Spatiotemporal Dynamics of Macrobenthic Communities and Environmental Factors in the Aquatic Vegetation Restoration Zone of Baimao Bay
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling and Index Determination
2.3. Statistical Analysis
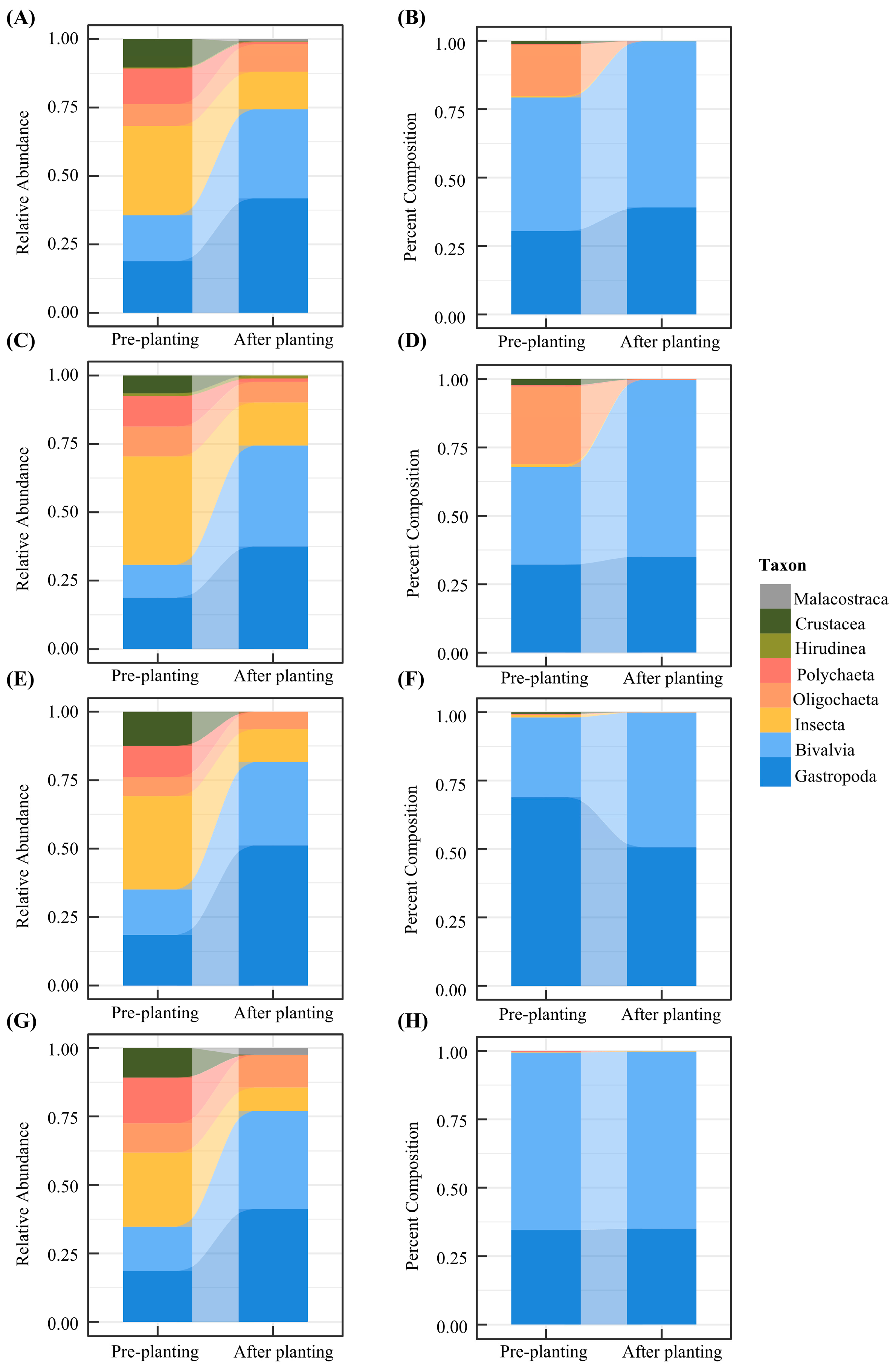
3. Results
3.1. Environmental Characteristics
3.2. Distribution of Macrobenthic Species
3.3. Correlation Analysis Between Benthos and Environmental Factors
4. Discussion
4.1. Will Aquatic Vegetation Restoration Significantly Improve Water Quality in Baimao Bay?
4.2. Will the Vegetation Restoration Project Alter the Composition of Macrobenthic Commu-Nities and Modify Their Habitats?
