Pore Characteristics of Deep-Sea Benthic Foraminifera
Abstract
1. Introduction
2. Location of Samples Used for Illustrations
2.1. Sulu Sea
2.2. Monterey Bay
2.3. California Bight
2.4. Northwest Atlantic Ocean: Nova Scotian Margin, Gulf of Maine, Bermuda Rise
3. Scanning Electron Micrographs
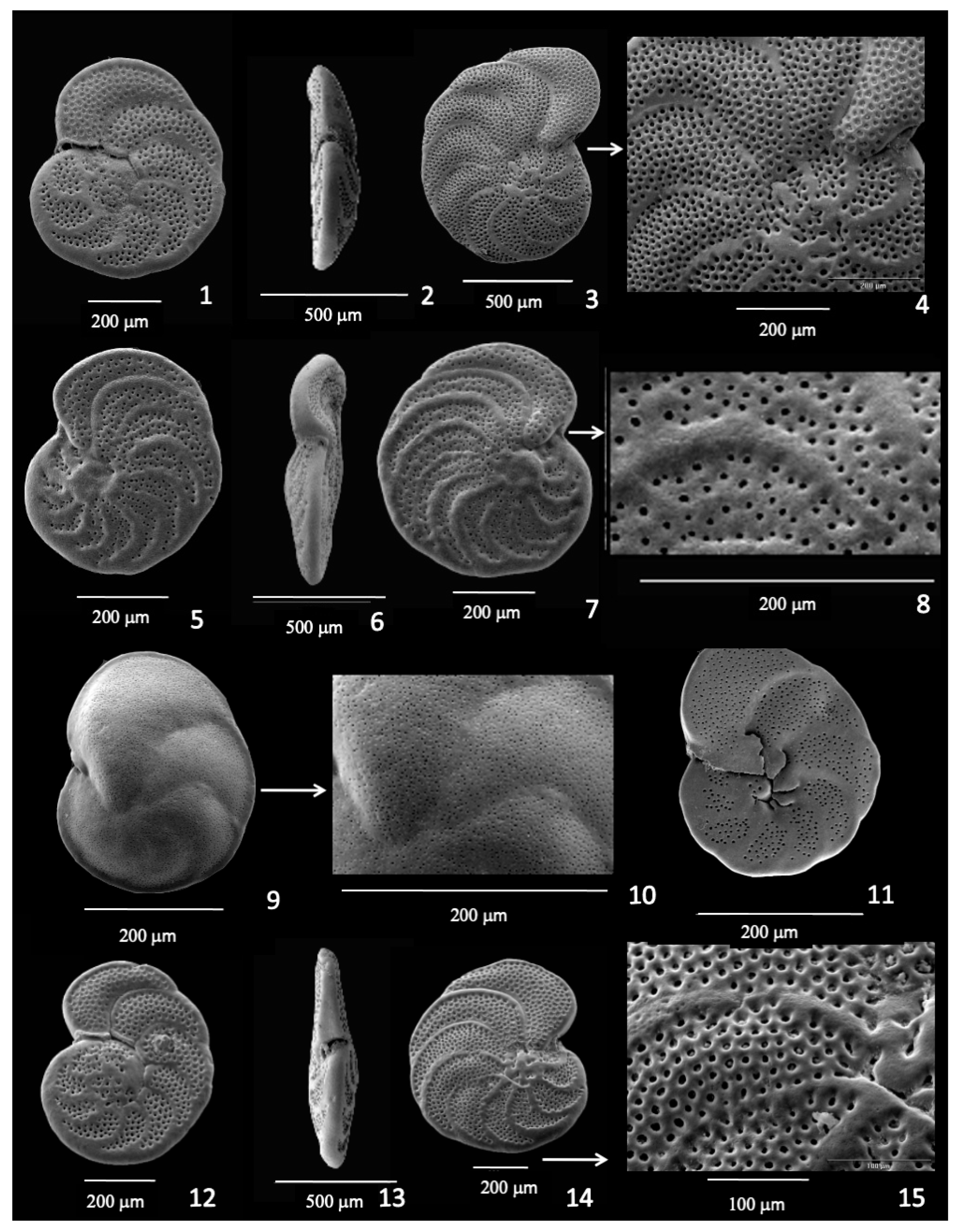
3.1. Cibicidoides (=Cibicides, Planulina, Fontbotia, Lobatula) wuellerstorfi
3.2. Planulina spp.
3.3. Discorbinella bertheloti
