The Impact of Vegetation Changes in Savanna Ecosystems on Tick Populations in Wildlife: Implications for Ecosystem Management
Abstract
1. Introduction
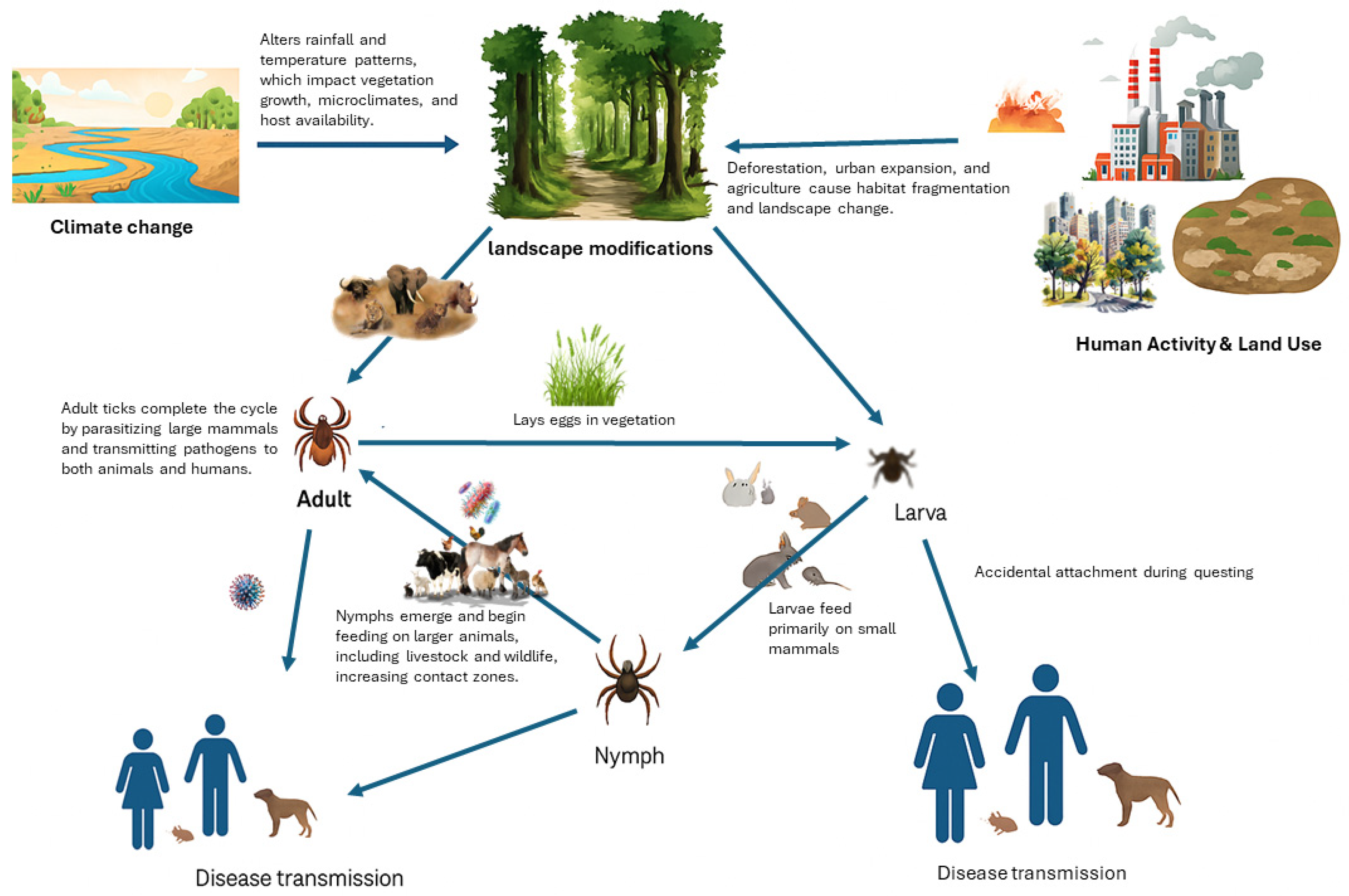
2. Vegetation Changes in Savanna Ecosystems
2.1. Temperature and Humidity Effects on Tick Survival
2.2. Habitat Modification and Tick Distribution
2.3. Seasonal Abundance and Tick Behavior
2.4. Climate Change and Vegetation Dynamics
2.5. Fire and Grazing Influences on Vegetation
2.6. Human Land Use and Habitat Fragmentation
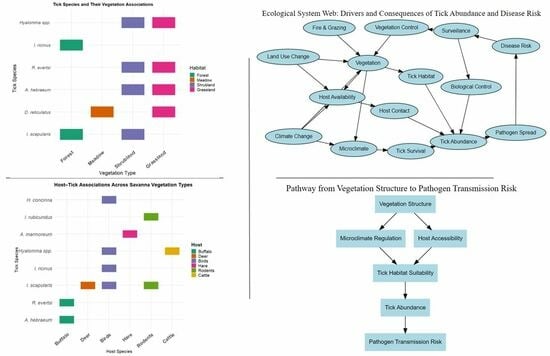
3. Vegetation Types and Tick Distribution
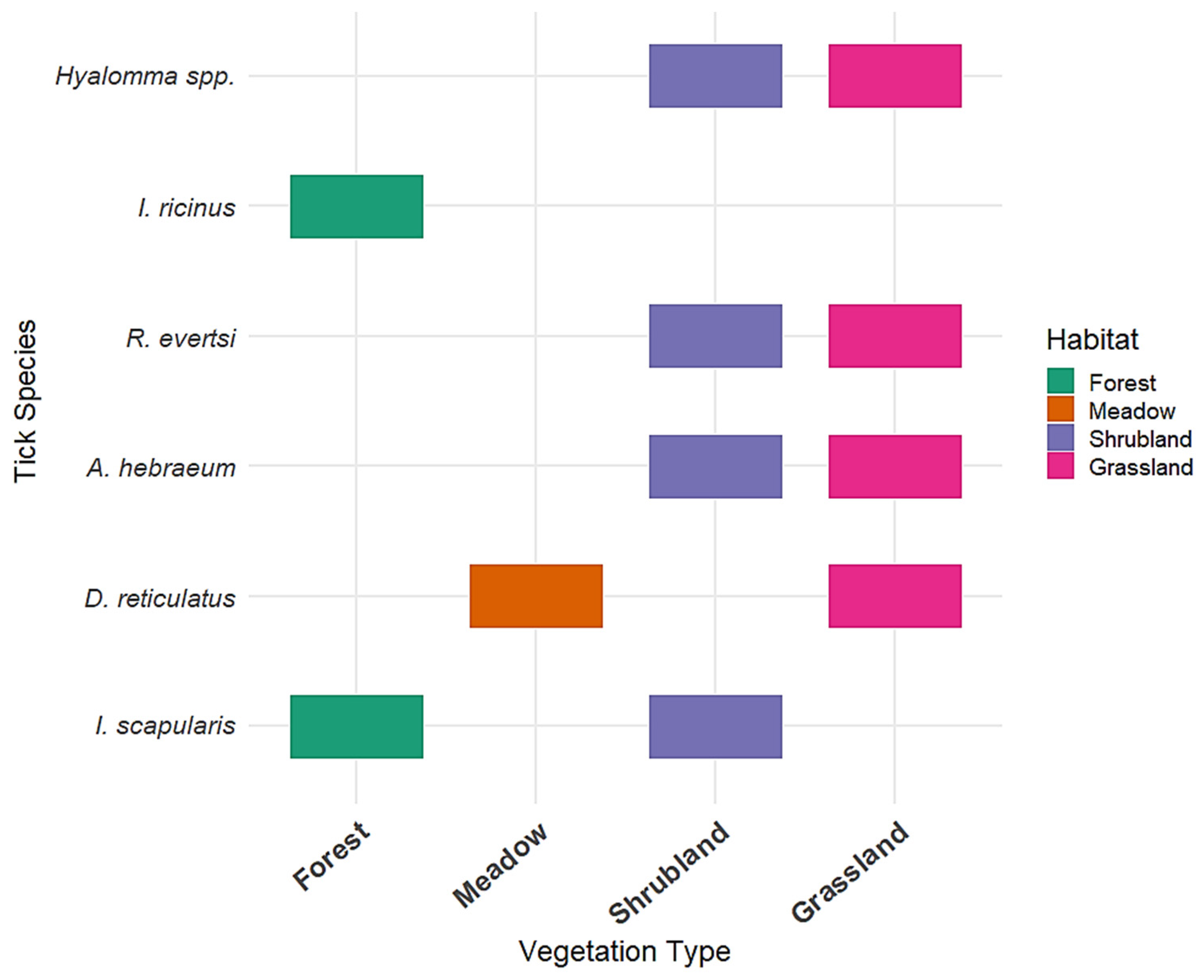
3.1. Tick Distribution in Different Vegetation Types
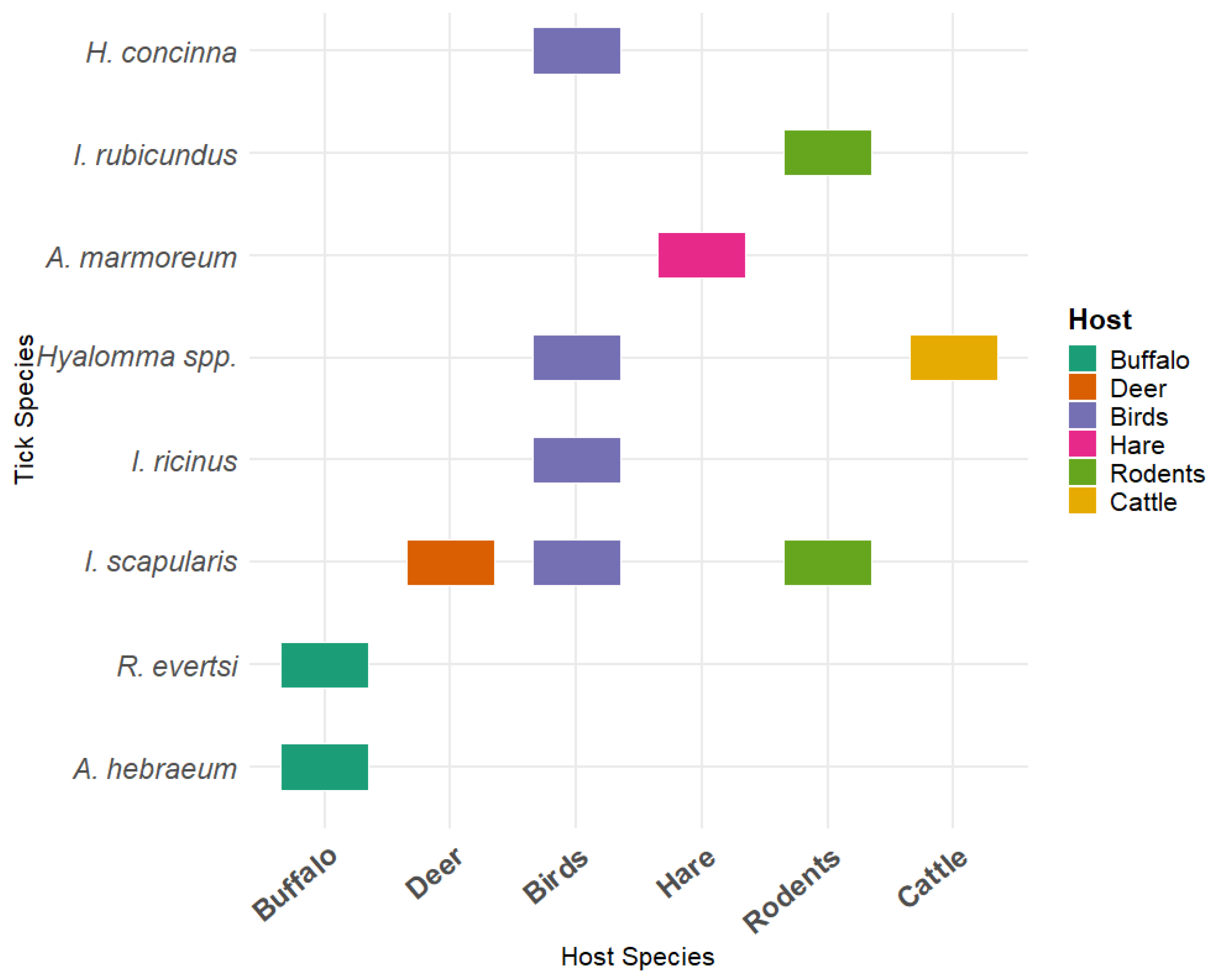
3.2. Tick Species and Habitat Associations
3.3. Host Availability and Tick Abundance
3.4. Vegetation and Disease Transmission Dynamics
4. Conservation and Disease Management Strategies
4.1. Integrated Tick Control and Vegetation Management
4.2. Wildlife Management and Host Control
4.3. Habitat and Vector Surveillance
4.4. Conservation Efforts and Biodiversity Protection
5. Conclusions and Recommendations
5.1. Recommendations
5.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.; Walker, A. Ticks of Domestic Animals in the Mediterranean Region; University of Zaragoza: Zaragoza, Spain, 2004; p. 131. [Google Scholar]
- Pegram, R.; Clifford, C.; Walker, J.B.; Keirans, J. Clarification of the Rhipicephalus sanguineus group (Acari: Ixodoidea: Ixodidae): R. sulcatus Neumann, 1908 and R. turanicus Pomerantsev, 1936. Syst. Parasitol. 1987, 10, 3–26. [Google Scholar] [CrossRef]
- Gilbert, L. The impacts of climate change on ticks and tick-borne disease risk. Annu. Rev. Entomol. 2021, 66, 373–388. [Google Scholar] [CrossRef]
- Tsao, J.I.; Hamer, S.A.; Han, S.; Sidge, J.L.; Hickling, G.J. The contribution of wildlife hosts to the rise of ticks and tick-borne diseases in North America. J. Med. Entomol. 2021, 58, 1565–1587. [Google Scholar] [CrossRef]
- Yessinou, R.E.; Koumassou, A.; Galadima, H.B.; Nanoukon-Ahigan, H.; Farougou, S.; Pfeffer, M. Tick diversity and distribution of pathogen in ticks collected from wild animals and vegetation in Africa. Pathogens 2025, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Ostfeld, R.S. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023540118. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Shi, Y.; Zhao, N.; Zhang, X.; Jin, X.; He, J.; Xu, B.; Qin, T. Molecular detection of tick-borne bacterial and protozoan pathogens in Haemaphysalis longicornis (Acari: Ixodidae) ticks from free-ranging domestic sheep in Hebei Province, China. Pathogens 2023, 12, 763. [Google Scholar] [CrossRef]
- Rocha, S.C.; Velásquez, C.V.; Aquib, A.; Al-Nazal, A.; Parveen, N. Transmission cycle of tick-borne infections and co-infections, animal models and diseases. Pathogens 2022, 11, 1309. [Google Scholar] [CrossRef]
- Schorn, S.; Pfister, K.; Reulen, H.; Mahling, M.; Silaghi, C. Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in Bavarian public parks, Germany. Parasites Vectors 2011, 4, 135. [Google Scholar] [CrossRef]
