Composition and Spatial Distribution of Benthic Foraminifera from Two Tropical Estuaries (7° S, Brazil)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area

2.2. Sampling
2.3. Laboratory Procedures
2.4. Data Analysis
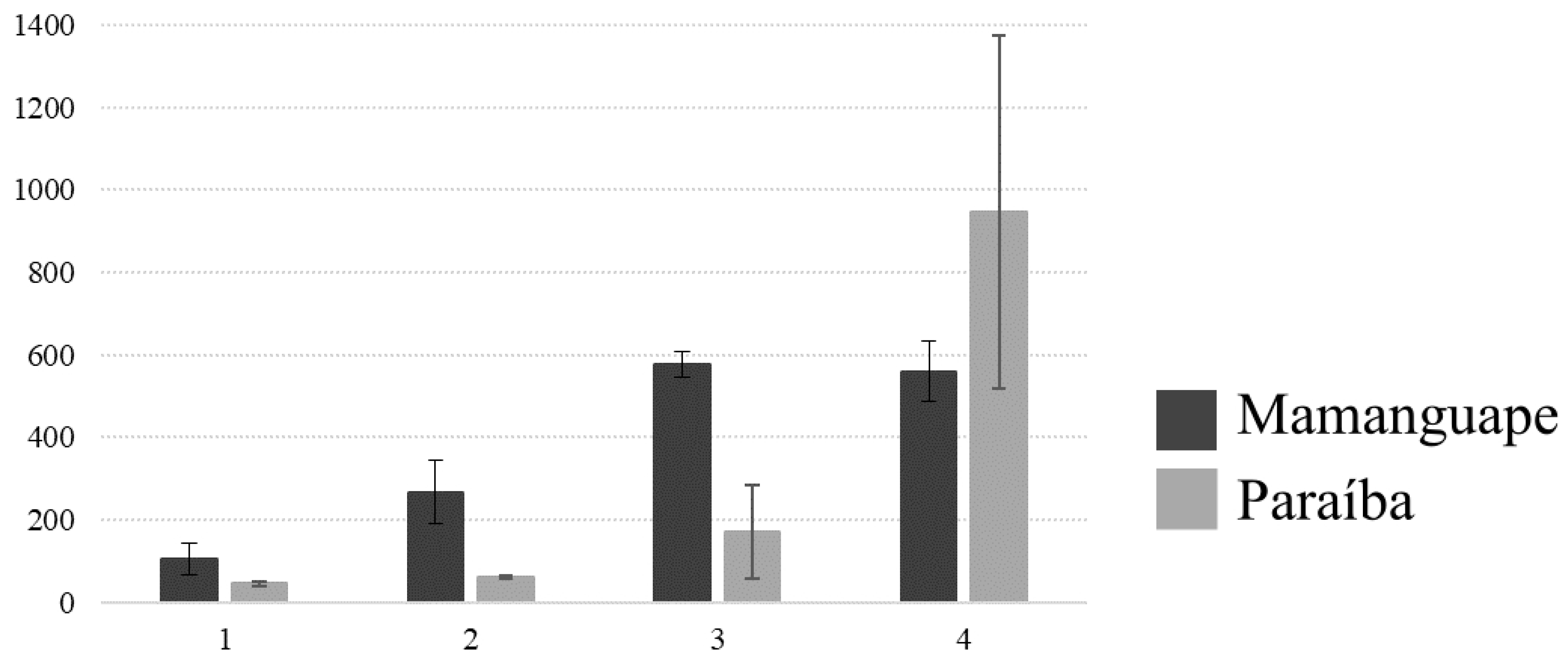
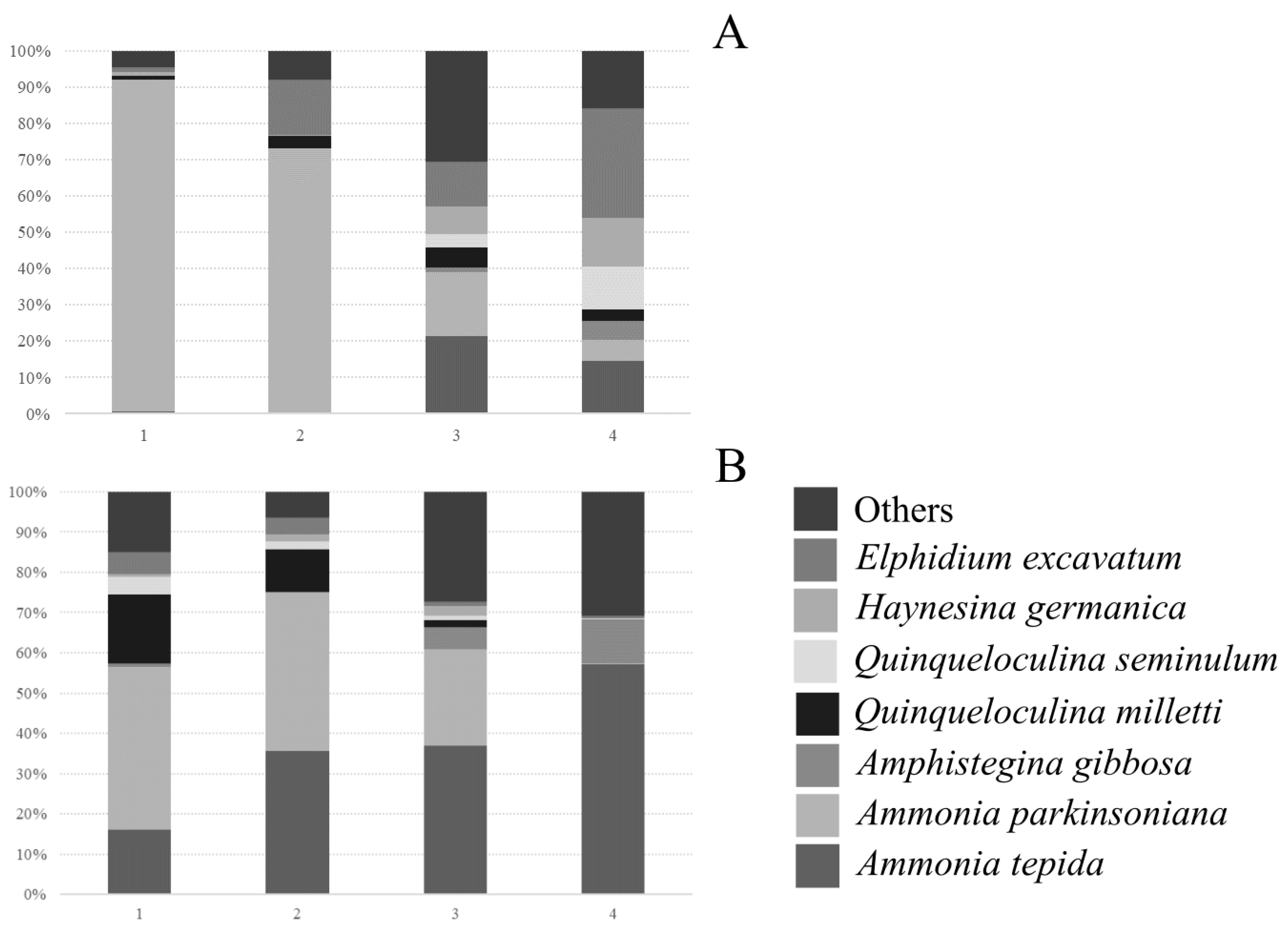
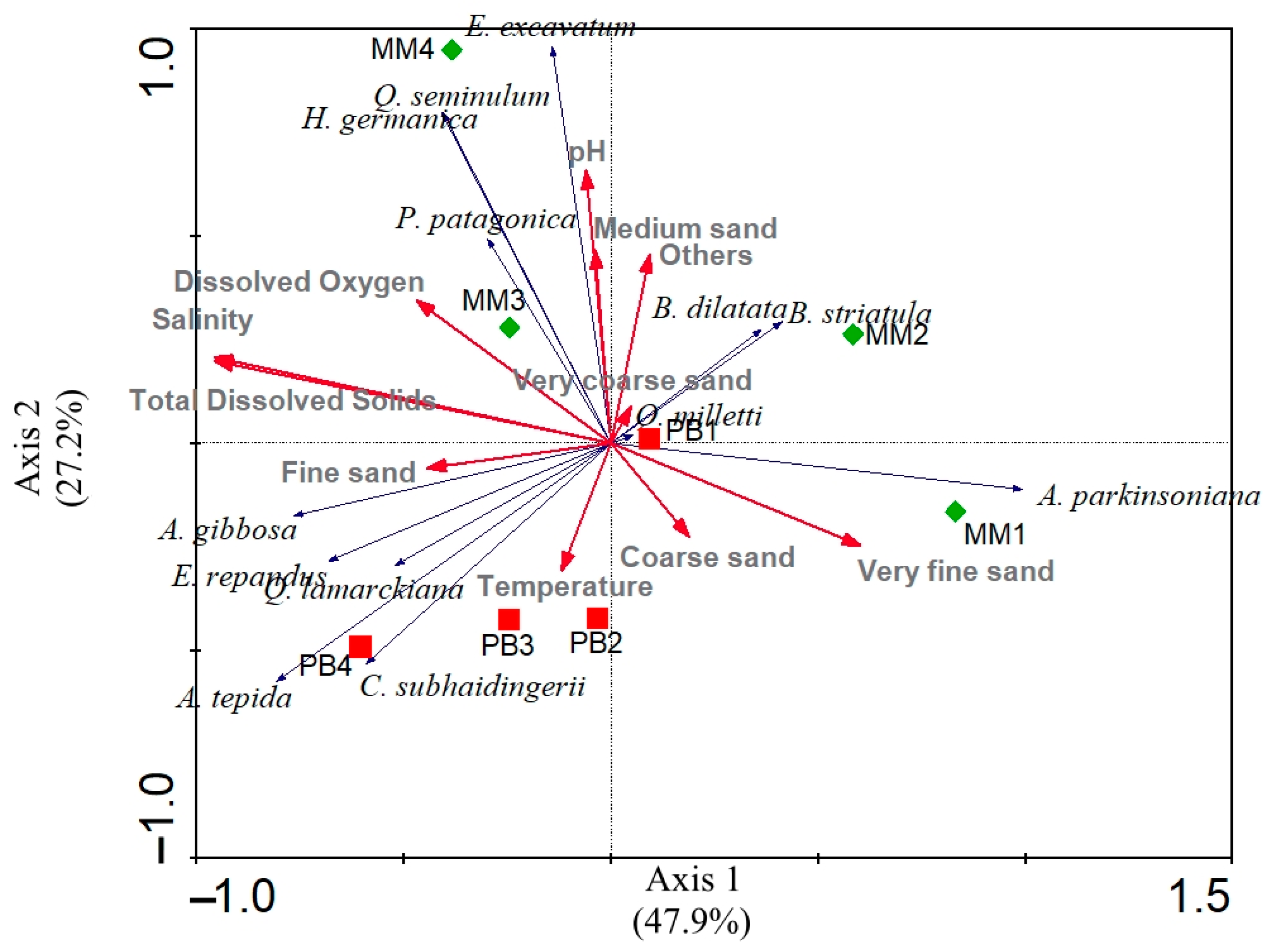
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boltovskoy, E.; Giussani, G.; Watanabe, S.; Wright, R. Atlas of Benthic Shelf Foraminifera of the Southwest Atlantic; Springer: Dordrecht, The Netherlands, 1980. [Google Scholar]
- Sen Gupta, B.K. Modern Foraminifera; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2003. [Google Scholar]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Siemensma, F.; Apothéloz-Perret-Gentil, L.; Holzmann, M.; Clauss, S.; Völcker, E.; Pawlowski, J. Taxonomic revision of freshwater foraminifera with the description of two new agglutinated species and genera. Eur. J. Protistol. 2017, 60, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Hayward, B.W.; Cedhagen, T.; Kaminski, M.; Gross, O. World Foraminifera Database. 2018. Available online: http://www.marinespecies.org/foraminifera (accessed on 18 October 2021).
- Kramer, K.J.M.; Botterweg, J. Bioindicators and Environmental Management; Jefrey, D.W., Madden, B., Eds.; Academic Press: London, UK, 1991; pp. 95–126. [Google Scholar]
- Schönfeld, J.; Alve, E.; Geslin, E.; Jorissen, F.; Korsun, S.; Spezzaferri, S.; Members of the FOBIMO Group. The FOBIMO (FOraminiferal BIo-MOnitoring) initiative—Towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies. Mar. Micropaleontol. 2012, 94, 1–13. [Google Scholar] [CrossRef]
- Francescangeli, F.; Milker, Y.; Bunzel, D.; Thomas, H.; Norbisrath, M.; Schönfeld, J.; Schmiedl, G. Recent benthic foraminiferal distribution in the Elbe Estuary (North Sea, Germany): A response to environmental stressors. Estuar. Coast. Shelf Sci. 2021, 251, 107198. [Google Scholar] [CrossRef]
- Hallock, P. Production of carbonate sediments by selected large benthic foraminifera on two Pacific coral reefs. J. Sediment. Res. 1981, 51, 467–474. [Google Scholar] [CrossRef]
- Langer, M.R.; Silk, M.T.; Lipps, J.H. Global ocean carbonate and carbon dioxide production; the role of reef Foraminifera. J. Foraminifer. Res. 1997, 27, 271–277. [Google Scholar] [CrossRef]