3.4. Cibicidoides mundulus
3.5. Cibicidoides bradyi
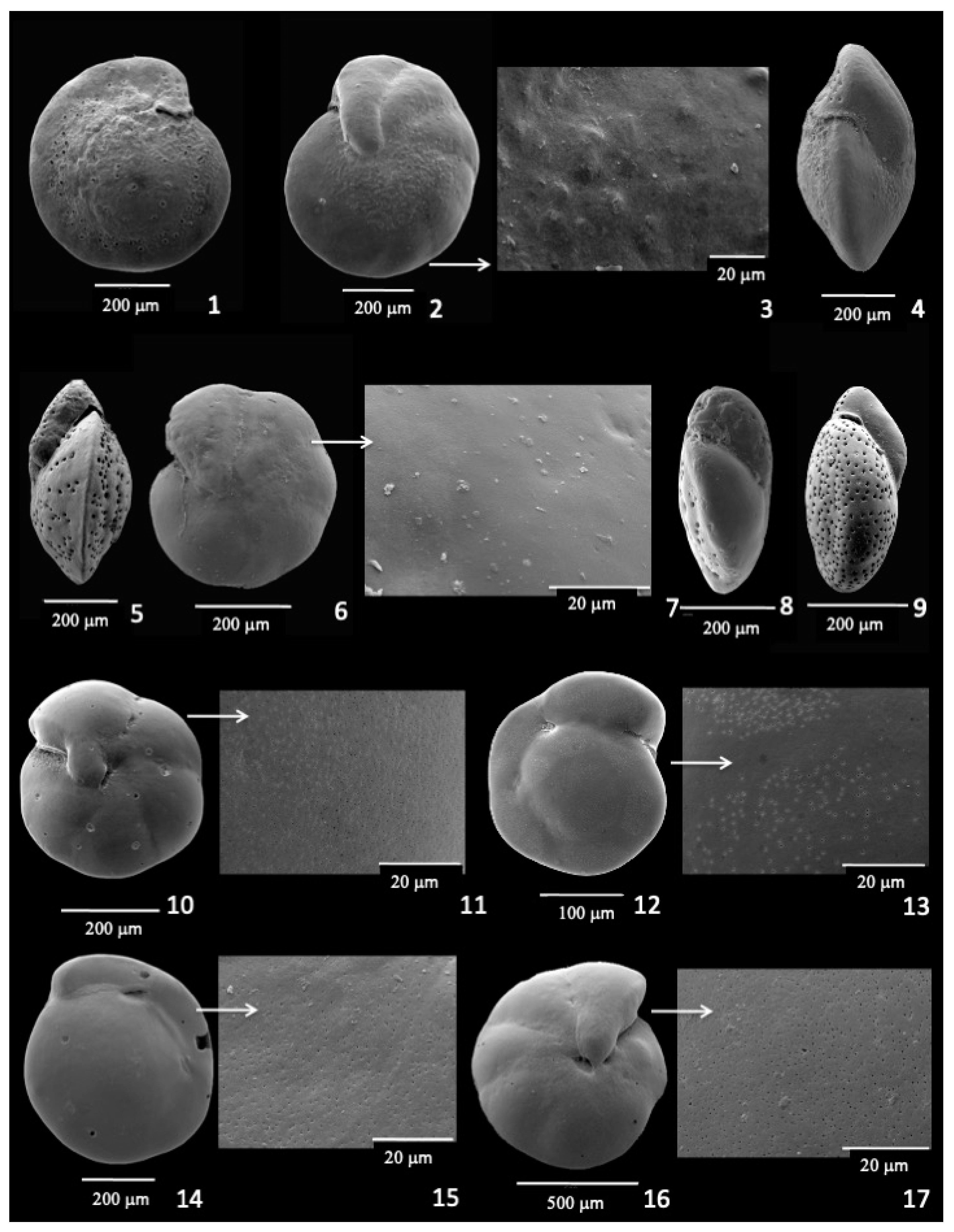
3.6. Oridorsalis umbonatus
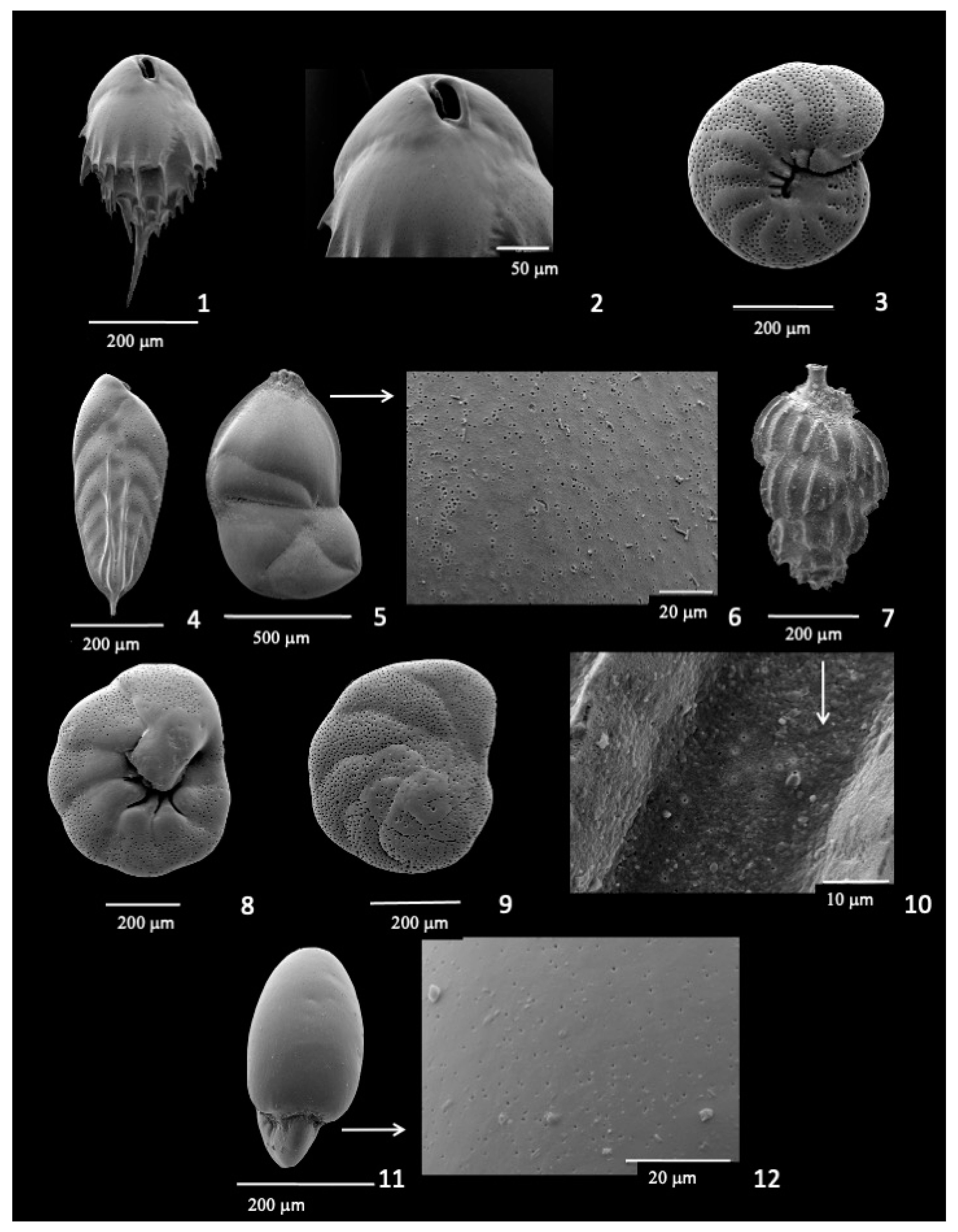
3.7. Hoeglundina elegans
3.8. Epistominella umbonifera
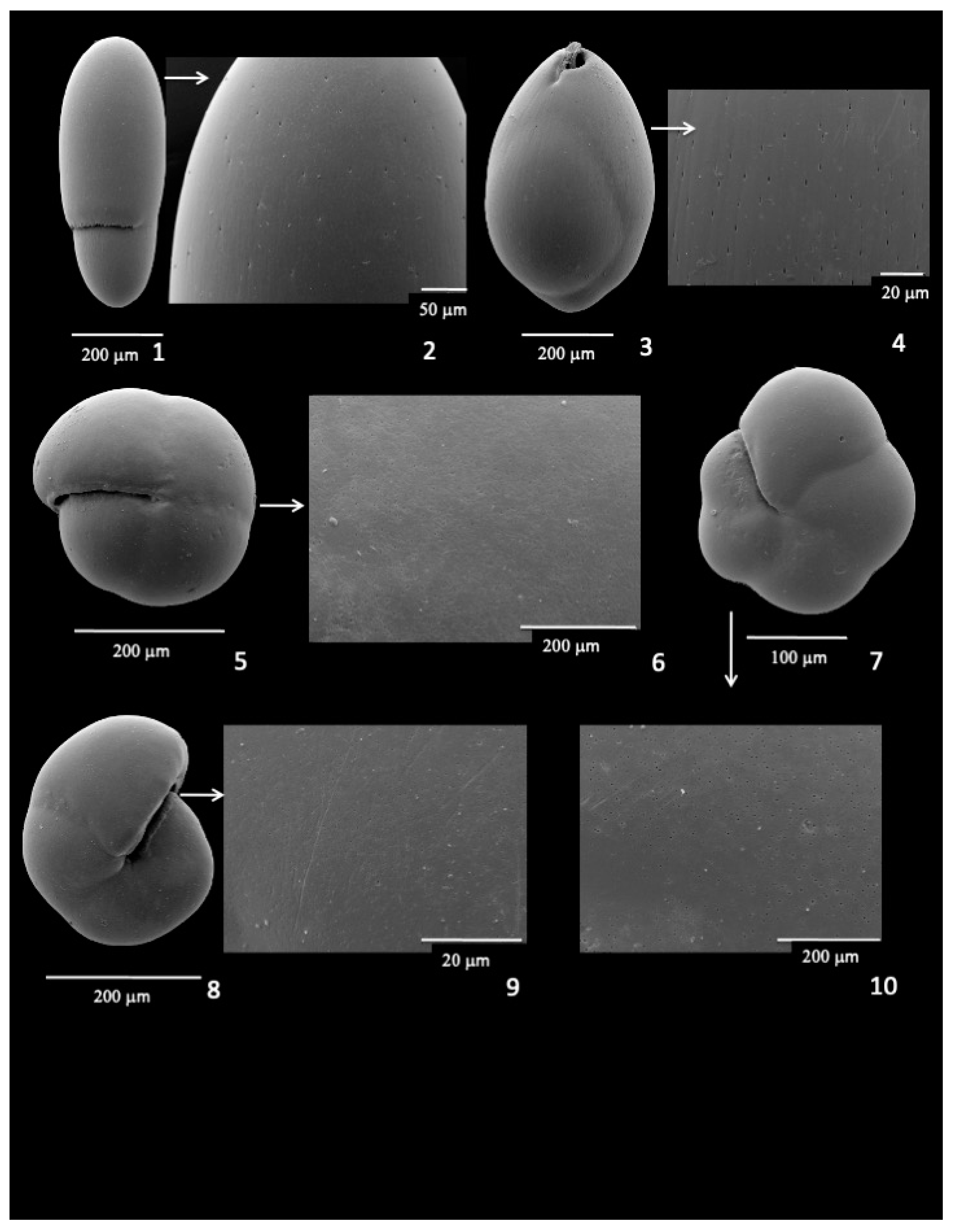
3.9. Infaunal Species
4. Discussion
5. Paleoceanographic Implications
6. Conclusions
- Pores are prominent features of many deep-sea benthic foraminifera and have been suggested to be conduits for gas exchange and, in particular, dissolved oxygen.
- Scanning electron micrographs are presented that illustrate different pore patterns in twenty species of deep-sea taxa to highlight pore patterns discussed in this review.
- Although pores were initially thought to be lacking in epifaunal species, recent studies show that both epifaunal and infaunal taxa have pores present in the tests in response to low-oxygen conditions.
