Abstract
Terrestrial small mammal species typically assemble according to plant communities, but multiple factors, including large-scale geographic patterns, influence their assemblage structure. Despite their ecological significance, small mammals are often underrepresented in biodiversity assessments, and many Polish national parks lack comprehensive surveys. This is also the case for Wolin National Park (WNP), Poland’s only national park on a coastal marine island, which is known for its unique bat fauna. Here, we surveyed small mammals in WNP using live and pitfall trapping, identifying only nine species—the lowest richness among the five regional national parks (which host 11–13 species based on trapping data alone). Rarefaction analysis indicated a very low probability of detecting additional species with further sampling. This unexpectedly low richness is likely linked to insular isolation and the park’s location at the edge of the regional distributions of three species. Cluster analysis revealed a key pattern in WNP’s small mammal assemblages: a division between two distinct landscape units—moraine hills and the alluvial delta—where Apodemus flavicollis and Apodemus agrarius were the predominant species, respectively. This division had a greater influence on assemblage clustering than local vegetation.
Keywords:
rodents; shrews; Rodentia; Soricidae; habitat preferences; species richness; Baltic; Poland 1. Introduction
Small land mammals, especially rodents and shrews, play crucial roles in trophic networks and soil processes within terrestrial ecosystems worldwide [1]. They also provide the largest contribution to mammalian diversity globally, along with bats [2,3]. Typically, different habitats—defined, for example, by plant communities—are occupied by distinct small mammal assemblages, usually consisting of several co-occurring species [4]. The qualitative and quantitative structure of these assemblages varies due to a combination of environmental factors. In temperate zones, these factors primarily include the composition of herbaceous [5] and tree layers [6], tree cover [7], the abundance of coarse woody debris [8], productivity [6], altitude [9], topography, micrometeorology, and the abundance of epigeic invertebrates [10]. Large-scale disturbances, such as fire [11,12], the activity of keystone species [13,14], successional stage [15], and land use [16,17,18,19], also influence these assemblages. However, the effects of these factors may be further modified by larger-scale geographical influences, such as insular isolation [20]. Consequently, the structure of small mammal assemblages can vary even among areas with similar habitat composition, and this variation remains only partially understood—likely due to numerous interactions between these factors.
National parks are crucial components of protected area networks worldwide, playing a significant role in biodiversity conservation [21]. In Poland, they are the only protected areas where all aspects of nature are subject to conservation. Therefore, they should be prioritized for broad-scale biodiversity inventories, particularly for taxonomic groups that serve keystone ecological functions. Unlike more charismatic large- and medium-sized mammals, which have been surveyed in all of Poland’s national parks and are continuously monitored [22], small rodents and shrews have received comparatively less attention. As a result, comprehensive inventories of this group have only recently been conducted in several national parks.
This lack of data also applies to Wolin National Park (WNP), located at the northwestern edge of Poland and the country’s only national park situated on a coastal marine island. Its unique geographic position among Polish national parks may result in distinct biogeographical characteristics that influence the structure of small mammal assemblages. WNP is known to host an unexpectedly depauperate bat fauna, with unusually uniform assemblages across habitats due to the dominance of a single, hyperabundant native species [23]. However, the small mammal fauna of this park has not been studied in the same detail.
This paper aims to present the structure of small terrestrial mammal assemblages in a Central European national park that exhibits an unexpectedly depauperate species composition compared to the regional fauna and local land cover variability. We also examine whether inter-assemblage similarity among small terrestrial mammals is shaped more by plant communities or by robust topographical features within the unique coastal landscape of the Southern Baltic Sea region.
2. Materials and Methods
2.1. Study Area
Wolin National Park (WNP) is located along the Baltic Sea coast in northwestern Poland at 53°57′15″ N, 14°29′20″ E (Figure 1). The park encompasses a significant portion of Wolin Island, the largest Polish island, which covers 265 km2. It is separated from the mainland by the Dziwna Channel, which narrows to 90 m at its narrowest point and expands to approximately 4.5 km at its widest.
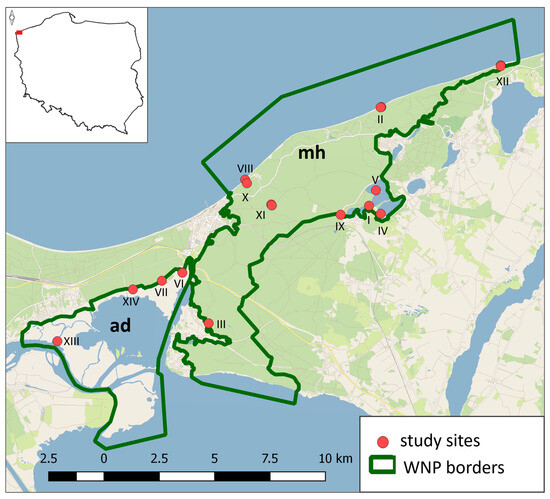
Figure 1.
Location of study sites at which small mammal trapping was conducted within the borders of Wolin National Park. The number of each site corresponds to Table 1. mh—moraine hills (uplands), ad—alluvial delta (lowlands).
Established in 1960, WNP covers 10,937 hectares, with 4648 hectares (42.5%) consisting of forest ecosystems. Most of the park’s woodlands form a continuous block that includes a range of moraine hills, with the highest point reaching 115 m above sea level (asl). To the north, these woodlands are bordered by active cliffs composed of clay, sand, and gravel, which rise to 93 m before dropping directly into the Baltic Sea. Lower, partially active cliffs mark the southern boundary and descend into the Szczecin Lagoon, while dunes are present in the northeast. The predominantly moraine, woodland-dominated section of the park is adjacent to the flat, alluvial region located in the regressive delta of the Świna Channel, which is 100–1000 m wide and separates Wolin from the neighboring Uznam Island. Seven lakes, including four postglacial and three post-mining ones, are located within the hilly part of the park, covering a total surface area of 163 hectares. Running waters are extremely scarce, with only four, short sections of a stream that connects and discharges the lakes. A significant component of the hydrological system in the delta is a network of brackish channels and the open waters of the Szczecin Lagoon, which form the estuary of the Oder River and divide parts of the alluvial land into seven smaller islands. Frequent backflows from the Baltic Sea cause periodic flooding in this part of WNP.
According to the Köppen climate classification, the region has a humid continental climate with a warm-summer subtype (Dfb). The mean annual temperature is 9.1 °C, with an average temperature of 0.8 °C in January and 18.2 °C in July. The mean annual precipitation is 585 mm, and the area typically experiences an average of 30 days of snow cover each year.
In WNP, the average age of the tree stands is 112 years. The dominant tree species are Scots pine (Pinus sylvestris), beech (Fagus sylvatica), and sessile oak (Quercus petraea). The predominant woodland communities are fertile beech forests (Galio odorati–Fagetum) and acid-poor beech forests (Luzulo pilosae–Fagetum), with significant contributions from acid beech–oak (Fago–Quercetum), birch–oak (Betulo–Quercetum), and suboceanic pine (Leucobryo–Pinetum) forests. Crowberry–pine forests (Empetro nigri–Pinetum) grow on coastal dunes, while sedge-beech forests (Carici–Fagetum) are found on the upper edges of marine cliffs. Swamp forests are dominated almost exclusively by black alder carrs (Ribeso nigri–Alnetum), occurring both around postglacial lakes and in the alluvial delta. Compared to other parts of northern Poland, particularly the Pomerania region, a notable characteristic of WNP’s vegetation is the near absence of moist Subatlantic oak–hornbeam (Stellario–Carpinetum) and alder–ash (Fraxino–Alnetum) forests, which are only found in small patches near postglacial lakes.
Among non-forest communities, saltmarsh pastures (Juncetum gerardi), located on the islands of the alluvial delta, are the most prominent. Significant areas are also covered by reed beds (Phragmitetum commune) and great fen-sedge (Cladietum marisci) communities. Glycophilous grasslands are primarily represented by false oatgrass (Arrhenatherion elatioris) and purple moor grass (Molinion) meadows. As one of only two Polish national parks protecting coastal habitats, WNP features diverse and well-developed cliff communities, which exist in various stages of succession as both swards and shrubs. In contrast, non-forest dune communities are poorly represented, with only a single, narrow patch along the central part of the park’s coastline, where the typical zonation from white dunes (Elymo–Ammophiletum) to grey dunes (Helichryso–Jasionetum) occurs [24,25].
Only a few buildings are present within WNP, including park offices, foresters’ lodges, and, in the delta, cow sheds. However, several settlements, including the city of Międzyzdroje, are located along the park’s borders.
2.2. Small Mammals Survey
Data for this study were collected during the first-ever broadscale survey of small mammals in Wolin National Park (WNP) in August and September 2023 at 14 localities (Figure 1), covering 17 different terrestrial and wetland habitats within the park (Table 1).

Table 1.
Habitat characteristics of the study sites at which small mammal trapping was conducted. Landscape units: mh—moraine hills (uplands) and ad—alluvial delta (lowlands).
The methodology was originally designed to maximize the completeness of the species inventory for the park; therefore, minor methodological differences were allowed among localities. In general, at each locality, 30 conical pitfall traps (diameter: 30 cm and depth: 50 cm) and 17 Sherman live traps (8 cm × 9 cm × 23 cm) were set. Pitfall trapping followed the methodology used in [14]. To improve thermal conditions, the bottom of each pitfall was filled with a ball of local herbaceous plants or moss, and 2–3 larvae of the mealworm Tenebrio molitor were added as a food source. Both plant stuffing and mealworms were provided to enhance the survival of captured shrews. Each live trap was baited with a mixture of oat flakes and peanut butter.
Traps were arranged in lines, with trap points located every 10 m. Each of the first 17 trap points included both a pitfall and a live trap, positioned 1 m apart; beyond this, only pitfall traps were set. When a locality was intended to include two different, neighboring habitats (II, XI, and XII), two parallel trap lines were used, each consisting of 15 pitfall traps and 8–9 live traps. Trapping at each site typically lasted two consecutive nights, with traps opened one hour before sunset and closed one hour after sunrise. Exceptions included localities II, VIII, IX, and XII, where traps were set for only one night. The total trapping effort amounted to 1098 trap-nights.