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef]
- Sri-in, C.; Thongmeesee, K.; Wechtaisong, W.; Yurayart, N.; Rittisornthanoo, G.; Akarapas, C.; Bunphungbaramee, N.; Sipraya, N.; Riana, E.; Bui, T.T.H.; et al. Tick diversity and molecular detection of Anaplasma, Babesia, and Theileria from Khao Kheow Open Zoo, Chonburi Province, Thailand. Front. Vet. Sci. 2024, 11, 1430892. [Google Scholar] [CrossRef]
- Rana, V.S.; Kitsou, C.; Dumler, J.S.; Pal, U. Immune evasion strategies of major tick-transmitted bacterial pathogens. Trends Microbiol. 2023, 31, 62–75. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.J. Can the Potential for Tick Infestation Influence Patterns of Resource Use by Eland (Taurotragus oryx)? Bachelor’s Thesis, University of Witwatersrand, Johannesburg, South Africa, 2016. [Google Scholar]
- Ledger, K.J.; Keenan, R.M.; Sayler, K.A.; Wisely, S.M. Multi-scale patterns of tick occupancy and abundance across an agricultural landscape in southern Africa. PLoS ONE 2019, 14, e0222879. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quirós, A.C.; Barrantes, G. Influence of vegetation structure and climatic conditions on abundance of host-seeking Amblyomma ticks (Acari: Ixodidae) in a Costa Rican dry forest. Acta Zool. Mex. 2024, 40, 1–16. [Google Scholar]
- Brenner, A.E.; Muñoz-Leal, S.; Sachan, M.; Labruna, M.B.; Raghavan, R. Coxiella burnetii and related tick endosymbionts evolved from pathogenic ancestors. Genome Biol. Evol. 2021, 13, evab108. [Google Scholar] [CrossRef]
- Kolo, A.O.; Raghavan, R. Impact of endosymbionts on tick physiology and fitness. Parasitology 2023, 150, 859–865. [Google Scholar] [CrossRef]
- Garcia-Vozmediano, A.; Krawczyk, A.I.; Sprong, H.; Rossi, L.; Ramassa, E.; Tomassone, L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in the Western Alps. Ticks Tick-Borne Dis. 2020, 11, 101489. [Google Scholar] [CrossRef]
- Tomassone, L.; Portillo, A.; Nováková, M.; De Sousa, R.; Oteo, J.A. Neglected aspects of tick-borne rickettsioses. Parasites Vectors 2018, 11, 263. [Google Scholar] [CrossRef]
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.A.; Lindsay, L.R. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019, 45, 83–89. [Google Scholar] [CrossRef]
- Makwarela, T.G.; Djikeng, A.; Masebe, T.M.; Nkululeko, N.; Nesengani, L.T.; Mapholi, N.O. Vector abundance and associated abiotic factors that influence the distribution of ticks in six provinces of South Africa. Vet. World 2024, 17, 1765–1777. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Ayllón, N.; De La Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef]
- Ogden, N.H.; Beard, C.B.; Ginsberg, H.S.; Tsao, J.I. Possible effects of climate change on ixodid ticks and the pathogens they transmit: Predictions and observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef]
- Elmieh, N. The Impacts of Climate and Land Use Change on Tick-Related Risks; National Collaborating Centre for Environmental Health: Vancouver, BC, Canada, 2022. [Google Scholar]