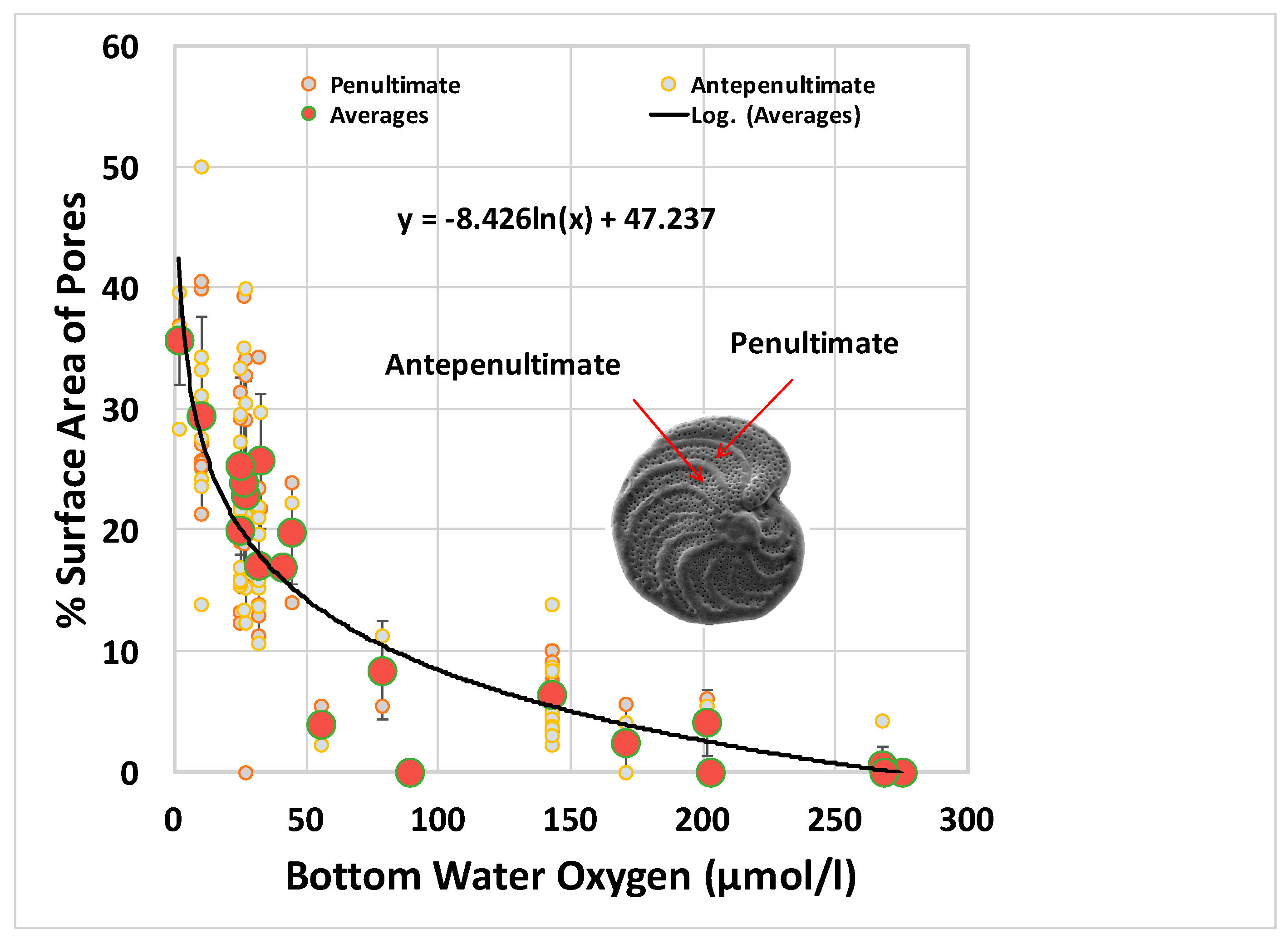
- The pore areas of epifaunal species show a significant inverse relationship with dissolved oxygen and can be used to reconstruct dissolved oxygen conditions during the Cenozoic.
- Infaunal species have pores present in response to low-oxygen conditions, but pore patterns vary between species and cannot always be simply related to oxygen conditions, since a number of infaunal taxa have been shown to respire nitrate.
- Three biconvex species illustrated here, E. umbonifera, H. elegans, and O. umbonatus, have pores found over most of the surface of the test. This pore pattern suggests that these species live infaunally for at least the part of their ontogeny.
