Biodiversity and Phytochemical Characterization of Adonis volgensis Populations from Central and Northern Kazakhstan: Insights into Bioactivity and Toxicity
Abstract
1. Introduction
- Assess the current ecological status of A. volgensis populations in Akmola and Kostanay regions of Kazakhstan.
- Conduct a comprehensive phytochemical analysis of extracts (chloroform, ethyl acetate, and ethanolic) from the aerial parts of the plant.
- Evaluate the biological activities of these extracts to determine their therapeutic potential.
- Compare the phytochemical profiles and biological activities between regional populations to identify geographical variations.
2. Materials and Methods
2.1. Study Area
2.2. Plant Material
2.3. Geobotanical and Phytocenotic Surveys
2.4. Extract Preparation
- Chloroform (CHCl3) (Ad.1), ethyl acetate (Ad.2), and ethanolic (EtOH) (Ad.3) extracts from the aerial parts.
- Chloroform (CHCl3) (Ad.Q1), ethyl acetate (Ad.Q2), and ethanolic (EtOH) (Ad.Q3) extracts from the aerial parts.
2.5. Phytochemical Composition
2.5.1. Gas Chromatography Coupled with Mass Spectrometry, GC–MS
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5.3. Determination of Total Phenolic and Flavonoid Contents
2.6. Biological Activity
2.6.1. Antioxidant Potential
2.6.2. Artemia salina Toxicity Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phytocenotic Characteristics of A. volgensis
3.2. Morphological and Quantitative Variation Across Populations of A. volgensis
3.3. Phytochemical Analysis
3.3.1. GC–MS Analysis
3.3.2. FTIR Analysis
3.3.3. Total Phenolic and Flavonoid Contents of A. volgensis
3.4. Biological Activity
3.4.1. Antioxidant Activity
- FRAP (Ferric Reducing Antioxidant Power) assay
- DPPH anti-radical scavenging activity
3.4.2. Artemia salina Toxicity Assay
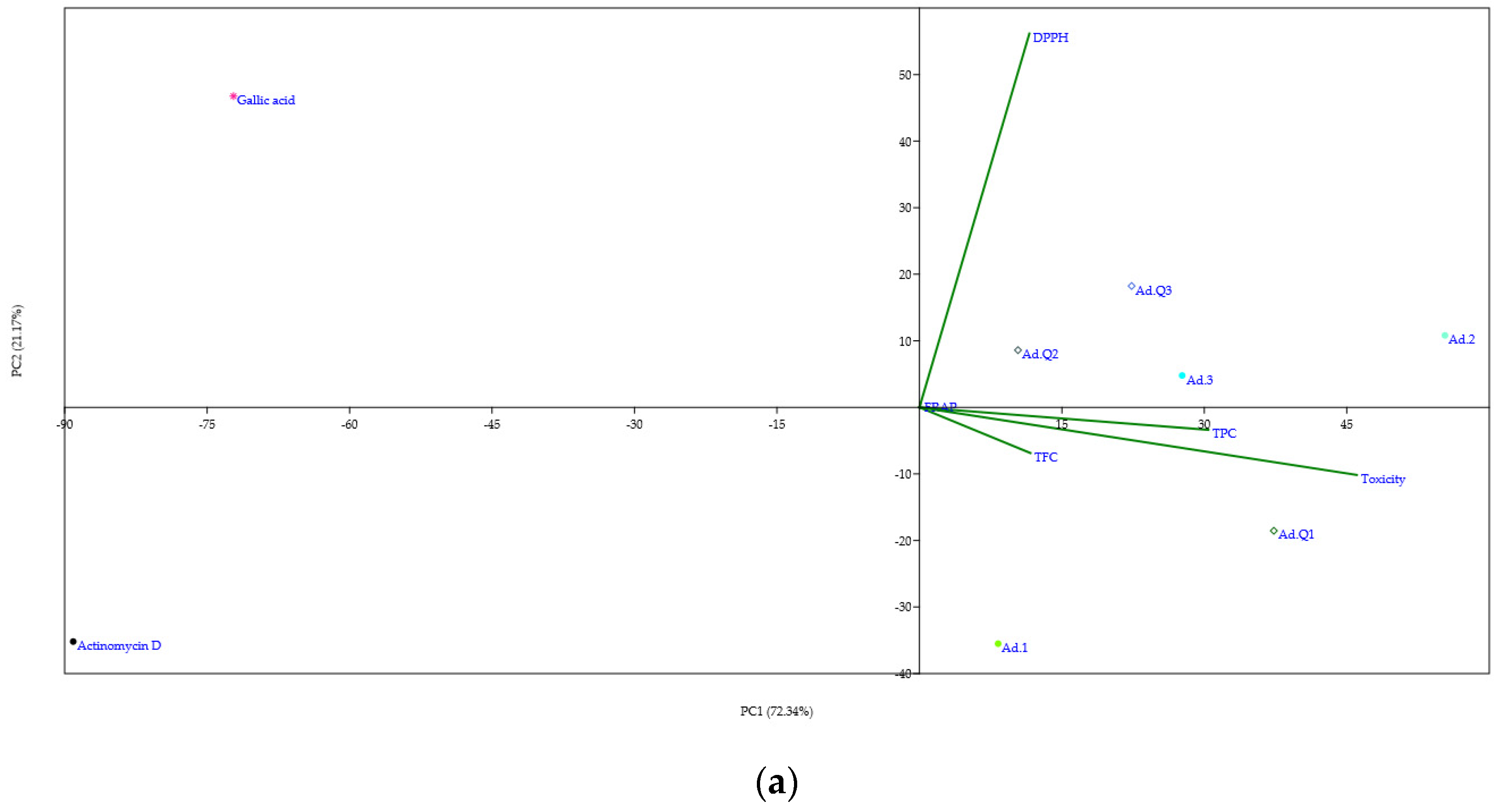
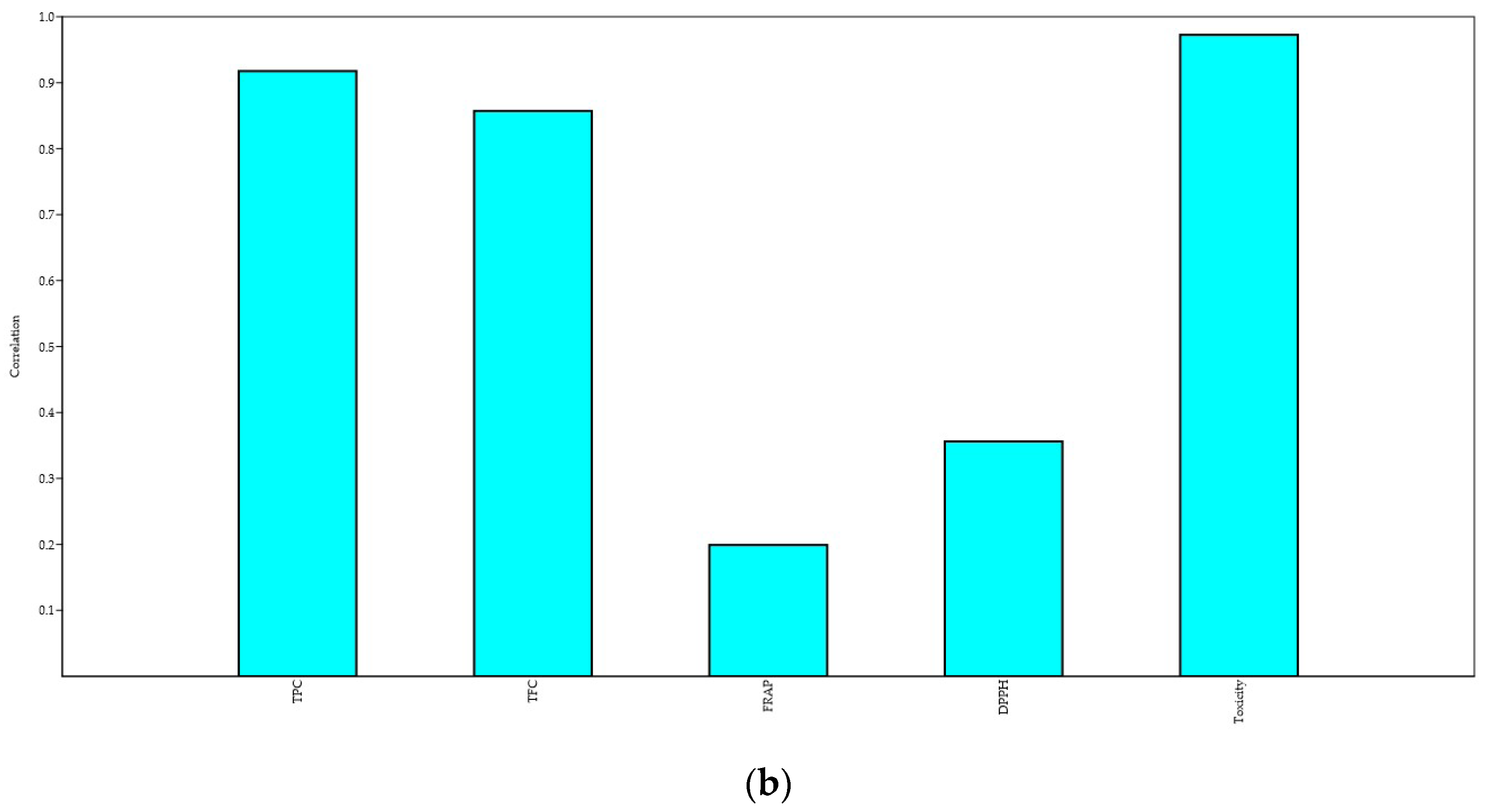
3.4.3. Principal Component Analysis (PCA)
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanova, T.; Marchev, A.; Chervenkov, M.; Bosseva, Y.; Georgiev, M.; Kozuharova, E.; Dimitrova, D. Catching the green—Diversity of ruderal spring plants traditionally consumed in bulgaria and their potential benefit for human health. Diversity 2023, 15, 435. [Google Scholar] [CrossRef]
