patterns of an unmanaged submontane mixed forest in Central Europe: 160 years of spontaneous dynamics. For. Ecol. Manag. 2011, 262, 873–885. [Google Scholar] [CrossRef]
- Wesołowski, T. “Lifespan” of woodpecker-made holes in a primeval temperate forest: A thirty year study. For. Ecol. Manag. 2011, 262, 1846–1852. [Google Scholar] [CrossRef]
- Seidling, W.; Travaglini, D.; Meyer, P.; Waldner, P.; Fischer, R.; Granke, O.; Chirici, G.; Corona, P. Dead wood and stand structure-relationships for forest plots across Europe. iFor.-Biogeosci. For. 2014, 7, 269. [Google Scholar] [CrossRef]
- Hurlbert, A.H. Species-energy relationships and habitat complexity in bird communities. Ecol. Lett. 2004, 7, 714–720. [Google Scholar] [CrossRef]
- Chettri, N.; Deb, D.C.; Sharma, E.; Jackson, R. The relationship between bird communities and habitat: A study along a trekking corridor in the Sikkim Himalaya. Mt. Res. Dev. 2005, 25, 235–243. [Google Scholar] [CrossRef]
- Kroodsma, R.L. Effect of edge on breeding forest bird species. Wilson Bull. 1984, 96, 426–436. [Google Scholar] [CrossRef]
- Yahner, R.H. Changes in wildlife communities near edges. Conserv. Biol. 1988, 2, 333–339. [Google Scholar] [CrossRef]
- Paquet, J.Y.; Vandevyvre, X.; Delahaye, L.; Rondeux, J. Bird assemblages in a mixed woodland–farmland landscape: The conservation value of silviculture-dependant open areas in plantation forest. For. Ecol. Manag. 2006, 227, 59–70. [Google Scholar] [CrossRef]
- Moussy, C.; Burfield, I.J.; Stephenson, P.J.; Newton, A.F.E.; Butchart, S.H.M.; Sutherland, W.J.; Gregory, R.D.; McRae, L.; Bubb, P.; Roesler, I.; et al. A quantitative global review of species population monitoring. Conserv. Biol. 2021, 36, e13721. [Google Scholar] [CrossRef]


| Species Composition of Vegetation Cover | Spatial Structure of Vegetation | |||||
|---|---|---|---|---|---|---|
| Effect | p | Tree Species | Forest Phytocenology | Target Forest Vegetation | Vertical Structure of Vegetation | Vegetation Height |
| increases diversity | <0.001 | European Beech | non-forest habitat | non-forest habitat | 20–30 m | |
| <0.01 | Pedunculate Oak, European Larch, Scots Pine, Aspen | non-forest habitat | multi-layered stand | 30–40 m | ||
| <0.05 | Wild Cherry, Alder, Goat Willow, Sycamore Maple, Sessile Oak, Rowan Tree | enriched rocky habitat, fertile lush habitat | exposed acid fir, exposed live fir, acid fir | |||
| reduces diversity | <0.001 | Norway Spruce | gley-poor habitat | watterlogged spruce, sour spruce | ||
| <0.01 | 0–0.5 m | |||||
| <0.05 | Dwarf Mountain Pine | gley habitat | dwarf and alpine spruce forests, scrub pine forests | |||
| Analysis | 1st Axis | 2nd Axis | 3rd Axis | 4th Axis | |
|---|---|---|---|---|---|
| RDA | Vegetation high | 8.61 | 1.86 | 1.25 | 0.58 |
| RDA | Target vegetation | 7.14 | 1.74 | 1.41 | 0.91 |
| RDA | Tree species composition | 6.88 | 2.1 | 1.44 | 1.36 |
| RDA | Forest phytocenology | 6.05 | 1.86 | 1.5 | 1.09 |
| RDA | Vertical stand structure | 6.01 | 1.62 | 1.05 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimurda, J.; Šmilauer, P.; Fuchs, R. Forestry Plans as the Source of Environmental Data for the Analysis of Bird Community Composition. Diversity 2025, 17, 351. https://doi.org/10.3390/d17050351
Šimurda J, Šmilauer P, Fuchs R. Forestry Plans as the Source of Environmental Data for the Analysis of Bird Community Composition. Diversity. 2025; 17(5):351. https://doi.org/10.3390/d17050351
Chicago/Turabian StyleŠimurda, Jakub, Petr Šmilauer, and Roman Fuchs. 2025. "Forestry Plans as the Source of Environmental Data for the Analysis of Bird Community Composition" Diversity 17, no. 5: 351. https://doi.org/10.3390/d17050351
APA StyleŠimurda, J., Šmilauer, P., & Fuchs, R. (2025). Forestry Plans as the Source of Environmental Data for the Analysis of Bird Community Composition. Diversity, 17(5), 351. https://doi.org/10.3390/d17050351





