Testing a Hump-Shaped Pattern with Increasing Elevation for Ant Species Richness in Daliang Mountain, Sichuan, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Design
2.2. Survey Method
2.3. Ant Specimen Processing and Identification
2.4. Statistical Analysis
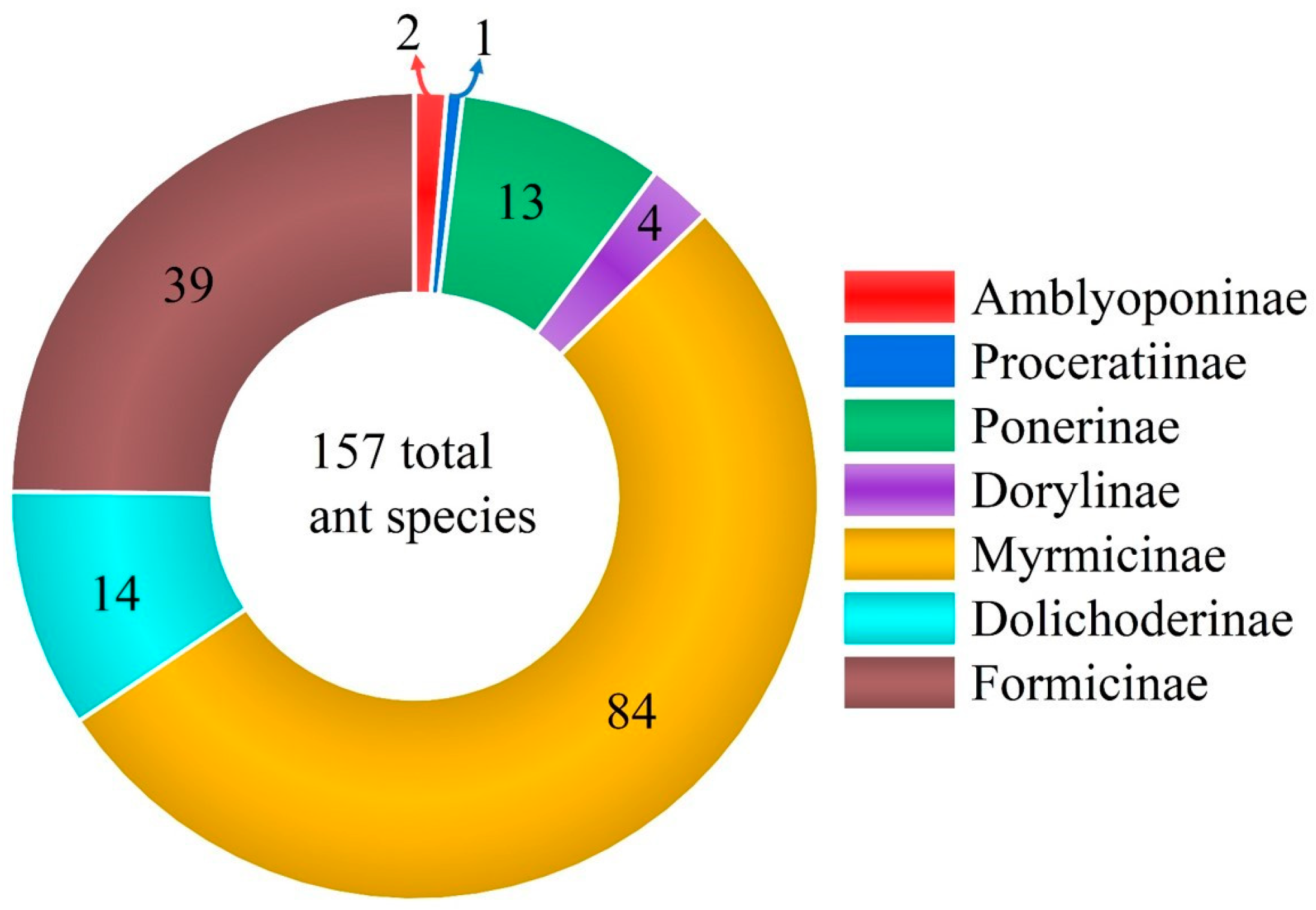
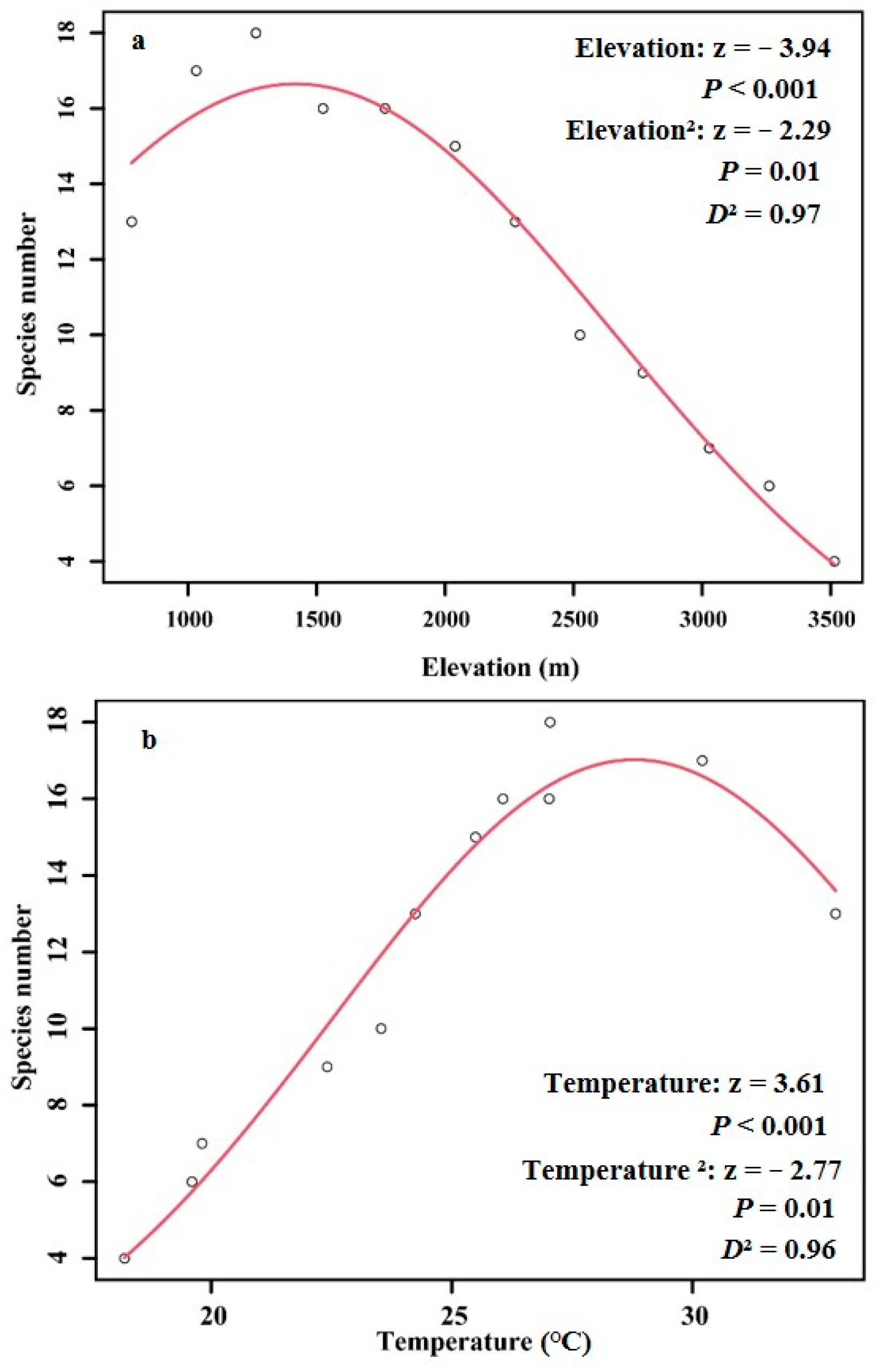
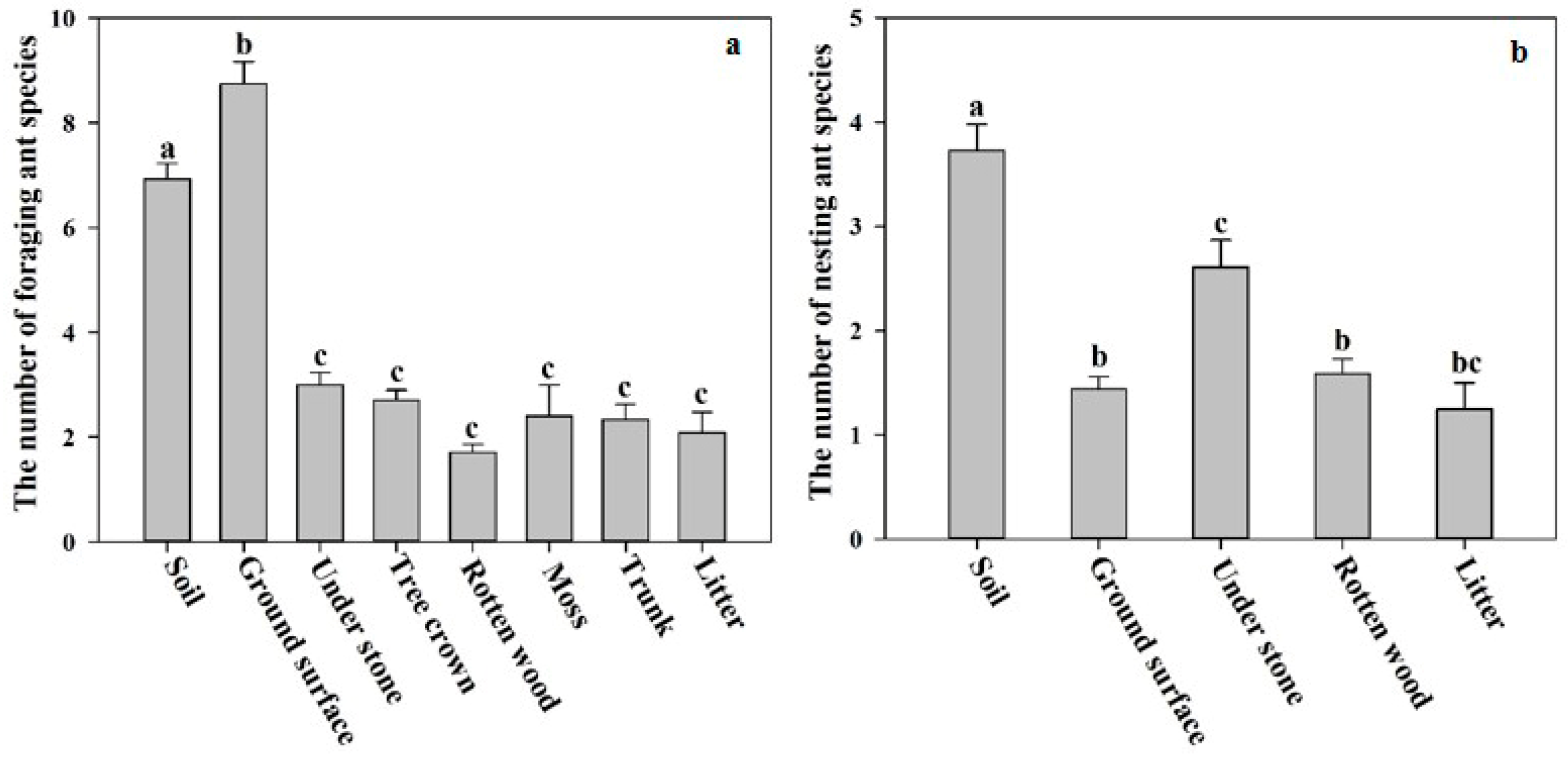
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guénard, B. An overview of the species and ecological diversity of ants. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Beattie, A.J.; Hughes, L. Ant-plant interactions. In Plant-Animal Interactions: An Evolutionary Approach; Herrera, C.M., Pellmyr, O., Eds.; Blackwell Science: Oxford, UK, 2002; p. 334. [Google Scholar]