- Groß, O. Sediment interactions of foraminifera: Implications for food degradation and bioturbation processes. J. Foraminifer. Res. 2002, 32, 414–424. [Google Scholar] [CrossRef]
- Langer, M.R. Assessing the contribution of foraminiferan protists to global ocean carbonate production 1. J. Eukaryot. Microbiol. 2008, 55, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Piña-Ochoa, E.; Høgslund, S.; Geslin, E.; Cedhagen, T.; Revsbech, N.P.; Nielsen, L.P.; Schweizer, M.; Jorissen, F.; Rysgaard, S.; Risgaard-Petersen, N. Widespread occurrence of nitrate storage and denitrification among Foraminifera and Gromiida. Proc. Natl. Acad. Sci. USA 2010, 107, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Cesbron, F.; Geslin, E.; Jorissen, F.J.; Delgard, M.L.; Charrieau, L.; Deflandre, B.; Jézéquel, D.; Anschutz, P.; Metzger, E. Vertical distribution and respiration rates of benthic foraminifera: Contribution to aerobic remineralization in intertidal mudflats covered by Zostera noltei meadows. Estuar. Coast. Shelf Sci. 2016, 179, 23–38. [Google Scholar] [CrossRef]
- Enge, A.J.; Wukovits, J.; Wanek, W.; Watzka, M.; Witte, U.F.; Hunter, W.R.; Heinz, P. Carbon and nitrogen uptake of calcareous benthic foraminifera along a depth-related oxygen gradient in the OMZ of the Arabian Sea. Front. Microbiol. 2016, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Wukovits, J.; Oberrauch, M.; Enge, A.J.; Heinz, P. The distinct roles of two intertidal foraminiferal species in phytodetrital carbon and nitrogen fluxes–results from laboratory feeding experiments. Biogeosciences 2018, 15, 6185–6198. [Google Scholar] [CrossRef]
- Li, T.; Zhang, M.; Li, B.; Cai, G.; Li, S.; Nie, X. Co-occurrence patterns and community assembly mechanisms of benthic foraminiferal communities in South Chinese bays. Ecol. Indic. 2022, 144, 109489. [Google Scholar] [CrossRef]
- Jorissen, F.J.; De Stigter, H.C.; Widmark, J.G.V. A conceptual model explaining benthic foraminiferal microhabitats. Mar. Micropaleontol. 1995, 26, 3–15. [Google Scholar] [CrossRef]