At site V, where the goal was to capture the hazel dormouse Muscardinus avellanarius, live traps were set in a separate trap line parallel to the pitfall traps, mounted on branches of hazelnut Corylus avellana at a height of 1–1.5 m above the ground. The bait was supplemented with sliced apples. At localities VIII and IX, only live traps were used due to the hardness of the soil, which made setting pitfall traps too time-consuming and potentially destructive to the plant cover.
All traps were checked every two hours. Captured mammals were identified based on external features, primarily using the key by Pucek [26] and the field guide by Aulagnier [27], and were then released on site. Some individuals of Apodemus and Microtus/Alexandromys were identified only to the genus or genus-pair level, as they escaped before measurements could be taken or before non-metric features could be examined. The unidentified Apodemus specimens exclusively belonged to the subgenus Sylvaemus [28], i.e., either A. flavicollis or A. sylvaticus, but not A. agrarius. After each capture, bait and food supplies were replenished.
We used capture data to estimate species richness based on individual-based rarefaction (species accumulation curve) using PAST ver. 5.1 [29]. This method allowed for a detailed comparison of small mammal species diversity among habitat classes, as well as among five national parks in northwestern Poland (Wolin, Słowiński, Warta Mouth, Drawieński) and northeastern Germany (Lower Oder Valley). In these four additional parks and their buffer zones, where data were obtained from the literature, between 8 and 37 sites were sampled, with total trapping efforts ranging from 540 to 3150 trap-nights [30,31,32,33,34].
To compare the species composition of WNP samples among sites, habitat classes, and topographical units (landscape units), we performed a cluster analysis using ClustVis 1.0, an open-access web tool for visualizing the clustering of multivariate data [35], provided by the University of Tartu [36]. Samples characterized by the relative abundances of small mammal species were clustered using the average linkage method, which calculates the average distance of all possible pairs, with Manhattan distance as the similarity measure and the “tightest cluster first” method for tree ordering. Since individuals were not marked, relative abundance was calculated based on the number of captures.
We divided the park and its habitats into two landscape units: one representing moraine hills (uplands) in the eastern part (10 sites, 13 habitats) and the other representing the alluvial regressive delta in the west (4 sites, 4 habitats) (Table 1). The sites were categorized into four habitat groups:
- Meso- and acidophilic forests on mineral soils (V, X, XI, and XII);
- Dry non-forest habitats (II, III, VIII, and IX), both exclusive to the upland part of the park;
- Wetland forests, mostly composed of black alder, associated with organic soils (I, IV, and VII);
- Non-forest wetland habitats, exclusive to the alluvial delta (VI, XIII, and XIV).
3. Results
In total, we captured nine species of small terrestrial mammals. Three of them belonged to the family Soricidae (order Eulipotyphla): the common shrew Sorex araneus Linnaeus, 1758, the Eurasian pygmy shrew Sorex minutus Linnaeus, 1766, and the Eurasian water shrew Neomys fodiens (Pennant, 1771). The remaining six species were rodents (order Rodentia), including four from the family Cricetidae—the bank vole Clethrionomys glareolus (Schreber, 1780), the root vole Alexandromys oeconomus (Pallas, 1776), the short-tailed field vole Microtus agrestis (Linnaeus, 1761), and the common vole Microtus arvalis (Pallas, 1778)—as well as two from the family Muridae: the striped field mouse Apodemus agrarius (Pallas, 1771) and the yellow-necked mouse Apodemus flavicollis (Melchior, 1834).
A total of 397 captures were recorded, with C. glareolus, A. flavicollis, and A. agrarius being the most numerous. Overall, rodents comprised 89.2% of the assemblage, with Cricetidae (45.6%) slightly outnumbering Muridae (43.6%). Shrews accounted for only 10.8% of all captured mammals, with S. minutus being the most abundant. N. fodiens was the rarest small mammal in our sample, represented by a single capture. The most widespread species were A. flavicollis (recorded at 10 sites across 12 habitats), C. glareolus (seven sites, seven habitats), and S. minutus (six sites, six habitats) (Table 2).

Table 2.
Results of small mammal trapping (number of captures of a particular taxon) in every sampled habitat of the Wolin National Park. Number of every site corresponds to Table 1. Species: Sara—S. araneus, Smin—S. minutus, Nfod—N. fodiens, Cgla—C. glareolus, Aoec—Alexandromys oeconomus, Magr—M. agrestis, Marv—M. arvalis, A/M—Alexandromys/Microtus, Aagr—A. agrarius, Afla—A. flavicollis, and Asp—Apodemus sp. (only representatives of subgenus Sylvaemus).
Samples from individual study plots clustered primarily based on their location within the park’s two landscape units (i.e., uplands and the alluvial delta). Notably, the alder swamp forest in the delta (site VII) appeared more similar to the nearby open habitats (sites XIII and XIV) than to swamp and riparian alder forests bordering the upland lakes (sites I and IV). The mosaic of freshwater meadows, sedge, and reed beds in the delta (site VI) formed a separate branch outside the cluster containing all of the other habitats but still shared greater resemblance with other delta habitats than with any site in the upland part of the park.
The two landscape units differed particularly in the relative abundance of the only two murid rodents recorded (A. flavicollis and A. agrarius) (Figure 2). Therefore, when presenting the dominance structure of small mammal assemblages, we merged the alder swamp forest at site VII with the other alluvial delta habitats, while the upland wetland forests were combined to form a separate sample (Figure 3).
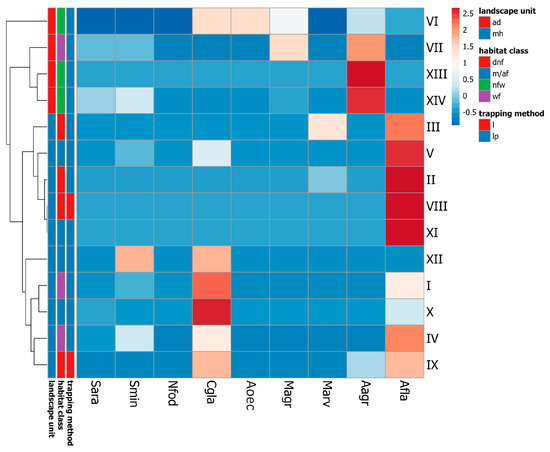
Figure 2.
Comparison of species composition of small mammals trapped at particular study sites in Wolin National Park, subjected to cluster analysis (clustering method—complete linkage, Manhattan distance, and average cluster first). Only individuals identified unambiguously to the species level were taken into account. Number of every site corresponds to Table 1. Species: Sara—S. araneus, Smin—S. minutus, Nfod—N. fodiens, Cgla—C. glareolus, Aoec—Alexandromys oeconomus, Magr—M. agrestis, Marv—M. arvalis, Aagr—A. agrarius, and Afla—A. flavicollis. Landscape unit: mh—moraine hills (upland) and ad—alluvial delta (lowland). Habitat class: dnf—deciduous non-forest habitats, m/af—meso- and acidophilic forests on mineral soils, nfw—non-forest wetlands, and wf—wetland forests. Trapping method: l—live traps only and lp—live- and pitfall traps. Data from Table S1.
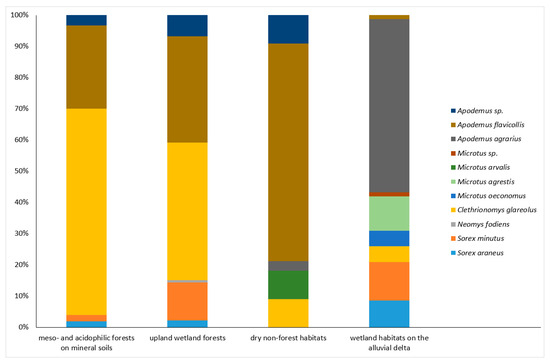
Figure 3.
Structure of small mammal assemblages hosted by four different groups of habitats, distinguished by plant communities and topography. Meso- and acidophilic forests on mineral soils: sites V, X, XI, and XII (N = 150 captures; 329 trap-nights). Upland wetland forests: sites I and IV (N = 132 captures; 188 trap-nights). Dry non-forest habitats: sites II, III, VIII, and IX (N = 33 captures; 205 trap-nights). Wetland habitats on the alluvial delta: sites VI, VII, XIII, and XIV (N = 81 captures; 376 trap-nights).
Small mammal assemblages in the upland, eastern part of the park were generally characterized by an abundance of A. flavicollis, regardless of tree cover and humidity. However, in mesophilous deciduous forests dominated by beech and oak in the tree layer, A. flavicollis was outranked by C. glareolus (66.0%) (Figure 3). This effect can be largely attributed to a single site (X, fertile beech forest, Table 2), which had the highest number of small mammal captures in the entire study.
Assemblages inhabiting the upland wetland forests (I and IV) exhibited a similar dominance of A. flavicollis (34.1%) and C. glareolus (43.9%) but also showed a relatively high proportion of shrews (15.2% in total, representing all three species) (Figure 3). The only capture of N. fodiens in the park was recorded in the alder–ash riparian forest (I). A. agrarius was documented in the upland part of the park based on a single capture in the garden surrounding the forester’s lodge (IX) (Table 2).
The only habitats occupied by M. arvalis were dry, non-forest areas (Figure 3), particularly grey dunes (IIa) and mesic meadows (III). The habitats closest to the seashore were occupied by only two species: A. flavicollis (white dunes, IIb, and treeless cliffs, VIII) and S. minutus (coastal dune pine forest, XIIa) (Table 2).
In contrast, the wetland habitats of the alluvial delta, located in the western part of the park, were almost devoid of A. flavicollis (Figure 3), with only a single capture recorded at site VI (Table 2), which was closest to the moraine, hilly uplands. Instead, small mammal assemblages in this area were characterized by an abundance of A. agrarius (55.6%) (Figure 3), which was present at all sites, including both woodland (VII) and treeless or ecotone habitats (VI, XIII, and XIV). In the halophytic mires (saltmarsh, XIII), it was the only small mammal recorded (Table 2).
A notable feature of the small mammal fauna in the delta wetlands was the presence of M. agrestis in three out of four habitats, along with the highest relative abundance of shrews among all four habitat groups in the park (20.9%). However, these shrews were exclusively of the genus Sorex (Figure 3). The only habitat occupied by M. oeconomus in the park was the complex of wet, freshwater meadows and sedge and reed beds (VI) (Table 2).