- Pore patterns of deep-sea taxa provide important information about the ecology of different species and have considerable potential for reconstructing dissolved oxygen conditions in past oceans.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boltovskoy, E.; Wright, R. Recent Foraminifera; Dr. W. Junk Publishers: The Hague, The Netherlands, 1976; p. 15. ISBN 9061930308. xvii + 515 pp., 133 figs, 17 tables. [Google Scholar]
- Sheehan, R.; Banner, F.T. The pseudopodia of Elphidium incertum (Will.). Rev. Esp. Micropaleontol. 1972, 4, 31–63. [Google Scholar]
- Dubicka, Z.; Złotnik, M.; Borszcz, T. Test morphology as a function of behavioral strategies-Inferences from benthic foraminifera. Mar. Micropaleontol. 2015, 116, 38–49. [Google Scholar] [CrossRef]
- Berthold, W.-U. Ultrastructure and function of wall perforations in Patellina corrugata Williamson, Foraminiferida. J. Foraminifer. Res. 1976, 6, 22–29. [Google Scholar] [CrossRef]
- Leutenegger, S.; Hansen, H.J. Ultrastructural and radiotracer studies of pore function in foraminifera. Mar. Biol. 1979, 54, 11–16. [Google Scholar] [CrossRef]
- Bernhard, J.M.; Martin, J.B.; Rathburn, A.E. Combined carbonate carbon isotopic and cellular ultrastructural studies of individual benthic foraminifera: 2. Toward an understanding of apparent disequilibrium in hydrocarbon seeps. Paleoceanography 2010, 25, PA4206. [Google Scholar] [CrossRef]
- Glock, N.; Schönfeld, J.; Mallon, J. The Functionality of Pores in Benthic Foraminifera in View of Bottom Water Oxygenation: A Review. In Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, Netherlands, 2012; pp. 537–552. [Google Scholar]
- Bernhard, J.M.; Alve, E. Survival, ATP pool, and ultrastructural characterization of benthic foraminifera from Drammensfjord (Norway): Response to anoxia. Mar. Micropaleontol. 1996, 28, 5–17. [Google Scholar] [CrossRef]
- Bernhard, J.M.; Sen Gupta, B.K. Foraminifera of oxygen-depleted environments. In Modern Foraminifera; Springer: Dordrecht, The Netherlands, 1999; pp. 201–216. [Google Scholar]
- Bernhard, J.M.; Goldstein, S.T.; Bowser, S.S. An ectobiont-bearing foraminiferan, Bolivina pacifica, that inhabits microxic pore waters: Cell-biological and paleoceanographic insights. Environ. Microbiol. 2010, 12, 2107–2119. [Google Scholar] [CrossRef]
- Jauffrais, T.; LeKieffre, C.; Koho, K.A.; Tsuchiya, M.; Schweizer, M.; Bernhard, J.M.; Meibom, A.; Geslin, E. Ultrastructure and distribution of kleptoplasts in benthic foraminifera from shallow-water (photic) habitats. Mar. Micropaleontol. 2017, 138, 46–62. [Google Scholar] [CrossRef]
- Gomaa, F.; Utter, D.R.; Powers, C.; Beaudoin, D.J.; Edgcomb, V.P.; Filipsson, H.L.; Hansel, C.M.; Wankel, S.D.; Zhang, Y.; Bernhard, J.M. Multiple integrated metabolic strategies allow foraminiferan protists to thrive in anoxic marine sediments. Sci. Adv. 2021, 7, eabf1586. [Google Scholar] [CrossRef]
- Ross, B.J.; Hallock, P. Dormancy in the Foraminifera: A Review. J. Foraminifer. Res. 2016, 46, 358–368. [Google Scholar] [CrossRef]
- LeKieffre, C.; Spangenberg, J.E.; Mabilleau, G.; Escrig, S.; Meibom, A.; Geslin, E. Surviving anoxia in marine sediments: The metabolic response of ubiquitous benthic foraminifera (Ammonia tepida). PLoS ONE 2017, 12, e0177604. [Google Scholar] [CrossRef] [PubMed]
- Glock, N.; Eisenhauer, A.; Milker, Y.; Liebetrau, V.; Schönfeld, J.; Mallon, J.; Sommer, S.; Hensen, C. Environmental Influences on the Pore Density of Bolivina spissa (Cushman). J. Foraminifer. Res. 2011, 41, 22–32. [Google Scholar] [CrossRef]
- Risgaard-Petersen, N.; Langezaal, A.M.; Ingvardsen, S.; Schmid, M.C.; Jetten, M.S.M.; Camp, H.J.M.O.D.; Derksen, J.W.M.; Piña-Ochoa, E.; Eriksson, S.P.; Nielsen, L.P.; et al. Evidence for complete denitrification in a benthic foraminifer. Nature 2006, 443, 93–96. [Google Scholar] [CrossRef]
- Piña-Ochoa, E.; Høgslund, S.; Geslin, E.; Cedhagen, T.; Revsbech, N.P.; Nielsen, L.P.; Schweizer, M.; Jorissen, F.; Rysgaard, S.; Risgaard-Petersen, N. Widespread occurrence of nitrate storage and denitrification among Foraminifera and Gromiida. Proc. Natl. Acad. Sci. USA 2010, 107, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Koho, K.A.; Piña-Ochoa, E.; Geslin, E.; Risgaard-Petersen, N. Vertical migration, nitrate uptake and denitrification: Survival mechanisms of foraminifers (Globobulimina turgida) under low oxygen conditions. FEMS Microbiol. Ecol. 2011, 75, 273–283. [Google Scholar] [CrossRef]
- Orsi, W.D.; Morard, R.; Vuillemin, A.; Eitel, M.; Wörheide, G.; Milucka, J.; Kucera, M. Anaerobic metabolism of Foraminifera thriving below the seafloor. ISME J. 2020, 14, 2580–2594. [Google Scholar] [CrossRef]
- Menon, A.G.; Davis, C.V.; Nürnberg, D.; Nomaki, H.; Salonen, I.; Schmiedl, G.; Glock, N. A deep-learning automated image recognition method for measuring pore patterns in closely related bolivinids and calibration for quantitative nitrate paleo-reconstructions. Sci. Rep. 2023, 13, 19628. [Google Scholar]