- Schmiedl, G.; Mackensen, A.; Müller, P.J. Recent benthic foraminifera from the eastern South Atlantic Ocean: Dependence on food supply and water masses. Mar. Micropaleontol. 1997, 32, 249–287. [Google Scholar] [CrossRef]
- Fontanier, C.; Jorissen, F.J.; Chaillou, G.; David, C.; Anschutz, P.; Lafon, V. Seasonal and internnual variability of benthic foraminiferal faunas at 550 m depth in the Bay of Biscay. Deep-Sea Res. I 2003, 50, 457–494. [Google Scholar] [CrossRef]
- Hess, S.; Jorissen, F.J. Distribution patterns of living benthic foraminifera from Cap Breton Canyon, Bay of Biscay: Faunal response to sediment instability. Deep-Sea Res. I 2009, 56, 1555–1578. [Google Scholar] [CrossRef]
- Magno, M.C.; Bergamin, L.; Finoia, M.G.; Pierfranceschi, G.; Venti, F.; Romano, E. Correlation between textural characteristics of marine sediments and benthic foraminifera in highly anthropogenically-altered coastal areas. Mar. Geol. 2012, 315, 143–161. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Palaeoecology of Benthic Foraminifera; Logman Scientific & Technical: London, UK, 1991. [Google Scholar]
- Scott, D.B.; Medioli, F.S.; Schafer, C.T. Monitoring in Coastal Environments Using Foraminifera and Thecamoebian Indicators; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Hallock, P.; Lidz, B.H.; Cockey-Burkhard, E.M.; Donnelly, K.B. Foraminifera as bioindicators in coral reef assessment and monitoring: The FORAM index. Environ. Monit. Assess. 2003, 81, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, M.; Martínez-Colón, M.; Hallock, P. Foraminifera as bioindicators of water quality: The FoRAM Index revisited. Environ. Pollut. 2020, 257, 113612. [Google Scholar] [CrossRef] [PubMed]
- Kaiho, K. Benthic foraminiferal dissolved-oxygen index and dissolved-oxygen levels in the modern ocean. Geology 1994, 22, 719–722. [Google Scholar] [CrossRef]
- Le Cadre, V.; Debenay, J.P. Morphological and cytological responses of ammonia (foraminifera) to copper contamination: Implication for the use of foraminifera as bioindicators of pollution. Environ. Pollut. 2006, 143, 304–317. [Google Scholar] [CrossRef]
- Nikulina, A.; Polovodova, I.; Schönfeld, J. Environmental response of living benthic foraminifera in Kiel Fjord, SW Baltic Sea. eEarth 2008, 3, 37–49. [Google Scholar] [CrossRef]