The species accumulation curves revealed the highest diversity in the wetland delta habitats (seven species), followed by the upland wetland forests (five species). In contrast, meso- and acidophilic forests on mineral soils had the lowest diversity, hosting only four species. In most habitat groups, the curves approached the asymptote, suggesting a low probability of detecting additional species with increased sampling effort. However, the dry non-forest habitats (four species) remained under-sampled (Figure 4).
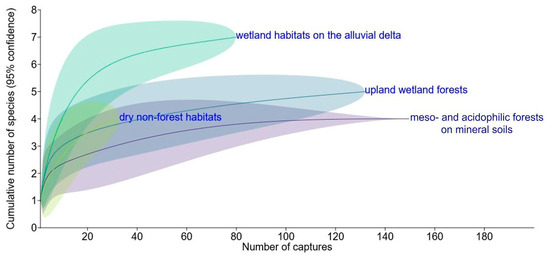
Figure 4.
Rarefaction curves for the cumulative numbers of species plotted against the numbers of small mammal captures in four different groups of habitats, distinguished by plant communities and topography. Meso- and acidophilic forests on mineral soils: sites V, X, XI, and XII (329 trap-nights). Upland wetland forests: sites I and IV (188 trap-nights). Dry non-forest habitats: sites II, III, VIII, and IX (205 trap-nights). Wetland habitats on the alluvial delta: sites VI, VII, XIII, and XIV (376 trap-nights). Data from Table 1, only individuals identified to the species level were included.
Wolin NP hosted the poorest small mammal fauna among the four nearby national parks compared using species accumulation curves. Lower Oder Valley and Drawieński NP hosted 11 species, while Słowiński NP hosted 12, though the difference between them was negligible, with nearly identical trends and strongly overlapping confidence intervals. None of these four parks appeared to be under-sampled, as their species accumulation curves approached the asymptote before the sample size reached half of the final number of captures. In the case of WNP, the final total of nine species had already been recorded by the 188th capture. Warta Mouth NP hosted the most diverse small mammal fauna, with 13 species, despite having the lowest sample size (N = 80). The species accumulation curve for that park was the only one that did not approach the asymptote, indicating that the sample is far from saturated (Figure 5).
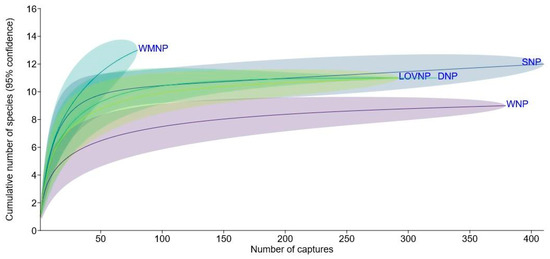
Figure 5.
Rarefaction curves for the cumulative numbers of species plotted against the numbers of small mammal captures in Wolin National Park (WNP) and four other national parks in the Polish Baltic Sea Coast and Lower Oder catchment. DNP—Drawieński National Park, LOVNP—Lower Oder Valley National Park, SNP—Słowiński National Park, and WMNP—Warta Mouth National Park (data in Table S2).
4. Discussion
4.1. Species Composition
The individual rarefaction curve for the small mammal fauna suggests that increasing the sampling effort would be unlikely to yield a significantly higher number of species. Therefore, the nine species recorded during our study likely represent the actual richness of the Park’s fauna, even though only the minimum richness was detected for each local assemblage at the site or habitat level. However, the fauna appears unusually depauperate compared to the four other national parks of the Lower Oder catchment and the Polish Baltic Sea Coast. Seven additional species have been recorded in these parks, specifically Neomys milleri Mottaz, 1907 (SNP), Crocidura suaveolens (Pallas, 1811) (WMNP, LOVNP), Arvicola amphibius (Linnaeus, 1758) (WMNP), Rattus norvegicus (Berkenhout, 1769) (SNP), Mus musculus Linnaeus, 1758 (DNP), Micromys minutus (Pallas, 1771), and Apodemus sylvaticus (Linnaeus, 1758) (all four national parks) [30,31,32,33,34].
Five of these species—A. amphibius, M. musculus, M. minutus, A. sylvaticus, and R. norvegicus—have previously been recorded on Wolin Island through trapping or owl pellet analysis [37,38,39]. The most recent published records for the first three species on the island date back to 1962 and 1966, based on an extensive survey conducted by the Mammal Research Institute, Polish Academy of Sciences. During that survey, these species were reported from three to seven localities, with M. minutus being the most widely distributed [38]. However, it is often difficult to determine whether these historical records originated from areas now within the boundaries of WNP, as the authors only provided the names of towns and villages where the material was collected, and these settlements are located outside the Park. The exception is A. sylvaticus, for which specimens stored in the zoological collection of the Mammal Research Institute PAS in Białowieża (Nos. 61932, 61819, 61723, and 61624) are explicitly labeled as collected in Wolin National Park in 1966 [40]. These specimens are likely the same individuals reported as trapped that year in Międzyzdroje [38].
R. norvegicus was recently recorded by us in August 2023, based on a dead individual found at the lakeshore near Site V [41]. The occurrence of Talpa europaea Linnaeus, 1758, on Wolin Island has been mentioned in two sources [42,43] and was confirmed within the Park itself by the presence of molehills near Sites I and V [41]. Additionally, two arboreal glirid rodents, Glis glis (Linnaeus, 1766) and Muscardinus avellanarius (Linnaeus, 1758), were reported from Wolin Island in the first half of the 20th century [42,44,45], despite not being trapped in any of the additional four national parks of the Lower Oder catchment and Polish Baltic Sea Coast [30,31,32,33,34]. However, G. glis was later observed directly in Słowiński National Park [46]. Lastly, the occurrence of Microtus subterraneus (de Sélys-Longchamps, 1836) was reported from the southeastern shore of Szczecin Lagoon, approximately 20 km from the WNP border [38], despite not being documented in any of the aforementioned national parks.
Based on these data, the potential small mammal fauna of WNP could consist of up to 19 species, yet only 11 have been recorded in recent years, and just nine were captured during our trapping survey, which covered 15 sites representing all major habitats. This raises questions about the factors contributing to the recent depauperation of small mammal assemblages in the Park, a pattern that mirrors the similarly impoverished bat assemblages recently discovered in the area [23].
4.2. Potential Methodological Biases Affecting the Structure of Small Mammal Samples
One of the factors contributing to differences in the structure of regional small mammal assemblages is the use of selective trapping methods that reduce the detection rate of certain taxa. Typically, small terrestrial mammals are sampled using two types of traps: baited traps with a trigger mechanism (live and snap traps) and pitfalls. Some studies suggest that live traps are more effective for capturing rodents, while pitfalls are better suited for shrews [47,48]. However, other research indicates that only snap and live traps underestimate the abundance and dominance of shrews, whereas the efficiency of rodent trapping remains similar across the trap types [49,50].
In some highly diverse biomes, pitfalls alone may yield higher estimates of overall small mammal abundance and species richness, including rodent abundance and species richness, compared to live traps alone or even a combination of both trap types [51]. A study conducted in the European boreal zone found that the use of cone traps provided a more balanced representation of small mammal assemblages, capturing both shrews and rodents in significant numbers. In contrast, snap traps captured not only three times fewer mammals but also produced a sample strongly biased toward rodents. The disparity was even more pronounced when examining individual species, with M. minutus, the smallest European rodent, being captured 70 times more frequently in pitfalls than in snap traps [52].
A study on small mammal assemblages in beaver-created wetlands in Poland yielded abundant captures of both Central European Neomys species (which were extremely scarce in WNP) and M. minutus (which was not detected at all in WNP), despite using only pitfalls as the trapping method [14]. Some species, notably A. flavicollis, may indeed be underrepresented in pitfall-only studies due to their ability to jump out of the traps [52,53]. However, even in pitfall-only studies, they are still regularly recorded [5,14]. To minimize this potential bias, we employed both live and pitfall traps at most sites where topography allowed.
Small mammal surveys in the three other national parks compared with WNP in our study used different trapping strategies: SNP and DNP relied exclusively on wooden live traps [30,32], LOVNP used a combination of wooden and metal live traps [34], and WMNP employed both wooden live traps and pitfalls [31]. Consequently, one might expect samples from SNP and LOVNP to underrepresent shrews compared to WNP. Despite this, all of the other parks exhibited a significantly higher proportion of shrews in their small mammal assemblages (SNP: 34.6% [32], WMNP: 28.8% [31], LOVNP: 21.23% [34], and DNP: 18.3% [30]) than WNP (10.8%), where pitfall trapping was the most widespread. Thus, the trap types used in our study were unlikely to be the primary reason for the lower species richness in WNP, including the scarcity of Neomys water shrews and the absence of M. minutus. Although some researchers suggest that above-ground live trapping is necessary for detecting M. minutus [54], this species is regularly captured in ground-level traps [5,14,32,52]. Therefore, the absence of specific trapping methods in our study is an unlikely explanation for its absence in WNP, even though elevated traps are more efficient for capturing M. minutus than ground-level ones [55].
Neither pitfalls nor live traps are particularly effective for detecting T. europaea and members of the family Gliridae, although they are occasionally captured in pitfalls [5,56]. Their absence in the WNP sample may be attributed to systematic bias in our methodology. However, these species were also not trapped in any other part of the region [30,31,32,33,34]. It is worth noting that even targeted live trapping for M. avellanarius (using live traps placed on hazel branches in its optimal habitat at site V, an oak–hornbeam forest [57]) failed to detect the species. Additionally, R. norvegicus is rarely captured in standard small mammal traps, likely due to its large body size, despite its widespread distribution across Poland [32,38,58]. However, even when considering only the species for which the applied methods are deemed optimal (i.e., epigeic taxa with a body size smaller than R. norvegicus), one would still expect to detect 15 species of small mammals based on their geographical ranges and habitat preferences.