- Polsomboon, S.; Hoel, D.F.; Murphy, J.R.; Linton, Y.-M.; Motoki, M.; Robbins, R.G.; Bautista, K.; Briceño, I.; Achee, N.L.; Grieco, J.P.; et al. Molecular detection and identification of Rickettsia species in ticks (Acari: Ixodidae) collected from Belize, Central America. J. Med. Entomol. 2017, 54, 1718–1726. [Google Scholar] [CrossRef]
- Usananan, P.; Kaenkan, W.; Sudsangiem, R.; Baimai, V.; Trinachartvanit, W.; Ahantarig, A. Phylogenetic studies of Coxiella-like bacteria and spotted fever group Rickettsiae in ticks collected from vegetation in Chaiyaphum Province, Thailand. Front. Vet. Sci. 2022, 9, 849893. [Google Scholar] [CrossRef] [PubMed]
- Reye, A.L.; Arinola, O.G.; Hübschen, J.M.; Muller, C.P. Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl. Environ. Microbiol. 2012, 78, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Di, C.; Sulkow, B.; Qiu, W.; Sun, S. Effects of micro-scale environmental factors on the quantity of questing black-legged ticks in suburban New York. Appl. Sci. 2023, 13, 11587. [Google Scholar] [CrossRef]
- van Oort, B.E.H.; Hovelsrud, G.K.; Risvoll, C.; Mohr, C.W.; Jore, S. A mini-review of Ixodes ticks climate sensitive infection dispersion risk in the Nordic region. Int. J. Environ. Res. Public Health 2020, 17, 5387. [Google Scholar] [CrossRef]
- Van Gestel, M.; Matthysen, E.; Heylen, D.; Verheyen, K. Survival in the understorey: Testing direct and indirect effects of microclimatological changes on Ixodes ricinus. Ticks Tick-Borne Dis. 2022, 13, 102035. [Google Scholar] [CrossRef]
- Nielebeck, C.; Kim, S.H.; Pepe, A.; Himes, L.; Miller, Z.; Zummo, S.; Tang, M.; Monzón, J.D. Climatic stress decreases tick survival but increases rate of host-seeking behavior. Ecosphere 2023, 14, e4369. [Google Scholar] [CrossRef]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Ogden, N.H.; Beard, C.B. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Entomol. 2016, 53, 250–261. [Google Scholar] [CrossRef]
- Ginsberg, H.S. Tick control. In Biology of Ticks, 2nd ed.; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2013; Volume 2, pp. 409–444. [Google Scholar]
- Burtis, J.C.; Sullivan, P.; Levi, T.; Oggenfuss, K.; Fahey, T.J.; Ostfeld, R.S. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasites Vectors 2016, 9, 606. [Google Scholar] [CrossRef]
- Ogden, N.H.; Lindsay, L.R. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Zahler, M.; Gothe, R. Effect of temperature and humidity on egg hatch, moulting and longevity of larvae and nymphs of Dermacentor reticulatus (Ixodidae). Appl. Parasitol. 1995, 36, 53–65. [Google Scholar] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Dyczko, D.; Błażej, P.; Kiewra, D. The influence of forest habitat type on Ixodes ricinus infections with Rickettsia spp. in south-western Poland. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 6, 100200. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.; Neitzel, D.F.; Moen, R.A.; Craft, M.E.; Hamilton, K.E.; Johnson, L.B.; Mulla, D.J.; Munderloh, U.G.; Redig, P.T.; Smith, K.E.; et al. Disease risk in a dynamic environment: The spread of tick-borne pathogens in Minnesota, USA. EcoHealth 2015, 12, 152–163. [Google Scholar] [CrossRef]
