Comparative Overview of Cave Biodiversity Research Activities in Southern Africa: Insights from Botswana, Namibia and South Africa
Abstract
1. Introduction
- To examine how temporal trends in cave ecology studies in southern Africa compare with trends from the rest of the world.
- To demonstrate the need for cave biodiversity research in southern Africa using Botswana, Namibia and South Africa as case studies.
- To identify subterranean research and conservation priorities for southern Africa.
2. Materials and Methods
3. Results
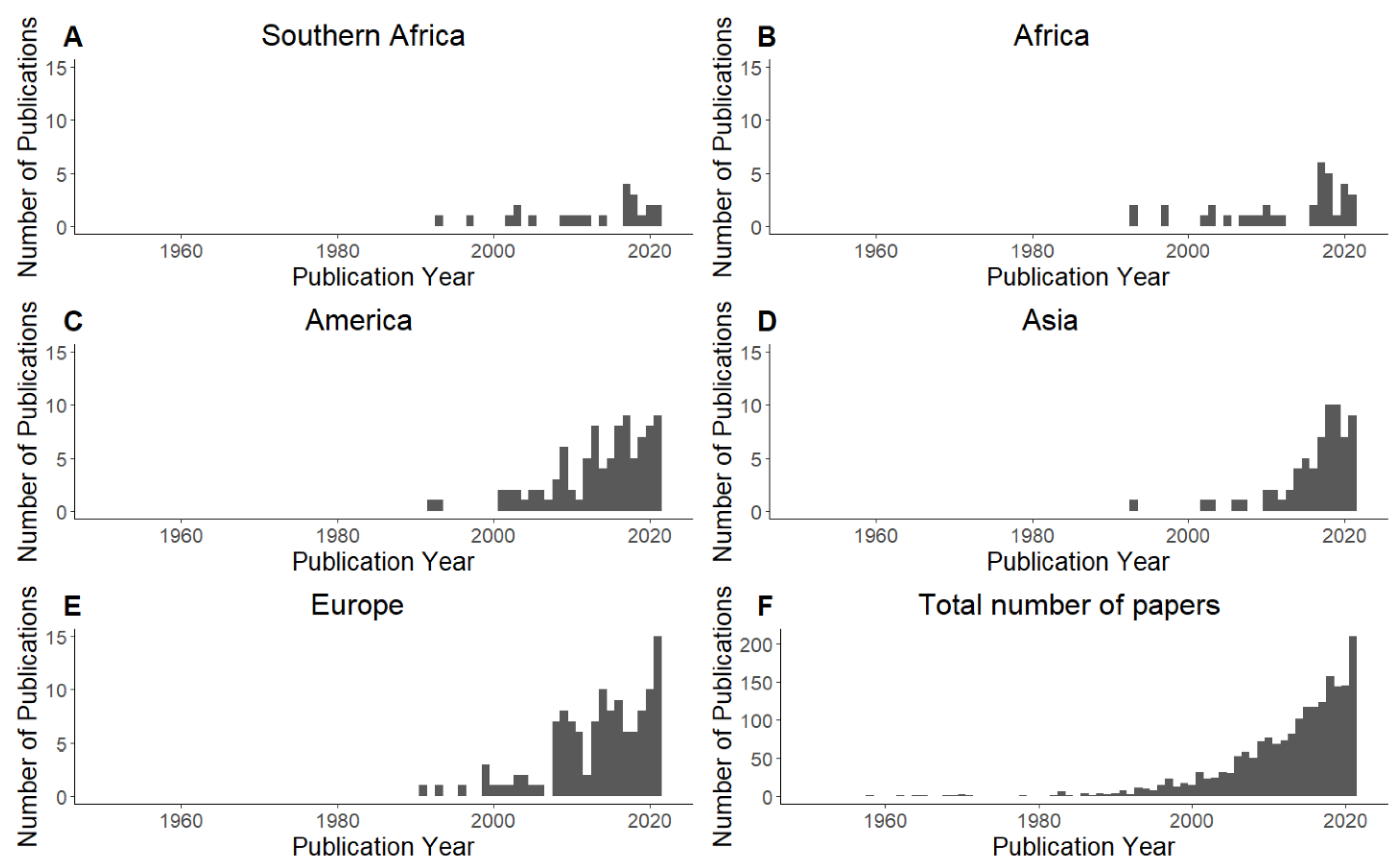
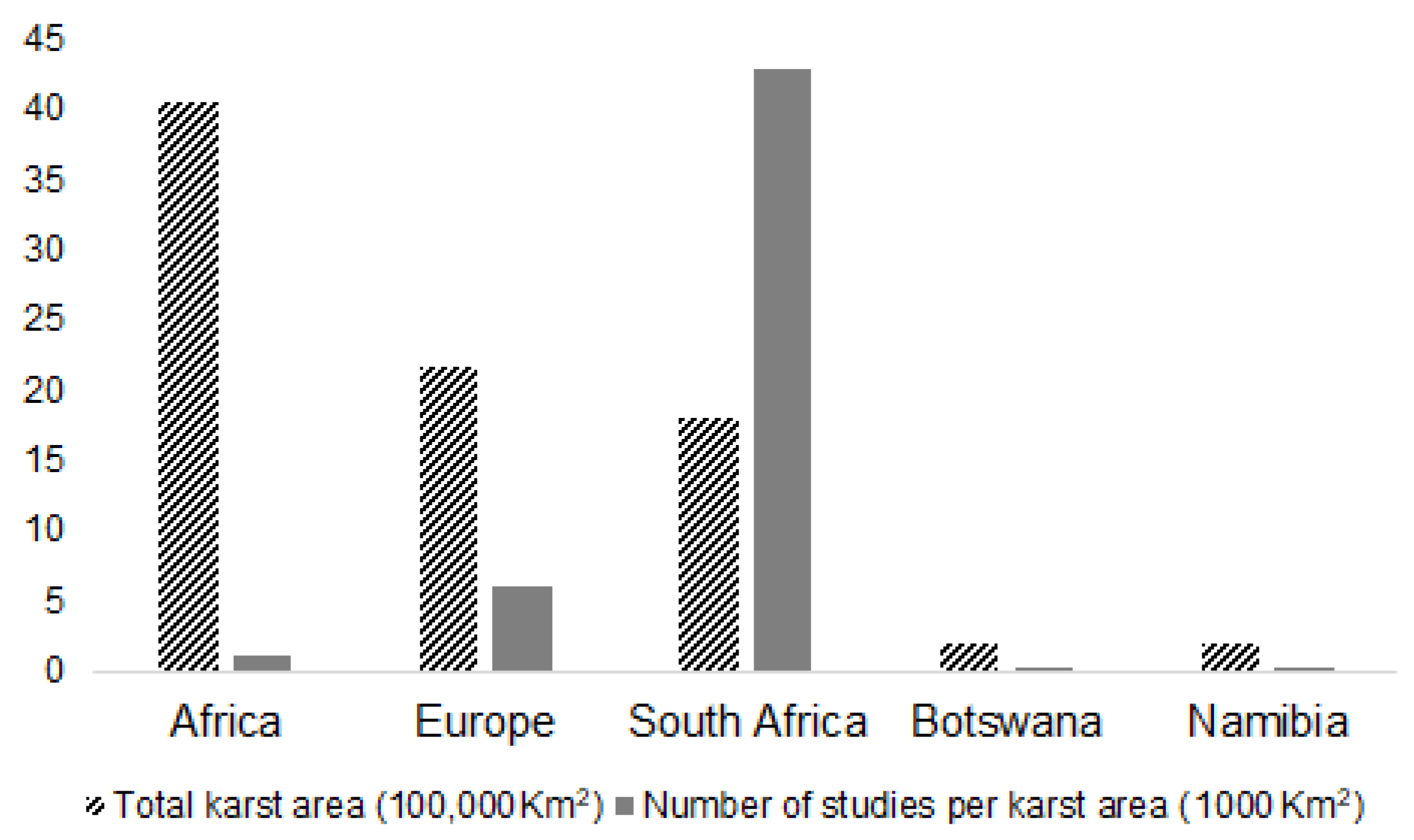
3.1. Temporal Patterns of Cave Ecological Studies in Southern Africa and the World
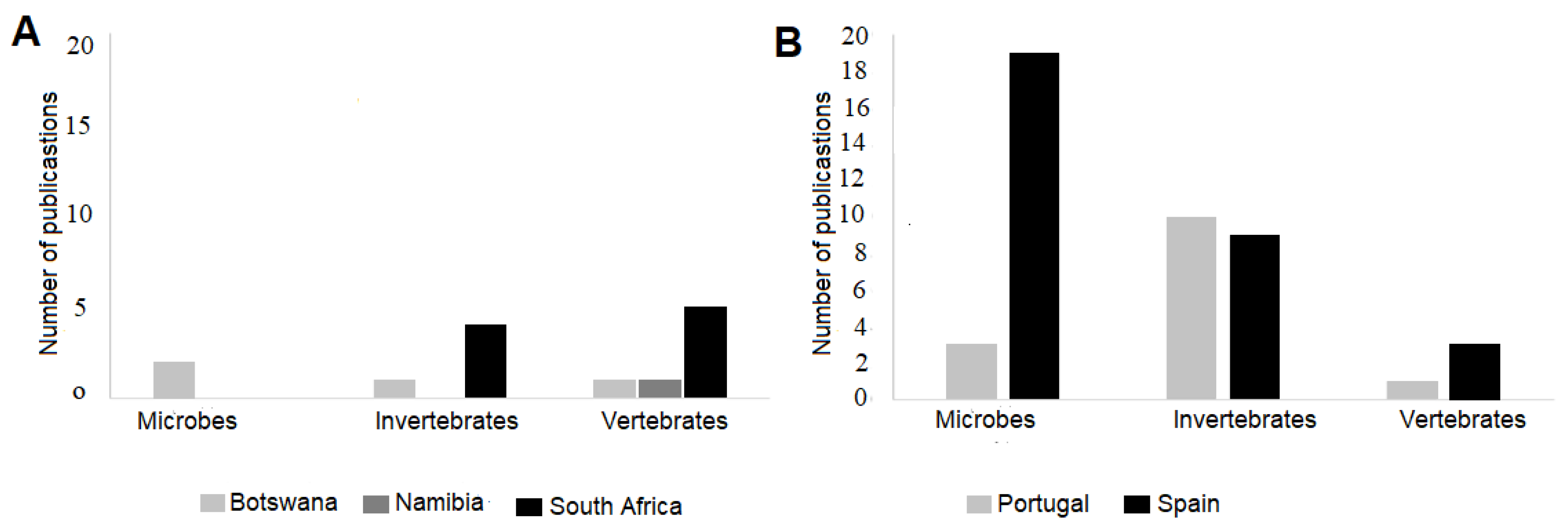
3.2. The Distribution of Ecological Surveys Between Three Different Cave Organisms in Botswana, Namibia, and South Africa
| Cave and Its Location | Show Cave? | Cave Length | No. of Visitors per Year | Studies Addressing Fundamental Questions in Subterranean Biology | ||||
|---|---|---|---|---|---|---|---|---|
| Q1: What are the main ecological and ecosystem services provided by subterranean populations and communities? | Q2. How do basic life-history characteristics differ among subterranean communities and between subterranean and surface communities? | Q3. How does climate change affect subterranean-adapted organisms? | Q4. How does the use of caves by humans (e.g., tourism, religious, therapeutic, and recreational activities) affect subterranean ecosystems? | Additional Q5. What are the organisms found in subterranean ecosystems? | ||||
| Gcwihaba cave 20°01′43′′ S, 21°21′22′′ E (Botswana) | Yes | 400 m | ~200 | None | None | None | None | Mazebedi and Hesselberg 2020 [37] Visagie et al. 2021 [38] Cardoso et al. 2021 [19] Harvey and Du Preez 2014 [39] |
| Arnhem cave 22°70′14′′ S, 18°09′65′′ E (Namibia) | Yes | ~4500 m | Unknown | None | None | None | None | Churchill et al. 1997 [40] Matos et al. 2023 [41] Kirk-Spriggs et al. 2010 [42] |
| Aigamas 19°27′33.9′′ S, 17°16′59.3′′ E (Namibia) | No | 250 m | Not open to tourists | None | None | None | None | Churchill et al. 1997 [40] Matos et al. 2023 [41] Jacobs et al. 2021 [24] Jacobs et al. 2019 [43] Kensley 1995 [44] |