Given the relatively limited trapping effort in our survey, one might suspect that the low species richness in WNP resulted primarily from under-sampling. Indeed, all fieldwork in WNP was conducted within a single season, with most sites sampled for only two nights, and three sites sampled for just one night. This contrasts sharply with small mammal surveys conducted in some national parks in eastern Poland, where trapping plots were operated continuously for several weeks to several years [5] or at least in five-day sessions spread across 3–5 years [59,60]. However, no national park in northwestern Poland or eastern Germany (which were compared to WNP) followed such extended sampling protocols. These parks were typically surveyed over one or two seasons, often with comparable or even lower trapping effort than our study [30,31,32,33,34] and Table S2. Among them, SPN was surveyed based on five-day trapping sessions at each site, making it an outlier, yet it was far from the most species-rich park [32]. Moreover, the highest species richness was observed in WMNP, where each site was sampled for only one night [31]. Finally, when comparing species diversity among samples using individual rarefaction curves, the number of trapping nights or trap-nights is a poor indicator of sampling effort, as it does not distinguish between actual differences in species richness and differences in trap efficiency [61]. Our study obtained the second-highest sample size (measured by the number of captured individuals) among the compared studies. Yet, the rarefaction curve for WNP reached the asymptote at the lowest number of species, suggesting that our estimate of species richness is accurate at the park level.
Despite the lower trapping effort compared to surveys of small mammals in Lithuanian national parks (which used 1550–2750 trap-days), the number of captures obtained in WNP (228–363) was similar to those studies [62]. However, individual habitats within WNP were likely under-sampled, and limiting the study to one season may have resulted in an overlap with minimum densities of species exhibiting cyclic or fluctuating populations (see Section 4.3).
Additionally, because we did not mark captured individuals, multiple captures went undetected, likely leading to an overrepresentation of the most abundant species and, consequently, an underestimation of overall diversity. However, this issue is not universal; for example, in SPN, where trapped individuals were marked, the numbers of individuals and total captures produced nearly identical assemblage dominance structures [32]. Finally, identifying microtine voles based only on external features rather than dental characteristics may have resulted in inaccuracies in species composition estimates, as juvenile individuals were particularly difficult or impossible to identify with certainty [63].
4.3. Factors Leading to the Depauperation of Small Mammal Assemblages
The notably low number of species in the small mammal fauna of WNP could be explained by the park’s geographical position—specifically, if it was located outside the geographical ranges of species that are present in other national parks of the Lower Oder catchment and the Polish Baltic Sea Coast. However, it is highly unlikely that large-scale biogeographical factors play a major role in this case. Most species that occur in the mentioned region but are absent in WNP have distribution ranges that encompass all of Poland and Germany. This applies to A. amphibius, M. minutus, M. musculus, and A. sylvaticus [38,64]. Nevertheless, three taxa reach the borders of their distribution near the Oder estuary, and their absence in WNP (and historically on Wolin and Uznam Islands in general) may be explained by this factor.
N. milleri exhibits a disjunct distribution pattern in Europe, with an isolated Pomeranian portion of its range [65], which terminates at the western Polish border (coinciding roughly with the Lower Oder Valley) and does not extend into northern Germany [64]. The nearest known localities of N. milleri have been reported about 30–40 km northeast and southeast of WNP’s borders, on the Pomeranian mainland [38]. Among the four compared national parks, only SNP, the easternmost one, appears to host a population of N. milleri [32]. Similarly, M. subterraneus does not occur in northern Germany, and the western limit of its distribution in the Central European lowlands nearly coincides with Poland’s western national border [64]. C. suaveolens is widely distributed across southern and western Poland, but it reaches the northern edge of its range in mainland western Pomerania. The northernmost record of this species in the Oder estuary region is approximately 30 km from WNP’s borders [66]. Furthermore, M. subterraneus exhibits a highly fragmented distribution in northern Poland, while A. sylvaticus is much rarer and more localized in the northern part of the country than in the south [38], which may contribute to their absence in recent small mammal samples from WNP. However, it is important to note that A. sylvaticus has previously been recorded in the park [40]. Even if regional distribution boundaries exclude three to four taxa from the potential fauna of WNP, local small mammal assemblages should still consist of at least 12 species.
Differences in habitat composition and land cover may influence species composition and diversity among the compared national parks. However, this explanation seems unlikely in the case of WNP. M. minutus, one of the most conspicuous rodent species in Poland and present in all of the other national parks of the region, is absent in WNP despite the apparent availability of its preferred habitats. The species selects non-forest habitats with tall herbaceous vegetation, including wetlands—especially reed beds [55,67] and sedge communities [5]—but prefers sparse reeds with sedge undergrowth [68], which are abundant in the lowland part of the park, particularly in alluvial delta sites XIV and VI. Similarly, A. amphibius, which inhabits water bodies and wetland habitats regardless of dominant vegetation type [69], should be well-represented in WNP, where such habitats cover more than half of the park and include six sampled sites (I, IV, VI, VII, XIII, and XIV).
The extreme scarcity of N. fodiens, which contributed to the overall low species diversity revealed by species accumulation curves, also requires explanation, as this species is associated with aquatic habitats [5,70] and various wetland types, both forested and non-forested. In Poland, N. fodiens is particularly numerous in black alder swamp forests [59,70], reed beds, and sedge communities [59,71]. Aside from site I, where it was captured, this species would be expected to occur abundantly in sites IV, VI, VII, and XIV. If N. milleri is present in WNP, at least some of these sites might also support its populations.
A. sylvaticus is typically associated with dry, open, or semi-open habitats, including xerothermic grasslands [56,72], shrubs (e.g., hedgerows), ruderal areas, and arable land [56,73]. Some of these habitat features are represented at sites VIII (active marine cliff) and III (mesic meadow). However, A. sylvaticus is also known to inhabit wetland environments [60], forests, and ecotones [74], albeit in lower numbers. Despite our broad sampling of potential habitats for this eurytopic species, all Sylvaemus specimens examined during our study exhibited unambiguous morphological features of A. flavicollis.
The only group of small mammals that may genuinely be limited by habitat scarcity are synanthropic species (M. musculus and C. suaveolens), as rural buildings and associated environments (e.g., gardens at site IX) are sparse within the park and may not support stable populations. General habitat parameters that are positively correlated with small mammal diversity and density, such as the percentage of deciduous forest cover [6] and the abundance of coarse woody debris [75,76], do not appear to be factors limiting species richness in WNP. Most of the forest cover consists of broadleaved trees, and restoration efforts combined with long-term passive protection in the oldest stands have resulted in an abundance of deadwood. Additionally, many sampled habitats belong to early successional stages or ecotones, which typically support the highest species richness in lowland Central Europe [74].
The entire study was conducted within a single season, while small terrestrial mammals often exhibit long-term population cycles or irregular fluctuations. Differences in population densities between the minima and maxima of these cycles can, in the case of rodents, vary by one or two orders of magnitude in the temperate broadleaved forest zone of the Palearctic [77,78]. Therefore, surveys conducted during the population minima of a particular species might not only reveal a low contribution to the quantitative structure of the small mammal assemblage but may even fail to detect these species altogether.
M. minutus exhibits irregular interannual fluctuations, sometimes with maximum increases exceeding five-fold and maximum decreases of approximately fifteen-fold [79]. Although our trapping may have been conducted during a year when M. minutus population numbers were low, it is important to note that in most locations, these minimum numbers do not appear to drop as significantly as those of cyclic microtine populations or woodland rodents that experience fluctuations associated with mast seeding [77,78]. In Lithuania, the long-term stability of M. minutus populations was confirmed through trapping and owl pellet analysis, showing only minor interannual variation [80].
Surveys conducted in the compared national parks, as well as other studies in optimal M. minutus habitats in northern Poland, were carried out in various years but did not fail to detect the species (1997 [81], 1998 [30], 2012–2013 [31], 2008–2014 [32], 2021 [33], 2022 [34], and 2020–2021 [14]). The exception to this pattern was the Lithuanian island of Rusnė, a patch of alluvial land separated from the mainland by two branches of the Nemunas River, where seasons with abundant M. minutus alternated with seasons in which the species remained undetected [82]. However, in the latter case, snap traps were used, which are a relatively ineffective tool for capturing M. minutus [52].
Shrews in Europe do not exhibit population cycles but instead show some irregular fluctuations, which are correlated across many distant areas and attributed primarily to the effects of the North Atlantic Oscillation [83]. In Lithuania, peaks in their abundance—sometimes tenfold higher—may occur even 20 years after population minima [84]. Conducting our trapping during one of these minima may partially explain the exceptionally low proportion of shrews compared to other national parks in the region. A population outbreak of A. flavicollis and C. glareolus following the mast year of beech in 2022 [41] led to their dominance in small mammal assemblages across several habitats, as high seed production typically triggers such outbreaks [85]. Consequently, this may have contributed to the low proportion of shrews in our samples. However, this does not explain the scarcity—or, in most cases, the absence—of N. fodiens in a park rich in aquatic and wetland environments.
Water shrews can also exhibit strong interannual variations in abundance, reflected in trappability fluctuations ranging from zero to thirty individuals per 100 pitfalls. However, such extreme fluctuations have only been documented in southwestern Siberia [86]. There are limited data on the population dynamics of N. fodiens in Europe, but in Lithuania, for example, the species does not follow a long-term population trend, and its numbers show only minor and irregular interannual variation. Nevertheless, there are years when fewer water shrews are captured than expected, though the difference typically does not exceed a factor of 1.3–1.4 [84]. It should be noted, however, that only two N. fodiens were captured in a single year out of nine years of trapping (N = 1359 small mammals in total) on the seasonally flooded meadows of the river delta, despite the clearly wetland nature of the habitat [82].
From a biogeographic and paleogeographic perspective, Wolin is a continental island, and a significant portion of WNP is located on smaller, alluvial islands of the regressive delta. The main island formed during the Atlantic period (5700–8900 BP) due to marine transgression of the Littorina Sea, which separated the moraine hills from the mainland and from their western counterparts on Uznam Island [87]. The surrounding brackish channels and lagoons act as barriers that limit small terrestrial mammal immigration. Although the Dziwna channel is only 90 m wide at its narrowest point, it may act as at least a filter to small mammal movements. A Slovakian river only 35 m wide restricted the mobility of small rodents, even though they occasionally crossed it [88], and most terrestrial small mammals are effective swimmers [89,90]. Limited immigration and, consequently, reduced population replenishment may lead to extinctions and species turnover, as predicted by the theory of island biogeography [91].