- Burkett, A.M.; Rathburn, A.E.; Pérez, M.E.; Levin, L.A.; Cha, H.; Rouse, G.W. Phylogenetic placement of Cibicidoides wuellerstorfi (Schwager, 1866) from methane seeps and non-seep habitats on the Pacific margin. Geobiology 2015, 13, 44–52. [Google Scholar] [CrossRef]
- Burkett, A.; Rathburn, A.; Pratt, R.B.; Holzmann, M. Insights into the ecology of epibenthic calcareous foraminifera from a colonization study at 4000 m (Station M) in the NE Pacific Ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2020, 173, 104709. [Google Scholar] [CrossRef]
- Rathburn, A.E.; Willingham, J.; Ziebis, W.; Burkett, A.M.; Corliss, B.H. A New biological proxy for deep-sea paleo-oxygen: Pores of epifaunal benthic foraminifera. Sci. Rep. 2018, 8, 9456. [Google Scholar] [CrossRef]
- Moodley, L.; HESS, C. Tolerance of Infaunal Benthic Foraminifera for Low and High Oxygen Concentrations. Biol. Bull. 1992, 183, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cruz, L.L.; Machain-Castillo, M.L. Benthic foraminifera of the oxygen minimum zone, continental shelf of the Gulf of Tehuantepec, Mexico. J. Foraminifer. Res. 1990, 20, 312–325. [Google Scholar] [CrossRef]
- Kuhnt, T.; Friedrich, O.; Schmiedl, G.; Milker, Y.; Mackensen, A.; Lückge, A. Relationship between pore density in benthic foraminifera and bottom-water oxygen content. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2013, 76, 85–95. [Google Scholar] [CrossRef]
- Kuhnt, T.; Schiebel, R.; Schmiedl, G.; Milker, Y.; Mackensen, A.; Friedrich, O. Automated and Manual Analyses of the Pore Density-to-Oxygen Relationship in Globobulimina turgida (Bailey). J. Foraminifer. Res. 2014, 44, 5–16. [Google Scholar] [CrossRef]
- Richirt, J.; Champmartin, S.; Schweizer, M.; Mouret, A.; Petersen, J.; Ambari, A.; Jorissen, F.J. Scaling laws explain foraminiferal pore patterns. Sci. Rep. 2019, 9, 9149. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Riedel, B.; Barras, C.; Pays, O.; Guihéneuf, A.; Mabilleau, G.; Schweizer, M.; Meysman, J.R.; Jorissen, F.J. Improved methodology for measuring pore patterns in the benthic foraminiferal genus Ammonia. Mar. Micropaleontol. 2016, 128, 1–13. [Google Scholar] [CrossRef]
- Lu, W.; Barbosa, C.F.; Rathburn, A.E.; Xavier, P.d.M.; Cruz, A.P.; Thomas, E.; Rickaby, R.E.; Zhang, Y.G.; Lu, Z. Proxies for paleo-oxygenation: A downcore comparison between benthic foraminiferal surface porosity and I./Ca. Palaeogeogr. Palaeoclim. Palaeoecol. 2021, 579, 110588. [Google Scholar] [CrossRef]
- Corliss, B.H. Microhabitats of benthic foraminifera within deep-sea sediments. Nature 1985, 314, 435–438. [Google Scholar] [CrossRef]
- Corliss, B.H. Morphology and microhabitat preferences of benthic foraminifera from the northwest Atlantic Ocean. Mar. Micropaleontol. 1991, 17, 195–236. [Google Scholar] [CrossRef]
- Jorissen, F.J.; Fontanier, C.; Thomas, E. Chapter Seven Paleoceanographical Proxies Based on Deep-Sea Benthic Foraminiferal Assemblage Characteristics. Develpments Mar. Geol. 2007, 1, 263–325. [Google Scholar]
- Burkett, A.M.; Rathburn, A.E.; Pérez, M.E.; Levin, L.A.; Martin, J.B. Colonization of over a thousand Cibicidoides wuellerstorfi (foraminifera: Schwager, 1866) on artificial substrates in seep and adjacent off-seep locations in dysoxic, deep-sea environments. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2016, 117, 39–50. [Google Scholar] [CrossRef]
- Venturelli, R.A.; Rathburn, A.E.; Burkett, A.M.; Ziebis, W. Epifaunal Foraminifera in an Infaunal World: Insights Into the Influence of Heterogeneity on the Benthic Ecology of Oxygen-Poor, Deep-Sea Habitats. Front. Mar. Sci. 2018, 5, 344. [Google Scholar] [CrossRef]
- Tyson, R.V.; Pearson, T.H. Modern and ancient continental shelf anoxia: An overview. Geol. Soc. Lond. Spec. Publ. 1991, 58, 1–24. [Google Scholar] [CrossRef]
- Bernhard, J.M.; Buck, K.R.; Barry, J.P. Monterey Bay cold-seep biota: Assemblages, abundance, and ultrastructure of living foraminifera. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 2233–2249. [Google Scholar] [CrossRef]
- Greene, H.D.; Maher, N.M.; Paull, C.K. Physiography of the Monterey Bay National Marine Sanctuary and implications about continental margin development. Mar. Geol. 2002, 181, 55–82. [Google Scholar] [CrossRef]
- Rathburn, A.E.; Pérez, M.E.; Martin, J.B.; Day, S.A.; Mahn, C.; Gieskes, J.; Ziebis, W.; Williams, D.; Bahls, A. Relationships between the distribution and stable isotopic composition of living benthic foraminifera and cold methane seep biogeochemistry in Monterey Bay, California. Geochem. Geophys. Geosystems 2003, 4. [Google Scholar] [CrossRef]
- Waggoner, J.; Rathburn, A.E.; Martin, J.B.; Bernhard, J.M.; Gieskes, J.; Ziebis, W. Foraminiferal Ecology and Stable Isotope Geochemistry of Methane Seeps in Monterey Bay, California. In Proceedings of the American Geophysical Union, Fall Meeting 2007, San Francisco, CA, USA, 10–14 December 2007. [Google Scholar]