- Way, M.J. Mutualism between ants and honeydew-producing Homoptera. Annu. Rev. Entomol. 1963, 8, 307–344. [Google Scholar] [CrossRef]
- Kronauer, D.J.; Pierce, N.E. Myrmecophiles. Curr. Biol. 2011, 21, R208–R209. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The fire ant (Solenopsis invicta): Still unvanquished. In Biological Pollution: The Control and Impact of Invasive Exotic Species; McKnight, B.N., Ed.; Indiana Academy of Science: Indianapolis, IN, USA, 1993; pp. 121–136. [Google Scholar]
- Stafford, C.T. Hypersensitivity to fire ant venom. Ann. Allergy Asthma Immunol. 1996, 77, 87–99. [Google Scholar] [CrossRef]
- Walker, D.H.; Barbour, A.G.; Oliver, J.H.; Lane, R.S.; Dumler, J.S.; Dennis, D.T.; Persing, D.H.; Azad, A.F.; McSweegan, E. Emerging Bacterial Zoonotic and Vector-Borne Diseases: Ecological and Epidemiological Factors. JAMA 1996, 275, 463–469. [Google Scholar] [CrossRef]
- Xu, Z.H. Study on the Biodiversity of Formicidae in Xishuangbanna Nature Reserve; Yunnan Science and Technology Press: Kunming, China, 2002. [Google Scholar]
- Kim, C.W.; Choi, S.Y.; Park, J.W.; Hong, C.S. Respiratory allergy to the indoor ant (Monomorium pharaonis) not related to sting allergy. Ann. Allergy Asthma Immunol. 2005, 94, 301–306. [Google Scholar] [CrossRef]
- Bolton, B. An Online Catalog of the Ants of the World. Available online: http://www.antcat.org/ (accessed on 22 June 2024).
