Preliminary Study on the Genetic Diversity of Sicilian Populations of Crataegus azarolus (Rosaceae) and Their Wild Relatives for Conservation and Valorisation Purposes
Abstract
1. Introduction
Ethnobotanical Significance and Potential Helth Benefits of Crataegus spp.
2. Materials and Methods
2.1. Plant Material
- C_1/3: Crataegus azarolus L. var. azarolus – Sicily: Custonaci (Trapani), Purgatorio locality, on red Mediterranean soil, 175 m a.s.l., 21.09.2022, Raimondo (PAL-Gr).
- C_e: Crataegus azarolus L. var. azarolus – Sicily: Custonaci (Trapani), Purgatorio locality, on red Mediterranean soil, 175 m a.s.l., 21.09.2022, Raimondo (PAL-Gr).
- C_4/3: Crataegus azarolus L. var. azarolus – Sicily: Castelluzzo-Macari road (Trapani), on red Mediterranean soil, 170 m a.s.l., 21.09.2022, Raimondo (PAL-Gr).
- C_b: Cratagus azarolus L. var. azarolus (cultivated) – Sicily: Pollina (Palermo), Carrara locality, cultivated plant on siliceous soil, 350 m a.s.l., 16.10.2022, Raimondo & Spadaro (PAL-Gr).
- C_5/3: Crataegus azarolus var. aronia (L.) DC. – Sicily: San Vito Lo Capo (Trapani), Tonnara del Secco locality, on carbonate lithosol, ca. 10 m a.s.l., 12.09.2022, Raimondo (PAL-Gr).
- C_f: Crataegs azarolus var. clorocarpa (Moris) K.I. Chr. – Sicily: Castellammare del Golfo (Trapani), Balata di Baida locality, ca. 150 m a.s.l, 23.09.2022, Raimondo & Spadaro (PAL-Gr).
- C_3: Crataegus laciniata Ucria – Sicily: Piano Pomo locality, Petralia Sottana (Palermo), on siliceous soil, 1400 m a.s.l., 10.10.2022, Raimondo (PAL-Gr).
- C_1: Crataegus aff. insengae (Tineo ex Guss.) Bertol. – Sicily: Petralia Sottana (Palermo), Piano Pomo locality, on siliceous soil, 1400 m a.s.l., 10.10.2022, Raimondo (PAL-Gr).
- C_2: Crataegus aff. insengae (Tineo ex Guss.) Bertol. – Sicily: Petralia Sottana (Palermo), Piano Pomo locality, on siliceous soil, 1400 m a.s.l., 10.10.2022, Raimondo (PAL-Gr).
- C_10: Crataegus aff. insengae (Tineo ex Guss.) Bertol. – Sicily: Petralia Sottana (Palermo), Piano Pomo locality, on siliceous soil, 1400 m a.s.l., 10.10.2022, Raimondo (PAL-Gr).
- C_11: Crataegus aff. insengae (Tineo ex. Guss.) Bertol. – Sicily: Petralia Sottana (Palermo), Piano Pomo locality, on siliceous soil, 1400 m a.s.l., 10.10.2022, Raimondo (PAL-Gr).
- C_12: Crataegus aff. insengae (Tineo ex Guss.) Bertol. – Sicily: Petralia Sottana (Palermo), Piano Pomo locality, on siliceous soil, 1400 m a.s.l., 10.10.2022, Raimondo (PAL-Gr).
- C_c: Crataegus monogyna L. – Sicily: Castelbuono (Palermo), Barraca locality, on siliceous soil, 750 m a.s.l., 16.10.2022, Raimondo & Spadaro (PAL-Gr).
- C_d: Crataegus x media Bechst. – Sicily: Pollina (Palermo), Carrara locality, on siliceous soil, 350 m a.s.l., 16.10.2022, Raimondo & Spadaro (PAL-Gr).
- C_2/3a: Crataegus drepanensis Raimondo, Marino & Scuderi – Sicily: San Vito Lo Capo (Trapani), Tonnara del Secco locality, on carbonate tithosol, 8 m a.s.l., 12.09.2022, Raimondo (PAL-Gr).
- C_2/3b: Crataegus drepanensis Raimondo, Marino & Scuderi – Sicily: San Vito Lo Capo (Trapani), Tonnara del Secco locality, on carbonate lithosol, 8 m a.s.l., 12.09.2022, Raimondo (PAL-Gr).