The diversity of insular mammals follows the same patterns observed in other taxa—that is, it is lower than on the mainland and is positively correlated with island size while being negatively correlated with island isolation [92,93]. These phenomena are not restricted to geologically old, marine islands far from the shore. Islands created by the flooding of surrounding land due to hydroelectric dam reservoirs follow a clear pattern of faunal impoverishment, similar to continental land-bridge islands, with a positive species–area relationship and a correlation between faunal nestedness and area resulting from selective extinction [94]. Both species diversity and the probability of occurrence for specific small mammal species inhabiting temperate and boreal zone islands—located on marine shelves and postglacial lakes—are influenced by area alone [95,96] or by both area and isolation [30,97]. Rodent and shrew populations on these islands experience regular extinctions, occurring over a timescale of 60 years [95] or even as little as 5 years [97].
To our knowledge, comparable data on small mammals from the land-bridge islands of the Baltic Sea have only been collected in Denmark. A dataset from 31 islands, covering 12 species, revealed a negative effect of isolation on species richness and the presence of five species. The nested distribution of species indicated interspecific differences in both dispersal abilities and extinction risks [98]. Small mammals of the North Sea Frisian Islands generally follow the species–area relationship, considered one of the two foundational principles of island biogeography [99]. However, the age of specific islands and the resulting habitat diversity appear to be crucial factors, with the youngest islands being devoid of small mammals [100].
Winter ice facilitates the colonization of temperate-zone islands on lakes or coastal marine waters by small, non-volant mammals [96]. However, the number of days with ice cover on the Szczecin Lagoon has declined significantly over the past 70 years due to rising temperatures [101], probably making it increasingly difficult for small mammals to disperse to the islands. It may be true even in the narrowest sections of the Dziwna channel, which are recently crossed by bridges but also located in urbanized areas.
Scarce but mostly quantitative historical data do indeed suggest species turnover and either local extinctions or shifts in the composition of the small mammal fauna on Wolin Island. Trapping and owl pellet data from 1962 and 1966 revealed not only the presence of three species that we failed to detect but also a much more widespread occurrence of A. sylvaticus (6/7 localities) compared to A. flavicollis (2/7 localities) [38]. In contrast, A. flavicollis is now the most widespread small mammal in WNP (10/14 localities). Since the older dataset includes owl pellet data, one might question whether direct comparisons with trapping surveys are valid, as pellet analysis has been documented as less selective and capable of detecting significantly more species than trapping [102]. However, that meta-analysis is based almost exclusively on cage-type live traps and snap traps, which, as mentioned previously, are much more selective than pitfalls—the predominant trapping method used in our study [42,43].
In conclusion, the low diversity of small mammals in WNP may be attributed to island isolation, which has likely intensified due to worsening ice conditions. The bat fauna of WNP is similarly depauperate, though this appears to result from a combination of natural and anthropogenic disturbances that leads to the expansion of single, hyperabundant species [23], as volant mammals on islands located so close to the mainland are not typically affected by area or isolation [97].
4.4. Structure of Small Mammal Assemblages and Habitat Selection by Particular Species
Despite the relative paucity of species and the absence of some taxa known elsewhere to be characteristic for specific habitats sampled in WNP, several features of small mammal assemblages and habitat selection by particular species resemble data from other regions of Central Europe, including Poland. In sufficiently sampled habitat groups (i.e., those in which rarefaction curves approached the asymptote), three species consistently comprised more than two-thirds of the entire assemblage, each representing a distinct ecomorphological type: a mouse (murid), a vole (arvicoline cricetid), and a shrew (soricid). This pattern aligns with findings on European small mammal assemblages in general [103].
The most numerous species, C. glareolus and A. flavicollis, usually predominate in mesic and acidophilic woodlands, including beech, oak, and hornbeam forests that provide abundant heavy seeds [5,56,104]. However, A. flavicollis, typically considered a woodland species, was also abundant—or even dominant—in non-forest habitats, yet all patches representing those habitats were located near forests. Such ecological plasticity of A. flavicollis has been observed in southeastern Poland, where the species regularly occurs in open habitats, even dominating on arable fields [56]. Additionally, this species is known to exhibit density-dependent spillovers into habitats outside woodlands during beech mast years [105].
Apart from C. glareolus, microtine rodents contributed surprisingly little to small mammal assemblages compared to previous studies from Polish lowlands, particularly those covering extensive open habitats and eutrophic wetlands [31,59,60,106]. This may be related to the low abundance of these mammals during the minimum phase of their population cycle [77]. However, their populations—and consequently their proportions in assemblages—may now be stabilizing at lower levels due to widespread cycle dampening across Europe [107]. Nonetheless, their dominance remains highest in habitats considered typical for these species in Central Europe. M. arvalis is often restricted to non-forest dry habitats, such as meadows [56,60,108] or coastal dunes [100], outside its preferred arable land—similar to its occurrence in WNP. In contrast, A. oeconomus is a wetland specialist, predominantly found in fen communities with sedge and reeds [5,59,60], resembling the site where it was captured in the park (VI).
M. agrestis, a hygrophilous species, occurs in various habitats, including humid meadows in forest glades [56], reed beds [103], and peat bogs [58], though in eastern Poland, it tends to be a woodland species [5]. This corresponds with its occurrence in wet meadows, reed beds, and black alder swamp forests of the delta (but not with its absence in respective upland forests). Its habitat preferences in WNP align closely with those of the much more abundant murid, A. agrarius, which, in addition to its preferred arable land, is known to inhabit various poorly defined wetland habitats. A. agrarius can reach high dominance indices in reed beds and meadows [56,59], which may explain its prevalence in the alluvial delta of Wolin Island.
The highest diversity of small mammals in the wetland delta, dominated by early succession stages, follows a general trend observed in the temperate zone [109,110]. One notable difference from previous studies is the significantly higher proportion of S. minutus compared to S. araneus in most habitats. In the majority of earlier studies, the latter species is the dominant shrew in Central Europe [5,32,104,111,112], except in either extremely oligotrophic [58] or very wet habitats [14]. However, several studies report high S. minutus abundance in black alder swamp forests, sometimes even surpassing S. araneus in dominance [32,56,59]. This aligns with our observations in upland wetland forests of WNP.
Data from salt marshes (halophytic meadows) require special attention, as small mammals are rarely sampled in this specific habitat in Europe. Unlike in North America, where a group of unique small mammal taxa is restricted to coastal salt marshes, no such specialists have evolved in Afro-Eurasia [113]. Few species have been recorded in these habitats in temperate Europe, which appear extremely depauperate compared to freshwater eutrophic wetlands, such as fens. M. arvalis, which was the most common species in a Dutch coastal salt marsh, accompanied only by scarce M. musculus [114], while on the Danish island of Sprogø, C. glareolus predominated, accompanied by a single A. sylvaticus [115]. To our knowledge, A. agrarius has never been reported in such a habitat before, yet in WNP, it appears to be the dominant—if not sole—small mammal there. Conversely, inland salt marshes in Slovakia harbor 15 small mammal species (including A. agrarius and five shrews), but trapping efforts there spanned 19 years across seven sites [116].
4.5. Is Small Mammal Zonation in the Wolin National Park Forced by Topography?
A unique feature of the small mammal fauna in WNP is the division of the park into two distinct zones: the moraine hills (upland), where A. flavicollis is widespread in both forest and non-forest habitats but A. agrarius is nearly absent, and the alluvial delta (lowland), where A. agrarius plays a similar role while A. flavicollis is almost absent, even in woodlands. Additionally, a secondary feature of the lowland assemblages is the significant presence of M. agrestis, found only in this zone, and the scarcity of C. glareolus, otherwise the most numerous species in the study area.
It is unclear whether this division is a short-term phenomenon—potentially influenced by population cycles or fluctuations desynchronized among habitats—or a more permanent aspect of the park’s biogeography. This pattern may be shaped and maintained by the presence of natural and anthropogenic barriers between the two parts of WNP, which restrict small mammal movements and reduce the likelihood of successful immigration. These barriers include the city of Międzyzdroje, with its densely built-up urban zone and associated road infrastructure—including an intensively used expressway and an interchange under construction [117]—as well as the waters of Lake Wicko Małe, an extension of the Szczecin Lagoon. Consequently, there is no ecological continuity between the forests of the island’s upland region and its lowland counterparts, which are located on a peninsula with a narrow base occupied by the city and access roads. The only natural terrestrial habitat connecting the two parts of the park is a narrow strip of reed beds.
Although urbanized areas and roads do not serve as absolute barriers for A. agrarius or A. flavicollis—both species are known to successfully colonize isolated green spaces in large cities [118,119]—they may act as selective filters for mammal species [120]. This is especially relevant during population outbreaks, such as those following mast years for beech and oak, when dominant woodland rodents tend to expand into adjacent suboptimal habitats [105], particularly open areas and wetlands.
5. Conclusions
Our study might reveal a unique quantitative structure of a small mammal assemblage in a Polish national park, characterized by low species richness and diversity. Some species that are typically abundant in certain habitats, particularly wetlands, were either extremely scarce or completely absent. Historical data indicate that some of these now-absent species previously inhabited the coastal marine island where the park is located.
We hypothesize that the observed species paucity, compared to other national parks in the region, can be explained within the framework of island biogeography—mainly the increased likelihood of local extinctions and consequent species turnover during population cycle minima or fluctuations. This effect appears to be significant, even though the water barrier separating the island from the mainland is, at its narrowest point, no wider than some large rivers in the region. Additionally, the park’s location at the edge of the regional distribution of three small mammal species may further contribute to the depauperation of local assemblages. However, this hypothesis remains speculative until equally extensive sampling is conducted in areas of the island outside the park’s borders.
Another distinct feature of the small mammal assemblages observed in our study is the effect of broad-scale topography, which appears to influence site clustering more than local vegetation. This is notable, as vegetation is typically considered a crucial factor in shaping the species composition of epigeic rodents and shrews. We attribute this pattern to intra-insular barriers that act as dispersal filters, at least partially linked to anthropogenic influences, particularly urbanization and the development of road infrastructure.
Our findings suggest that even young land-bridge islands—formed after the last glaciation and located very close to the mainland—may develop faunal specificity absent from similar mainland areas. On Wolin Island, the situation of small terrestrial mammals superficially resembles that of bats; however, we propose a completely different set of factors driving these patterns.