- Tian, D.; Cui, X.-M.; Ye, R.-Z.; Li, Y.-Y.; Wang, N.; Gao, W.-Y.; Wang, B.-H.; Lin, Z.-T.; Zhu, W.-J.; Wang, Q.-S.; et al. Distribution and diversity of ticks determined by environmental factors in Ningxia, China. One Health 2024, 19, 100897. [Google Scholar] [CrossRef]
- Janzén, T.; Choudhury, F.; Hammer, M.; Petersson, M.; Dinnétz, P. Ticks—Public health risks in urban green spaces. BMC Public Health 2024, 24, 1031. [Google Scholar] [CrossRef]
- Butler, R.A.; Randolph, K.C.; Vogt, J.T.; Paulsen, D.J.; Fryxell, R.T.T. Forest-associated habitat variables influence human–tick encounters in the southeastern United States. Environ. Entomol. 2023, 52, 1033–1041. [Google Scholar] [CrossRef]
- Kopsco, H.L.; Gronemeyer, P.; Mateus-Pinilla, N.; Smith, R.L. Current and future habitat suitability models for four ticks of medical concern in Illinois, USA. Insects 2023, 14, 213. [Google Scholar] [CrossRef]
- Makwarela, T.G.; Seoraj-Pillai, N.; Nangammbi, T.C. Tick control strategies: Critical insights into chemical, biological, physical, and integrated approaches for effective hard tick management. Vet. Sci. 2025, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Kim, H.; Won, S.; Kim, H.-C.; Chong, S.-T.; Klein, T.A.; Kim, K.-G.; Seo, H.-Y.; Chae, J.-S. Ticks collected from wild and domestic animals and natural habitats in the Republic of Korea. Korean J. Parasitol. 2014, 52, 281–285. [Google Scholar] [CrossRef]
- Park, S.-W.; Song, B.G.; Shin, E.-H.; Yun, S.-M.; Han, M.-G.; Park, M.Y.; Park, C.; Ryou, J. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks Tick-Borne Dis. 2014, 5, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Grelle, C. The Atlantic Forest History, Biodiversity, Threats and Opportunities of the Mega-Diverse Forest; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Joly, C.A.; Metzger, J.P.; Tabarelli, M. Experiences from the Brazilian Atlantic Forest: Ecological findings and conservation initiatives. New Phytol. 2014, 204, 459–473. [Google Scholar] [CrossRef]
- Ribeiro, M.; Martensen, A.; Metzger, J.; Tabarelli, M.; Scarano, F.; Fortin, M.-J. The Brazilian Atlantic Forest: A shrinking biodiversity hotspot. In Biodiversity Hotspots; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin, Germany, 2011; pp. 405–434. [Google Scholar]
- de Lima, R.A.F.; Oliveira, A.A.; Pitta, G.R.; de Gasper, A.L.; Vibrans, A.C.; Chave, J.; ter Steege, H.; Prado, P.I. The erosion of biodiversity and biomass in the Atlantic Forest biodiversity hotspot. Nat. Commun. 2020, 11, 6347. [Google Scholar] [CrossRef]
- Szabó, M.P.J.; Martins, T.F.; Barbieri, A.R.M.; Costa, F.B.; Soares, H.S.; Tolesano-Pascoli, G.V.; Torga, K.; Saraiva, D.G.; do Nascimento Ramos, V.; Osava, C.F.; et al. Ticks biting humans in the Brazilian savannah: Attachment sites and exposure risk in relation to species, life stage and season. Ticks Tick-Borne Dis. 2020, 11, 101328. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Garcia, M.; Matias, J.; Aguirre, A.; Csordas, B.; Szabó, M.; Andreotti, R. Successful feeding of Amblyomma coelebs (Acari: Ixodidae) nymphs on humans in Brazil: Skin reactions to parasitism. J. Med. Entomol. 2015, 52, 117–119. [Google Scholar] [CrossRef]