4.3. Which Environmental Factors Will Emerge as Key Drivers Restructuring Macrobenthic Communities?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oluwaseun, A.S. Eutrophication: Causes, Consequences, Physical, Chemical and Biological Techniques for Mitigation Strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar]
- Luminita, L.; Vlas, O.; Pantea, E.; Boicenco, L.; Marin, O.; Abaza, V.; Filimon, A.; Bisinicu, E. Black Sea Eutrophication Comparative Analysis of Intensity between Coastal and Offshore Waters. Sustainability 2024, 16, 5146. [Google Scholar] [CrossRef]
- Luminița, L.; Boicenco, L.; Marin, O.; Culcea, O.; Pantea, E.; Bișinicu, E.; Timofte, F.; Abaza, V.; Spînu, A. Black Sea Eutrophication Status-the Integrated Assessment Limitations and Obstacles. Cercet. Mar.-Rech. Mar. 2019, 49, 57–73. [Google Scholar]
- Krutov, A.; BSC. State of the Environment of the Black Sea (2009–2014/5); Publications of the Commission on the Protection of the Black Sea Against Pollution (BSC): Istanbul, Turkey, 2019; 811p, Chapter 1.2.6. Publisher: © 2019, Commission on the Protection of the Black Sea Against Pollution. [Google Scholar]
- Meng, J.-N.; Fang, H.; Huang, L.; He, G.; Liu, X.; Xu, C.; Wu, X.; Scavia, D. Multidimensional Ecosystem Assessment of Poyang Lake under Anthropogenic Influences. Ecol. Model. 2022, 473, 110134. [Google Scholar] [CrossRef]
- Cheng, X.; Li, S. An Analysis on the Evolvement Processes of Lake Eutrophication and Their Characteristics of the Typical Lakes in the Middle and Lower Reaches of Yangtze River. Chin. Sci. Bull. 2006, 51, 1603–1613. [Google Scholar] [CrossRef]
- Xue, Z.; Zhu, W.; Cheng, L.; Lv, Y.; Feng, G. Wind-Driven Hydrodynamic Characteristics of Lake Taihu, a Large Shallow Lake in China. Environ. Sci. Pollut. Res. 2024, 31, 26123–26140. [Google Scholar] [CrossRef]
- Cai, G.; Ge, Y.; Dong, Z.; Liao, Y.; Chen, Y.; Wu, A.; Li, Y.; Liu, H.; Yuan, G.; Fu, H.; et al. Temporal Shifts in the Phytoplankton Network in a Large Eutrophic Shallow Freshwater Lake Subjected to Major Environmental Changes due to Human Interventions. Water Res. A J. Int. Water Assoc. 2024, 261, 122054. [Google Scholar] [CrossRef]
- Uherek, C.B.; Gouveia, F.B.P. Biological Monitoring Using Macroinvertebrates as Bioindicators of Water Quality of Maroaga Stream in the Maroaga Cave System, Presidente Figueiredo, Amazon, Brazil. Int. J. Ecol. 2014, 2014, 308149. [Google Scholar] [CrossRef]
- Wallace, J.B. The Role of Macroinvertebrates in Stream Ecosystem Function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef]
- Selvanayagam, M.; Abril, R. Use of Benthic Macro Invertebrates as a Biological Indicator in Assessing Water Quality of River Puyo, Puyo, Pastaza, Ecuador. Am. J. Life Sci. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Paul, D.K.; Kumari, D. Assessing the Role of Bioindicators in Freshwater Ecosystem. J. Interdiscip. Cycle Res. 2020, 12, 17. [Google Scholar]
- Wang, W.; Zhang, Y.; Ye, Z.; Li, T.; Fang, H. Water Ecological Status of the Wenyu River Park Based on Macrobenthos. Environ. Prot. Sci. 2023, 49, 96–102. [Google Scholar]