- AntWiki. 2024. Available online: https://www.antwiki.org/ (accessed on 9 December 2024).
- Marathe, A.; Priyadarsanan, D.R.; Krishnaswamy, J.; Shanker, K. Spatial and climatic variables independently drive elevational gradients in ant species richness in the Eastern Himalaya. PLoS ONE 2020, 15, e0227628. [Google Scholar] [CrossRef]
- Stephens, S.S.; Wagner, M.R. Using ground foraging ant (Hymenoptera: Formicidae) functional groups as bioindicators of forest health in northern Arizona ponderosa pine forests. Environ. Entomol. 2006, 35, 937–949. [Google Scholar] [CrossRef]
- Majer, J.D.; Orabi, G.; Bisevac, L. Ants (Hymenoptera: Formicidae) pass the bioindicator scorecard. Myrmecol. News 2007, 10, 69–76. [Google Scholar]
- Tiede, Y.; Schlautmann, J.; Donoso, D.A.; Wallis, C.I.B.; Bendix, J.; Brandl, R.; Farwig, N. Ants as indicators of environmental change and ecosystem processes. Ecol. Indic. 2017, 83, 527–537. [Google Scholar] [CrossRef]
- Dunn, R.R.; Sanders, N.J.; Fitzpatrick, M.C.; Laurent, E.; Lessard, J.P.; Agosti, D.; Andersen, A.N.; Bruhl, C.; Cerda, X.; Ellison, A.M.; et al. Global ant (Hymenoptera: Formicidae) biodiversity and biogeography-a new database and its possibilities. Myrmecol. News 2007, 10, 77–83. [Google Scholar]
- Guénard, B.; Weiser, M.D.; Dunn, R.R. Global models of ant diversity suggest regions where new discoveries are most likely are under disproportionate deforestation threat. Proc. Natl. Acad. Sci. USA 2012, 109, 7368–7373. [Google Scholar] [CrossRef]
- Guénard, B.; Weiser, M.D.; Gómez, K.; Narula, N.; Economo, E.P. The Global Ant Biodiversity Informatics (GABI) database: Synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae). Myrmecol. News 2017, 24, 83–89. [Google Scholar]
- Gibb, H.; Dunn, R.R.; Sanders, N.J.; Grossman, B.F.; Photakis, M.; Abril, S.; Agosti, D.; Andersen, A.N.; Angulo, E.; Armbrecht, I.; et al. A global database of ant species abundances. Ecology 2017, 98, 883–884. [Google Scholar] [CrossRef]
- Economo, E.P.; Narula, N.; Friedman, N.R.; Weiser, M.D.; Guénard, B. Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 2018, 9, 1778. [Google Scholar] [CrossRef]
- Kass, J.M.; Guénard, B.; Dudley, K.L.; Jenkins, C.N.; Azuma, F.; Fisher, B.L.; Parr, C.L.; Gibb, H.; Longino, J.T.; Ward, P.S.; et al. The global distribution of known and undiscovered ant biodiversity. Sci. Adv. 2022, 8, eabp9908. [Google Scholar] [CrossRef]
- Schultheiss, P.; Nooten, S.S.; Wang, R.; Wong, M.K.L.; Brassard, F.; Guénard, B. The abundance, biomass, and distribution of ants on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2201550119. [Google Scholar] [CrossRef]
- Sanders, N.J.; Lessard, J.; Fitzpatrick, M.C.; Dunn, R.R. Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Glob. Ecol. Biogeogr. 2007, 16, 640–649. [Google Scholar] [CrossRef]
- Sabu, T.K.; Vineesh, P.J.; Vinod, K.V. Diversity of forest litter-inhabiting ants along elevations in the Wayanad region of the Western Ghats. J. Insect Sci. 2008, 8, 69. [Google Scholar] [CrossRef][Green Version]
- Olson, D.M. The distribution of leaf litter invertebrates along a Neotropical altitudinal gradient. J. Trop. Ecol. 1994, 10, 129–150. [Google Scholar] [CrossRef]