- Bourdin, A.; Dokhelar, T.; Bord, S.; van Halder, I.; Stemmelen, A.; Scherer-Lorenzen, M.; Jactel, H. Forests harbor more ticks than other habitats: A meta-analysis. For. Ecol. Manag. 2023, 541, 121081. [Google Scholar] [CrossRef]
- Rodriguez, I.A.; Rasoazanabary, E.; Godfrey, L.R. Seasonal variation in the abundance and distribution of ticks that parasitize Microcebus griseorufus at the Bezà Mahafaly Special Reserve, Madagascar. Int. J. Parasitol. Parasites Wildl. 2015, 4, 408–413. [Google Scholar] [CrossRef]
- Janzen, D.H. Tropical dry forests. In Biodiversity; Wilson, E.O., Peter, F.M., Eds.; National Academy Press: Washington, DC, USA, 1988; pp. 130–137. [Google Scholar]
- Norval, R. Ecology of the tick Amblyomma hebraeum Koch in the Eastern Cape Province of South Africa. I. Distribution and seasonal activity. J. Parasitol. 1977, 63, 734–739. [Google Scholar] [CrossRef]
- Eisen, L. Seasonal activity patterns of Ixodes scapularis and Ixodes pacificus in the United States. Ticks Tick-Borne Dis. 2025, 16, 102433. [Google Scholar] [CrossRef] [PubMed]
- Synodinos, A.D.; Tietjen, B.; Lohmann, D.; Jeltsch, F. The impact of inter-annual rainfall variability on African savannas changes with mean rainfall. J. Theor. Biol. 2018, 437, 92–100. [Google Scholar] [CrossRef]
- Wikel, S.K. Ticks and tick-borne infections: Complex ecology, agents, and host interactions. Vet. Sci. 2018, 5, 60. [Google Scholar] [CrossRef]
- Olwoch, J.; Reyers, B.; Engelbrecht, F.; Erasmus, B. Climate change and the tick-borne disease, Theileriosis (East Coast fever) in sub-Saharan Africa. J. Arid Environ. 2008, 72, 108–120. [Google Scholar] [CrossRef]
- Nyangiwe, N.; Harrison, A.; Horak, I. Displacement of Rhipicephalus decoloratus by Rhipicephalus microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa. Exp. Appl. Acarol. 2013, 61, 371–382. [Google Scholar] [CrossRef]
- Copeland, S.; Sambado, S.; Orr, D.; Bui, A.; Swei, A.; Young, H.S. Variable effects of wildlife and livestock on questing tick abundance across a topographical–climatic gradient. Ecosphere 2025, 16, e70190. [Google Scholar] [CrossRef]
- Perret, J.-L.; Rais, O.; Gern, L. Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. J. Med. Entomol. 2004, 41, 361–365. [Google Scholar] [CrossRef]
- Randolph, S.E.; Sonenshine, D.; Roe, R. Ecology of non-nidicolous ticks. In Biology of Ticks; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 2, pp. 3–39. [Google Scholar]
- Needham, G.R.; Teel, P.D. Off-host physiological ecology of ixodid ticks. Annu. Rev. Entomol. 1991, 36, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, P.A. Climate change impacts on ticks and tick-borne infections. Biologia 2022, 77, 1503–1512. [Google Scholar] [CrossRef]
- Morrison, W. Theileriosis in Animals. Available online: https://www.msdvetmanual.com/circulatory-system/blood-parasites/theileriosis-in-animals (accessed on 14 April 2025).