| Cango Caves 33°23′34″ S, 22°12′53″ E (South Africa) | Yes | 4 km | ~250,000 | None | None | None | Craven 1992 [45], Baker and Genty 1998 [46] | Cipola and Bellini 2016 [47] Babalola et al. 2024 [48] |
| Sterkfontein Caves 26°00′57″ S, 27°44′05″ E (South Africa) | Yes | 5.23 km | Unknown | None | None | Hopley and Maslin 2010 [49] | None | Zumpt, 1950 [50] |
| Wonderwerk cave 27°50′44.7″ S, 23°33′12.3″ E (South Africa) | Yes | 140 m | Unknown | None | None | None | None | None |
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Domínguez-Villar, D.; Culver, D.C.; Isaia, M. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Souza, M.F.V.R.; Alvarenga, D.A.; Souza-Silva, M.; Ferreira, R.L. Do different relevance attributes indicate the same conservation priorities? A case study in caves of southeastern Brazil. Int. J. Speleol. 2021, 50, 223–238. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; de la Montaña, E. Identifying areas of high-value vertebrate diversity for strengthening conservation. Biol. Conserv. 2003, 114, 357–370. [Google Scholar] [CrossRef]
- Duke, J.M.; Dundas, S.J.; Messer, K.D. Cost-effective conservation planning: Lessons from economics. J. Environ. Manag. 2013, 125, 126–133. [Google Scholar] [CrossRef]
- Hughes, A.C. Mapping priorities for conservation in Southeast Asia. Biol. Conserv. 2017, 209, 395–405. [Google Scholar] [CrossRef]
- Conenna, I.; Rocha, R.; Russo, D.; Cabeza, M. Insular bats and research effort: A review of global patterns and priorities. Mammal Rev. 2017, 47, 169–182. [Google Scholar] [CrossRef]
- Hernández-Quiroz, N.S.; Badano, E.I.; Barragán-Torres, F.; Flores, J.; Pinedo-Álvarez, C. Habitat suitability models to make conservation decisions based on areas of high species richness and endemism. Biodivers. Conserv. 2018, 27, 3185–3200. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Bekessy, S.A. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef]
- Chandra, A.; Idrisova, A. Convention on Biological Diversity: A review of national challenges and opportunities for implementation. Biodivers. Conserv. 2011, 20, 3295–3316. [Google Scholar] [CrossRef]