- Fisher, B.L. Ant diversity patterns along an elevational gradient in the Reserve Naturelle Integrale d’s Andringitra, Madagascar. Fieldiana Zool. 1996, 85, 93–108. [Google Scholar]
- Samson, D.A.; Rickart, E.A.; Gonzales, P.C. Ant Diversity and Abundance along an Elevational Gradient in the Philippines. Biotropica 1997, 29, 349–363. [Google Scholar] [CrossRef]
- Brühl, C.A.; Mohamed, M.; Linsenmair, K.E. Altitudinal distribution of leaf litter ants along a transect in primary forests on Mount Kinabalu, Sabah, Malaysia. J. Trop. Ecol. 1999, 15, 265–277. [Google Scholar] [CrossRef]
- Majer, J.; Kitching, R.; Heterick, B.; Hurley, K.; Brennan, K. North-South Patterns within Arboreal Ant Assemblages from Rain Forests in Eastern Australia. Biotropica 2001, 33, 643–661. [Google Scholar] [CrossRef]
- Araújo, L.M.; Fernandes, G.W. Altitudinal patterns in a tropical ant assemblage and variation in species richness between habitats. Lundiana 2003, 4, 103–109. [Google Scholar] [CrossRef]
- Longino, J.T.; Colwell, R.K. Density compensation, species composition, and richness of ants on a neotropical elevational gradient. Ecosphere 2011, 2, 1–20. [Google Scholar] [CrossRef]
- Munyai, T.; Foord, S. Ants on a mountain: Spatial, environmental and habitat associations along an altitudinal transect in a centre of endemism. J. Insect Conserv. 2012, 16, 677–695. [Google Scholar] [CrossRef]
- Sanders, N.J.; Rahbek, C. The patterns and causes of elevational diversity gradients. Ecography 2012, 35, 1–3. [Google Scholar] [CrossRef]
- Li, Z.Y.; Xu, Z.H.; Zhang, X.M.; Li, T.; Yin, X.D.; Duan, J.H. Ant Species Diversity in the Central and Northern Parts of the Western Sichuan Plateau in China. Diversity 2023, 15, 935. [Google Scholar] [CrossRef]
- Yin, X.D.; Xu, Z.H.; Zhang, X.M.; Li, T.; Han, X. Ant diversity and distribution patterns along an elevational gradient in the northern part of western Sichuan Plateau, China. Orient. Insects 2023, 58, 278–292. [Google Scholar] [CrossRef]
- Subedi, I.P.; Budha, P.B.; Kunwar, R.M.; Charmakar, S.; Ulak, S.; Pradhan, D.K.; Pokharel, Y.P.; Velayudhan, S.T.; Sathyapala, S.; Animon, I. Diversity and Distribution of Forest Ants (Hymenoptera: Formicidae) in Nepal: Implications for Sustainable Forest Management. Insects 2021, 12, 1128. [Google Scholar] [CrossRef]
- Szewczyk, T.; McCain, C.M. A Systematic Review of Global Drivers of Ant Elevational Diversity. PLoS ONE 2016, 11, e0155404. [Google Scholar] [CrossRef]
- Bharti, H.; Sharma, Y.P.; Bharti, M.; Pfeiffer, M. Ant species richness, endemicity and functional groups, along an elevational gradient in the Himalayas. Asian Myrmecol. 2013, 5, 79–101. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Korner, C. Why are there global gradients in species richness? Mountains might hold the answer. Trends Ecol. Evol. 2000, 15, 513–514. [Google Scholar] [CrossRef]
- Carina, H.; Allison, P.; Alexandre, A. Mountains, Climate and Biodiversity; John Wiley and Sons: New York, NY, USA, 2018. [Google Scholar]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Jankowski, J.E.; Ciecka, A.L.; Meyer, N.Y.; Rabenold, K.N. Beta diversity along environmental gradients: Implications of habitat specialization in tropical montane landscapes. J. Anim. Ecol. 2009, 78, 315–327. [Google Scholar] [CrossRef]