- Kerteshev, T.; van Zyl, H. Biodiversity Finance Initiative Kazakhstan (BIOFIN). 2021. Available online: https://www.biofin.org/kazakhstan (accessed on 8 September 2024).
- Nyamgerel, N.; Baasanmunkh, S.; Oyuntsetseg, B.; Bayarmaa, G.A.; Erst, A.; Park, I.; Choi, H.J. Insight into chloroplast genome structural variation of the Mongolian endemic species Adonis mongolica (Ranunculaceae) in the Adonideae tribe. Sci. Rep. 2023, 13, 22014. [Google Scholar] [CrossRef]
- Taldybay, A.; Aydarbayeva, D.; Aksoy, A.; Sitpayeva, G.; Baiseitova, A.; Jenis, J. Ajania fastigiata in Zhetysu Alatau: Distribution, morphological characterization, phytochemical profiles, and optimization of extraction of bioactive constituents. J. Appl. Res. Med. Arom. Plants 2024, 40, 100540. [Google Scholar] [CrossRef]
- Poshkurlat, A.P. Rod Goritsvet—Adonis L. Sistematika, Rasprostraneniye, Biologiya [The Genus Adonis L. Systematics, Distribution, Biology]; Nauka: Moscow, Russia, 2000; 199p. [Google Scholar]
- Luferov, A.N. Genus Adonis L. In Flora of the Lower Volga Region, 1st ed.; Reshetnikov, N.M., Ed.; Dicotyledonous flowering plants. Part 1. Salicaceae—Droseraceae. (Resp. Ed.); Main Botanical Garden named after N. V. Tsitsin RAS; KMK Scientific Press: Moscow, Russia, 2018; Volume 2, pp. 347–350. [Google Scholar]
- Karahan, F.; Avşar, C.; Turkmen, M.; Gezici, S.; Ilcim, A. Comparative study on phytochemical profiles, antiproliferative, antimicrobial and antioxidant activities of Adonis species from Turkey. Pharm. Chem. J. 2022, 56, 667–678. [Google Scholar] [CrossRef]
- Karahan, F.; İlcim, A.; Türkoğlu, A.; Haliloğlu, K. Molecular phylogeny based on its sequences of nrDNA ITS of Adonis (Linnaeus, 1753) (Ranunculaceae) from various ecological sites of Turkey. Mol. Biol. Rep. 2022, 52, 1075–1086. [Google Scholar]
- Wang, W.T. Revision of Adonis (Ranunculaceae) (1). Bull. Bot. Res. 1994, 14, 1–31. [Google Scholar]
- Wang, W.T. Revision of Adonis (Ranunculaceae) (2). Bull. Bot. Res. 1994, 14, 105–138. [Google Scholar]
- Son, D.C.; Park, B.K.; Chang, K.S.; Choi, K.; Shin, C.H. Cladistic analysis of the section Adonanthe under genus Adonis L. (Ranunculaceae) from East Asia. J. Asia-Pac. Biodivers. 2017, 10, 232–236. [Google Scholar] [CrossRef]
- POWO Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 4 August 2024).