- C_2/3c: Crataegus drepanensis Raimondo, Marino & Scuderi – Sicily: San Vito Lo Capo (Trapani), Tonnara del Secco locality, on carbonate lithosol, 8 m a.s.l., 12.09, 2022, Raimondo (PAL-Gr).
- C_a: Crataegus zichichii Raimondo, Venturella & Spadaro – Sicily: Paceco-Marsala road (Trapani), on carbonate lithosol, ca. 15 m a.s.l., 04.10.2022, Raimondo (PAL-Gr).
- C_g: Crataegus zichichii Raimondo, Venturella & Spadaro – Sicily: Castellammare del Golfo (Trapani), Balata di Baida locality, on carbonate soil, ca. 150 m a.s.l., 23.09.2022, Raimondo & Spadaro (PAL-Gr).
2.2. Molecular Analysis
2.3. Cluster Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, X.; Zhang, X.; Bu, H.; Zhang, T.; Lao, Y.; Dong, W. Molecular analysis of evolution and origins of cultivated Hawthorn (Crataegus spp.) and related species in China. Front. Plant Sci. 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, S.; Salvini, D.; Turchini, D.; Pastorelli, R.; Vendramin, G.G. Crataegus monogyna Jacq. and C. laevigata (Poir.) DC. (Rosaceae, Maloideae) display low level of genetic diversity assessed by chloroplast markers. Plant Syst. Evol. 2005, 250, 187–196. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org (accessed on 14 April 2024).
- Koljanin, D.; Brujić, J.; Čarni, A.; Milanović, Đ.; Škvorc, Ž.; Stupar, V. Classification of wetland forests and scrub in the western Balkans. Diversity 2023, 15, 370. [Google Scholar] [CrossRef]
- Spadaro, V.; Marino, P.; Scuderi, L.; Venturella, G.; Raimondo, F.M. Biodiversity in some populations of Crataegus (Rosaceae) from western Sicily: Description of two new species and notes on conservation and valorisation. Flora Medit. 2024, 34, 239–255. [Google Scholar] [CrossRef]
- Chang, Q.; Zuo, Z.; Harrison, F.; Chow, M.S.S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora native to Italy. Plant Biosyst. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Traverso, O. Botanica Orticola; Edagricole: Bologna, Italy, 1990. [Google Scholar]
- Galasso, G.; Conti, F.; Peruzzi, L.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora alien to Italy. Plant Biosyst. 2024, 158, 297–340. [Google Scholar] [CrossRef]
- Portal to the Flora of Italy. Available online: http://dryades.units.it/floritaly (accessed on 3 May 2024).
- Raimondo, F.M.; Arena, M.P.; Marino, P.; Spadaro, V.; Zizzo, G.V. Note tassonomiche, corologiche e fitogeografiche sul genere Crataegus (Rosaceae) in Sicilia. Quad. Bot. Amb. Appl. 2023, 33, 113–118. [Google Scholar]
- Christensen, K.I.; Zieliński, J. Notes on the genus Crataegus (Rosaceae–Pyreae) in southern Europe, the Crimea and western Asia. Nord. J. Bot. 2008, 26, 344–360. [Google Scholar] [CrossRef]
- Barbieri, C.; Bignami, C.; Cristofori, V.; Paolocci, M.; Bertazza, G. Characterization and exploitation of minor pome fruits in Italy. Acta Hort. 2010, 918, 953–959. [Google Scholar] [CrossRef]
- Fiori, A. Nuova Flora Analitica d’Italia; Ricci: Firenze, Italy, 1924; pp. 641–800. [Google Scholar]
- Franco, J.A. Crataegus L. In Flora Europaea; Cambridge University Press: Cambridge, UK, 1968; Volume 2, pp. 73–77. [Google Scholar]
- Sydora, N. Morphological and taxonomic study of Oxyacanthae Zbl. section of Crataegus L. genus by vegetative characteristics. Sci. Pharm. Sci 2018, 1, 36–41. [Google Scholar] [CrossRef][Green Version]
- Cimò, G.; Marchese, A.; Germanà, M.A. Microspore embryogenesis induced through in vitro anther culture of almond (Prunus dulcis Mill.). Plant Cell Tissue Organ Cult. 2017, 128, 85–95. [Google Scholar] [CrossRef]
- Emami, A.; Shabanian, N.; Rahmani, M.S.; Khadivi, A.; Mohammad-Panah, N. Genetic characterization of the Crataegus genus: Implications for in situ conservation. Sci. Hortic. 2018, 231, 56–65. [Google Scholar] [CrossRef]