- Laut, L.; Da Matta, G.; Camara, G.; Belart, P.; Clemente, I.; Ballalai, J.; Volino, E.; Couto, E.D.C.G. Living and dead foraminifera assemblages as environmental indicators in the Almada River Estuary, Ilheus, northeastern Brazil. J. S. Am. Earth Sci. 2021, 105, 102883. [Google Scholar] [CrossRef]
- Lauchlan, S.S.; Nagelkerken, I. Species range shifts along multistressor mosaics in estuarine environments under future climate. Fish Fish. 2020, 21, 32–46. [Google Scholar] [CrossRef]
- Colombano, D.D.; Litvin, S.Y.; Ziegler, S.L.; Alford, S.B.; Baker, R.; Barbeau, M.A.; Cebrián, J.; Connolly, R.M.; Currin, C.A.; Deegan, L.A.; et al. Climate change implications for tidal marshes and food web linkages to estuarine and coastal nekton. Estuaries Coasts 2021, 44, 1–12. [Google Scholar] [CrossRef]
- Eichler, P.P.; Barker, C.P.; De Miranda, L.B. Benthic foraminifera as indicators of water renewal in Bertioga estuarine channel (SP, Brazil). Environ. Monit. Assess. 2021, 193, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Flemer, D.A.; Champ, M.A. What is the future fate of estuaries given nutrient over-enrichment, freshwater diversion and low flows? Mar. Pollut. Bull. 2006, 52, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Burone, L.; Venturini, N.; Sprechmann, P.; Valente, P.; Muniz, P. Foraminiferal responses to polluted sediments in the Montevideo coastal zone, Uruguay. Mar. Pollut. Bull. 2006, 52, 61–73. [Google Scholar] [CrossRef]
- Sousa, S.H.D.M.; Yamashita, C.; Semensatto, D.L.; Santarosa, A.C.A.; Iwai, F.S.; Omachi, C.Y.; Disaró, S.T.; Martins, M.V.A.; Barbosa, C.F.; Bonetti, C.H.C.; et al. Opportunities and challenges in incorporating benthic foraminifera in marine and coastal environmental biomonitoring of soft sediments: From science to regulation and practice. J. Sediment. Environ. 2020, 5, 257–265. [Google Scholar] [CrossRef]
- Włodarska-Kowalczuk, M.; Pawłowska, J.; Zajączkowski, M. Do foraminifera mirror diversity and distribution patterns of macrobenthic fauna in an Arctic glacial fjord? Mar. Micropaleontol. 2013, 103, 30–39. [Google Scholar] [CrossRef]
- Debenay, J.P.; Bicchi, E.; Goubert, E.; Armynot Du Châtelet, E. Spatio-temporal distribution of benthic foraminifera in relation to estuarine dynamics (Vie estuary, Vendée, W France). Estuar. Coast. Shelf Sci. 2006, 67, 181–197. [Google Scholar] [CrossRef]