- Abdulina, S.A. Spisok Sosudistykn Rastenii Kazakhstana; Academy of Sciences: Almaty, Kazakhstan, 1999; pp. 1–187. [Google Scholar]
- Kulymbet, K.; Mukhitdinov, N.; Kubentayev, S.A.; Tynybayeva, K.; Tastanbekova, A.; Kurmanbayeva, M.; Gafforov, Y.; Kaparbay, R.; Zhumagul, M. The current state of the cenopopulations of Adonis tianschanica (Adolf) Lipsch. (Ranunculaceae) in Southeast Kazakhstan. Biodiversitas 2023, 24, 4359–4372. [Google Scholar] [CrossRef]
- Institute of Zoology, Ministry of Education and Science of the Republic of Kazakhstan. The Red Book of Kazakhstan; Astana Ltd.: Astana, Kazakhstan, 2014; Volume 2, p. 452. (In Russian) [Google Scholar]
- Gemejiyeva, N.G.; Grudzinskaya, L.M. Current state and prospects for studies on the diversity of medicinal flora in Kazakhstan. In Vegetation of Central Asia and Environs, 1st ed.; Egamberdieva, D., Öztürk, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 239–262. [Google Scholar]
- Min, B.M. The characteristics of seed production in an Adonis multiflora (Ranunculaceae) population. J. Ecol. Environ. 2014, 37, 165–175. [Google Scholar] [CrossRef]
- Gafforov, Y.; Rašeta, M.; Zafar, M.; Makhkamov, T.; Yarasheva, M.; Chen, J.-J.; Zhumagul, M.; Wang, M.; Ghosh, S.; Abbasi, A.M.; et al. Exploring biodiversity and ethnobotanical significance of Solanum species in Uzbekistan: Unveiling the cultural wealth and ethnopharmacological uses. Front. Pharmacol. 2024, 14, 1287793. [Google Scholar] [CrossRef]
- Grudzinskaia, L.M.; Gemedzhieva, N.G.; Nelina, N.V.; Karzhaubekova, Z.Z. Annotirovannyi Spisok Lechebnykh Rastenii Kazakhstana, Almaty, Kazakhstan. 2014; 200p.
- Mohadjerani, M.; Tavakoli, R.; Hosseinzadeh, R. Fatty acid composition, antioxidant and antibacterial activities of Adonis wolgensis L. extract. Avicenna J. Phytomed. 2014, 4, 24–30. [Google Scholar]
- Komissarenko, N.F.; Yatsyuk, V.Y.; Korzennikova, E.P. Flavonoids of Adonis wolgensis. Chem. Nat. Compd. 1973, 9, 417. [Google Scholar] [CrossRef]
- Komissarenko, N.F.; Korzennikova, É.P.; Yatsyuk, V.Y. Cardenolides of Adonis wolgensis. Chem. Nat. Compd. 1974, 10, 838. [Google Scholar] [CrossRef]
- Tavakoli, R.; Mohadjerani, M.; Hosseinzadeh, R.; Tajbakhsh, M.; Naqinezhad, A. Determination of chemical composition of essential oil from aerial parts of Adonis wolgensis grown in North of Iran by GC-MS. Anal. Chem. Lett. 2013, 2, 125–128. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Hu, X.; Zhang, H.; Lu, C.; Cui, G.; Luo, X. Orientin and neuropathic pain in rats with spinal nerve ligation. Int. Immunopharmacol. 2018, 58, 72–79. [Google Scholar] [CrossRef]
- Kim, S.J.; Pham, T.H.; Bak, Y.; Ryu, H.W.; Oh, S.R.; Yoon, D.Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKC/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF7 breast cancer cells. Phytomedicine 2018, 50, 35–42. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Shang, X.; Miao, X.; Yang, F.; Wang, C.; Li, B.; Wang, W.; Pan, H.; Guo, X.; Zhang, Y.; Zhang, J. The genus Adonis as an important cardiac folk medicine: A review of the ethnobotany, phytochemistry and pharmacology. Front. Pharmacol. 2019, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Zhang, X.W.; Xu, W.J.; Huang, H.Y.; Ma, Y.; Bai, H.; Gong, J.; Ni, S. Overview of pharmacological research on Adonis L. Agric. Sci. Technol. 2015, 16, 626–628. [Google Scholar]