- Phalan, B.; Onial, M.; Balmford, A.; Green, R.E. Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science 2011, 333, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Pullin, A.S.; Knight, T.M.; Stone, D.A.; Charman, K. Do conservation managers use scientific evidence to support their decision-making? Biol. Conserv. 2004, 119, 245–252. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.H.; Ouyang, Z.Y.; Zhu, C.Q. Determination of priority nature conservation areas and human disturbances in the Yangtze River Basin, China. J. Nat. Conserv. 2014, 22, 326–336. [Google Scholar] [CrossRef]
- Tanalgo, K.C.; Hughes, A.C. Important but not a priority? Conservation concerns & priorities for Philippine bats in the Anthropocene. PeerJ Preprints 2018, 6, e27169v1. [Google Scholar]
- Deltshev, C.; Blagoev, G.; Hubenov, Z. Conservation priorities on biodiversity of invertebrates (non-Insecta) in Bulgarian Mountains. Ambio 1998, 27, 330–334. [Google Scholar]
- Sharratt, N.J.; Picker, M.D.; Samways, M.J. The invertebrate fauna of the sandstone caves of the Cape Peninsula (South Africa): Patterns of endemism and conservation priorities. Biodivers. Conserv. 2000, 9, 107–143. [Google Scholar] [CrossRef]
- Munasinghe, C.S.; Ranawana, K.B. Ecology, Diversity and Conservation Priorities of Cave Dwelling Fauna in Mandaramnuwara Cave, Nuwara Eliya District. In Proceedings of the 18th International Forestry and Environment Symposium, Thulhiriya, Sri Lanka, 10–14 January 2014. [Google Scholar]
- Cardoso, G.M.; Du Preez, G.; Taiti, S.; Ferreira, R.L. New troglobitic species of Niambia from Botswana and Namibia (Crustacea, Isopoda, Oniscidea). Subterr. Biol. 2021, 40, 91–108. [Google Scholar] [CrossRef]
- Engel, A.S. Chemolithoautotrophy. In Encyclopedia of Caves; Academic Press: Cambridge, MA, USA, 2019; pp. 267–276. [Google Scholar]
- Horváth, G.; Kerekes, K.; Nyitrai, V.; Balazs, G.; Berisha, H.; Herczeg, G. Exploratory behaviour divergence between surface populations, cave colonists and a cave population in the water louse, Asellus aquaticus. Behav. Ecol. Sociobiol. 2023, 77, 15. [Google Scholar] [CrossRef]