- Gupta, B.K.S.; Platon, E. Tracking Past Sedimentary Records of Oxygen Depletion in Coastal Waters. J. Coast. Res. 2006, III, 1351–1355. Available online: https://www.jstor.org/stable/25742974 (accessed on 27 November 2024).
- Frontalini, F.; Buosi, C.; Da Pelo, S.; Coccioni, R.; Cherchi, A.; Bucci, C. Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Mar. Pollut. Bull. 2009, 58, 858–877. [Google Scholar] [CrossRef]
- Moksness, E.; Dahl, E.; Støttrup, J. Integrated Coastal Zone Management, Integrated Coastal Zone Management; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Antonarakou, A.; Kontakiotis, G.; Zarkogiannis, S.; Mortyn, P.G.; Drinia, H.; Koskeridou, E.; Anastasakis, G. Planktonic foraminiferal abnormalities in coastal and open marine eastern Mediterranean environments: A natural stress monitoring approach in recent and early Holocene marine systems. J. Mar. Syst. 2018, 181, 63–78. [Google Scholar] [CrossRef]
- Bergamin, L.; Pierfranceschi, G.; Romano, E. Anthropogenic impact due to mining from a sedimentary record of a marine coastal zone (SW Sardinia, Italy). Mar. Micropaleontol. 2021, 169, 102036. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.E.N.; Patrício, J.; Dolbeth, M.; Pessanha, A.; Palma, A.R.T.; Dantas, E.W.; Vendel, A.L. Do different degrees of human activity affect the diet of Brazilian silverside Atherinella brasiliensis? J. Fish Biol. 2016, 89, 1239–1257. [Google Scholar] [CrossRef]
- CPTEC/INPE. Proclima: Programa de Monitoramento Climático em Tempo Real da Região Nordeste—Análise Municipal das Componentes do Balanço Hídrico. Available online: http://proclima.cptec.inpe.br/ (accessed on 1 November 2015).
- Nishida, A.K.; Nordi, N.; Alves, R.R.N. Abordagem Etnoecológica Da Coleta De Moluscos No Litoral Paraibano. Trop. Oceanogr. 2004, 32, 53–68. [Google Scholar] [CrossRef]
- AESA. Agência Executiva de Gestão das Aguás do Estado da Paraíba. Available online: http://www.aesa.pb.gov.br/aesa-website/ (accessed on 17 October 2020).
- AESA. Relação dos Açudes Monitorados. Available online: https://site2.aesa.pb.gov.br/aesa/volumesAcudes.do?metodo=listarAcudesUltimaCota/ (accessed on 1 November 2015).