- Kupriyanov, A.N. Summary of the Flora of the Kazakh Small Hills; Russian Academy of Sciences, Siberian Department, Research Center of Coal and Coal Chemistry, Institute of Human Ecology, Kuzbass Botanical Garden—Academic Publishing House “Geo”: Novosibirsk, Russia, 2020; 358p. [Google Scholar]
- Bykov, B.A. Introduction to Phytocenology; ANKazSSR Publishing House: Alma-Ata, Kazakhstan, 1970. [Google Scholar]
- Rabotnov, T.A. Determination of the Age Composition of Species Populations in a Community. Field Geobotany; M-LPublishing, House of the Academy of Sciences of the USSR: Moscow, Russia, 1964. [Google Scholar]
- Kubentayev, S.A.; Suleimenov, A.N.; Kotukhov, J.A.; Danilova, A.N.; Sumbembayev, A.A. Phytocenotic characteristics and stocks of the main medicinal plants of the South-Western Altai (East Kazakhstan). Eurasia J. Biosci. 2018, 12, 355–356. [Google Scholar]
- Zhumagul, M.; Kurmanbayeva, M.; Kubentayev, S.; Kurmantayeva, A.; Turgumbayeva, A.; Nurpeissova, I.; Cherepkova, N.; Moldakaryzova, A. Studies on the biological activity of different populations of the medicinal plant Rhodiola rosea L. (Golden root). Pak. J. Bot. 2023, 55, 1857–1865. [Google Scholar] [CrossRef]
- Shvidchenko, A.V.; Odinokov, A.S.; Primachenko, O.N.; Gofman, I.V.; Yevlampieva, N.P.; Marinenko, E.A.; Lebedev, V.T.; Kuklin, A.I.; Kulvelis, Y.V. Improving PFSA membranes using sulfonated nanodiamonds. Membranes 2023, 13, 712. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid and concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Parra, A.L.; Yhebra, R.S.; Sardiñas, I.G.; Buela, L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar]
- Yerezhepova, N.; Kurmanbayeva, M.; Terletskaya, N.; Zhumagul, M.; Kebert, M.; Rašeta, M.; Gafforov, Y.; Jalmakhanbetova, R.; Razhanov, M. New data on phytochemical and morphophysiological characteristics of Platycladus orientalis L. Franco and Thuja occidentalis L. conifer trees in polluted urban areas of Kazakhstan. Forests 2024, 15, 790. [Google Scholar] [CrossRef]
- Lal, R.; Reicosky, D.C.; Hanson, J.D. Evolution of the plow over 10,000 years and the rationale for no-till farming. Soil Till. Res. 2007, 93, 1–12. [Google Scholar] [CrossRef]
- Janišová, M.; Bojko, I.; Ivașcu, C.M.; Iuga, A.; Biro, A.; Magnes, M. Grazing hay meadows: History, distribution, and ecological context. Appl. Veg. Sci. 2023, 26, e12723. [Google Scholar] [CrossRef]
- Sultangazina, G.Z.; Khrustaleva, I.A.; Kupriyanov, A.N. Redkie rasteniia NATSIONAL’NOGO prirodnogo parka “Burabai”. Vestnik Kazakhskogo natsional’nogo universiteta. Seriia Ekol. 2013, 39, 264–270. [Google Scholar]
- Beishova, I.S.; Sultangazina, G.; Ulyanov, V.A.; Beishov, R.S.; Beltyukova, N.N. Assessment of the state of gene pools of the North Kazakhstan populations of Adonis wolgensis Stev. based on polymorphism of ISSR markers. Ann. Agri Bio Res. 2019, 24, 160–164. [Google Scholar]
- Beishova, I.S.; Sultangazina, G.; Ulyanov, V.A.; Beyshov, R.S.; Beltyukova, N.N.; Kutinskaia, A.M. Genetic diversity of the coenopopulations of Adonis wolgensis Stev. growing in the Northern regions of Kazakhstan. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2019, 94, 17–25. [Google Scholar]
- Sultangazina, G.J.; Kuprijanov, A.N.; Kuprijanov, O.A.; Steshenko, M.Y. The structure of Adonis wolgensis Stev. coenopopulation in the conditions of Northern Kazakhstan. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2020, 99, 134–139. [Google Scholar] [CrossRef]