- Du, X.; Song, D.; Ming, K.; Jin, X.; Wang, H.; Wang, L.; Liu, H.; Zhao, C.; Huo, T. Response of Macroinvertebrate Communities to Land Use and Water Quality in Wudalianchi Lake. Ecol. Evol. 2021, 11, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Bazzanti, M.; Della Bella, V.; Grezzi, F. Functional Characteristics of Macroinvertebrate Communities in Mediterranean Ponds (Central Italy): Influence of Water Permanence and Mesohabitat Type. Ann. De Limnol.-Int. J. Limnol. 2009, 45, 29–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Wan, X.; Wang, Y.; Wang, W. Community Characteristics of Benthic Macroinvertebrates and Identification of Environmental Driving Factors in Rivers in Semi-Arid Areas–A Case Study of Wei River Basin, China. Ecol. Indic. 2021, 121, 107153. [Google Scholar] [CrossRef]
- Iliopoulou-Georgudaki, J.; Kantzaris, V.; Katharios, P.; Kaspiris, P.; Montesantou, B. An Application of Different Bioindicators for Assessing Water Quality: A Case Study in the Rivers Alfeios and Pineios (Peloponnisos, Greece). Ecol. Indic. 2003, 2, 345–360. [Google Scholar] [CrossRef]
- Ghosh, D.; Biswas, J.K. Macroinvertebrate Diversity Indices: A Quantitative Bioassessment of Ecological Health Status of an Oxbow Lake in Eastern India. Kurd. Univ. Med. Sci. 2015, 3, 78–90. [Google Scholar]
- Wijesiri, B.; Deilami, K.; Goonetilleke, A. Evaluating the Relationship between Temporal Changes in Land Use and Resulting Water Quality. Environ. Pollut. 2018, 234, 480–486. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic Shifts in Ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Wang, Q.; Liu, Y. The Effects of Aquatic Vegetation on Ecological Restoration of Eutrophic Lakes. J. Nat. 2002, 24, 33–36. [Google Scholar]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative Equilibria in Shallow Lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Chao, C.; Yu, H.; Yu, D.; Liu, C. Submerged Macrophytes Successfully Restored a Subtropical Aquacultural Lake by Controlling Its Internal Phosphorus Loading. Environ. Pollut. 2021, 268, 115949. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.P.; Wikström, S.A.; Axemar, H.; Kautsky, L. Distribution Differences and Active Habitat Choices of Invertebrates between Macrophytes of Different Morphological Complexity. Aquat. Ecol. 2011, 45, 11–22. [Google Scholar] [CrossRef]
- Liu, E.; Birch, G.F.; Shen, J.; Yuan, H.; Zhang, E.; Cao, Y. Comprehensive Evaluation of Heavy Metal Contamination in Surface and Core Sediments of Taihu Lake, the Third Largest Freshwater Lake in China. Environ. Earth Sci. 2012, 67, 39–51. [Google Scholar] [CrossRef]
- Zhou, F.; Cheng, J. Freshwater Micro-Organisms and Benthic Map, 2nd ed.; Chemical Industry Press: Amersham, UK, 2011. [Google Scholar]
- Hammer, O. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Rai, A.; Shah, D.N.; Shah, R.D.T.; Milner, C. Influence of Environmental Parameters on Benthic Macroinvertebrate Assemblages in the Headwaters of Bagmati River, Kathmandu Valley, Nepal. Banko Janakari 2019, 29, 53–61. [Google Scholar] [CrossRef]
- Tan, S.Y.; Li, Z.; Cheng, S. Ecological Interaction between Submerged Macrophytes and Zoobenthos. SDRP J. Earth Sci. Environ. Stud. 2017, 2, 173–182. [Google Scholar]