- BRASIL, Ministério do Meio Ambiente/Secretaria de Biodiversidade e Florestas. Áreas Prioritárias para Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira: Atualização—Portaria MMA n°9, de 23 de Janeiro de 2007; MMA: Brasília, Brazil, 2007. [Google Scholar]
- Sassi, R.; Watanabe, T. Estudos ecológicos básicos no estuário do Rio Paraíba do Norte, Paraíba, Brasil. Fitoplâncton e fatores hidrológicos. Simpósio Nac. Ecol. 1980, 2, 305–313. [Google Scholar]
- Marcelino, R.L.; Sassi, R.; Cordeiro, T.A.; Costa, C.F. Uma abordagem sócio-econômica e sócio-ambiental dos pescadores artesanais e outros usuários ribeirinhos do Estuário do Rio Paraíba do Norte, estado da Paraíba. Trop. Oceanogr. 2005, 33, 183–197. [Google Scholar] [CrossRef]
- Oliveira, I.S. Uso do Conhecimento Tradicional dos Catadores de Caranguejo-Uçá Ucides cordatus, (LINNAEUS, 1763) para a Identificação dos Principais Locais de Catação, no Estuário do Rio Mamanguape-PB. Master’s Thesis, Universidade Federal da Paraíba, João Pessoa, Brazil, 2009. [Google Scholar]
- Rocha, M.S.P.; Mourão, J.S.; Souto, W.M.S.; Barboza, R.R.D.; Alves, R.R.N. Use of fishing resources in the Mamanguape river estuary, Paraiba state, Brazil. Interciencia 2008, 33, 903–909. [Google Scholar] [CrossRef]
- Schlitzer, R. Ocean Data View. 2021. Available online: http://odv.awi.de (accessed on 31 May 2022).
- UNESCO. Background papers and supporting data on the Practical Salinity Scale 1978. UNESCO Tech. Pap. Mar. Sci. 1981, 37, 1–145. [Google Scholar]
- Boltovskoy, E.; Giussani, G.; Watanabe, S.; Wright, R.C. Atlas of Benthic Shelf foraminifera of the Southwest Atlantic; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Laut, L.L.M.; De Almeida Cabral, I.; Da Conceição Rodrigues, M.A.; Silva, F.S.; Martins, M.V.A.; Boski, T.; Gomes, A.; Dias, J.M.A.; Fontana, L.F.; Laut, V.M.; et al. Compartimentos ambientais do Estuário do Rio Arade, Sul de Portugal, com base na distribuição e ecologia de foraminíferos. Anu. Inst. Geociênc. 2014, 37, 60–74. [Google Scholar] [CrossRef]
- Laut, L.L.M.; Clemente, I.M.M.M.; Belart, P.; Martins, M.V.A.; Frontalini, F.; Laut, V.M.; Gomes, A.; Boski, T.; Lorini, M.L.; Fortes, R.R.; et al. Multiproxies (benthic foraminifera, ostracods and biopolymers) approach applied to identify the environmental partitioning of the Guadiana River Estuary (Iberian Peninsula). J. Sediment. Environ. 2016, 1, 178–195. [Google Scholar] [CrossRef]
- Lei, Y.; Li, T. Atlas of Benthic Foraminifera from China Seas; Springer: Berlin/Heidelberg, Germany, 2016; pp. 89–134. [Google Scholar]
- Symphonia, T.K.; Senthil, N.D. Taxonomic notes on Recent Foraminifera from the Continental Shelf-Slope Region of Southwestern Bay of Bengal, East Coast of India. Palaeont. Electr. 2019, 22, 1–89. [Google Scholar] [CrossRef] [PubMed]
- Damasio, B.V.; Timoszczuk, C.T.; Kim, B.S.M.; E Sousa, S.H.D.M.; Bícego, M.C.; Siegle, E.; Figueira, R.C.L. Impacts of hydrodynamics and pollutants on foraminiferal fauna distribution in the Santos Estuary (SE Brazil). J. Sediment. Environ. 2020, 5, 61–86. [Google Scholar] [CrossRef]
- Suguio, K. Introdução à Sedimentologia; Edgard Bliicher: São Paulo, Brazil, 1973. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Anderson, M.I. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in marine communities. Approach Stat. Anal. Interpret. 2001, 2, 1–168. [Google Scholar]
- Leps, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER V6. User Manual/Tutorial; Plymouth Mariner Laboratory: Plymouth, UK, 2006. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Statsoft. Statistica (Data Analysis Software System) Version 8.0; StatSoft, Inc. CD-ROM: Tulsa, OK, USA, 2007. [Google Scholar]
- Eichler, P.P.B.; Eichler, B.B.; Miranda, L.B.; Rodrigues, A.R. Modern foraminiferal facies in a subtropical estuarine channel, Bertioga, São Paulo, Brazil. J. Foramin. Res. 2007, 37, 234–247. [Google Scholar] [CrossRef]