- Guney, M.; Kafkas, S.; Keles, H.; Aras, S.; Ercisli, S. Characterization of hawthorn (Crataegus spp.) genotypes by SSR markers. Physiol. Mol. Biol. Plants 2018, 24, 1221–1230. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Bras. De Farmacogn. 2012, 22, 1187–1200. [Google Scholar]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, M.A.; Severino, P.; Souto, B.E.; Santini, A. Hawthorn (Crataegus spp.): An updated overview on its beneficial properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Rafeeq, J.; Qaisar, K.N.; Khan, P.A.; Mugloo, J.A.; Singh, A.; Hassan, I.; Mir, J.I.; Malik, A.R.; Dutt, V.; Mushtaq, T.; et al. Regulation of Phytochemical Properties of Hawthorn: A Crataegus Species. In Genetic Manipulation of Secondary Metabolites in Medicinal Plant; Springer Nature: Singapore, 2023; pp. 179–203. [Google Scholar]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2023, 87, 23–142. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. 2009: Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Azaizeh, H.; Cogan, U.; Bomzon, A. The Effects of a Decoction Prepared from the Leaves and Unripe Fruits of Crataegus aronia in Streptozotocin-Induced Diabetic Rats. J. Complement. Integr. Med. 2006, 3. [Google Scholar] [CrossRef]
- Miller, A.L. Botanical influences on cardiovascular disease. Altern. Med. Rev. 1998, 3, 422–431. [Google Scholar] [PubMed]
- Kao, E.S.; Wang, C.J.; Lin, W.L.; Chu, C.Y.; Tseng, T.H. Effects of polyphenols derived from fruit of Crataegus pinnatifida on cell transformation, dermal edema and skin tumor formation by phorbol ester application. Food Chem. Toxicol. 2007, 45, 1795–1804. [Google Scholar]
- Boukef, M.K. Les Plantes dans la Médecine Traditionnelle Tunisienne, Médecine Traditionnelle et Pharmacopée; Agence de Coopération Culturelle et Technique: Paris, France, 1986. [Google Scholar]
- Bouaziz, A.; Khennouf, S.; Abdalla, S.; Djidel, S.; Abu Zarga, M.; Bentahar, A.; Dahamna, S.; Baghiani, A.; Amira, S. Phytochemical analysis, antioxidant activity and hypotensive effect of Algerian azarole (Crataegus azarolus L.) leaves extracts. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 286. [Google Scholar]
- Nadia, M.; Imen, M.; Mounira, K.; Fadwa, C.; Zied, G.; Kamel, G. Antibacterial activity and modulation of antibiotic resistance by Crataegus azarolus extracts. J. Nat. Prod. 2014, 7, 131–140. [Google Scholar]
- Amina, B.; Ahmed, T.; Hamdi, B.; Chawki, B.; Salah, R. Phytochemical constituents, phenolic contents, and antioxidant activity of Crataegus azarolus extracts. Asian J. Pharm. Clin. Res. 2018, 11, 133–137. [Google Scholar] [CrossRef]
- Cherifa, H.; Sourour, Z. Contribution to the study of the antidiabetic effect of local medicinal plant: Crataegus azarolus. Curr. Opin. Biotechnol. 2011, 22, S137. [Google Scholar] [CrossRef]
- Mohammedsaeed, A.A.; Mohamad, T.S. Inhibitory and anti-cancer effects of Crataegus azarolus extracts on gastric cancer cell line (AGS). Zanco J. Pure Appl. Sci. 2023, 35, 211–220. [Google Scholar] [CrossRef]
- Mustapha, N.; Mokdad-Bzéouich, I.; Sassi, A.; Abed, B.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Immunomodulatory potencies of isolated compounds from Crataegus azarolus through their antioxidant activities. Tumor Biol. 2016, 37, 7967–7980. [Google Scholar] [CrossRef]