Our conclusions are based on limited data, as our survey covered only one season and does not reflect long-term population fluctuations. Furthermore, the relatively small number of sampled sites, compared to similar studies in other protected areas, may limit the generalizability of our findings. Future research is needed to assess whether the observed faunal depauperation is a lasting trend.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17040246/s1. Table S1: Species composition of small mammals trapped at particular study sites, presented as proportion of captures of each species, used for cluster analysis (Figure 2); individuals identified only to genus or genus-group were excluded. Table S2: Species composition of small mammals in the five national parks of northwestern Poland and northeastern Germany, presented as number of captures of each species, used to plot rarefaction curves (Figure 4).
Author Contributions
Conceptualization: M.C.; methodology, M.C.; small mammal trapping, M.C., Z.W., T.K., M.W., J.B., B.S., K.B., and A.R.; preparation of databases, M.C.; statistical analysis, M.C.; literature research, M.C., T.K., A.R., and B.S.; writing—original draft preparation, M.C.; writing—review and editing, M.C. and Z.W.; data visualization, M.C. and Z.W.; graphic abstract, map and spatial data, Z.W.; preparation of references, Z.W. and T.K.; project administration, M.C. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Forest Fund of State Forests National Forest Holding (Fundusz Leśny Państwowego Gospodarstwa Leśnego Lasy Państwowe), Grant No. MZ.0290.1.22.2023, through Contract No. 97/2023 between Wolin National Park (the recipient of the grant) and the Polish Society for Nature Protection ‘Salamandra’.
Institutional Review Board Statement
This study was conducted based on the license to trap and handle protected species by the Ministry of Climate and Environment and the permission to conduct research issued by the Director of the Wolin National Park.
Data Availability Statement
All data are provided in the Supplementary Material.
Acknowledgments
We would like to thank the staff of Wolin National Park for their permission to conduct the study, organization of funding and accommodation, and help with logistics: Wioletta Nawrocka, Alicja Łepek, Konrad Wrzecionkowski, Marek Szwarc, Tomasz Bajor, and Krzysztof Liszka. We express our gratitude also to anonymous referees for comments and advice that improved the quality of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WNP | Wolin National Park |
| DNP | Drawieński National Park |
| LOVNP | Lower Oder Valley National Park |
| SNP | Słowiński National Park |
| WMNP | Warta Mouth National Park |
| NP | National Park |
References
- Hayward, G.F.; Phillipson, J. Community structure and functional role of small mammals in ecosystems. In Ecology of Small Mammals; Springer: Dordrecht, The Netherlands, 1979; pp. 135–211. [Google Scholar]
- Gardezi, T.; da Silva, J. Diversity in relation to body size in mammals: A comparative study. Am. Nat. 1999, 153, 110–123. [Google Scholar] [PubMed]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Stephens, R.B.; Anderson, E.M. Habitat associations and assemblages of small mammals in natural plant communities of Wisconsin. J. Mammal. 2014, 95, 404–420. [Google Scholar] [CrossRef]
- Aulak, W. Small mammal communities of the Białowieża National Park. Acta Theriol. 1970, 15, 465–515. [Google Scholar]
- Niedziałkowska, M.; Kończak, J.; Czarnomska, S.; Jędrzejewska, B. Species diversity and abundance of small mammals in relation to forest productivity in northeast Poland. Ecoscience 2010, 17, 109–119. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I.; Lazăr, A. Responses of small mammals to habitat characteristics in Southern Carpathian forests. Sci. Rep. 2021, 11, 12031. [Google Scholar] [CrossRef]
- Fauteux, D.; Imbeau, L.; Drapeau, P.; Mazerolle, M.J. Small mammal responses to coarse woody debris distribution at different spatial scales in managed and unmanaged boreal forests. For. Ecol. Manag. 2012, 266, 194–205. [Google Scholar] [CrossRef]
- Kamenišťák, J.; Baláž, I.; Tulis, F.; Jakab, I.; Ševčík, M.; Poláčiková, Z.; Klimant, P.; Ambros, M.; Rychlik, L. Changes of small mammal communities with the altitude gradient. Biologia 2020, 75, 713–722. [Google Scholar] [CrossRef]
- Chirichella, R.; Ricci, E.; Armanini, M.; Gobbi, M.; Mustoni, A.; Apollonio, M. Small mammals in a mountain ecosystem: The effect of topographic, micrometeorological, and biological correlates on their community structure. Community Ecol. 2022, 23, 289–299. [Google Scholar] [CrossRef]
- Tomassini, O.; Aghemo, A.; Baldeschi, B.; Bedini, G.; Petroni, G.; Giunchi, D.; Massolo, A. Some like it burnt: Species differences in small mammal assemblage in a Mediterranean basin nearly 3 years after a major fire. Mamm. Res. 2024, 69, 283–302. [Google Scholar] [CrossRef]
- Torre, I.; Ribas, A.; Puig-Gironès, R. Effects of post-fire management on a Mediterranean small mammal community. Fire 2023, 6, 34. [Google Scholar] [CrossRef]
- Jasiulionis, M.; Balčiauskas, L.; Balčiauskienė, L. Size Matters: Diversity and Abundance of Small Mammal Community Varies with the Size of Great Cormorant Colony. Diversity 2023, 15, 220. [Google Scholar] [CrossRef]
- Wikar, Z.; Ciechanowski, M.; Zwolicki, A. The positive response of small terrestrial and semi-aquatic mammals to beaver damming. Sci. Total Environ. 2024, 906, 167568. [Google Scholar] [CrossRef] [PubMed]
- Churchfield, S.; Hollier, J.; Brown, V.K. Community structure and habitat use of small mammals in grasslands of different successional age. J. Zool. 2009, 242, 519–530. [Google Scholar] [CrossRef]
- Gentili, S.; Sigura, M.; Bonesi, L. Decreased small mammals species diversity and increased population abundance along a gradient of agricultural intensification. Hystrix 2014, 25, 39–44. [Google Scholar] [CrossRef]
- Wołk, E.; Wołk, K. Responses of small mammals to the forest management in the Białowieża Primeval Forest. Acta Theriol. 1982, 27, 45–59. [Google Scholar]
- Pearce, J.; Venier, L. Small mammals as bioindicators of sustainable boreal forest management. For. Ecol. Manag. 2005, 208, 153–175. [Google Scholar] [CrossRef]
- Ważna, A.; Cichocki, J.; Bojarski, J.; Gabryś, G. Impact of sheep grazing on small mammals diversity in lower mountain coniferous forest glades. Appl. Ecol. Environ. Res. 2016, 14, 115–127. [Google Scholar] [CrossRef]
- Adler, G.H.; Wilson, M.L. Small mammals on Massachusetts islands: The use of probability functions in clarifying biogeographic relationships. Oecologia 1985, 66, 178–186. [Google Scholar]
- Haq, S.M.A. Multi-benefits of national parks and protected areas: An integrative approach for developing countries. Environ. Socio-Econ. S. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Jamroży, G. Ssaki Polskich Parków Narodowych: Drapieżne, Kopytne, Zajęczaki, Duże Gryzonie; Instytut Bioróżnorodności Leśnej Uniwersytetu Rolniczego.Adv. Tomasz Müller: Kraków, Poland, 2014; 432p. [Google Scholar]
- Ciechanowski, M.; Wikar, Z.; Borzym, K.; Janikowska, E.; Brachman, J.; Jankowska-Jarek, M.; Bidziński, K. Exceptionally Uniform Bat Assemblages across Different Forest Habitats Are Dominated by Single Hyperabundant Generalist Species. Forests 2024, 15, 337. [Google Scholar] [CrossRef]
- Piotrowska, H. Stosunki geobotaniczne wysp Wolina i południowo-wschodniego Uznamu [Geobotanical study of the Wolin and south-east Uznam Isles]. Monogr. Bot. 1966, 22, 1–157. [Google Scholar]
- Piotrowska, H. Wyspa Wolin ze szczególnym uwzględnieniem Wolińskiego Parku Narodowego. In Szata Roślinna Pomorza—Zróżnicowanie, Dynamika, Zagrożenia, Ochrona; Przewodnik Sesji Terenowych 51. Zjazdu PTB 15-19 IX 1998; Herbich, J., Herbichowa, M., Eds.; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 1998; pp. 9–21. [Google Scholar]
- Pucek, Z. Keys to Vertebrates of Poland: Mammals; Polish Scientific Publishers: Warszawa, Poland, 1981; pp. 1–367. [Google Scholar]
- Aulagnier, S.; Mitchell-Jones, A.J.; Zima, J.; Haffner, P.; Moutou, F.; Chevalier, J. Mammals of Europe, North Africa and the Middle East; Bloomsbury Publishing: London, UK, 2009; pp. 1–272. [Google Scholar]
- Michaux, J.R.; Chevret, P.; Filippucci, M.G.; Macholan, M. Phylogeny of the genus Apodemus with a special emphasis on the subgenus Sylvaemus using the nuclear IRBP gene and two mitochondrial markers: Cytochrome b and 12S rRNA. Mol. Phyl. Evol. 2002, 23, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Hammer, R.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Piłacińska, B.; Ziomek, J.; Bajaczyk, R. Drobne ssaki Drawieńskiego Parku Narodowego [Small mammals of the Drawieński National Park]. Bad. Fizjogr. Pol. Zach. 1999, 46, 95–106. [Google Scholar]
- Wojtaszyn, G.; Rutkowski, T.; Lesiński, G.; Stephan, W.; Salamandra, P.T.O.P. Soricomorphs and rodents of the Ujście Warty National Park and the surrounding area. Chrońmy Przyr. Ojczystą 2015, 71, 179–191. [Google Scholar]
- Rychlik, L.S.; Eichert, U.M.; Kowalski, K. Diversity of small mammal assemblages in natural forests and other habitats of the Słowiński National Park, northern Poland—Preliminary results. Natl. Jahrb. Unteres Odertal 2020, III, 66–71. [Google Scholar]
- Decher, J.; Bakarr, I.; Hoffmann, A.; Jentke, T.; Klappert, A.; Kowalski, G.; Kuzdrowska, K.; Malinowska, B.; Rychlik, L.S. Aktualisierung unserer Kenntnisse über die Kleinsäugergemeinschaften im Nationalpark Unteres Odertal. Natl.-Jahrb. Unteres Odertal 2021, 18, 145–150. [Google Scholar]
- Hoffmann, A.; Jankowiak, Ł.; Modelska, Z.; Piórkowska, K.; Decher, J.; Jentke, T.; Klappert, A.; Kuzdrowska, K.; Malinowska, B.; Sęk, O.W.; et al. Diversität von Kleinsäugern im nördlichen Teil des Nationalparks Unteres Odertal. Natl.-Jarbuch Unteres Odertal 2022, 19, 37–45. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar]
- ClustVis. Available online: https://biit.cs.ut.ee/clustvis/ (accessed on 1 July 2024).