- Gupta, B.K.S.; Smith, L.E.; Lobegeier, M.K. Attachment of Foraminifera to vestimentiferan tubeworms at cold seeps: Refuge from seafloor hypoxia and sulfide toxicity. Mar. Micropaleontol. 2007, 62, 1–6. [Google Scholar] [CrossRef]
- Jackson, G.A. Physical oceanography of the southern California Bight, Chapter 2. In Lecture Notes on Coastal and Estuarine Studies; Springer: New York, NY, USA, 1986; pp. 13–83. [Google Scholar]
- Bernhard, J.M.; Reimers, C.E. Benthic foraminiferal population fluctuations related to anoxia: Santa Barbara Basin. Biogeochemistry 1991, 15, 127–149. [Google Scholar] [CrossRef]
- Silva, K.A.; Corliss, B.H.; Rathburn, A.E.; Thunell, R.C. Seasonality of living benthic Foraminifera from the San Pedro Basin, California Borderland. J. Foraminifer. Res. 1996, 26, 71–93. [Google Scholar] [CrossRef]
- Rathburn, A.E.; Perez, M.E.; Lange, C.B. Benthic-pelagic coupling in the Southern California Bight: Relationships between sinking organic material, diatoms and benthic foraminifera. Mar. Micropaleontol. 2001, 43, 261–271. [Google Scholar] [CrossRef]
- Shepherd, A.S.; Rathburn, A.E.; Pérez, M.E. Living foraminiferal assemblages from the Southern California margin: A comparison of the >150, 63–150, and >63 μm fractions. Mar. Micropaleontol. 2007, 65, 54–77. [Google Scholar] [CrossRef]
- Corliss, B.H.; Emerson, S. Distribution of rose bengal stained deep-sea benthic foraminifera from the Nova Scotian continental margin and Gulf of Maine. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 381–400. [Google Scholar] [CrossRef]
- Schweizer, M.; Pawlowski, J.; Kouwenhoven, T.J.; Guiard, J.; van der Zwaan, B. Molecular phylogeny of Rotaliida (Foraminifera) based on complete small subunit rDNA sequences. Mar. Micropaleontol. 2008, 66, 233–246. [Google Scholar] [CrossRef][Green Version]
- Schweizer, M.; Pawlowski, J.; Kouwenhoven, T.; van der Zwaan, B. Molecular phylogeny of common Cibicidids and related Rotaliida (Foraminifera) based on small subunit rDNA sequences. J. Foraminifer. Res. 2009, 39, 300–315. [Google Scholar] [CrossRef]
- Hoogakker, B.; Ishimura, T.; de Nooijer, L.; Rathburn, A.; Schmiedl, G. A review of benthic foraminiferal oxygen and carbon isotopes. Quat. Sci. Rev. 2024, 342, 108896. [Google Scholar] [CrossRef]
- Lutze, G.F.; Thiel, H. Epibenthic foraminifera from elevated microhabitats; Cibicidoides wuellerstorfi and Planulina ariminensis. J. Foraminifer. Res. 1989, 19, 153–158. [Google Scholar] [CrossRef]
- Rathburn, A.E.; Corliss, B.H. The ecology of living (stained) deep-sea benthic foraminifera from the Sulu Sea. Paleoceanography 1994, 9, 87–150. [Google Scholar] [CrossRef]
- Rathburn, A.E.; Corliss, B.H.; Tappa, K.D.; Lohmann, K.C. Comparisons of the ecology and stable isotopic compositions of living (stained) benthic foraminifera from the Sulu and South China Seas. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1996, 43, 1617–1646. [Google Scholar] [CrossRef]
- Fontanier, C.; Jorissen, F.; Anschutz, P.; Gwena, A.; Chaillou, L. Seasonal variability of benthic foraminiferal faunas at 1000 M depth in the Bay of Biscay. J. Foraminifer. Res. 2006, 36, 61–76. [Google Scholar] [CrossRef]
- Bremer, M.L.; Lohmann, G.P. Evidence for primary control of the distribution of certain Atlantic Ocean benthonic foraminifera by degree of carbonate saturation. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1982, 29, 987–998. [Google Scholar] [CrossRef]
- Corliss, B.H. Distribution of Holocene deep-sea benthonic foraminifera in the southwest Indian Ocean. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1983, 30, 95–117. [Google Scholar] [CrossRef]
- Darling, K.F.; Wade, C.M.; Kroon, D.; Brown, A.J.L.; Bijma, J. The Diversity and Distribution of Modern Planktic Foraminiferal Small Subunit Ribosomal RNA Genotypes and their Potential as Tracers of Present and Past Ocean Circulations. Paleoceanography 1999, 14, 3–12. [Google Scholar] [CrossRef]
- Holzmann, M.; Pawlowski, J. Taxonomic relationships in the genus Ammonia (Foraminifera) based on ribosomal DNA sequences. J. Micropalaeontol. 2000, 19, 85–95. [Google Scholar] [CrossRef]
- Pawlowski, J.; Fahrni, J.; Lecroq, B.; Longet, D.; Cornelius, N.; Excoffier, L.; Cedhagen, T.; Gooday, A.J. Bipolar gene flow in deep-sea benthic foraminifera. Mol. Ecol. 2007, 16, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gooday, A.J.; Brandão, S.N.; Brix, S.; Brökeland, W.; Cedhagen, T.; Choudhury, M.; Cornelius, N.; Danis, B.; De Mesel, I.; et al. First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 2007, 447, 307–311. [Google Scholar] [CrossRef]
- Schweizer, M. Evolution and molecular phylogeny of Cibicides and Uvigerina (Rotaliida, Foraminifera). Geol. Ultraiectina 2006, 261, 1–168. [Google Scholar]