- Sultangazina, G.J.; Steshenko, M.Y.; Novak, Y.O. Cenopopulations of Adonis wolgensis stev. in the conditions of northern Kazakhstan. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2022, 107, 123–126. [Google Scholar]
- Karimova, O.A.; Abramova, L.M.; Mustafina, A.N.; Golovanov, Y.M. State of coenopopulations of Anthemis trotzkiana (Asteraceae) in Orenburg region. Bot. Zhurnal 2018, 103, 740–754. [Google Scholar]
- He, J.; Ning, C.; Zhang, W.; Halik, Ü.; Shen, Z. The effect of elevation on the population structure, spatial patterning and intraspecific interactions of Picea schrenkiana in the Eastern Tianshan Mountains: A test of the stress gradient hypothesis. Forests 2023, 14, 2092. [Google Scholar] [CrossRef]
- McNichol, B.H.; Russo, S.E. Plant species’ capacity for range shifts at the habitat and geographic scales: A trade-off-based framework. Plants 2023, 12, 1248. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.K.; Campbell, D.R. Pollinator visitation rate and effectiveness vary with flowering phenology. Am. J. Bot. 2020, 107, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Poethig, R.S. Vegetative phase change and shoot maturation in plants. Curr. Top. Dev. Biol. 2013, 105, 125–152. [Google Scholar]
- Chen, R.; Shi, C.; Zhang, L.; Tu, C.; Weiner, J. Potential role of kin selection in the transition from vegetative to reproductive allocation in plants. J. Plant Ecol. 2023, 6, rtad025. [Google Scholar] [CrossRef]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional health players in the management of obesity and its related disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Bae, Y.J.; Rarison, R.H.G.; Bang, J.H.; Park, S.Y.; Jeong, W.S. Anti-inflammatory and antioxidant activities of lipophilic fraction from Liriope platyphylla seeds using network pharmacology, molecular docking, and in vitro experiments. Int. J. Mol. Sci. 2023, 24, 14958. [Google Scholar] [CrossRef]
- Obeidnejad, E.; Kavoosi, G.; Saharkhiz, M.J. Antioxidant, anti-amylase, anti-lipase, and efficiency of Satureja fatty acid on the anti-inflammatory parameters in lipopolysaccharide-stimulated macrophage through Nrf2/NF-kB/NADH oxidase pathway. Sci. Rep. 2024, 14, 12490. [Google Scholar] [CrossRef]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and applications-A review. Front. Sustain. 2022, 3, 826190. [Google Scholar] [CrossRef]
- Shahzad, N.; Khan, W.; Md, S.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.; Passos, T.M.; Quilty, B.; Thiré, R.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Materia 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Ponomarenko, A.A.; Komissarenko, N.F.; Stukkei, K.L. Coumarins from Adonis amurensis. Khim. Prir. Soedin. 1971, 5, 661–662. [Google Scholar]
- Komissarenko, N.F.; Yatsyuk, Y.; Korzennikova, P. Flavonoids of Adonis wolgensis. Khim. Prir. Soedin. 1973, 3, 439. [Google Scholar] [CrossRef]
- Evdokimov, P.K. Composition of Adonis leiosepala. Khim. Prir. Soedin. 1979, 5, 736. [Google Scholar]
- Lamzhav, A. Coumarins of Adonis mongolica. Khim. Prir. Soedin. 1983, 3, 402. [Google Scholar] [CrossRef]
- Ucuncu, O.; BaltacI, C.; Akar, Z.; Duzgun, A.O.; Cuce, M.; Kandemir, A. Biological activities and phytochemical screening of ethanol extracts from Adonis paryadrica (Ranunculaceae). Farmacia 2020, 68, 1062–1068. [Google Scholar] [CrossRef]