- Sammari, H.; Jedidi, S.; Selmi, H.; Rtibi, K.; Jabri, M.A.; Jridi, M.; Sebai, H. Protective effects of Crataegus azarolus L. berries aqueous extract against castor oil–induced diarrhea, oxidative stress, and inflammation in rat. Neurogastroenterol. Motil. 2021, 33, e14065. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Prgomet, Ž.; Cerutti, A.K.; Beccaro, G.L. Phytochemical characterization and antioxidant activity evaluation of Mediterranean medlar fruit (Crataegus azarolus L.): Preliminary study of underutilized genetic resources as a potential source of health-promoting compound for food supplements. J. Food Nutr. Res. 2017, 56, 18–31. [Google Scholar]
- Bignami, C.; Poalocci, A.; Scossa, A.; Bertazza, G. Preliminary evaluation of nutritional and medicinal components of Crataegus azarolus fruits. J. Food Eng. 2000, 5, 241–247. [Google Scholar] [CrossRef]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 1–12. [Google Scholar] [CrossRef]
- Mirabile, S.; D’Angelo, V.; Germanò, M.P.; Arabi, S.P.; Parisi, V.; Raimondo, F.M.; Rosa, E. Chemical Profile and Health-Promoting Activities of Crataegus laciniata (Rosaceae) Flowers. Plants 2024, 13, 34. [Google Scholar] [CrossRef]
- Cacciola, A.; D’Angelo, V.; Germanò, M.P.; Mirabile, S.; Raimondo, F.M.; Arabi, S.B.; Rosa, E.; Santoro, V.; De Tommasi, N. Polyphenolic characterization, antioxidant and antidiabetic activities of Crataegus spp. fruit extracts. In Proceedings of the X International Plant Science Conference (IPSC), 119° Congresso della Società Botanica Italiana Onlus, Università di Teramo, Abruzzo, Italy, 11–13 September 2024. [Google Scholar]
- Yilmaz, K.U.; Yanar, M.; Ercisli, S.; Sahiner, H.; Taskin, T.; Zengin, Y. Genetic Relationships Among Some Hawthorn (Crataegus spp.) Species and Genotypes. Biochem. Genet. 2010, 48, 73–878. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Wang, A.; Aldwinckle, H.; Forsline, P.; Main, D.; Fazio, G.; Brown, S.; Xu, K. EST contig-based SSR linkage maps for Malus x domestica cv Royal Gala and an apple scab resistant accession of M. sieversii, the progenitor species of domestic apple. Mol. Breed. 2012, 29, 379–397. [Google Scholar] [CrossRef]
- Liebhard, R.; Gianfranceschi, L.; Koller, B.; Ryder, C.D.; Tarchini, R.; Van de Weg, E.; Gessler, C. Development and characterisation of 140 new microsatellites in apple (Malus x domestica Borkh.). Mol. Breed. 2002, 10, 217–241. [Google Scholar] [CrossRef]
- Silfverberg-Dilworth, E.; Matasci, C.L.; Van de Weg, W.E.; Van Kaauwen, M.P.W.; Walser, M.; Kodde, L.P.; Patocchi, A. Microsatellite markers spanning the apple (Malus x domestica Borkh.) genome. Tree Genet. Genomes 2006, 2, 202–224. [Google Scholar] [CrossRef]
- Hemmat, M.; Weeden, N.F.; Brown, S.K. Mapping and evaluation of Malus × domestica microsatellites in apple and pear. J. Amer. Soc. Hort. Sci. 2003, 128, 515–520. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Ullah, F.; Gao, Y.; Sari, İ.; Jiao, R.F.; Saqib, S.; Gao, X.F. Macro-morphological and ecological variation in Rosa sericea complex. Agronomy 2022, 12, 1078. [Google Scholar] [CrossRef]
- Talent, N.; Dickinson, T.A. Polyploidy in Crataegus and Mespilus (Rosaceae, Maloideae): Evolutionary inferences from flow cytometry of nuclear DNA amounts. Canad. J. Bot. 2005, 83, 1268–1304. [Google Scholar] [CrossRef]
- Vašková, D.; Kolarčik, V. Breeding Systems in Diploid and Polyploid Hawthorns (Crataegus): Evidence from Experimental Pollinations of C. monogyna, C. subsphaerica, and Natural Hybrids. Forests 2019, 10, 1059. [Google Scholar] [CrossRef]
- Protopopova, M.; Pavlichenko, V.; Chepinoga, V.; Gnutikov, A.; Adelshin, R. Waldsteinia within Geum s.l. (Rosaceae): Main Aspects of Phylogeny and Speciation History. Diversity 2023, 15, 479. [Google Scholar] [CrossRef]
- IPNI International Plant of Name Index. Available online: https://www.ipni.org/n/722954-1 (accessed on 10 June 2024).