- Sany, S.B.T.; Tajfard, M.; Hashim, R.; Rezayi, M.; Rahman, M.A.; Karlen, D.J. The Response of Macrobenthic Communities to Environmental Variability in Tropical Coastal Waters. Estuaries Coasts 2018, 41, 1178–1192. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.-l.; Ye, Q.-f.; Wang, H.-y. Study on the Removal of Nitrogen, Phosphorus and Inhibiting Effect of Algae Growth by Aquatic Plants. J. Nucl. Agric. Sci. 2007, 21, 393–396+332. [Google Scholar]
- Enuo, W.U.; Zhu, M.; Tang, L.; Zhu, G.; Wang, Q.A.; Zhang, J. Dynamics of Chlorophyll-a and Analysis of Environmental Factors in Lake Dianshan During Summer and Autumn. J. Lake Ences 2011, 23, 67–72. [Google Scholar] [CrossRef]
- Wu, L.; Feng, W.; Yu, Y.; Shen, Y. Temporal and Spatial Variations in the Species Composition, Distribution, and Abundance of Protozoa in East Dongting Lake, China. J. Freshw. Ecol. 2007, 22, 655–665. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Zhang, Y.-M.; Gao, Y.-X.; Zhang, Z.-W.; Zhu, Y.-M.; Kong, M.; Zhao, Y.; Qian, W.-H. Temporal and Spatial Variation Characteristics of Chlorophyll a and Analysis of Related Environmental Factors in the Estuary Area of Gehu Lake. J. Ecol. Rural. Environ. 2021, 37, 733–739. [Google Scholar]
- Dong, B.; Zhou, Y.; Jeppesen, E.; Qin, B.; Shi, K. Six Decades of Field Observations Reveal How Anthropogenic Pressure Changes the Coverage and Community of Submerged Aquatic Vegetation in a Eutrophic Lake. Sci. Total Environ. 2022, 842, 156878. [Google Scholar] [CrossRef]
- Christiansen, N.H.; Andersen, F.Ø.; Jensen, H.S. Phosphate Uptake Kinetics for Four Species of Submerged Freshwater Macrophytes Measured by a 33p Phosphate Radioisotope Technique. Aquat. Bot. 2016, 128, 58–67. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, C.; Zhao, Y.; Liu, Z.; Guan, B. Effects of Diversity, Coverage and Biomass of Submerged Macrophytes on Nutrient Concentrations, Water Clarity and Phytoplankton Biomass in Two Restored Shallow Lakes. Water 2020, 12, 1425. [Google Scholar] [CrossRef]
- Ye, C.; Yu, H.C.; Kong, H.N.; Song, X.F.; Zou, G.Y.; Xu, Q.J.; Liu, J. Community Collocation of Four Submerged Macrophytes on Two Kinds of Sediments in Lake Taihu, China. Ecol. Eng. 2009, 35, 1656–1663. [Google Scholar] [CrossRef]
- Nalepa, T.F.; Hartson, D.J.; Fanslow, D.L.; Lang, G.A.; Lozano, S.J. Declines in Benthic Macroinvertebrate Populations in Southern Lake Michigan, 1980–1993. J. Can. Des Sci. Halieut. Et Aquat. 1998, 55, 2402–2413. [Google Scholar] [CrossRef]
- Inagaki, Y.; Takatsu, T.; Ashida, Y.; Takahashi, T. Annual Changes in Macrobenthos Abundance in Funka Bay, Japan. Fish. Sci. 2012, 78, 647–659. [Google Scholar] [CrossRef]
- Donohue, I.; Donohue, L.A.; Ainín, B.N.; Irvine, K. Assessment of Eutrophication Pressure on Lakes Using Littoral Invertebrates. Hydrobiologia 2009, 633, 105–122. [Google Scholar] [CrossRef]
- Cheruvelil, K.S.; Soranno, P.A.; Serbin, R.D. Macroinvertebrates Associated with Submerged Macrophytes: Sample Size and Power to Detect Effects. Hydrobiologia 2000, 441, 133–139. [Google Scholar] [CrossRef]
- Human, L.R.D.; Snow, G.C.; Adams, J.B.; Bate, G.C.; Yang, S.-C. The Role of Submerged Macrophytes and Macroalgae in Nutrient Cycling: A Budget Approach. Estuar. Coast. Shelf Sci. 2015, 154, 169–178. [Google Scholar] [CrossRef]