- Lunghi, E.; Mammola, S.; Martínez, A.; Hesselberg, T. Behavioural adjustments enable the colonization of subterranean environments. Zool. J. Linn. Soc. 2024, 201, 549–559. [Google Scholar] [CrossRef]
- Simonsen, D.; Hesselberg, T. Unique behavioural modifications in the web structure of the cave orb spider Meta menardi (Araneae, Tetragnathidae). Sci. Rep. 2021, 11, 92. [Google Scholar] [CrossRef]
- Jacobs, F.J.; Jacobs, P.G.; Hay, C.J.; Naesje, T.F. Status update of the endemic and critically endangered cave catfish Clarias cavernicola Trewavas 1936, from the Aigamas Cave system, Namibia. Afr. J. Aquat. Sci. 2021, 46, 18–21. [Google Scholar] [CrossRef]
- Chiarini, V.; Duckeck, J.; De Waele, J. A global perspective on sustainable show cave tourism. Geoheritage 2022, 14, 82. [Google Scholar] [CrossRef]
- Ramaano, A.I. The potential role of cultural heritage resources in tourism and community development at Musina municipality, Limpopo province, South Africa. J. Cult. Herit. Manag. Sustain. Dev. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Cigna, A.A.; Burri, E. Development, management and economy of show caves. Int. J. Speleol. 2000, 29, 1–27. [Google Scholar] [CrossRef]
- Baquedano Estévez, C.; Moreno Merino, L.; de la Losa Román, A.; Duran Valsero, J.J. The lampenflora in show caves and its treatment: An emerging ecological problem. Int. J. Speleol. 2019, 48, 4. [Google Scholar] [CrossRef]
- Duval, M.; Smith, B.; Hœrlé, S.; Bovet, L.; Khumalo, N.; Bhengu, L. Towards a holistic approach to heritage values: A multidisciplinary and cosmopolitan approach. Int. J. Herit. Stud. 2019, 25, 1279–1301. [Google Scholar] [CrossRef]
- Rüther, H.; Chazan, M.; Schroeder, R.; Neeser, R.; Held, C.; Walker, S.J.; Horwitz, L.K. Laser scanning for conservation and research of African cultural heritage sites: The case study of Wonderwerk Cave, South Africa. J. Archaeol. Sci. 2009, 36, 1847–1856. [Google Scholar] [CrossRef]
- Pule, O.J.; Lindsay, R. Exposure of tour guides to Radon at the Cango caves. In Proceedings of the 12. International Congress of the International Radiation Protection Association (IRPA 12), Buenos Aires, Argentina, 19–24 October 2008. [Google Scholar]