- Souza, V.M.; Laut, L.M.; Da Silva, F.S.; De Figueiredo Jr, A.G.; Vital, H.; Frazão, E. Benthic foraminifera and bacterial activity as a proxy for environmental characterization in Potengi Estuary, Rio Grande do Norte, Brazil. Anu. Inst. Geociênc. 2010, 33, 20–34. [Google Scholar] [CrossRef]
- Farias, C.L.C.; Beck Eichler, P.P.; Vital, H.; Praxedes Gomes, M. Influência de Variações Ambientais e da Dinâmica Sedimentar na Distribuição de Foraminíferos no Estuário do Rio Potengi (RN, Brasil). Anu. Inst. Geociênc. 2019, 42, 112–128. [Google Scholar] [CrossRef]
- Lal, K.K.; Bonetti, C.; Woodroffe, C.D.; Rogers, K. Contemporary distribution of benthic foraminiferal assemblages in coastal wetlands of south-eastern Australia. Estuar. Coast. Shelf Sci. 2020, 245, 106949. [Google Scholar] [CrossRef]
- Dias, B.B.; Hart, M.B.; Smart, C.W.; Hall-Spencer, J.M. Modern seawater acidification: The response of foraminifera to high-CO2 conditions in the Mediterranean Sea. J. Geol. Soc. 2010, 167, 843–846. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Eichler, P.B.; Eichler, B.B. Utilização de foraminíferos no monitoramento do Canal de Bertioga (SP, BRASIL). Revista Atlântica 2003, 25, 35–51. [Google Scholar]
- Silva, S.T.Â.D. Diversidade e Estrutura da Nematofauna em Regiões Estuarinas Tropicais (~7° S). Master’s Thesis, Universidade Federal da Paraíba, João Pessoa, Brazil, 2017. [Google Scholar]
- Pereira, E.D.A.A.; De Paiva, W.; Molozzi, J.; Lopes, W.S. Sediment and tissue analysis for metals in a tropical estuary. Reg. Stud. Mar. Sci. 2020, 38, 101358. [Google Scholar] [CrossRef]
- Veronez Júnior, P.; Bastos, A.C.; Quaresma, V.D.S. Morfologia e distribuição sedimentar em um sistema estuarino tropical: Baía de Vitória, ES. Braz. J. Geophys. 2009, 27, 609–624. [Google Scholar] [CrossRef]
- Barcellos, R.L.; Figueira, R.C.L.; França, E.J.; Schettini, C.A.; De Arruda Xavier, D. Changes of estuarine sedimentation patterns by urban expansion: The case of Middle Capibaribe Estuary, Northeastern Brazil. Int. J. Geosci. 2017, 8, 514–535. [Google Scholar] [CrossRef][Green Version]
- Nagendra, R.; Reddy, A.N. Benthic foraminifera response to ecosystem pollution in the Uppanar Estuary, Tamil Nadu Coast, India. J. Geol. Soc. India 2019, 93, 555–566. [Google Scholar] [CrossRef]
- Guerra, L.; Veiga-Pires, C.; González-Regalado, M.L.; Abad, M.; Toscano, A.; Muñoz, J.M.; Ruiz, F.; Vidal, J.R.; Cáceres, L.M.; Izquierdo, T.; et al. Relationship between substrate, physico-chemical parameters and foraminiferal tests in the Doñana National Park, a Biosphere Reserve in SW Spain. J. Iber. Geol. 2020, 46, 21–38. [Google Scholar] [CrossRef]
- Sariaslan, N.; Langer, M.R. Atypical, high-diversity assemblages of foraminifera in a mangrove estuary from Northern Brazil. Biogeosciences Discuss. 2021, 18, 1–29. [Google Scholar] [CrossRef]
- Pregnolato, L.A.; De Almeida Viana, R.; Passos, C.C.; Misailidis, M.L.; Duleba, W. Ammonia-Elphidium index as a proxy for marine pollution assessment, Northeast Brazil. J. Sediment. Environ. 2018, 3, 176–186. [Google Scholar] [CrossRef]
- Belart, P.; Laut, V.M.; Fortes, R.; Clemente, I.M.M.M.; Raposo, D.S.; Martins, V.; Frontalini, F.; Lorini, M.L.; Fortes, R.R.; Laut, L. Living benthic Foraminifera from the Saquarema lagoonal system (Rio de Janeiro, southeastern Brazil). Check List 2017, 13, 1. [Google Scholar] [CrossRef][Green Version]
- Laut, L.; Clemente, I.; Martins, M.V.A.; Frontalini, F.; Raposo, D.; Belart, P.; Habib, R.; Fortes, R.; Lorini, M.L. Benthic Foraminifera and Thecamoebians of Godineau River Estuary, Gulf of Paria, Trinidad Island. Anu. Inst. Geociênc. 2017, 40, 118–143. [Google Scholar]
- Barbieri, G.; Vaiani, S.C. Benthic foraminifera or Ostracoda? Comparing the accuracy of palaeoenvironmental indicators from a Pleistocene lagoon of the Romagna coastal plain (Italy). J. Micropala 2018, 37, 203–230. [Google Scholar] [CrossRef]