- Hunter, P.J.; Teakle, G.R.; Bending, G.D. Root Traits and Microbial Community Interactions in Relation to Phosphorus Availability and Acquisition, with Particular Reference to Brassica. Front. Plant Sci. 2014, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bai, G.; Liu, Y.; Zou, Y.; Ding, Z.; Wang, R.; Chen, D.; Kong, L.; Wang, C.; Liu, L.; et al. Long-Term Study of Ecological Restoration in a Typical Shallow Urban Lake. Sci. Total Environ. 2022, 846, 157505. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qiu, D.; Wu, Z. Effects of Reestablishment of Aquatic Macrophytes on Zoobenthos in a Eutrophic Shallow Lake. Chin. J. Appl. Environ. Biol. 1997, 3, 323–327. [Google Scholar]
- Batzer, D.P.; Palik, B.J.; Buech, R. Relationships between Environmental Characteristics and Macroinvertebrate Communities in Seasonal Woodland Ponds of Minnesota. J. N. Am. Benthol. Soc. 2004, 23, 50–68. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Yuan, X.-Z.; Liu, H.; Ren, H.-Q. Research Advances in Macroinvertebrate Ecology of the Stream Hyporheic Zone. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2014, 25, 3357–3365. [Google Scholar]
- Liu, B.; Hu, D. An Evaluation on Pollution in the Xiang Jiang River by Using Zoobenthos. Acta Hydrobiol. Sin. 1984, 8, 225–236. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.N.; Yu, Z.S. Study on the Macrobenthic Abundance and Biomass in Bohai Sea. J. Ocean. Univ. Qingdao 2001, 31, 889–896. [Google Scholar]




| pH *** | SD *** | TN *** | TP *** | NH4+-N *** | CODMn *** | Chl-a *** | |
|---|---|---|---|---|---|---|---|
| cm | mg/L | mg/L | mg/L | mg/L | μg/L | ||
| Pre-planting | 8.37 ± 0.44 | 35.68 ± 18.36 | 0.75 ± 0.22 | 0.07 ± 0.04 | 0.23 ± 0.12 | 5.24 ± 0.87 | 30.94 ± 20.01 |
| After planting | 8.45 ± 0.41 | 64.66 ± 21.77 | 0.96 ± 0.84 | 0.04 ± 0.02 | 0.15 ± 0.06 | 4.91 ± 0.79 | 9.48 ± 4.51 |
| Abundance *** | Biomass | d | H′ | J ** | |
|---|---|---|---|---|---|
| (ind./m2) | (g/m2) | ||||
| Pre-planting | 202.37 ± 197.60 | 83.61 ± 105.79 | 0.39 ± 0.32 | 0.74 ± 0.53 | 0.57 ± 0.21 |
| After planting | 182.26 ± 132.16 | 222.51 ± 177.46 | 0.34 ± 0.22 | 0.75 ± 042 | 0.68 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Hu, N.; Li, C.; Ye, C.; Miao, K.; Wang, Y.; Xiao, X.; Zhao, Y.; Yang, Y.; Lai, L. Spatiotemporal Dynamics of Macrobenthic Communities and Environmental Factors in the Aquatic Vegetation Restoration Zone of Baimao Bay. Diversity 2025, 17, 349. https://doi.org/10.3390/d17050349
Wei W, Hu N, Li C, Ye C, Miao K, Wang Y, Xiao X, Zhao Y, Yang Y, Lai L. Spatiotemporal Dynamics of Macrobenthic Communities and Environmental Factors in the Aquatic Vegetation Restoration Zone of Baimao Bay. Diversity. 2025; 17(5):349. https://doi.org/10.3390/d17050349
Chicago/Turabian StyleWei, Weiwei, Ning Hu, Chunhua Li, Chun Ye, Kexin Miao, Yang Wang, Xian Xiao, Yuan Zhao, Youde Yang, and Liangkui Lai. 2025. "Spatiotemporal Dynamics of Macrobenthic Communities and Environmental Factors in the Aquatic Vegetation Restoration Zone of Baimao Bay" Diversity 17, no. 5: 349. https://doi.org/10.3390/d17050349
APA StyleWei, W., Hu, N., Li, C., Ye, C., Miao, K., Wang, Y., Xiao, X., Zhao, Y., Yang, Y., & Lai, L. (2025). Spatiotemporal Dynamics of Macrobenthic Communities and Environmental Factors in the Aquatic Vegetation Restoration Zone of Baimao Bay. Diversity, 17(5), 349. https://doi.org/10.3390/d17050349