- Clarke, R.J.; Kuman, K. The skull of StW 573, a 3.67 ma Australopithecus prometheus skeleton from Sterkfontein Caves, South Africa. J. Hum. Evol. 2019, 134, 102634. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Giribet, G.; Du Preez, G.; Ventouras, O.; Janion, C.; Silva, M.S. The Wynberg cave system, the most important site for cave fauna in South Africa at risk. Subterr. Biol. 2020, 36, 73–81. [Google Scholar] [CrossRef]
- Mumbengegwi, P.; Odmell, C.; Doreen, M.; Candida, C. Cave tourism as an alternative form of sustainable tourism and heritage preservation: Insights from chirorodziva calabash festival. Gph-Int. J. Soc. Sci. Humanit. Res. 2023, 6, 33–46. [Google Scholar]
- Goldscheider, N.; Chen, Z.; Auler, A.S.; Bakalowicz, M.; Broda, S.; Drew, D.; Veni, G. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 2020, 28, 1661–1677. [Google Scholar] [CrossRef]
- Masilela, M.; Beckedahl, H. Karst geomorphology and related environmental problems in Southern Africa—A review. J. Afr. Earth Sci. 2022, 196, 104686. [Google Scholar] [CrossRef]
- Mazebedi, R.; Hesselberg, T. A preliminary survey of the abundance, diversity and distribution of terrestrial macroinvertebrates of Gcwihaba cave, northwest Botswana. Subterr. Biol. 2020, 35, 49–63. [Google Scholar] [CrossRef]
- Visagie, C.M.; Goodwell, M.; Nkwe, D.O. Aspergillus diversity from the Gcwihaba Cave in Botswana and description of one new species. Fungal Syst. Evol. 2021, 8, 81–89. [Google Scholar] [CrossRef]
- Harvey, M.S.; Du Preez, G. A new troglobitic ideoroncid pseudoscorpion (Pseudoscorpiones: Ideoroncidae) from southern Africa. J. Arachnol. 2014, 42, 105–110. [Google Scholar] [CrossRef]
- Churchill, S.; Draper, R.; Marais, E. Cave utilisation by Namibian bats: Population, microclimate and roost selection. South Afr. J. Wildl. Res. 1997, 27, 44–50. [Google Scholar]
- Matos, D.D.; Zastrow, J.; Val, A.; Mendelsohn, J. Caves and their fauna in Highlands and Escarpments of Angola and Namibia. Namibian Journal of Environment. Endemism in the Highlands and Escarpments of Angola and Namibia. Monograph 2023, 8, 323–330. [Google Scholar]