- Browicz, K. Crataegus L. (Rosaceae). In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1972; Volume 4, pp. 133–147. [Google Scholar]
- Zohary, M. Crataegus L. In Flora Palaestina 2 (Text); Feinbrun-Dothan, N., Ed.; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1972; pp. 19–20. [Google Scholar]
- Danin, A.; Fragman-Sapir, O. Flora of Israel and Adjacent Areas. 2016. Available online: https://flora.org.il/en/plants/ (accessed on 10 May 2024).
- Christensen, K.I. Revision of Crataegus Sect. Crataegus and Nothosect. Crataeguineae (Rosaceae-Maloideae) in the old world. Syst. Bot. Monogr. 1992, 35, 1–199. [Google Scholar] [CrossRef]
- MirAli, N.; Al-Odat, M.; Haider, N.; Nabulsi, I. The genus Crataegus L.: An ecological and molecular study. Russ. J. Gen. 2011, 47, 26–34. [Google Scholar] [CrossRef]
- Calvo, J.; Ufimov, R.; Aedo, C. Proposal to conserve the name Crataegus laciniata (Rosaceae) with a conserved type. Taxon 2015, 64, 175–176. [Google Scholar] [CrossRef]
- Sargsyan, M.V. The genus Crataegus (Rosaceae) in Armenia (an updated review). Biosyst. Divers. 2022, 30, 270–273. [Google Scholar] [CrossRef]
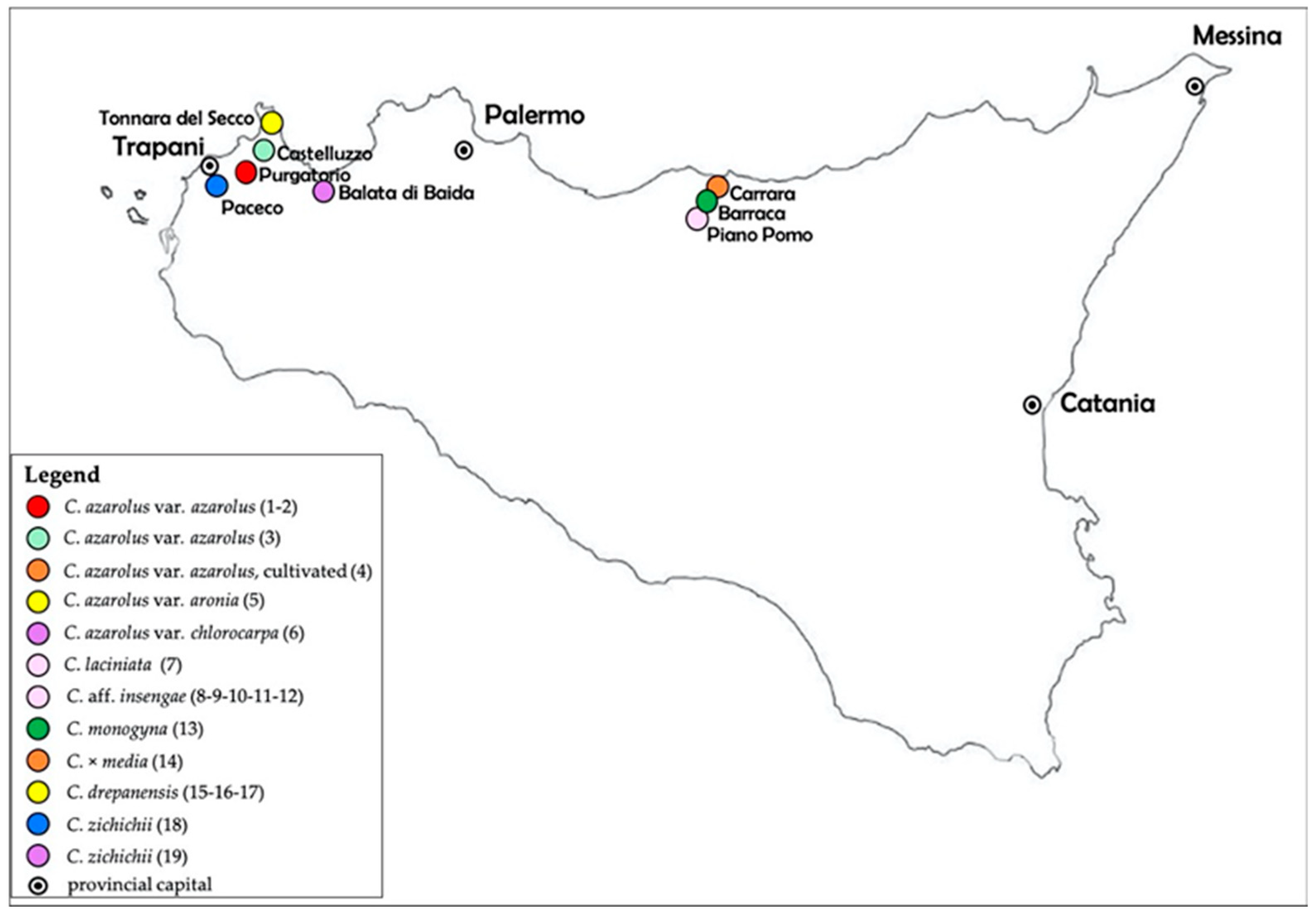
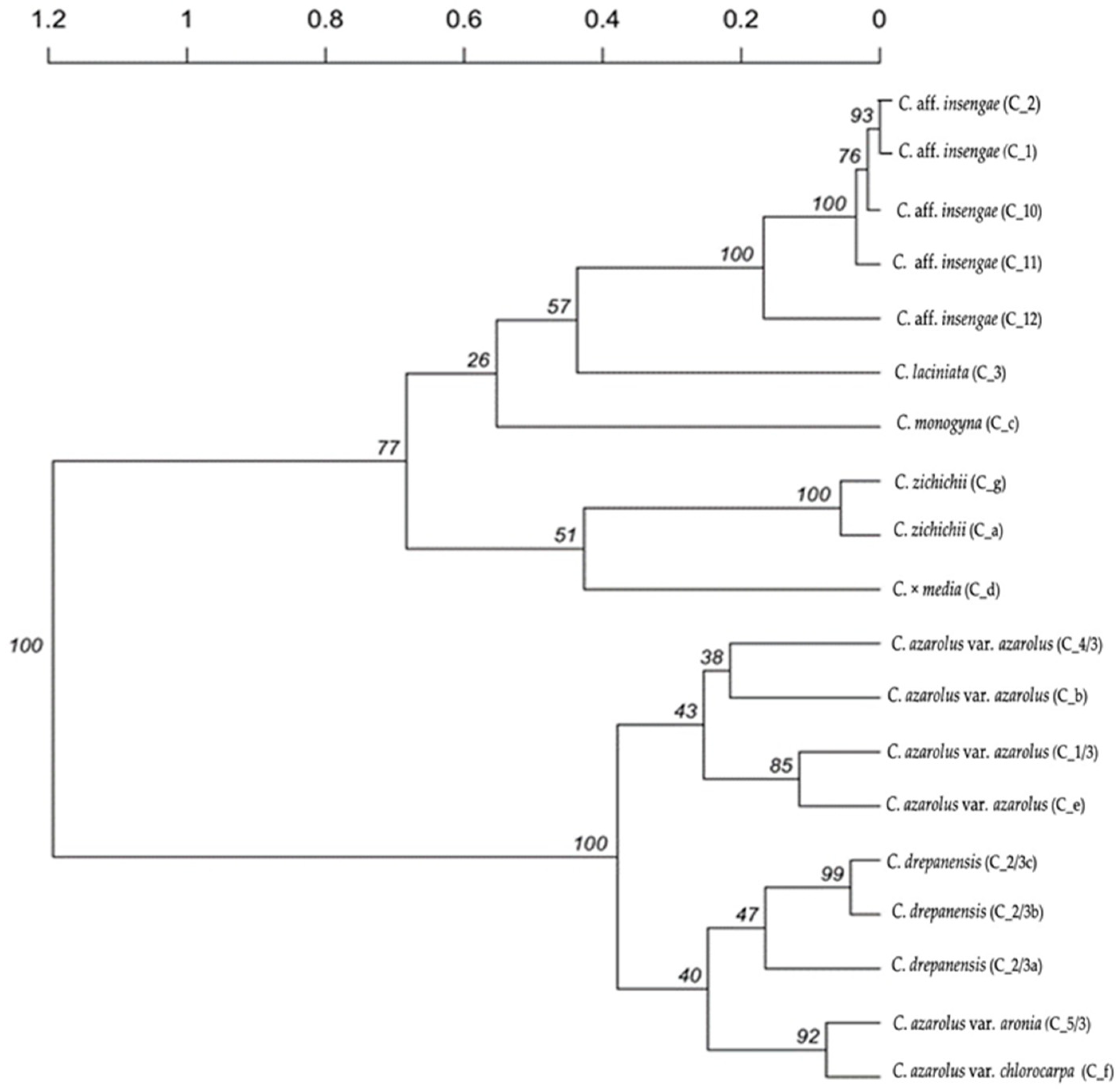
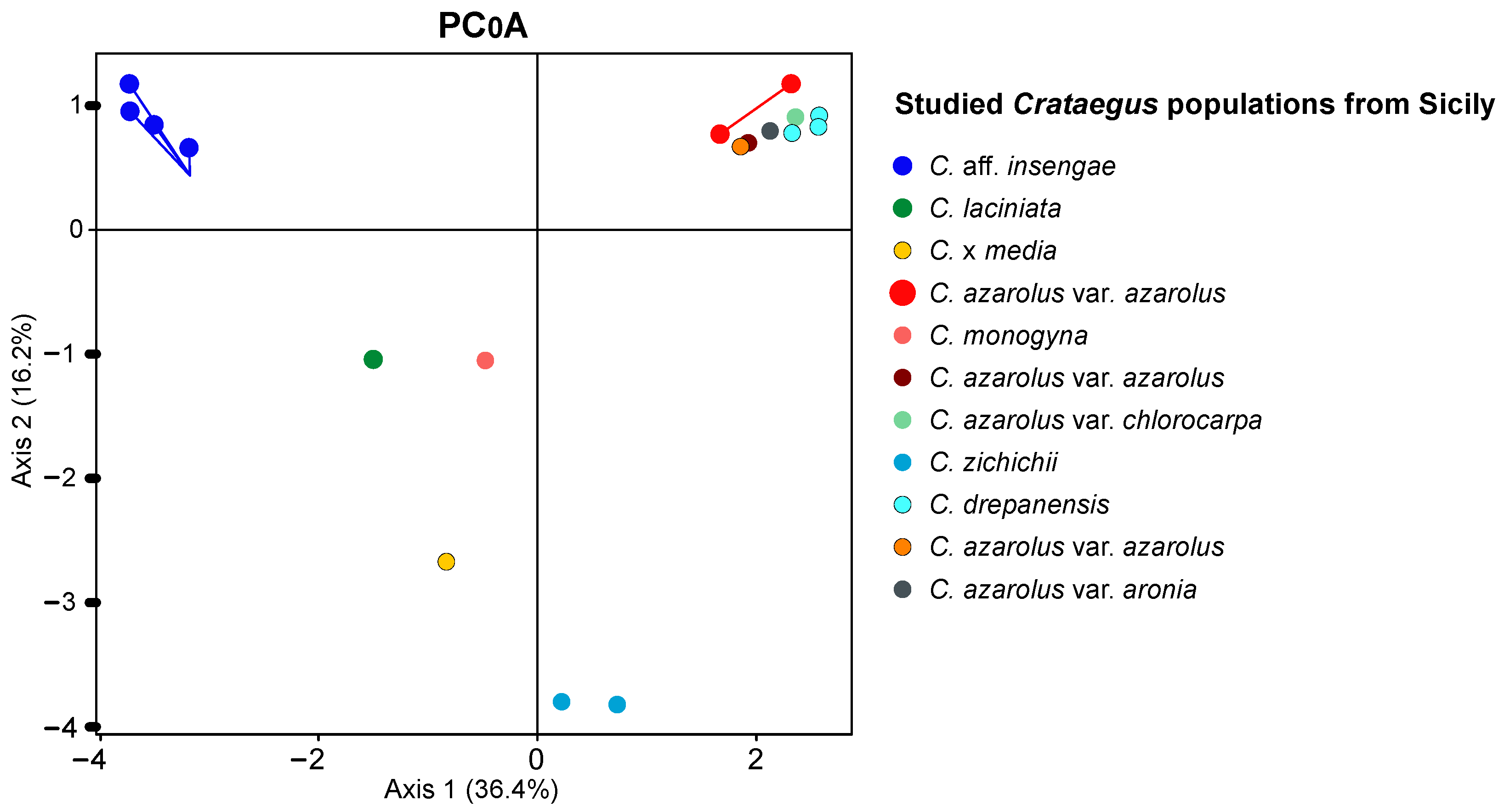
| Sample No. | Taxon | Sample ID | Collection Location |
|---|---|---|---|
| 1 | C. azarolus var. azarolus | C_1/3 | Purgatorio, Custonaci (Trapani) |
| 2 | C. azarolus var. azarolus | C_e | Purgatorio, Custonaci (Trapani) |
| 3 | C. azarolus var. azarolus | C_4/3 | Castelluzzo–Macari road (Trapani) |
| 4 | C. azarolus var. azarolus (cultivated) | C_b | Carrara, Pollina (Palermo) |
| 5 | C. azarolus var. aronia | C_5/3 | Tonnara del Secco, San Vito Lo Capo (Trapani) |
| 6 | C. azarolus var. chlorocarpa | C_f | Balata di Baida, Castellammare del Golfo (Trapani) |
| 7 | C. laciniata | C_3 | Piano Pomo, Petralia Sottana (Palermo) |
| 8 | C. aff. insengae | C_1 | Piano Pomo, Petralia Sottana (Palermo) |
| 9 | C. aff. insengae | C_2 | Piano Pomo, Petralia Sottana (Palermo) |