- Herold, W. Zur Kleinsäugerfauna der Insel Usedom und Wolin. Dohrniana 1934, 13, 176–196. [Google Scholar]
- Pucek, Z.; Raczyński, J. (Eds.) Atlas of Polish Mammals; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1983; pp. 1–188 (text), pp. 1–175 (maps). [Google Scholar]
- Herold, W. Beiträge zur Säugetierfauna Usedom-Wollins. I Abch. Ber. Pommerschen Nat-forsch. Ges. Stettin. 1921, 2, 75–79. [Google Scholar]
- Open Forest Data. Available online: https://dataverse.openforestdata.pl/dataverse/zoo (accessed on 5 May 2024).
- Ciechanowski, M.; Wikar, Z.; Kowalewska, T.; Wojtkiewicz, M.; Brachman, J.; Sarnowski, B.; Borzym, K.; Rydzyńska, A. Department of Vertebrate Ecology and Zoology, University of Gdańsk, Gdańsk, Poland. 2023; unpublished data. [Google Scholar]
- Gaffrey, G. Die Rezenten Wildlebenden Säugetiere Pommerns. Ph.D. Thesis, Universität Greifswald, Greifswald, Germany, 1944. [Google Scholar]
- Skuratowicz, W. Materiały do fauny pcheł (Aphaniptera) Polski. Acta Parasitol. Pol. 1954, 2, 65–96. [Google Scholar]
- Herold, W. Zur Verbreitung der Schlagmäuse in Pommern. I Abch. Ber. Pommerschen Nat-Forsch. Ges. Stettin 1922, 3, 43–50. [Google Scholar]
- Herold, W. Zum Vorkommen von Glis glis (L.). Dohrniana 1939, 18. [Google Scholar]
- Goc, M. Stanowisko popielicy szarej Glis glis w Słowińskim Parku Narodowym. Przegląd Przyr. 2019, 30, 114–115. [Google Scholar]
- Rathke, D.; Bröring, U. Colonization of post-mining landscapes by shrews and rodents (Mammalia: Rodentia, Soricomorpha). Ecol. Eng. 2005, 24, 149–156. [Google Scholar] [CrossRef]
- Nicolas, V.; Colyn, M. Relative efficiency of three types of small mammal traps in an African rainforest. Belg. J. Zool. 2006, 136, 107. [Google Scholar]
- Pucek, Z. Trap response and estimation of numbers of shrews in removal catches. Acta Theriol. 1969, 14, 403–426. [Google Scholar] [CrossRef]
- O’Brien, C.; McShea, W.J.; Guimondou, S.; Barriere, P.; Carleton, M.D. Terrestrial small mammals (Soricidae and Muridae) from the Gamba Complex in Gabon: Species composition and comparison of sampling techniques. Bull. Biol. Soc. Wash. 2006, 12, 353–363. [Google Scholar]
- Bovendorp, R.S.; Mccleery, R.A.; Galetti, M. Optimising sampling methods for small mammal communities in Neotropical rainforests. Mamm. Rev. 2017, 47, 148–158. [Google Scholar] [CrossRef]
- Pankakoski, E. The cone trap—A useful tool for index trapping of small mammals. Ann. Zool. Fenn. 1979, 16, 144–150. [Google Scholar]
- Pelikan, J.; Zejda, J.; Holisova, V. Efficiency of different traps in catching small mammals. Folia Zool. 1977, 26, 1–13. [Google Scholar]
- Darinot, F. Dispersal and Genetic Structure in a Harvest Mouse (Micromys minutus Pallas, 1771) Population, Subject to Seasonal Flooding. PhD Thesis, PSL University, Paris, 2019. [Google Scholar]
- Occhiuto, F.; Mohallal, E.; Gilfillan, G.D.; Lowe, A.; Reader, T. Seasonal patterns in habitat use by the harvest mouse (Micromys minutus) and other small mammals. Mammalia 2021, 85, 325–335. [Google Scholar] [CrossRef]
- Ziomek, J. Drobne ssaki (Micromammalia) Roztocza. Część I. Micromammalia wybranych biotopów Roztocza Środkowego. Fragm. Faunist. 1998, 41, 93–123. [Google Scholar] [CrossRef]
- Juskaitis, R. Peculiarities of habitats of the common dormouse, Muscardinus avellanarius, within its distributional range and in Lithuania: A review. Folia Zool. 2007, 56, 337. [Google Scholar]
- Ciechanowski, M.; Cichocki, J.; Ważna, A.; Piłacińska, B. Small-mammal assemblages inhabiting Sphagnum peat bogs in various regions of Poland. Biol. Lett. 2012, 49, 115–133. [Google Scholar] [CrossRef][Green Version]
- Raczyński, J.; Fedyk, S.; Gębczyńska, Z.; Pucek, M. Drobne ssaki środkowego i dolnego basenu Biebrzy. Zesz. Probl. Post. Nauk Roln. 1983, 255, 297–328. [Google Scholar]
- Gębczyńska, Z.; Raczyński, J. Fauna i ekologia drobnych ssaków Narwiańskiego Parku Narodowego. Park. Nar. Rez. Przyr. 1997, 16, 37–61. [Google Scholar]
- Willott, S.J. Species accumulation curves and the measure of sampling effort. J. Appl. Ecol. 2001, 38, 484–486. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Juškaitis, R. Diversity of small mammal community in Lithuania (1. A review). Acta Zool. Litu. 1997, 7, 29–45. [Google Scholar] [CrossRef]
- da Luz Mathias, M.; Hart, E.B.; da Graca Ramalhinho, M.; Jaarola, M. Microtus agrestis (Rodentia: Cricetidae). Mamm. Species 2017, 49, 23–39. [Google Scholar] [CrossRef]
- Mitchell-Jones, A.J.; Mitchell, J.; Amori, G.; Bogdanowicz, W.; Spitzenberger, F.; Krystufek, B.; Reijnders, P.J.H.; Spitzenberger, E.; Stubbe, M.; Thissen, J.B.M.; et al. The Atlas of European Mammals; T & AD Poyser: London, UK, 2000; Volume 3. [Google Scholar] [CrossRef]
- Igea, J.; Aymerich, P.; Bannikova, A.A.; Gosálbez, J.; Castresana, J. Multilocus species trees and species delimitation in a temporal context: Application to the water shrews of the genus Neomys. BMC Evol. Biol. 2015, 15, 209. [Google Scholar] [CrossRef]
- Cichocki, J.; Kościelska, A.; Piłacińska, B.; Kowalski, M.; Ważna, A.; Dobosz, R.; Nowakowski, K.; Lesiński, G.; Gabrys, G. Occurrence of lesser white-toothed shrew Crocidura suaveolens (Pallas, 1811) in Poland. Zesz. Naukowe. Acta Biol. Uniw. Szczeciński 2014, 21, 149–168. [Google Scholar]
- Haberl, W.; Kryštufek, B. Spatial distribution and population density of the harvest mouse Micromys minutus in a habitat mosaic at Lake Neusiedl, Austria. Mammalia 2003, 67, 355–366. [Google Scholar] [CrossRef]
- Surmacki, A.; Gołdyn, B.; Tryjanowski, P. Location and habitat characteristics of the breeding nests of the harvest mouse (Micromys minutus) in the reed-beds of an intensively used farmland. Mammalia 2005, 69, 5–9. [Google Scholar] [CrossRef]
- Brzeziński, M.; Jedlikowski, J.; Komar, E. Space use, habitat selection and daily activity of water voles Arvicola amphibius co-occurring with the invasive American mink Neovison vison. Folia Zool. 2019, 68, 21–28. [Google Scholar] [CrossRef]
- van der Putten, T.A.; Verhees, J.J.; Koma, Z.; van Hoof, P.H.; Heijkers, D.; de Boer, W.F.; Esser, H.J.; Hoogerwerf, G.; Lemmers, P. Insights into the fine-scale habitat use of Eurasian Water Shrew (Neomys fodiens) using radio tracking and LiDAR. J. Mammal. 2025, gyae146. [Google Scholar] [CrossRef]
- Rychlik, L. Habitat preferences of four sympatric species of shrews. Acta Theriol. 2000, 45 (Suppl. S1), 173–190. [Google Scholar]
- Łopucki, R.; Mróz, I.; Klich, D.; Kitowski, I. Small mammals of xerothermic grasslands of south-eastern Poland. Ann. Wars. Univ. Life Sci.—SGGW. Anim. Sci. 2018, 57, 257–267. [Google Scholar] [CrossRef]
- Tattersall, F.H.; Macdonald, D.W.; Hart, B.J.; Manley, W.J.; Feber, R.E. Habitat use by wood mice (Apodemus sylvaticus) in a changeable arable landscape. J. Zool. 2001, 255, 487–494. [Google Scholar] [CrossRef]
- Suchomel, J.; Purchart, L.; Čepelka, L. Structure and diversity of small-mammal communities of lowland forests in the rural central European landscape. Eur. J. For. Res. 2012, 131, 1933–1941. [Google Scholar] [CrossRef]
- Loeb, S.C. Responses of small mammals to coarse woody debris in a southeastern pine forest. J. Mammal. 1999, 80, 460–471. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S. Maintenance of small mammals using post-harvest woody debris structures on clearcuts: Linear configuration of piles is comparable to windrows. Mamm. Res. 2018, 63, 11–19. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Jędrzejewska, B. Rodent cycles in relation to biomass and productivity of ground vegetation and predation in the Palearctic. Acta Theriol. 1996, 41, 1–34. [Google Scholar] [CrossRef]
- Andreassen, H.P.; Sundell, J.; Ecke, F.; Halle, S.; Haapakoski, M.; Henttonen, H.; Huitu, O.; Jacob, J.; Johnsen, K.; Koskela, E.; et al. Population cycles and outbreaks of small rodents: Ten essential questions we still need to solve. Oecologia 2021, 195, 601–622. [Google Scholar] [CrossRef] [PubMed]
- Trout, R.C. A review of studies on populations of wild harvest mice (Micromys minutus (Pallas). Mamm. Rev. 1978, 8, 143–158. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Long-Term Stability of Harvest Mouse Population. Diversity 2023, 15, 1102. [Google Scholar] [CrossRef]