- Kirk-Spriggs, A.H.; Barraclough, D.A.; Meier, R. The immature stages of Katacamilla cavernicola Papp, the first described for the Camillidae (Diptera: Schizophora), with comparison to other known Ephydroidea larvae, and notes on biology. J. Nat. Hist. 2002, 36, 1105–1128. [Google Scholar] [CrossRef]
- Jacobs, F.; Hay, C.; Jacobs, G.; Næsje, T. Preliminary technical report: A baseline study of the critically endangered. Perspectives 2019, 52, 473–481. [Google Scholar]
- Kensley, B.F. A new genus of aquatic cave-dwelling isopod from Namibia (Crustacea: Isopoda: Asellota). Cimbebasia 1995, 14, 1–15. [Google Scholar]
- Craven, S.A. Cango Cave, Oudtshoorn District of the Cape Province, South Africa: An Assessment of Its Development and Management 1780–1992: Short Title, Management Problems at Cango Cave. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1992. [Google Scholar]
- Baker, A.; Genty, D. Environmental pressures on conserving cave speleothems: Effects of changing surface land use and increased cave tourism. J. Environ. Manag. 1998, 53, 165–175. [Google Scholar] [CrossRef]
- Cipola, N.G.; Bellini, B.C. A new cave species of Coecobrya Yosii (Collembola, Entomobryidae, Entomobryinae) from South Africa, with an identification key to the genus. Zootaxa 2016, 4200, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O.; Adedayo, A.A.; Akinola, S.A. High-throughput metagenomic assessment of Cango Cave microbiome–A South African limestone cave. Data Brief 2024, 54, 110381. [Google Scholar] [CrossRef] [PubMed]
- Hopley, P.J.; Maslin, M.A. Climate-averaging of terrestrial faunas: An example from the Plio-Pleistocene of South Africa. Paleobiology 2010, 36, 32–50. [Google Scholar] [CrossRef]
- Zumpt, F. Ectoparasites of bats from the Sterkfontein caves, Transvaal. J. Entomol. Soc. S. Afr. 1950, 13, 87–98. [Google Scholar]
- Hollingsworth, E.; Brahana, V.; Inlander, E. Karst Regions of the World (KROW): Global Karst Datasets and Maps to Advance the Protection of Karst Species and Habitats Worldwide; USGS Scientific Investigations Report 2008-5023; USGS Publication Warehouse: Reston, VA, USA, 2008.