| 10 | C. aff. insengae | C_10 | Piano Pomo, Petralia Sottana (Palermo) |
| 11 | C. aff. insengae | C_11 | Piano Pomo, Petralia Sottana (Palermo) |
| 12 | C. aff. insengae | C_12 | Piano Pomo, Petralia Sottana (Palermo) |
| 13 | C. monogyna | C_c | Barraca, Castelbuono (Palermo) |
| 14 | C. x media | C_d | Carrara, Pollina (Palermo) |
| 15 | C. drepanensis | C_2/3a | Tonnara del Secco, San Vito Lo Capo (Trapani) |
| 16 | C. drepanensis | C_2/3b | Tonnara del Secco, San Vito Lo Capo (Trapani) |
| 17 | C. drepanensis | C_2/3c | Tonnara del Secco, San Vito Lo Capo (Trapani) |
| 18 | C. zichichii | C_a | Paceco-Marsala road (Trapani) |
| 19 | C. zichichii | C_g | Balata di Baida, Castellammare del Golfo (Trapani) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanno, F.; Aprile, S.; Spadaro, V.; Raimondo, F.M.; Giovino, A. Preliminary Study on the Genetic Diversity of Sicilian Populations of Crataegus azarolus (Rosaceae) and Their Wild Relatives for Conservation and Valorisation Purposes. Diversity 2025, 17, 258. https://doi.org/10.3390/d17040258
Bonanno F, Aprile S, Spadaro V, Raimondo FM, Giovino A. Preliminary Study on the Genetic Diversity of Sicilian Populations of Crataegus azarolus (Rosaceae) and Their Wild Relatives for Conservation and Valorisation Purposes. Diversity. 2025; 17(4):258. https://doi.org/10.3390/d17040258
Chicago/Turabian StyleBonanno, Floriana, Simona Aprile, Vivienne Spadaro, Francesco M. Raimondo, and Antonio Giovino. 2025. "Preliminary Study on the Genetic Diversity of Sicilian Populations of Crataegus azarolus (Rosaceae) and Their Wild Relatives for Conservation and Valorisation Purposes" Diversity 17, no. 4: 258. https://doi.org/10.3390/d17040258
APA StyleBonanno, F., Aprile, S., Spadaro, V., Raimondo, F. M., & Giovino, A. (2025). Preliminary Study on the Genetic Diversity of Sicilian Populations of Crataegus azarolus (Rosaceae) and Their Wild Relatives for Conservation and Valorisation Purposes. Diversity, 17(4), 258. https://doi.org/10.3390/d17040258