- Pryke, J.; Samways, M. Significant variables for the conservation of mountain invertebrates. J. Insect Conserv. 2010, 14, 247–256. [Google Scholar] [CrossRef]
- Wu, F.; Yang, X.J.; Yang, J.X. Additive diversity partitioning as a guide to regional montane reserve design in Asia: An example from Yunnan Province, China. Divers. Distrib. 2010, 16, 1022–1033. [Google Scholar] [CrossRef]
- Ruggiero, A.; Hawkins, B.A. Why do mountains support so many species of birds? Ecography 2008, 31, 306–315. [Google Scholar] [CrossRef]
- Ding, B.; Feng, L.; Ba, S.; Jiang, X.; Liu, G.; Liu, W. Temperature drives elevational diversity patterns of different types of organisms in Qinghai-Tibetan Plateau wetlands. iScience 2023, 26, 107252. [Google Scholar] [CrossRef] [PubMed]
- Sanders, N.J. Elevational gradients in ant species richness: Area, geometry, and Rapoport’s rule. Ecography 2002, 25, 25–32. [Google Scholar] [CrossRef]
- Paraskevopoulos, A.W.; Sanders, N.J.; Resasco, J. Temperature-driven homogenization of an ant community over 60 years in a montane ecosystem. Ecology 2024, 05, e4302. [Google Scholar] [CrossRef] [PubMed]
- Brassard, F.; Leong, C.M.; Chan, H.H.; Guénard, B. High Diversity in Urban Areas: How Comprehensive Sampling Reveals High Ant Species Richness within One of the Most Urbanized Regions of the World. Diversity 2021, 13, 358. [Google Scholar] [CrossRef]
- Brassard, F.; Murphy, B.P.; Andersen, A.N. The Impacts of Fire Vary among Vertical Strata: Responses of Ant Communities to Long-Term Experimental Burning. Ecol. Appl. 2024, 34, e3025. [Google Scholar] [CrossRef]
- Qian, Y.S.; Chen, C.; Qi, B.; Guo, N.Y.; Xu, Z.H.; Zhang, X.M. Distribution patterns of formicidae insects from middle Daliangshan of Sichuan province. J. Yangzhou Univ. 2022, 43, 111–120. [Google Scholar] [CrossRef]
- Zhu, S.Z. Forest changes of Liangshan Moutain areas in Sichuan Province in history. J. Chin. Hist. Geogr. 2007, 22, 43–52. [Google Scholar]
- Luo, C.L.; Xu, Z.H.; Xiong, Z.P.; Qi, B.; Yuan, D.Y.; Ran, M.J. Distribution patterns of ant species from Wanglang Nature Reserve and adjacent areas of Sichuan Province. J. Zhejiang AF Univ. 2019, 36, 638–645. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, J.H.; Liu, H.; Huang, H.X.; Zhang, T.J.; Huang, Y. Classification of soil parent materials in mountain areas of Southwest China based on geological formations: A case study of Daliangshan region. Geol. Surv. China 2021, 8, 51–62. [Google Scholar] [CrossRef]
- Wang, W.X.; Wang, J.Y.; Chen, W.D. Diversity and Flora Analysis of Spermatophyte in Liangshan Mountains, Sichuan Province. J. Lishui Univ. 2024, 46, 20–30. [Google Scholar]
- Agosti, D.; Majer, J.; Alonso, L.E.; Schultz, T.R. (Eds.) Ants: Standard Methods for Measuring and Monitoring Biodiversity; Biological Diversity Handbook Series; Smithsonian Institution Press: Washington, DC, USA, 2000; p. 280. [Google Scholar]
- R Core. Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.r-project.org/index.html (accessed on 10 October 2022).