- Ciechanowski, M.; Fałtynowicz, W.; Zieliński, S. The nature of the planned reserve “Dolina Mirachowskiej Strugi” in the Kaszubskie Lakeland (northern Poland). Acta Bot. Cassubica 2004, 4, 5–137. [Google Scholar]
- Balčiauskas, L.; Skipitytė, R.; Balčiauskienė, L.; Jasiulionis, M. Resource partitioning confirmed by isotopic signatures allows small mammals to share seasonally flooded meadows. Ecol. Evol. 2019, 9, 5479–5489. [Google Scholar] [CrossRef]
- Dokulilová, M.; Krojerová-Prokešová, J.; Heroldová, M.; Čepelka, L.; Suchomel, J. Population dynamics of the common shrew (Sorex araneus) in Central European forest clearings. Eur. J. Wildl. Res. 2023, 69, 54. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Habitat and Body Condition of Small Mammals in a Country at Mid-Latitude. Land 2024, 13, 1214. [Google Scholar] [CrossRef]
- Pucek, Z.; Jędrzejewski, W.; Jędrzejewska, B.; Pucek, M. Rodent population dynamics in a primeval deciduous forest (Białowieża National Park) in relation to weather, seed crop, and predation. Acta Theriol. 1993, 38, 199–232. [Google Scholar]
- Panov, V.V.; Karpenko, S.V. The population dynamics of the water shrew Neomys fodiens (Mammalia, Soricidae) and its helminthes fauna in the northern Baraba. Parazitologiia 2004, 38, 448–456. [Google Scholar]
- Strzelczyk, J.; Łabuz, T. Zmiany linii brzegowej oraz powierzchni wyspy Wolin w holocenie i ich wpływ na osadnictwo od mezolitu do czasów współczesnych (Coastline and the surface of the Wolin Island changes in the Holocene and their impact on settlement from the Mesolithic to modern times). In Najnowsze Doniesienia z Zakresu Ochrony Środowiska i Nauk Pokrewnych; Danielewska, A., Maciag, M., Eds.; Wydawnictwo Naukowe TYGIEL: Lublin, Poland, 2020. [Google Scholar]
- Bohdal, T.; Navrátil, J.; Sedláček, F. Small terrestrial mammals living along streams acting as natural landscape barriers. Ekológia 2016, 35, 191–204. [Google Scholar] [CrossRef]
- Schenk, F. Comparison of spatial learning in woodmice (Apodemus sylvaticus) and hooded rats (Rattus norvegicus). J. Comp. Psychol. 1987, 101, 150. [Google Scholar] [CrossRef]
- Stawski, C.; Koteja, P.; Sadowska, E.T.; Jefimow, M.; Wojciechowski, M.S. Selection for high activity-related aerobic metabolism does not alter the capacity of non-shivering thermogenesis in bank voles. J. Comp. Physiol. A 2015, 180, 51–56. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2010; pp. 509–619. [Google Scholar]
- Lomolino, M.V. Mammalian island biogeography: Effects of area, isolation and vagility. Oecologia 1984, 61, 376–382. [Google Scholar]
- Barreto, E.; Rangel, T.F.; Pellissier, L.; Graham, C.H. Area, isolation and climate explain the diversity of mammals on islands worldwide. Proc. R. Soc. B 2021, 288, 20211879. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Y.; Yu, M.; Xu, G.; Ding, P. Nestedness for different reasons: The distributions of birds, lizards and small mammals on islands of an inundated lake. Div. Distrib. 2010, 16, 862–873. [Google Scholar] [CrossRef]
- Mallinger, E.C.; Khadka, B.; Farmer, M.J.; Morrison, M.; Van Stappen, J.; Van Deelen, T.R.; Olson, E.R. Longitudinal trends of the small mammal community of the Apostle Islands archipelago. Comm. Ecol. 2021, 22, 55–67. [Google Scholar] [CrossRef]
- Pichler, T.R.; Mallinger, E.C.; Farmer, M.J.; Morrison, M.J.; Khadka, B.; Matzinger, P.J.; Kirschbaum, A.; Goodwin, K.R.; Route, W.T.; Van Stappen, J.; et al. Comparative biogeography of volant and nonvolant mammals in a temperate island archipelago. Ecosphere 2022, 13, e3911. [Google Scholar] [CrossRef]
- Peltonen, A.; Hanski, I. Patterns of island occupancy explained by colonization and extinction rates in shrews. Ecology 1991, 72, 1698–1708. [Google Scholar] [CrossRef]
- Christiansen, T.S. Island Biogeography of Small Mammals in Denmark: Effects of Area, Isolation and Habitat Diversity. Master’s Thesis, University of Aarhus, Aarhus, Denmark, 2005. [Google Scholar]
- Kleinekuhle, J.; Bach, L.; Donning, A.; Berns, S. Die freilebenden Säugetiere (Mammalia) der Ostfriesischen Inseln unter besonderer Berücksichtigung der Insel Norderney, der Raubsäuger (Carnivora) und der Fledermäuse (Chiroptera). Abh. Naturwiss Ver Brem. 2023, 48, 1–19. [Google Scholar]
- Wijngaarden, A. The terrestrial mammal-fauna of the Dutch Wadden-Islands. Z. Saugetiere 1964, 29, 359–368. [Google Scholar]
- Girjatowicz, J.P.; Świątek, M. Relationship between air temperature change and southern Baltic coastal lagoons ice conditions. Atmosphere 2021, 12, 931. [Google Scholar] [CrossRef]
- Heisler, L.M.; Somers, C.M.; Poulin, R.G. Owl pellets: A more effective alternative to conventional trapping for broad-scale studies of small mammal communities. Methods Ecol. Evol. 2016, 7, 96–103. [Google Scholar] [CrossRef]
- Schröpfer, R. The Structure of European Small Mammal Communities. Zool. Jb. Syst. 1990, 117, 355–367. [Google Scholar]
- Suchomel, J.; Purchart, L.; Čepelka, L.; Heroldová, M. Structure and diversity of small mammal communities of mountain forests in Western Carpathians. Eur. J. For. Res. 2014, 133, 481–490. [Google Scholar] [CrossRef]
- Zwolak, R.; Witczuk, J.; Bogdziewicz, M.; Rychlik, L.; Pagacz, S. Simultaneous population fluctuations of rodents in montane forests and alpine meadows suggest indirect effects of tree masting. J. Mammal. 2018, 99, 586–595. [Google Scholar] [CrossRef]
- Romanowski, J.; Dudek-Godeau, D.; Lesiński, G. The Diversity of Small Mammals along a Large River Valley Revealed from Pellets of Tawny Owl Strix aluco. Biology 2023, 12, 1118. [Google Scholar] [CrossRef]
- Cornulier, T.; Yoccoz, N.G.; Bretagnolle, V.; Brommer, J.E.; Butet, A.; Ecke, F.; Framstad, E.; Henttonen, H.; Hörnfeldt, B.; Huitu, O.; et al. Europe-wide dampening of population cycles in keystone herbivores. Science 2013, 340, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Juškaitis, R.; Ulevičius, A. Kuršių Nerijos nacionalinio parko smulkieji žinduoliai. Theriol. Litu. 2002, 2, 34–46. [Google Scholar]
- Grodziński, W. The succession of small mammal communities on an overgrown clearing and landslip in the Western Carpathians. Bull. Acad. Pol. Sc. Cl. II 1958, 6, 10. [Google Scholar]
- Bogdziewicz, M.; Zwolak, R. Responses of small mammals to clear-cutting in temperate and boreal forests of Europe: A meta-analysis and review. Eur. J. For. Res. 2014, 133, 1–11. [Google Scholar] [CrossRef]
- Mažeikytė, R. Small mammals in the mosaic landscape of eastern Lithuania: Species composition, distribution and abundance. Acta Zool. Litu. 2002, 12, 381–391. [Google Scholar] [CrossRef]
- Šinkūnas, R.; Balčiauskas, L. Small mammal communities in the fragmented landscape in Lithuania. Acta Zool. Litu. 2006, 16, 130–136. [Google Scholar] [CrossRef]
- Greenberg, R.; Maldonado, J.E.; Droege, S.A.M.; McDonald, M.V. Tidal marshes: A global perspective on the evolution and conservation of their terrestrial vertebrates. BioScience 2006, 56, 675–685. [Google Scholar] [CrossRef]
- Verkuil, Y.I.; van Guldener, W.E.; Lagendijk, D.G.; Smit, C. Molecular identification of temperate Cricetidae and Muridae rodent species using fecal samples collected in a natural habitat. Mamm. Res. 2018, 63, 379–385. [Google Scholar] [CrossRef]
- Christensen, J.T.; Jensen, T.S. Småpattedyrfaunaen på Anholt og Sprogø. Flora Og Fauna 2024, 129, 3–7. [Google Scholar]
- Ambros, M. Drobné cicavce (Mammalia: Soricomorpha, Rodentia) území európskeho významu: Slaniská a slané lúky. Naturae Tutela 2018, 22, 203–214. [Google Scholar]
- Rico, A.; Kindlmann, P.; Sedlacek, F. Barrier effects of roads on movements of small mammals. Folia Zool. 2007, 56, 1. [Google Scholar]
- Andrzejewski, R.; Babińska-Werka, J.; Gliwicz, J.; Goszczyński, J. Synurbization processes in population of Apodemus agrarius. I. Characteristics of populations in an urbanization gradient. Acta Theriol. 1978, 23, 341–358. [Google Scholar]
- Lesiński, G.; Gryz, J.; Krauze-Gryz, D.; Stolarz, P. Population increase and synurbization of the yellow-necked mouse Apodemus flavicollis in some wooded areas of Warsaw agglomeration, Poland, in the years 1983–2018. Urban Ecosyst. 2021, 24, 481–489. [Google Scholar] [CrossRef]
- Suárez-Tangil, B.D.; Rodríguez, A. Environmental filtering drives the assembly of mammal communities in a heterogeneous Mediterranean region. Ecol. Appl. 2023, 33, e2801. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).