- Padmapriya, R.; Ashwini, S.; Raveendran, R. In vitro antioxidant and cytotoxic potential of different parts of Tephrosia purpurea. Res. Pharm. Sci. 2017, 12, 31–37. [Google Scholar]
- Grabowska, K.; Pietrzak, W.; Paśko, P.; Sołtys, A.; Galanty, A.; Żmudzki, P.; Nowak, R.; Podolak, I. Antihyaluronidase and antioxidant potential of Atriplex sagittata Borkh. in relation to phenolic compounds and triterpene saponins. Molecules 2023, 28, 982. [Google Scholar] [CrossRef]
- Rašeta, M.; Popović, M.; Beara, I.; Šibul, F.; Zengin, G.; Krstić, S.; Karaman, M. Anti-inflammatory, antioxidant and enzyme inhibition activities in correlation with mycochemical profile of selected indigenous Ganoderma spp. from Balkan region (Serbia). Chem. Biodivers. 2021, 18, e2000828. [Google Scholar] [CrossRef]
- Rašeta, M.; Mišković, J.; Berežni, S.; Kostić, S.; Kebert, M.; Matavulj, M.; Karaman, M. Antioxidant proficiency in Serbian mushrooms: A comparative study on Hydnum repandum L. 1753 from mycorrhizal and edible niches. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljević, S.; Rašeta, M.; Berezni, S.; Passamonti, S.; Tramer, F. Four selected commercial seaweeds: Biologically active compounds, antioxidant and cytotoxic properties. Int. J. Food Sci. Nutr. 2021, 72, 757–766. [Google Scholar] [CrossRef]
- Rašeta, M.; Kebert, M.; Mišković, J.; Rakić, M.; Kostić, S.; Čapelja, E.; Karaman, M. Polyamines in edible and medicinal fungi from Serbia: A novel perspective on neuroprotective properties. J. Fungi 2024, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Zhu, R.; Kang, T.; Tong, L.; Xie, H.; Wang, H.; University-Lishui, T. Cytotoxic activities of some selected medicinal plants of the genus Euphorbia. J. Med. Plants Res. 2011, 5, 6766–6769. [Google Scholar]
- Nuringtyas, T.R.; Isromarina, R.; Septia, Y.; Hidayati, L.; Wijayanti, N.; Moeljopawiro, S. The antioxidant and cytotoxic activities of the chloroform extract of agarwood (Gyrinops versteegii (Gilg.) Domke) leaves on HeLa cell lines. In Proceedings of the 5th International Conference on Biological Science, Bucharest, Romania, 7–9 March 2018; Volume 2002, p. 020067. [Google Scholar]
- Cholich, L.A.; Pistán, M.E.; Torres, A.M.; Ortega, H.H.; Gardner, D.R.; Bustillo, S. Characterization and cytotoxic activity on glial cells of alkaloid-enriched extracts from pods of the plants Prosopis flexuosa and Prosopis nigra (Fabaceae). Rev. Biol. Trop. 2020, 69, 197–206. [Google Scholar] [CrossRef]
- König, S. The composition and biochemical properties of Strophantus (Apocynaceae), with a focus on S. sarmentosus. Molecules 2024, 29, 2847. [Google Scholar] [CrossRef]
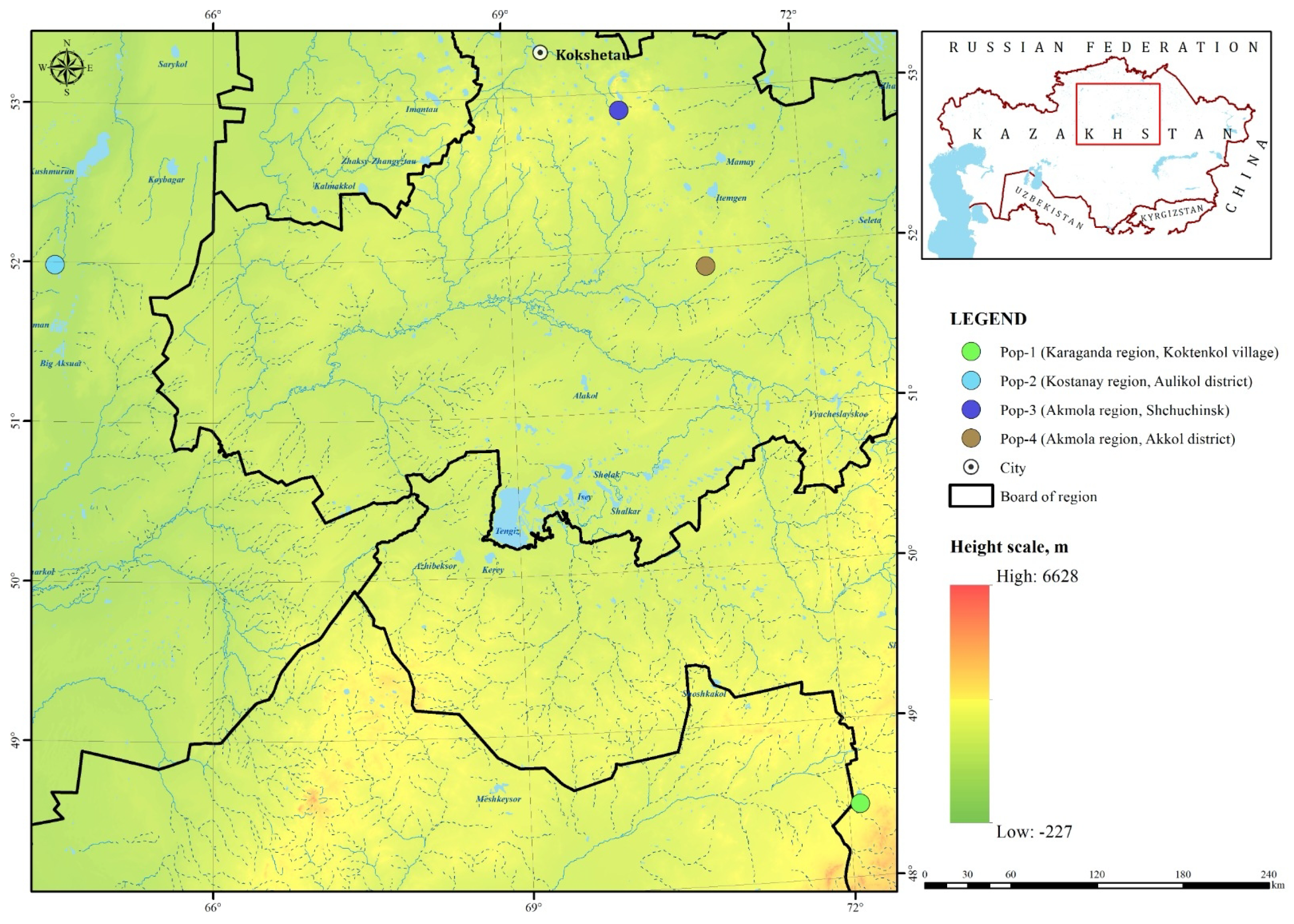
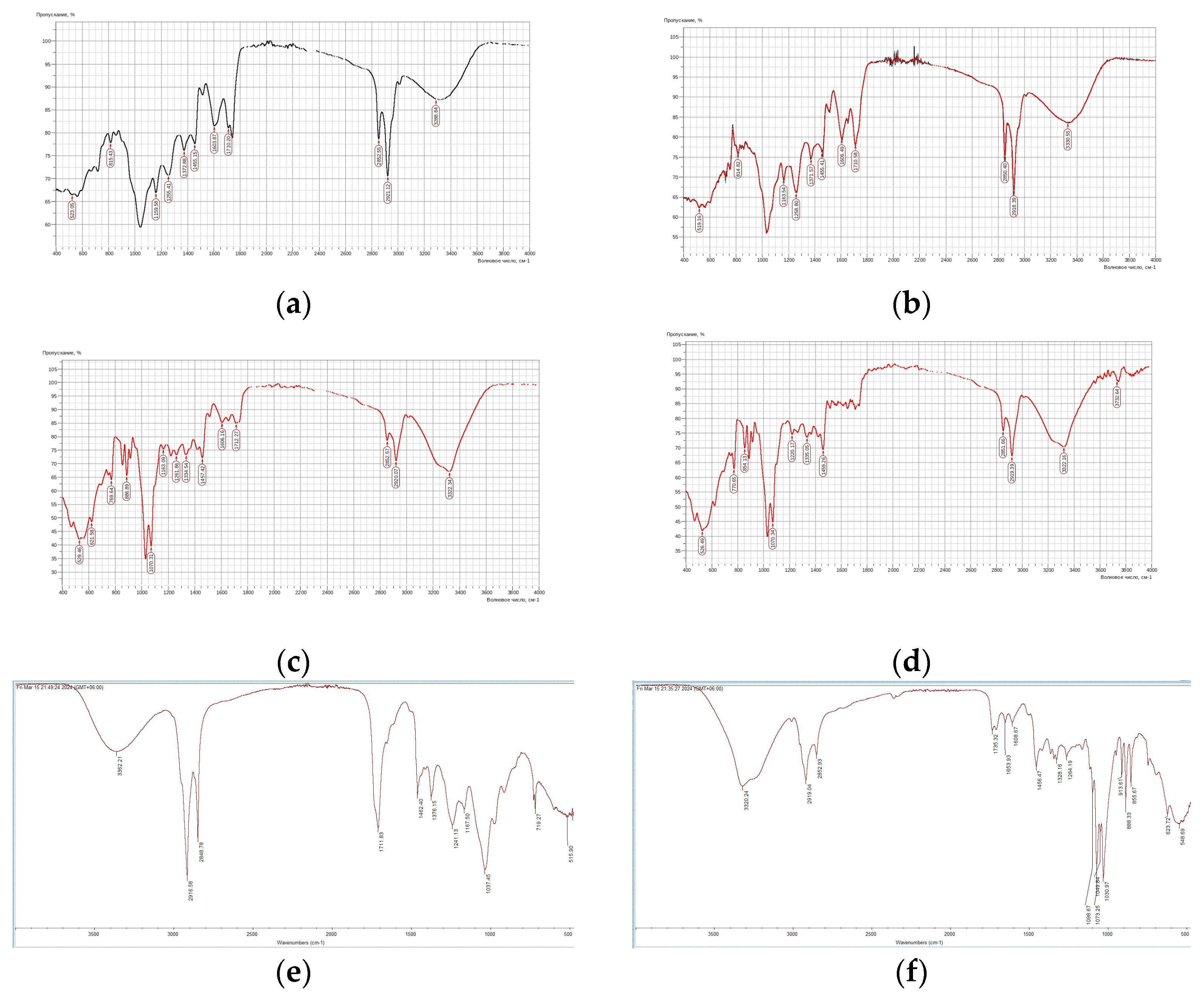

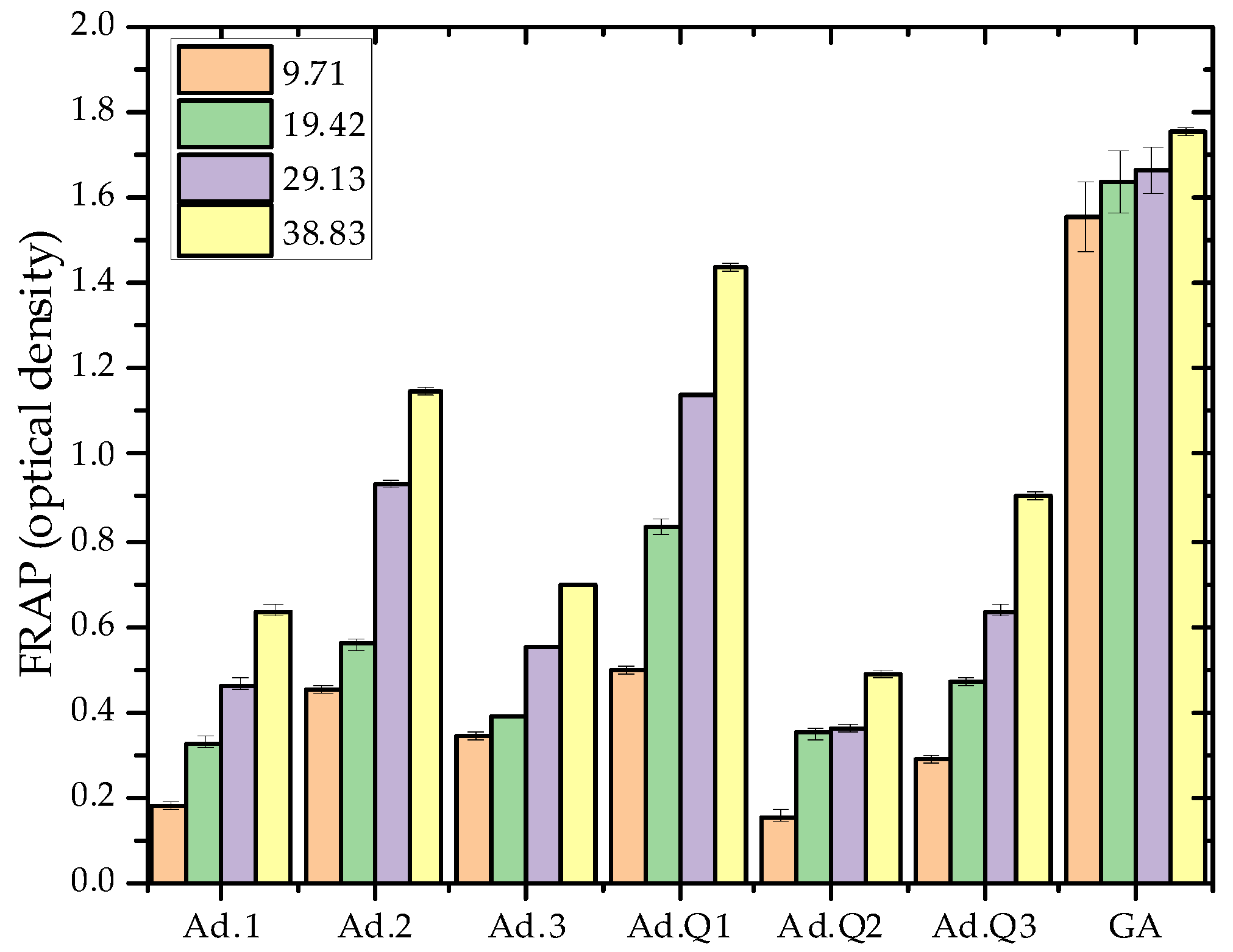
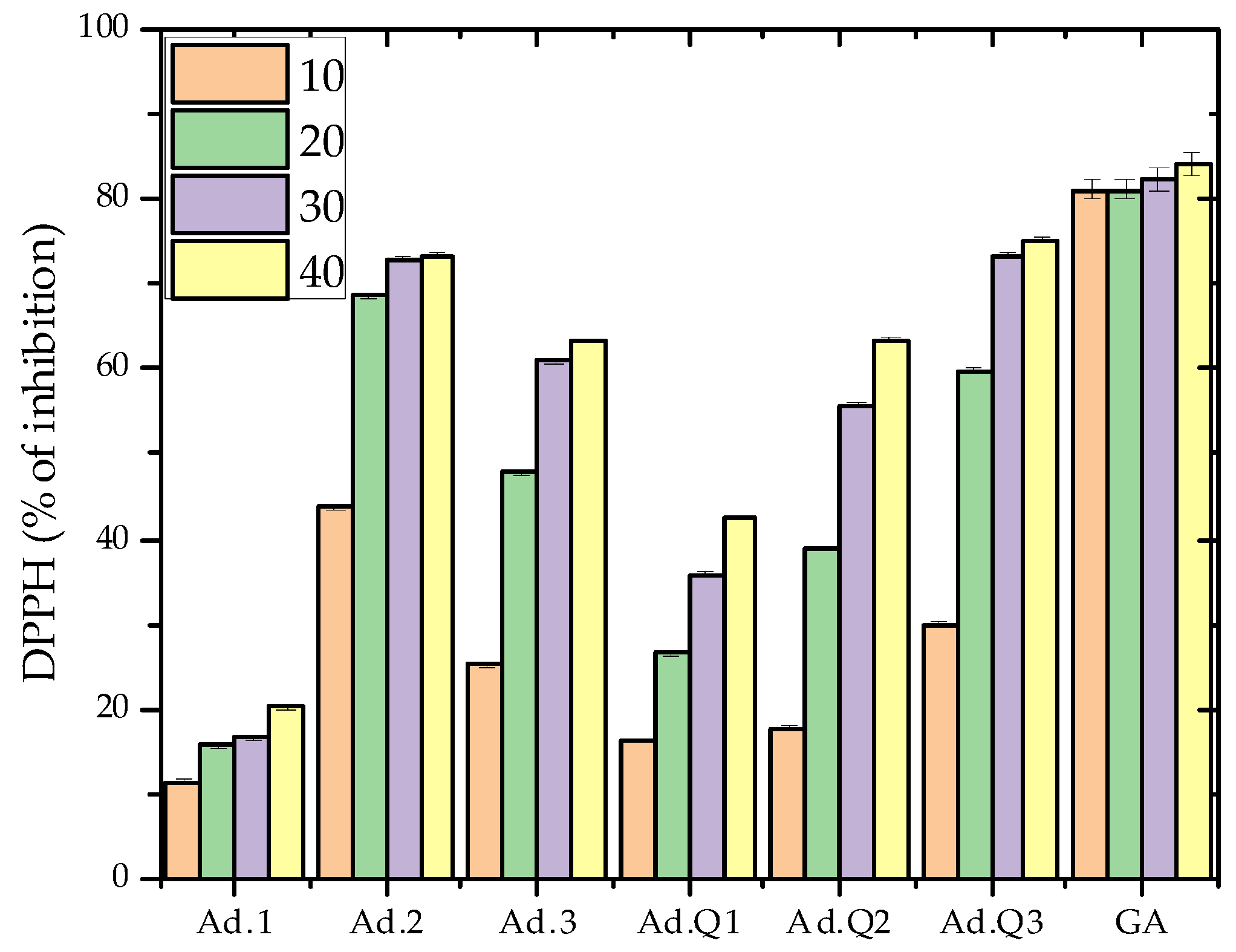
| Characteristic | Pop-1 (Karaganda, Koktenkol) | Pop-2 (Kostanay, Aulikol) | Pop-3 (Akmola, Shchuchinsk) | Pop-4 (Akmola, Akkol) |
|---|---|---|---|---|
| Average Scores | ||||
| Plant height (cm) | 14.36 ± 0.58 b | 17.23 ± 1.04 a | 9.07 ± 0.37 d | 12.87 ± 0.95 c |
| Bush diameter (cm) | 22.40 ± 1.60 b | 24.87 ± 1.55 a | 9.70 ± 0.42 d | 12.60 ± 1.24 c |
| No. of generative shoots per plant | 12.80 ± 1.68 b | 19.93 ± 2.69 a | 2.70 ± 0.37 d | 4.60 ± 0.46 c |
| No. of vegetative shoots per plant | 9.20 ± 1.41 a | 9.00 ± 1.70 a | 3.13 ± 0.50 b | 1.93 ± 0.32 c |