- Pfeiffer, M.; Mezger, D.; Hosoishi, S.; Bakhtiar, E.; Kohout, R.J. The Formicidae of Borneo (Insecta: Hymenoptera): A preliminary species list. Asian Myrmecol. 2011, 4, 9–58. [Google Scholar] [CrossRef]
- Liu, C.; Guénard, B.; Hita Garcia, F.; Yamane, S.; Blanchard, B.; Yang, D.; Economo, E. New records of ant species from Yunnan, China. ZooKeys 2015, 477, 17–78. [Google Scholar] [CrossRef]
- Fellowes, J.R. Ant (Hymenoptera: Formicidae) genera in southern China: Observations on the Oriental-Palaearctic boundary. Myrmecol. Nachrichten 2006, 8, 239–249. [Google Scholar]
- Fontanilla, A.M.; Nakamura, A.; Xu, Z.; Cao, M.; Kitching, R.L.; Tang, Y.; Burwell, C.J. Taxonomic and functional ant diversity along tropical, subtropical, and subalpine elevational transects in Southwest China. Insects 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Bharti, H.; Wachkoo, A.A.; Kumar, R. First inventory of ants (Hymenoptera: Formicidae) in northwestern Shivalik, India. Halteres 2017, 8, 33–68. [Google Scholar] [CrossRef]
- Wachkoo, A.A.; Akbar, S.A.; Jan, U.; Shah, G.M. Taxonomic inventory of ants (Hymenoptera: Formicidae) in Jammu and Kashmir state. In Biodiversity of the Himalaya: Jammu and Kashmir State; Springer Nature: Basingstoke, UK, 2020; pp. 733–747. [Google Scholar]
- Wilson, E.O. Which are the most prevalent ant genera. Stud. Entomol. 1976, 19, 187–200. [Google Scholar]
- Karaman, M.G. Zoogeography, diversity and altitudinal distribution of ants (Hymenoptera: Formicidae) in the Mediterranean and the oro-Mediterranean parts of Montenegro. North West. J. Zool. 2011, 7, 26–34. [Google Scholar]
- Burwell, C.J.; Nakamura, A. Distribution of ant species along an altitudinal transect in continuous rainforest in subtropical Queensland, Australia. Mem. Qld. Mus. 2011, 55, 391–411. [Google Scholar]
- Longino, J.T.; Branstetter, M.G.; Colwell, R.K. How ants drop out: Ant abundance on tropical mountains. PLoS ONE 2014, 9, e104030. [Google Scholar] [CrossRef]
- Yusah, K.M.; Turner, E.C.; Yahya, B.E.; Fayle, T.M. An elevational gradient in litter-dwelling ant communities in Imbak Canyon, Sabah, Malaysia. J. Trop. Biol. Conserv. JTBC 2012, 9, 192–199. [Google Scholar]
- Liu, C.; Dudley, K.L.; Xu, Z.H.; Economo, E.P. Mountain meta communities: Climate and spatial connectivity shape ant diversity in a complex landscape. Ecography 2018, 41, 101–112. [Google Scholar] [CrossRef]
- Subedi, I.P.; Budha, P.B. Diversity and distribution patterns of ants along elevational gradients. Nepal. J. Zool. 2020, 4, 44–49. [Google Scholar] [CrossRef]
- Cao, K. Analysis and Prediction of Climate and Water Resources Changes in Sichuan Region. Water Resour. Hydropower Northeast China 2018, 4, 28–30. [Google Scholar] [CrossRef]
- Vetaas, O.R.; Grytnes, J.A. Distribution of vascular plant species richness and endemic richness along the Himalayan elevation gradient in Nepal. Glob. Ecol. Biogeogr. 2002, 11, 291–301. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Vetaas, O.R. Can Rapoport’s rule explain tree species richness along the Himalayan elevation gradient, Nepal? Divers. Distrib. 2006, 12, 373–378. [Google Scholar] [CrossRef]
- Acharya, K.P.; Vetaas, O.R.; Birks, H. Orchid species richness along Himalayan elevational gradients. J. Biogeogr. 2011, 38, 1821–1833. [Google Scholar] [CrossRef]
- Manish, K.; Pandit, M.K. Phylogenetic diversity, structure and diversification patterns of endemic plants along the elevational gradient in the Eastern Himalaya. Plant Ecol. Divers. 2018, 11, 501–513. [Google Scholar] [CrossRef]
- Sun, L.; Luo, J.; Qian, L.S.; Deng, T.; Sun, H. The relationship between elevation and seed-plant species richness in the Mt. Namjagbarwa region (Eastern Himalayas) and its underlying determinants. Glob. Ecol. Conserv. 2020, 23, e01053. [Google Scholar] [CrossRef]
- Barry, R.G. Mountain Weather and Climate; Routledge: London, UK, 1992. [Google Scholar]