- Quesada, M.; Stoner, K.E. Threats to the conservation of the tropical dry forest in Costa Rica. In Biodiversity Conservation in Costa Rica: Learning the Lessons in a Seasonal Dry Forest; Frankie, G.W., Ed.; University of California Press: Berkeley, CA, USA, 2004; pp. 266–280. [Google Scholar]
- Bishop, R.P.; Kappmeyer, L.S.; Onzere, C.K.; Odongo, D.O.; Githaka, N.; Sears, K.P.; Knowles, D.P.; Fry, L.M. Equid-infective Theileria cluster in distinct 18S rRNA gene clades comprising multiple taxa with unusually broad mammalian host ranges. Parasites Vectors 2020, 13, 261. [Google Scholar] [CrossRef]
- Peralbo-Moreno, A.; Baz-Flores, S.; Cuadrado-Matías, R.; Barroso, P.; Triguero-Ocaña, R.; Jiménez-Ruiz, S.; Herraiz, C.; Ruiz-Rodríguez, C.; Acevedo, P.; Ruiz-Fons, F. Environmental factors driving fine-scale ixodid tick abundance patterns. Sci. Total Environ. 2022, 853, 158633. [Google Scholar] [CrossRef] [PubMed]
- Fill, J.M.; Miller, H.M.; Crandall, R. Prescribed fire as a tool for controlling tick populations in the Southeastern United States: FOR398/FR469, 8/2023. EDIS 2023, 2023. [Google Scholar] [CrossRef]
- Glass, G.E.; Schwartz, B.S.; Morgan, J.M., III; Johnson, D.T.; Noy, P.M.; Israel, E. Environmental risk factors for Lyme disease identified with geographic information systems. Am. J. Public Health 1995, 85, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Tiffin, H.S.; Rajotte, E.G.; Sakamoto, J.M.; Machtinger, E.T. Tick control in a connected world: Challenges, solutions, and public policy from a United States border perspective. Trop. Med. Infect. Dis. 2022, 7, 388. [Google Scholar] [CrossRef]
- Esser, H.J.; Herre, E.A.; Kays, R.; Liefting, Y.; Jansen, P.A. Local host–tick coextinction in neotropical forest fragments. Int. J. Parasitol. 2019, 49, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Tardy, O.; Acheson, E.S.; Bouchard, C.; Chamberland, É.; Fortin, A.; Ogden, N.H.; Leighton, P.A. Mechanistic movement models to predict geographic range expansions of ticks and tick-borne pathogens: Case studies with Ixodes scapularis and Amblyomma americanum in eastern North America. Ticks Tick-Borne Dis. 2023, 14, 102161. [Google Scholar] [CrossRef]
- Machtinger, E.T.; Poh, K.C.; Pesapane, R.; Tufts, D.M. An integrative framework for tick management: The need to connect wildlife science, One Health, and interdisciplinary perspectives. Curr. Opin. Insect Sci. 2024, 61, 101131. [Google Scholar] [CrossRef]
- Ortiz, D.I.; Piche-Ovares, M.; Romero-Vega, L.M.; Wagman, J.; Troyo, A. The impact of deforestation, urbanization, and changing land use patterns on the ecology of mosquito and tick-borne diseases in Central America. Insects 2021, 13, 20. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Roe, R.M. Biology of Ticks; Oxford University Press: New York, NY, USA, 2014; Volume 2. [Google Scholar]
- Van Gestel, M.; Heylen, D.; Verheyen, K.; Fonville, M.; Sprong, H.; Matthysen, E. Recreational hazard: Vegetation and host habitat use correlate with changes in tick-borne disease hazard at infrastructure within forest stands. Sci. Total Environ. 2024, 919, 170749. [Google Scholar] [CrossRef]
- Gömer, A.; Lang, A.; Janshoff, S.; Steinmann, J.; Steinmann, E. Epidemiology and global spread of emerging tick-borne Alongshan virus. Emerg. Microbes Infect. 2024, 13, 2404271. [Google Scholar] [CrossRef]
- Mathisson, D.C.; Kross, S.M.; Palmer, M.I.; Diuk-Wasser, M.A. Effect of vegetation on the abundance of tick vectors in the Northeastern United States: A review of the literature. J. Med. Entomol. 2021, 58, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, M.; Verheyen, K.; Matthysen, E.; Heylen, D. Danger on the track? Tick densities near recreation infrastructures in forests. Urban For. Urban Green. 2021, 59, 126994. [Google Scholar] [CrossRef]
- Zając, Z.; Bartosik, K.; Woźniak, A. Monitoring Dermacentor reticulatus host-seeking activity in natural conditions. Insects 2020, 11, 264. [Google Scholar] [CrossRef]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasites Vectors 2016, 9, 314. [Google Scholar] [CrossRef]
- Szabó, M.P.J.; Martins, M.M.; de Castro, M.B.; Pacheco, R.C.; Tolesano-Pascoli, G.V.; Dos Santos, K.T.; Martins, T.F.; de Souza, L.G.A.; May-Junior, J.A.; Yokosawa, J.; et al. Ticks (Acari: Ixodidae) in the Serra da Canastra National Park in Minas Gerais, Brazil: Species, abundance, ecological and seasonal aspects with notes on rickettsial infection. Exp. Appl. Acarol. 2018, 76, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Ezenwa, V.O.; Jolles, A.E. Tick infestation patterns in free-ranging African buffalo (Syncercus caffer): Effects of host innate immunity and niche segregation among tick species. Int. J. Parasitol. Parasites Wildl. 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Busi, A.; Martínez-Sánchez, E.T.; Alvarez-Londoño, J.; Rivera-Páez, F.A.; Ramírez-Chaves, H.E.; Fontúrbel, F.E.; Castaño-Villa, G.J. Environmental and ecological factors affecting tick infestation in wild birds of the Americas. Parasitol. Res. 2024, 123, 254. [Google Scholar] [CrossRef]
- Keve, G.; Csörgő, T.; Kováts, D.; Hornok, S. Long term evaluation of factors influencing the association of ixodid ticks with birds in Central Europe, Hungary. Sci. Rep. 2024, 14, 4958. [Google Scholar] [CrossRef]