- Haller, C.; Smith, C.G.; Hallock, P.; Hine, A.C.; Osterman, L.E.; McCloskey, T. Distribution of modern salt-marsh foraminifera from the eastern Mississippi Sound, USA. J. Foramin. Res. 2019, 49, 29–47. [Google Scholar] [CrossRef]
- Ruiz, F.; González-Regalado, M.L.; Pendón, J.G.; Abad, M.; Olías, M.; Muñoz, J.M. Correlation between foraminifera and sedimentary environments in recent estuaries of southwestern Spain: Applications to Holocene reconstructions. Quatern. Int. 2005, 140, 21–36. [Google Scholar] [CrossRef]
- Edwards, P.G. Aspects of Micropalaeontology; Springer: Dordrecht, The Netherlands, 1982; pp. 111–141. [Google Scholar]
- Alves, B.M.; Nogueira Junior, M. Foraminiferal assemblage structure from Brazilian tropical urbanized beaches (~7° S). An. Acad. Bras. Ciênc. 2020, 92, 1–15. [Google Scholar] [CrossRef]
- Buragohain, D.; Ghosh, A. Seasonal Distribution Trends of Benthic Foraminiferal Assemblages from the Saurashtra Coast, Western India. J. Geol. Soc. India 2021, 97, 61–69. [Google Scholar] [CrossRef]
- Nagendra, R.; Sathiyamoorthy, P.; Reddy, A.N.; Ramachandran, A. Spatial distribution of benthic foraminifera in the Palar estuary, Tamil Nadu. J. Geol. Soc. India 2015, 86, 305–316. [Google Scholar] [CrossRef]




| DF | MS | F | p | Turkey HSD | |
|---|---|---|---|---|---|
| S (species number) | |||||
| Estuary | 1 | 228.17 | 23.913 | 0.000163 | See below a |
| Sampling point | 3 | 471.61 | 49.426 | 0.000000 | See below b |
| Estuary × Sampling point | 3 | 41.39 | 4.338 | 0.020364 | See below c |
| H’ (diversity) | |||||
| Estuary | 1 | 0.64383 | 28.646 | 0.000065 | See below d |
| Sampling point | 3 | 2.15051 | 95.682 | 0.000000 | See below e |
| Estuary × Sampling point | 3 | 1.17449 | 52.256 | 0.000000 | See below f |
| J’ (evenness) | |||||
| Estuary | 1 | 0.034740 | 20.952 | 0.000310 | See below g |
| Sampling point | 3 | 0.101752 | 61.368 | 0.000000 | See below h |
| Estuary × Sampling point | 3 | 0.114939 | 69.321 | 0.000000 | See below i |
| Factor | DF | Pseudo-F | p (Perm) | Exclusive Permutations |
|---|---|---|---|---|
| Estuary | 1 | 20.8 | 0.001 | 999 |
| Sampling point | 3 | 17.9 | 0.001 | 999 |
| Estuary × Sampling point | 3 | 8.5 | 0.001 | 999 |
| Species/Contribution | Sampling Points | Estuary | ||||
|---|---|---|---|---|---|---|
| SP1 (71.7) | SP2 (79.6) | SP3 (76.9) | SP4 (76.0) | MM (75.5) | PB (76.6) | |
| Ammonia tepida | 10.73 | 10.44 | 11.29 | 12.30 | 7.32 | 15.0 |
| Ammonia parkinsoniana | 33.28 | 25.02 | 10.43 | 3.97 | 23.79 | 12.33 |
| Amphistegina gibbosa | 5.42 | 8.43 | 2.76 | 4.42 | ||
| Bolivina dilatata | 8.31 | 4.95 | 2.44 | 6.46 | 1.65 | |
| Bolivina striatula | 4.61 | 3.25 | 5.80 | 1.10 | ||
| Elphidium excavatum | 6.41 | 15.07 | 5.88 | 7.68 | 11.58 | 6.18 |
| Eponides repandus | 4.44 | 4.45 | 1.42 | 3.03 | ||
| Haynesina germanica | 5.22 | 3.39 | 6.26 | 5.53 | 6.50 | 3.69 |
| Quinqueloculina lamarckiana | 3.57 | 4.54 | 3.12 | |||
| Quinqueloculina milletti | 7.07 | 13.07 | 5.39 | 3.35 | 6.68 | 7.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, B.M.; Eichler-Barker, P.B.; Nogueira Júnior, M. Composition and Spatial Distribution of Benthic Foraminifera from Two Tropical Estuaries (7° S, Brazil). Diversity 2025, 17, 142. https://doi.org/10.3390/d17030142
Alves BM, Eichler-Barker PB, Nogueira Júnior M. Composition and Spatial Distribution of Benthic Foraminifera from Two Tropical Estuaries (7° S, Brazil). Diversity. 2025; 17(3):142. https://doi.org/10.3390/d17030142
Chicago/Turabian StyleAlves, Bruna Marinho, Patricia Beck Eichler-Barker, and Miodeli Nogueira Júnior. 2025. "Composition and Spatial Distribution of Benthic Foraminifera from Two Tropical Estuaries (7° S, Brazil)" Diversity 17, no. 3: 142. https://doi.org/10.3390/d17030142
APA StyleAlves, B. M., Eichler-Barker, P. B., & Nogueira Júnior, M. (2025). Composition and Spatial Distribution of Benthic Foraminifera from Two Tropical Estuaries (7° S, Brazil). Diversity, 17(3), 142. https://doi.org/10.3390/d17030142