| Leaf blade length (cm) | 3.78 ± 0.20 a | 3.47 ± 0.17 b | 2.63 ± 0.23 c | 3.44 ± 0.19 b |
| Leaf blade width (cm) | 3.49 ± 0.22 b | 3.35 ± 0.17 b | 2.55 ± 0.15 c | 3.78 ± 0.24 a |
| Flower diameter (cm) | 2.38 ± 0.15 b | 3.27 ± 0.18 a | 2.60 ± 0.12 b | 3.40 ± 0.23 a |
| Stem thickness (cm) | 0.27 ± 0.02 a | 0.29 ± 0.02 a | 0.27 ± 0.07 a | 0.33 ± 0.03 a |
| No. of generative individuals per 100 m2 | 12.07 ± 5.34 a | 8.27 ± 1.10 b | 4.67 ± 0.43 d | 6.00 ± 0.75 c |
| No. | Retention Time (min) | Compound Name | Identification Probability (%) 1 | Composition (%) |
|---|---|---|---|---|
| 1 | 12.59 | Acetic acid | 80 | 0.15 |
| 2 | 12.81 | Propanoic acid, 2-oxo-, methyl ester | 82 | 0.37 |
| 3 | 20.04 | 2-Cyclopenten-1-one, 2-hydroxy- | 80 | 0.13 |
| 4 | 20.15 | 4-Pyranone, 2,3-dihydro- | 71 | 0.18 |
| 5 | 20.95 | 2-Furanmethanol, tetrahydro-, acetate | 76 | 0.10 |
| 6 | 21.84 | Phenol, 2-methoxy- | 88 | 0.30 |
| 7 | 24.07 | 3-Buten-1-ol, 2-methyl- | 70 | 0.06 |
| 8 | 24.62 | Phenol | 87 | 0.15 |
| 9 | 25.11 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | 88 | 0.17 |
| 10 | 25.30 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 86 | 0.29 |
| 11 | 25.56 | Phytol, acetate | 87 | 0.16 |
| 12 | 26.84 | Tetradecanoic acid, ethyl ester | 88 | 0.21 |
| 13 | 27.19 | 4-Chloro-3-methylbut-2-en-1-ol | 79 | 0.75 |
| 14 | 27.38 | 2-Hydroxy-gamma-butyrolactone | 88 | 0.22 |
| 15 | 27.94 | Nonanoic acid | 66 | 0.07 |
| 16 | 28.28 | 2-Methoxy-4-vinylphenol | 91 | 0.51 |
| 17 | 28.75 | Pentadecanoic acid, ethyl ester | 65 | 0.13 |
| 18 | 28.96 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 82 | 0.23 |
| 19 | 29.53 | Phenol, 2,6-dimethoxy- | 89 | 0.27 |
| 20 | 29.85 | Glycerin | 94 | 1.78 |
| 21 | 30.61 | Hexadecanoic acid, ethyl ester | 91 | 2.29 |
| 22 | 30.90 | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | 80 | 0.09 |
| 23 | 31.21 | Ethyl 9-hexadecenoate | 78 | 0.17 |
| 24 | 31.60 | Benzofuran, 2,3-dihydro- | 81 | 0.08 |
| 25 | 31.97 | Benzoic acid | 71 | 0.15 |
| 26 | 32.19 | Methyl 8,11,14-heptadecatrienoate | 84 | 0.36 |
| 27 | 32.37 | Heptadecanoic acid, ethyl ester | 72 | 0.12 |
| 28 | 33.20 | 5-Hydroxymethylfurfural | 81 | 0.12 |
| 29 | 34.15 | Ethyl oleate | 86 | 0.87 |
| 30 | 34.61 | 2-Propenoic acid, 2-methyl-, pentyl ester | 74 | 5.96 |
| 31 | 34.73 | 9,12-Octadecadienoic acid, ethyl ester | 92 | 2.38 |
| 32 | 35.40 | Apocynin | 85 | 0.12 |
| 33 | 35.62 | 9,12,15-Octadecatrienoic acid, ethyl ester, (Z,Z,Z)- | 96 | 4.34 |
| 34 | 36.00 | Phytol | 93 | 1.69 |
| 35 | 38.04 | 3-(1-Methylhept-1-enyl)-5-methyl-2,5-dihydrofuran-2-one | 71 | 0.09 |
| 36 | 38.78 | 2,5-Monomethylene-l-rhamnitol | 78 | 1.20 |
| 37 | 39.54 | Sorbitol | 71 | 0.22 |
| 38 | 39.74 | Hexadecanoic acid | 93 | 4.31 |
| 39 | 40.19 | 2,6,8-Trimethylbicyclo[4,2,0]oct-2-ene-1,8-diol | 68 | 0.60 |
| 40 | 41.18 | Heptadecanoic acid | 77 | 0.18 |
| 41 | 42.59 | Octadecanoic acid | 82 | 0.36 |
| 42 | 42.86 | Oleic acid | 87 | 0.78 |
| 43 | 43.48 | 9,12-Octadecadienoic acid (Z,Z)- | 93 | 2.97 |
| 44 | 43.84 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | 86 | 0.33 |
| 45 | 44.30 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | 93 | 13.51 |
| 46 | 44.43 | D-Allose | 77 | 0.58 |
| 47 | 45.26 | Eicosanoic acid | 72 | 0.74 |
| 48 | 47.78 | Docosanoic acid | 79 | 1.45 |
| 49 | 48.57 | Xylitol | 91 | 37.81 |