- Burkett, A.; Rathburn, A.; Acevedo, A.G.; Acevedo, C.G.; Ezpeleta, J. Foraminiferal population dynamics on elevated plastic substrates and in sediments at 4000 m in the Eastern Pacific. Mar. Ecol. Prog. Ser. 2023, 723, 1–18. [Google Scholar] [CrossRef]
- Bernhard, J.M. Characteristic assemblages and morphologies of benthic foraminifera from anoxic, organic-rich deposits; Jurassic through Holocene. J. Foraminifer. Res. 1986, 16, 207–215. [Google Scholar] [CrossRef]
- Tetard, M.; Licari, L.; Tachikawa, K.; Ovsepyan, E.; Beaufort, L. Toward a global calibration for quantifying past oxygenation in oxygen minimum zones using benthic Foraminifera. Biogeosciences 2021, 18, 2827–2841. [Google Scholar] [CrossRef]
- Cai-Li, R.-Y.; Ren, H.; Fang, W.-N.; Yang, E.-W.; Chen, W.-H.; LeKieffre, C.; Branson, O.; Fehrenbacher, J.; Vetter, L.; Jeng, M.-S.; et al. Symbiont regulation of nitrogen metabolism and excretion in tropical planktonic foraminifera. Geochim. et Cosmochim. Acta 2025, 396, 135–145. [Google Scholar] [CrossRef]
- Fujita, K.; Okai, T.; Hosono, T. Oxygen metabolic responses of three species of large benthic foraminifers with algal symbionts to temperature stress. PLoS ONE 2014, 9, e90304. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Dang, Z.; Li, C.; Zhou, Y.; Yang, B.; Huang, S. Simultaneous oxygen and nitrate respiration for nitrogen removal driven by aeration: Carbon/nitrogen metabolism and metagenome-based microbial ecology. J. Water Process. Eng. 2022, 50, 103196. [Google Scholar] [CrossRef]
- Wollenburg, J.E.; Zittier, Z.M.; Bijma, J. Insight into deep-sea life-Cibicidoides pachyderma substrate and pH-dependent behaviour following disturbance. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2018, 138, 34–45. [Google Scholar] [CrossRef]
- Bornmalm, L.; Corliss, B.H.; Tedesco, K. Laboratory observations of rates and patterns of movement of continental margin benthic foraminifera. Mar. Micropaleontol. 1997, 29, 175–184. [Google Scholar] [CrossRef]
- Jorissen, F.; Gupta, B. Benthic foraminiferal microhabitats below the sediment-water interface. In Modern Foraminifera; Springer: Dordrecht, The Netherlands, 2003; pp. 161–180. [Google Scholar]
- Glock, N.; Erdem, Z.; Wallmann, K.; Somes, C.J.; Liebetrau, V.; Schönfeld, J.; Gorb, S.; Eisenhauer, A. Coupling of oceanic carbon and nitrogen facilitates spatially resolved quantitative reconstruction of nitrate inventories. Nat Commun. 2018, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, S.; Zhai, B.; Gong, S.; Wang, J.; Fu, X.; Yi, J.; Ning, Z. Pore density of the benthic foraminiferal test responded to the hypoxia off the Changjiang estuary in the East China Sea. Front. Mar. Sci. 2023, 10, 1159614. [Google Scholar] [CrossRef]
- Kaiho, K. Benthic foraminiferal dissolved-oxygen index and dissolved-oxygen levels in the modern ocean. Geology 1994, 22, 719–722. [Google Scholar] [CrossRef]
- Harzhauser, M.; Beer, C.; Auer, G.; Piller, W.E.; Kranner, M. Calculating dissolved marine oxygen values based on an enhanced Benthic Foraminifera Oxygen Index. Sci. Rep. 2022, 12, 1376. [Google Scholar] [CrossRef]


| Site | Core | Latitude | Longitude | Depth (m) | Temp. C | Oxygen (μM) | Salinity |
|---|---|---|---|---|---|---|---|
| NW Atlantic | K104 | ||||||
| 1 | 41°40.65′ N | 64°11.43′ W | 2975 | 3–4° | >260–290 | 34.90–34.95 | |
| 2 | 41°59.56′ N | 64°39.07′ W | 2225 | 3–4° | >260–290 | 34.90–34.95 | |
| 4 | 42°14.99′ N | 65°02.65′ W | 1075 | 3–4° | >260–290 | 34.90–34.95 | |
| 8 | 42°34.38′ N | 69°52.61′ W | 202 | 4–7° | >260–290 | 34.25–34.75 | |
| Bermuda Rise | OC86/2:5-3 | 35°22.8′ N | 65°20′ W | 4800 | 2.3° | >260–290 | 34.9 |
| Sulu Sea | |||||||
| 10 | 8°20.6 N | 118°57.6 E | 1980 | 10.15° | 57.2 | 34.46 | |
| 12 | 12 8°02.9 N | 118°22.4 E | 510 | 11.06° | 78.5 | 34.46 | |
| Monterey Bay | |||||||
| Core 37 | 36°44.757 N | 122°16.690 W | 1006 | 4° | 44.5 | 34.5 | |
| STC52 | 36°44.73 N | 122°16.673 W | 1002 | 4° | 44.5 | 34.5 | |
| LTC49 | 36°44.249 N | 122°16.678 W | 1006 | 4° | 44.5 | 34.5 | |
| California Bight | |||||||
| Core 7-1B | 32°33.89 N | 118°37.27 W | 1050 | 3.13–3.23° | 38.4–43.3 | 33.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corliss, B.H.; Rathburn, A.E. Pore Characteristics of Deep-Sea Benthic Foraminifera. Diversity 2025, 17, 343. https://doi.org/10.3390/d17050343
Corliss BH, Rathburn AE. Pore Characteristics of Deep-Sea Benthic Foraminifera. Diversity. 2025; 17(5):343. https://doi.org/10.3390/d17050343
Chicago/Turabian StyleCorliss, Bruce H., and Anthony E. Rathburn. 2025. "Pore Characteristics of Deep-Sea Benthic Foraminifera" Diversity 17, no. 5: 343. https://doi.org/10.3390/d17050343
APA StyleCorliss, B. H., & Rathburn, A. E. (2025). Pore Characteristics of Deep-Sea Benthic Foraminifera. Diversity, 17(5), 343. https://doi.org/10.3390/d17050343