- Lassau, S.; Hochuli, D. Effects of habitat complexity on ant assemblages. Ecography 2004, 2, 157–164. [Google Scholar] [CrossRef]
- Sarty, M.; Abbott, K.L.; Lester, P.J. Habitat complexity facilitates coexistence in a tropical ant community. Oecologia 2006, 149, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Vasconcelos, H.L. Habitat diversity enhances ant diversity in a naturally heterogeneous Brazilian landscape. Biodivers. Conserv. 2011, 21, 797–809. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.H.; Zhang, C.L.; Yu, N.N.; Xu, G.L. Distribution patterns of ants from west slope of Mount Demula and Bomi Valley. J. Northwest For. Univ. 2012, 27, 77–82. [Google Scholar] [CrossRef]
- Zhai, J.; Qian, Y.H.; Xu, Z.H.; Zhang, X.; Li, X.Y.; Huang, X.R. Distribution patterns of ant species in the Anzihe Nature Reserve of Sichuan Province. J. For. Environ. 2020, 40, 612–618. [Google Scholar] [CrossRef]
- Xu, Z.H.; Chu, J.J.; Zhang, C.L.; Yu, N.N. Ant species and distribution pattern in Gongbo Nature Reserve in Southeastern Tibet. Sichuan J. Zool. 2011, 30, 118–123. [Google Scholar] [CrossRef]
- Yu, N.N.; Xu, Z.H.; Zhang, C.L.; Chu, J.J.; Yang, B.L.; Liu, X. Distribution patterns of ant species from Mount Sejila, southeastern Tibet. J. Beijing For. Univ. 2011, 33, 75–80. [Google Scholar] [CrossRef]
- Zhang, C.L.; Xu, Z.H.; Yu, N.N.; He, Q.J. Distribution patterns of ant species on east slope of Mount Demola and Zayu Valley in Southeastern Tibet. J. Northeast. For. Univ. 2012, 40, 87–92. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.H.; Yu, N.N.; Zhang, C.L. Distribution patterns of ant species (Hymenoptera: Formicidae) in Galongla Mountains and Medog Valley of Southestern Tibet. Sci. Silvae Sin. 2016, 52, 88–95. [Google Scholar] [CrossRef]
- Li, W.Q.; Xu, Z.H.; Zhou, X.Y.; Li, A.N.; Xu, G.L. Distribution patterns of ant species from Mount Everest Section of Mt. Himalaya. J. Enivron. Entomol. 2017, 39, 1054–1062. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Van Pelt, A. High altitude ants of the Southern Blue Ridge. Am. Midl. Nat. 1963, 69, 205–223. [Google Scholar] [CrossRef]
- Billick, I. Density dependence and colony growth in the ant species Formica neorufibarbis. J. Anim. Ecol. 2001, 70, 895–905. [Google Scholar] [CrossRef]
- McCaffrey, J.; Galen, C. Between a rock and a hard place: Impact of nest selection behavior on the altitudinal range of an alpine ant, Formica neorufibarbis. Environ. Entomol. 2011, 40, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Brian, M.V. Production Ecology of Ants and Termites, International Biological Programme; Cambridge University Press: Cambridge, UK, 1978; p. 409. [Google Scholar]
- Blüthgen, N.; Feldhaar, H. Food and Shelter: How Resources Influence Ant Ecology. In Ant Ecology; Lach, L., Parr, C., Abbott, K., Eds.; Oxford Academic: Oxford, UK, 2010; Chapter 7; pp. 115–136. [Google Scholar] [CrossRef]
- Kaspari, M.; Yuan, M.; Alonso, L. Spatial grain and the causes of regional diversity gradients in ants. Am. Nat. 2003, 161, 459–477. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.-J.; Xu, Z.-H.; Zhang, X.-M. Testing a Hump-Shaped Pattern with Increasing Elevation for Ant Species Richness in Daliang Mountain, Sichuan, China. Diversity 2025, 17, 308. https://doi.org/10.3390/d17050308
You S-J, Xu Z-H, Zhang X-M. Testing a Hump-Shaped Pattern with Increasing Elevation for Ant Species Richness in Daliang Mountain, Sichuan, China. Diversity. 2025; 17(5):308. https://doi.org/10.3390/d17050308
Chicago/Turabian StyleYou, Shi-Jia, Zheng-Hui Xu, and Xin-Min Zhang. 2025. "Testing a Hump-Shaped Pattern with Increasing Elevation for Ant Species Richness in Daliang Mountain, Sichuan, China" Diversity 17, no. 5: 308. https://doi.org/10.3390/d17050308
APA StyleYou, S.-J., Xu, Z.-H., & Zhang, X.-M. (2025). Testing a Hump-Shaped Pattern with Increasing Elevation for Ant Species Richness in Daliang Mountain, Sichuan, China. Diversity, 17(5), 308. https://doi.org/10.3390/d17050308