- Teng-Zeng, F.K. Financing Science and Innovation in Africa: Institutional Development and Challenges; Science and Technology Innovation for Public Health in Africa; AUDA-NEPAD: Lusaka, Zambia, 2009; pp. 167–197. [Google Scholar]
- Davies, R. Economic growth in a post-apartheid South Africa: Its significance for relations with other African countries. J. Contemp. Afr. Stud. 1992, 11, 51–71. [Google Scholar] [CrossRef]
- Mammola, S.; Martinez, A. Let research on subterranean habitats resonate! Subterr. Biol. 2020, 36, 63–71. [Google Scholar] [CrossRef]
- Klopper, R.R.; Smith, G.F.; Van Rooy, J. The biodiversity of Africa. In Rebirth of Science in Africa: A Shared Vision for Life and Environmental Sciences; Umdaus Press: Pretoria, South Africa, 2002; pp. 60–86. [Google Scholar]
- Titley, M.A.; Snaddon, J.L.; Turner, E.C. Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PLoS ONE 2017, 12, e0189577. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Du Preez, G.; Theron, P.; Fourie, D. Terrestrial mesofauna biodiversity in unique karst environments in southern Africa. In Proceedings of the 16th International Congress of Speleology, Brno, Czech Republic, 21–28 July 2013; pp. 386–390. [Google Scholar]
- Edgecombe, G.D.; Akkari, N.; Netherlands, E.C.; Du Preez, G. A troglobitic species of the centipede Cryptops (Chilopoda, Scolopendromorpha) from northwestern Botswana. ZooKeys 2020, 977, 25. [Google Scholar] [CrossRef]
- Dandurand, G.; Duranthon, F.; Jarry, M.; Stratford, D.J.; Bruxelles, L. Biogenic corrosion caused by bats in Drotsky’s Cave (the Gcwihaba Hills, NW Botswana). Geomorphology 2019, 327, 284–296. [Google Scholar] [CrossRef]
- van der Schyff, V.; Serfontein, C.; Theron, P.; van Rooyen, D.; Dickie, B.; hew Dickie, M.; Stander, R. Armageddon Cave Survey. Redmayne-Smith, C., Ed.; South African Spelaeological Associacion: Pretoria, South Africa, 2015; Volume 41, pp. 39–48. [Google Scholar]
- Mbaiwa, J.E.; Sakuze, L.K. Cultural tourism and livelihood diversification: The case of Gcwihaba Caves and XaiXai village in the Okavango Delta, Botswana. J. Tour. Cult. Change 2009, 7, 61–75. [Google Scholar] [CrossRef]
- Reimold, W.U.; Whitfield, G.; Wallmach, T. Geotourism potential of southern Africa. In Geotourism; Routledge: Oxfordshire, UK, 2006; pp. 42–62. [Google Scholar]
- Marope, M.T. Namibia Human Capital and Knowledge Development for Economic Growth with Equity; World Bank: Washington, DC, USA, 2005. [Google Scholar]
- Geingos-Onuegbu, I.L. Technology and innovation landscapes in the context of a knowledge-based economy. J. Namib. Stud. Hist. Politics Cult. 2015, 18, 111–121. [Google Scholar]
- Dowling, R.; Pforr, C. Geotourism–a sustainable development option for Namibia. J. Ecotour. 2021, 20, 371–385. [Google Scholar] [CrossRef]
- Magnussen, A.; Visser, G. Developing a World Heritage Site: The cradle of humankind. Afr. Insight 2003, 33, 78–86. [Google Scholar] [CrossRef]