- Hoffman, T.; Carra, L.G.; Öhagen, P.; Fransson, T.; Barboutis, C.; Piacentini, D.; Figuerola, J.; Kiat, Y.; Onrubia, A.; Jaenson, T.G.T.; et al. Association between guilds of birds in the African–Western Palaearctic region and the tick species Hyalomma rufipes, one of the main vectors of Crimean–Congo hemorrhagic fever virus. One Health 2021, 13, 100349. [Google Scholar] [CrossRef]
- Ledwaba, M.B.; Nozipho, K.; Tembe, D.; Onyiche, T.E.; Chaisi, M.E. Distribution and prevalence of ticks and tick-borne pathogens of wild animals in South Africa: A systematic review. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100088. [Google Scholar] [CrossRef]
- Makwarela, T.G.; Nyangiwe, N.; Masebe, T.; Mbizeni, S.; Nesengani, L.T.; Djikeng, A.; Mapholi, N.O. Tick diversity and distribution of hard (Ixodidae) cattle ticks in South Africa. Microbiol. Res. 2023, 14, 42–59. [Google Scholar] [CrossRef]
- Thutwa, K. Comparison of Genetic and Immunological Responses to Tick Infestation Between Three Breeds of Sheep in South Africa. Master’s Thesis, University of the Free State, Bloemfontein, South Africa, 2016. [Google Scholar]
- Espinaze, M.P.A.; Hellard, E.; Horak, I.G.; Cumming, G.S. Domestic mammals facilitate tick-borne pathogen transmission networks in South African wildlife. Biol. Conserv. 2018, 221, 228–236. [Google Scholar] [CrossRef]
- O’Neill, X.; White, A.; Gortázar, C.; Ruiz-Fons, F. The impact of host abundance on the epidemiology of tick-borne infection. Bull. Math. Biol. 2023, 85, 30. [Google Scholar] [CrossRef] [PubMed]
- Hofmeester, T.R.; Rowcliffe, J.M.; Jansen, P.A. Quantifying the availability of vertebrate hosts to ticks: A camera-trapping approach. Front. Vet. Sci. 2017, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Babayani, N.D.; Makati, A. Predictive analytics of cattle host and environmental and micro-climate factors for tick distribution and abundance at the livestock–wildlife interface in the Lower Okavango Delta of Botswana. Front. Vet. Sci. 2021, 8, 698395. [Google Scholar] [CrossRef]
- Esser, H.J.; Foley, J.E.; Bongers, F.; Herre, E.A.; Miller, M.J.; Prins, H.H.T.; Jansen, P.A. Host body size and the diversity of tick assemblages on Neotropical vertebrates. Int. J. Parasitol. Parasites Wildl. 2016, 5, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Watari, Y.; Furukawa, T.; Okabe, K. Importance of host abundance and microhabitat in tick abundance. J. Med. Entomol. 2022, 59, 2110–2119. [Google Scholar] [CrossRef]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef]
- Bourdin, A.; Bord, S.; Durand, J.; Galon, C.; Moutailler, S.; Scherer-Lorenzen, M.; Jactel, H. Forest diversity reduces the prevalence of pathogens transmitted by the tick Ixodes ricinus. Front. Ecol. Evol. 2022, 10, 891908. [Google Scholar] [CrossRef]
- Wimms, C.; Aljundi, E.; Halsey, S.J. Regional dynamics of tick vectors of human disease. Curr. Opin. Insect Sci. 2023, 55, 101006. [Google Scholar] [CrossRef]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef]
- Shaw, M.T.; Keesing, F.; McGrail, R.; Ostfeld, R.S. Factors influencing the distribution of larval blacklegged ticks on rodent hosts. Am. J. Trop. Med. Hyg. 2003, 68, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L. Rodent-targeted approaches to reduce acarological risk of human exposure to pathogen-infected Ixodes ticks. Ticks Tick-Borne Dis. 2023, 14, 102119. [Google Scholar] [CrossRef]
- Eisen, L.; Stafford, K.C. Barriers to effective tick management and tick-bite prevention in the United States (Acari: Ixodidae). J. Med. Entomol. 2021, 58, 1588–1600. [Google Scholar] [CrossRef]
- Jori, F.; Hernandez-Jover, M.; Magouras, I.; Dürr, S.; Brookes, V.J. Wildlife–livestock interactions in animal production systems: What are the biosecurity and health implications? Anim. Front. 2021, 11, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Makovska, I.; Dhaka, P.; Chantziaras, I.; Pessoa, J.; Dewulf, J. The role of wildlife and pests in the transmission of pathogenic agents to domestic pigs: A systematic review. Animals 2023, 13, 1830. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Mangold, A.J.; Nava, S.; Venzal, J.M.; Labruna, M.; Guglielmone, A.A. A review of the systematics of the tick family Argasidae (Ixodida). Acarologia 2010, 50, 317–333. [Google Scholar] [CrossRef]
- Nava, S.; Venzal, J.M.; Labruna, M.B.; Mastropaolo, M.; González, E.M.; Mangold, A.J.; Guglielmone, A.A. Hosts, distribution and genetic divergence (16S rDNA) of Amblyomma dubitatum (Acari: Ixodidae). Exp. Appl. Acarol. 2010, 51, 335–351. [Google Scholar] [CrossRef]
- Labruna, M.B.; Terassini, F.; Camargo, L.M.A. Notes on population dynamics of Amblyomma ticks (Acari: Ixodidae) in Brazil. J. Parasitol. 2009, 95, 1016–1018. [Google Scholar] [CrossRef]
- Bursali, A.; Keskin, A.; Tekin, S. A review of the ticks (Acari: Ixodida) of Turkey: Species diversity, hosts and geographical distribution. Exp. Appl. Acarol. 2012, 57, 91–104. [Google Scholar] [CrossRef]
- Mather, T.N.; Nicholson, M.C.; Hu, R.; Miller, N.J. Entomological correlates of Babesia microti prevalence in an area where Ixodes scapularis (Acari: Ixodidae) is endemic. J. Med. Entomol. 1996, 33, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Hofmann-Lehmann, R.; Radford, A.D.; Tasker, S.; Belák, S.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2017, 19, 542–548. [Google Scholar] [CrossRef]