| 50 | 49.41 | Stigmasterol | 77 | 5.86 |
| 51 | 51.43 | γ-Sitosterol | 86 | 2.72 |
| 52 | 52.79 | Phytol, acetate | 72 | 1.34 |
| Test Substances | Concentration, mg/mL | No. of Larvae in Control | No. of Larvae in the Test Sample | % of Surviving Larvae in Control | % of Surviving Larvae in the Sample | Mortality, A, % | Presence of Neurotoxicity, % | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Survived | Dead | Survived | Dead | Par. | ||||||
| Actinomycin D | 0.10 | 24 | 1 | 0 | 22 | 0 | 96 | 0 | 96 | 0 |
| 0.05 | 24 | 1 | 1 | 25 | 0 | 96 | 4 | 92 | 0 | |
| 0.01 | 24 | 1 | 9 | 18 | 0 | 96 | 33 | 63 | 0 | |
| Extracts of A. volgensis plants from the Akmola region | ||||||||||
| CHCl3 extract (Ad.1) | 0.10 | 24 | 1 | 23 | 3 | 0 | 96 | 88 | 8 | 0 |
| 0.05 | 24 | 1 | 30 | 1 | 0 | 96 | 96 | 0 | 0 | |
| 0.01 | 24 | 1 | 28 | 0 | 0 | 96 | 96 | 0 | 0 | |
| Ethyl acetate extract (Ad.2) | 0.10 | 24 | 1 | 22 | 1 | 0 | 96 | 96 | 0 | 0 |
| 0.05 | 24 | 1 | 26 | 0 | 0 | 96 | 96 | 0 | 0 | |
| 0.01 | 24 | 1 | 26 | 0 | 0 | 96 | 96 | 0 | 0 | |
| EtOH extract (Ad.3) | 0.10 | 24 | 1 | 24 | 0 | 0 | 96 | 96 | 0 | 0 |
| 0.05 | 24 | 1 | 22 | 0 | 0 | 96 | 96 | 0 | 0 | |
| 0.01 | 24 | 1 | 24 | 0 | 0 | 96 | 96 | 0 | 0 | |
| Extracts of A. volgensis plants from the Kostanay region | ||||||||||
| CHCl3 extract (Ad.Q1) | 0.10 | 24 | 1 | 25 | 0 | 0 | 96 | 96 | 0 | 0 |
| 0.05 | 24 | 1 | 21 | 0 | 0 | 96 | 96 | 0 | 0 | |
| 0.01 | 24 | 1 | 24 | 0 | 0 | 96 | 96 | 0 | 0 | |
| Ethyl acetate extract (Ad.Q2) | 0.10 | 24 | 1 | 18 | 5 | 0 | 96 | 78 | 18 | 0 |
| 0.05 | 24 | 1 | 20 | 3 | 0 | 96 | 87 | 9 | 0 | |
| 0.01 | 24 | 1 | 26 | 0 | 0 | 96 | 96 | 0 | 0 | |
| EtOH extract (Ad.Q3) | 0.10 | 24 | 1 | 26 | 1 | 0 | 96 | 96 | 0 | 0 |
| 0.05 | 24 | 1 | 24 | 0 | 0 | 96 | 96 | 0 | 0 | |
| 0.01 | 24 | 1 | 26 | 1 | 0 | 96 | 96 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhumagul, M.; Rašeta, M.; Iskakova, Z.; Kubentayev, S.; Myrzagaliyeva, A.; Tleubergenova, G.; Mukhtubayeva, S.; Mišković, J.; Gafforov, Y. Biodiversity and Phytochemical Characterization of Adonis volgensis Populations from Central and Northern Kazakhstan: Insights into Bioactivity and Toxicity. Diversity 2025, 17, 352. https://doi.org/10.3390/d17050352
Zhumagul M, Rašeta M, Iskakova Z, Kubentayev S, Myrzagaliyeva A, Tleubergenova G, Mukhtubayeva S, Mišković J, Gafforov Y. Biodiversity and Phytochemical Characterization of Adonis volgensis Populations from Central and Northern Kazakhstan: Insights into Bioactivity and Toxicity. Diversity. 2025; 17(5):352. https://doi.org/10.3390/d17050352
Chicago/Turabian StyleZhumagul, Moldir, Milena Rašeta, Zhanar Iskakova, Serik Kubentayev, Anar Myrzagaliyeva, Gulnara Tleubergenova, Saule Mukhtubayeva, Jovana Mišković, and Yusufjon Gafforov. 2025. "Biodiversity and Phytochemical Characterization of Adonis volgensis Populations from Central and Northern Kazakhstan: Insights into Bioactivity and Toxicity" Diversity 17, no. 5: 352. https://doi.org/10.3390/d17050352
APA StyleZhumagul, M., Rašeta, M., Iskakova, Z., Kubentayev, S., Myrzagaliyeva, A., Tleubergenova, G., Mukhtubayeva, S., Mišković, J., & Gafforov, Y. (2025). Biodiversity and Phytochemical Characterization of Adonis volgensis Populations from Central and Northern Kazakhstan: Insights into Bioactivity and Toxicity. Diversity, 17(5), 352. https://doi.org/10.3390/d17050352