- Caruana, M.V.; Stratford, D.J. Historical perspectives on the significance of archaeology in the Cradle of Humankind, South Africa. S. Afr. Archaeol. Soc. Goodwin Ser. 2019, 12, 44–55. [Google Scholar]
- Dirks, P.H.; Berger, L.R. Hominin-bearing caves and landscape dynamics in the Cradle of Humankind, South Africa. J. Afr. Earth Sci. 2013, 78, 109–131. [Google Scholar] [CrossRef]
- Gommery, D.; Thackeray, J.F.; Sénégas, F.; Potze, S.; Kgasi, L. The earliest primate (Parapapio sp.) from the Cradle of Humankind World Heritage site (Waypoint 160, Bolt’s Farm, South Africa). S. Afr. J. Sci. 2008, 104, 405–408. [Google Scholar]
- Adams, J.W.; Hemingway, J.; Kegley AD, T.; Thackeray, J.F. Luleche, a new paleontological site in the Cradle of Humankind, North-West Province, South Africa. J. Hum. Evol. 2007, 53, 751–754. [Google Scholar] [CrossRef]
- Leichliter, J. Micromammal paleoecology: Theory, Methods, and Application to Modern and Fossil Assemblages in the Cradle of Humankind World Heritage Site, South Africa. Ph.D. Thesis, University of Colorado at Boulder, Boulder, CO, USA, 2011. [Google Scholar]
- Pavia, M.; Val, A.; Carrera, L.; Steininger, C.M. Fossil birds from Cooper’s D aid in reconstructing the Early Pleistocene paleoenvironment in the Cradle of Humankind (Gauteng, South Africa). J. Hum. Evol. 2022, 167, 103185. [Google Scholar] [CrossRef]
- Pavia, M. Palaeoenvironmental reconstruction of the Cradle of Humankind during the Plio-Pleistocene transition, inferred from the analysis of fossil birds from Member 2 of the hominin-bearing site of Kromdraai (Gauteng, South Africa). Quat. Sci. Rev. 2020, 248, 106532. [Google Scholar] [CrossRef]
- Matshusa, K. The Potential for Geotourism at the Kruger National Park for Social Sustainability. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazebedi, R.; Majoka, K.; Hesselberg, T. Comparative Overview of Cave Biodiversity Research Activities in Southern Africa: Insights from Botswana, Namibia and South Africa. Diversity 2025, 17, 342. https://doi.org/10.3390/d17050342
Mazebedi R, Majoka K, Hesselberg T. Comparative Overview of Cave Biodiversity Research Activities in Southern Africa: Insights from Botswana, Namibia and South Africa. Diversity. 2025; 17(5):342. https://doi.org/10.3390/d17050342
Chicago/Turabian StyleMazebedi, Richard, Kefeletswe Majoka, and Thomas Hesselberg. 2025. "Comparative Overview of Cave Biodiversity Research Activities in Southern Africa: Insights from Botswana, Namibia and South Africa" Diversity 17, no. 5: 342. https://doi.org/10.3390/d17050342
APA StyleMazebedi, R., Majoka, K., & Hesselberg, T. (2025). Comparative Overview of Cave Biodiversity Research Activities in Southern Africa: Insights from Botswana, Namibia and South Africa. Diversity, 17(5), 342. https://doi.org/10.3390/d17050342