- Kauffmann, M.; Rehbein, S.; Hamel, D.; Lutz, W.; Heddergott, M.; Pfister, K.; Silaghi, C. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol. Cell. Probes 2017, 31, 46–54. [Google Scholar] [CrossRef]
- Falco, R.C.; Fish, D. Ticks parasitizing humans in a Lyme disease endemic area of southern New York State. Am. J. Epidemiol. 1988, 128, 1146–1152. [Google Scholar] [CrossRef]
- Guo, E.; Agusto, F.B. Baptism of fire: Modeling the effects of prescribed fire on Lyme disease. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 5300887. [Google Scholar] [CrossRef] [PubMed]
- Little, I.T.; Hockey, P.A.; Jansen, R. Impacts of fire and grazing management on South Africa’s moist highland grasslands: A case study of the Steenkampsberg Plateau, Mpumalanga, South Africa. Bothalia-Afr. Biodivers. Conserv. 2015, 45, a1786. [Google Scholar] [CrossRef]
- Rapiya, M.; Hawkins, H.-J.; Muchenje, V.; Mupangwa, J.F.; Marufu, M.C.; Dzama, K.; Mapiye, C. Rotational grazing approaches reduce external and internal parasite loads in cattle. Afr. J. Range Forage Sci. 2019, 36, 151–159. [Google Scholar]
- Abate, T.; Abebe, T.; Treydte, A. Do we need post-tree thinning management? Prescribed fire and goat browsing to control woody encroacher species in an Ethiopian savanna. Pastoralism 2024, 14, 13039. [Google Scholar] [CrossRef]
- Oundo, J.W.A.A.; Hartemink, N.; de Jong, M.C.M.; Koenraadt, C.J.M.; Kalayou, S.; Masiga, D.; ten Bosch, Q. Biological control of ticks in domestic environments: Modeling the potential impact of entomopathogenic fungi on the transmission of East Coast fever in cattle. Ticks Tick-Borne Dis. 2025, 16, 102435. [Google Scholar] [CrossRef]
- Barroso, P.; Gortázar, C. The wildlife–livestock interface: A general perspective. Anim. Front. 2024, 14, 3–4. [Google Scholar] [CrossRef]
- Okal, M.N.; Odhiambo, B.K.; Otieno, P.; Bargul, J.L.; Masiga, D.; Villinger, J.; Kalayou, S. Anaplasma and Theileria pathogens in cattle of Lambwe Valley, Kenya: A case for pro-active surveillance in the wildlife–livestock interface. Microorganisms 2020, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Allan, F.; Sindoya, E.; Adam, K.; Byamungu, M.; Lea, R.; Lord, J.; Mbata, G.; Paxton, E.; Mramba, F.; Torr, S.; et al. A cross-sectional survey to establish Theileria parva prevalence and vector control at the wildlife–livestock interface, Northern Tanzania. Prev. Vet. Med. 2021, 196, 105491. [Google Scholar] [CrossRef]
- Rajput, M.; Sajid, M.S.; Rajput, N.A.; George, D.R.; Usman, M.; Zeeshan, M.; Iqbal, O.; Bhutto, B.; Atiq, M.; Rizwan, H.M.; et al. Entomopathogenic fungi as alternatives to chemical acaricides: Challenges, opportunities and prospects for sustainable tick control. Insects 2024, 15, 1017. [Google Scholar] [CrossRef] [PubMed]
- Murigu, M.M.; Nana, P.; Waruiru, R.M.; Nga’nga’, C.J.; Ekesi, S.; Maniania, N.K. Laboratory and field evaluation of entomopathogenic fungi for the control of amitraz-resistant and susceptible strains of Rhipicephalus decoloratus. Vet. Parasitol. 2016, 225, 12–18. [Google Scholar] [CrossRef]
- Motloung, R.F.; Chaisi, M.; Sibiya, M.S.; Nyangiwe, P.N.; Shivambu, D.T.C. Predicting tick distributions in a changing climate: An ensemble approach for South Africa. SSRN 2024, 5035415. [Google Scholar] [CrossRef]
- Dziedziech, A.; Krupa, E.; Persson, K.E.M.; Paul, R.; Bonnet, S. Tick exposure biomarkers: A One Health approach to new tick surveillance tools. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 6, 100212. [Google Scholar] [CrossRef]
- Alale, T.Y.; Sormunen, J.J.; Nzeh, J.; Agjei, R.O.; Vesterinen, E.J.; Klemola, T. Public knowledge and awareness of tick-borne pathogens and diseases: A cross-sectional study in Ghana. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 6, 100228. [Google Scholar] [CrossRef]
- Namgyal, J.; Tenzin, T.; Checkley, S.; Lysyk, T.J.; Rinchen, S.; Gurung, R.B.; Dorjee, S.; Couloigner, I.; Cork, S.C. A knowledge, attitudes, and practices study on ticks and tick-borne diseases in cattle among farmers in a selected area of eastern Bhutan. PLoS ONE 2021, 16, e0247302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makwarela, T.G.; Seoraj-Pillai, N.; Nangammbi, T.C. The Impact of Vegetation Changes in Savanna Ecosystems on Tick Populations in Wildlife: Implications for Ecosystem Management. Diversity 2025, 17, 314. https://doi.org/10.3390/d17050314
Makwarela TG, Seoraj-Pillai N, Nangammbi TC. The Impact of Vegetation Changes in Savanna Ecosystems on Tick Populations in Wildlife: Implications for Ecosystem Management. Diversity. 2025; 17(5):314. https://doi.org/10.3390/d17050314
Chicago/Turabian StyleMakwarela, Tsireledzo Goodwill, Nimmi Seoraj-Pillai, and Tshifhiwa Constance Nangammbi. 2025. "The Impact of Vegetation Changes in Savanna Ecosystems on Tick Populations in Wildlife: Implications for Ecosystem Management" Diversity 17, no. 5: 314. https://doi.org/10.3390/d17050314
APA StyleMakwarela, T. G., Seoraj-Pillai, N., & Nangammbi, T. C. (2025). The Impact of Vegetation Changes in Savanna Ecosystems on Tick Populations in Wildlife: Implications for Ecosystem Management. Diversity, 17(5), 314. https://doi.org/10.3390/d17050314