Effect of Drought and Salinity on Water Relations and Photosynthetic Responses of Tamarix elongata and Haloxylon ammodendron in Wutonggou Desert Tourist Area, Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Greenhouse Conditions
2.2. Measurements of Soil Water Content, pH and Electrical Conductivity
2.3. Water Potentials
2.4. Sodium and Potassium Concentration
2.5. Leaf Gas Exchange
2.6. Chlorophyll Contents and Chlorophyll Fluorescence
2.7. Statistical Analysis
3. Results
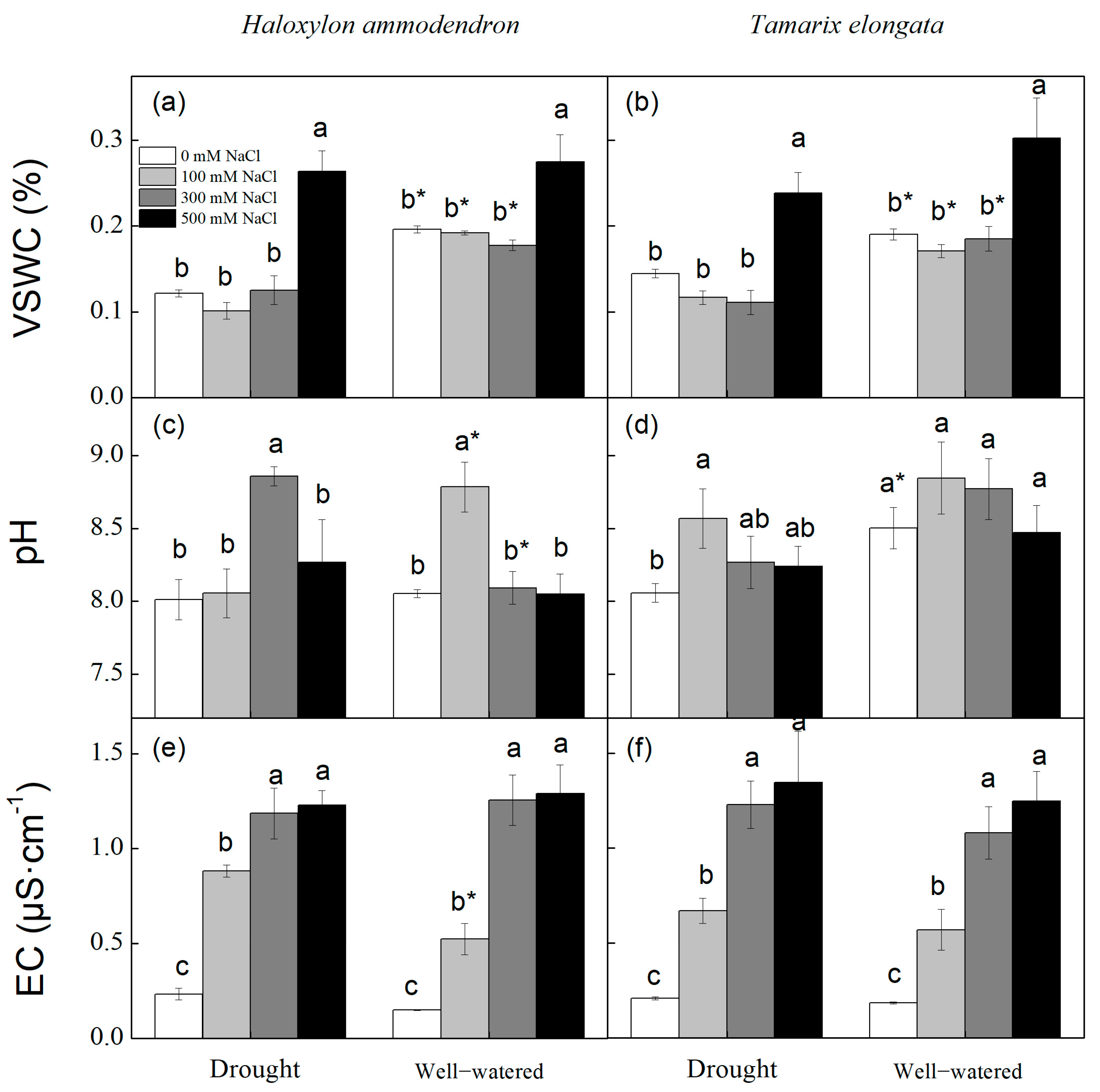
3.1. Soil Water Content, pH and Electrical Conductivity
3.2. Plant Height
3.3. Water Potentials and Leaf Ion Contents
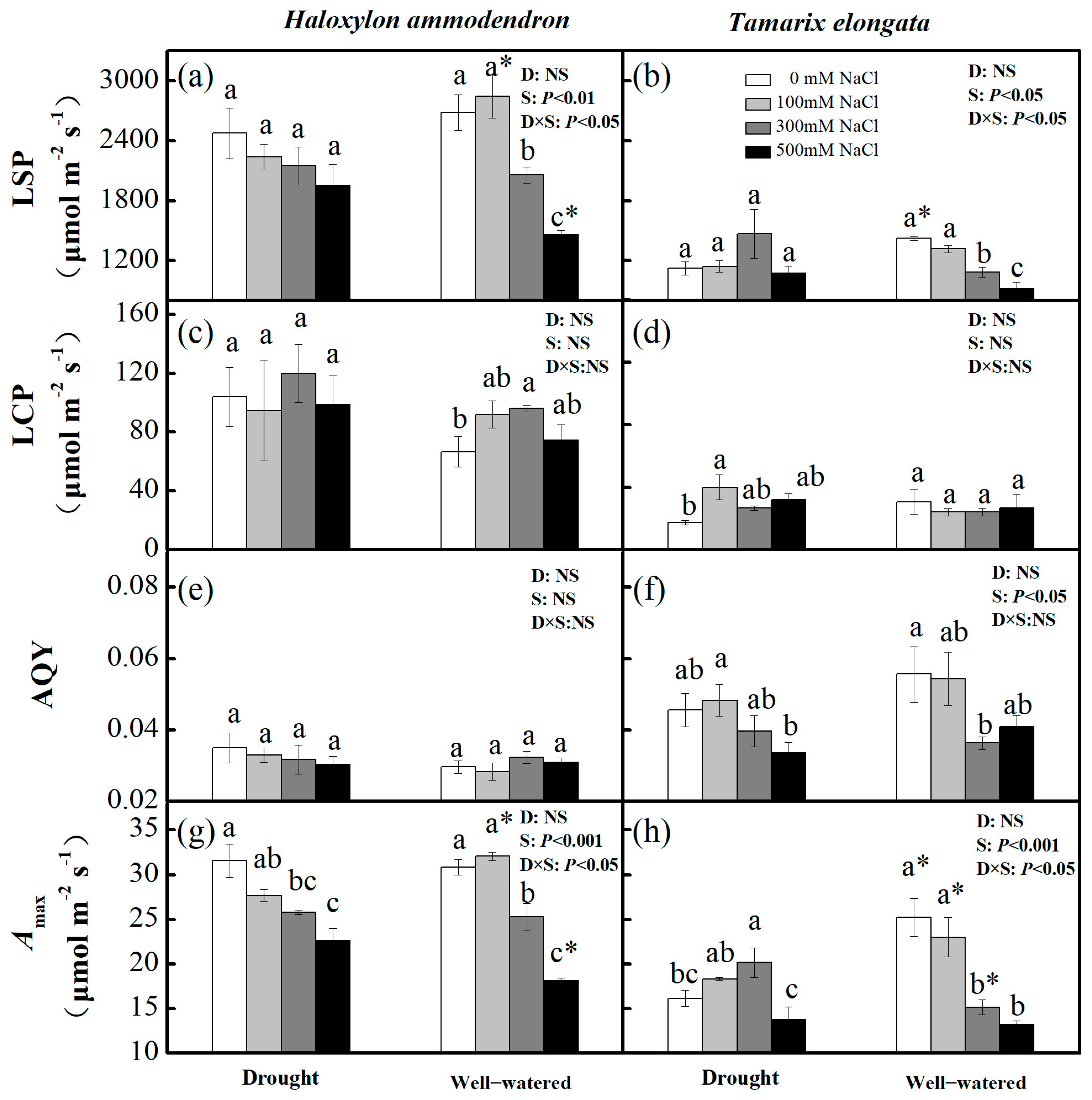
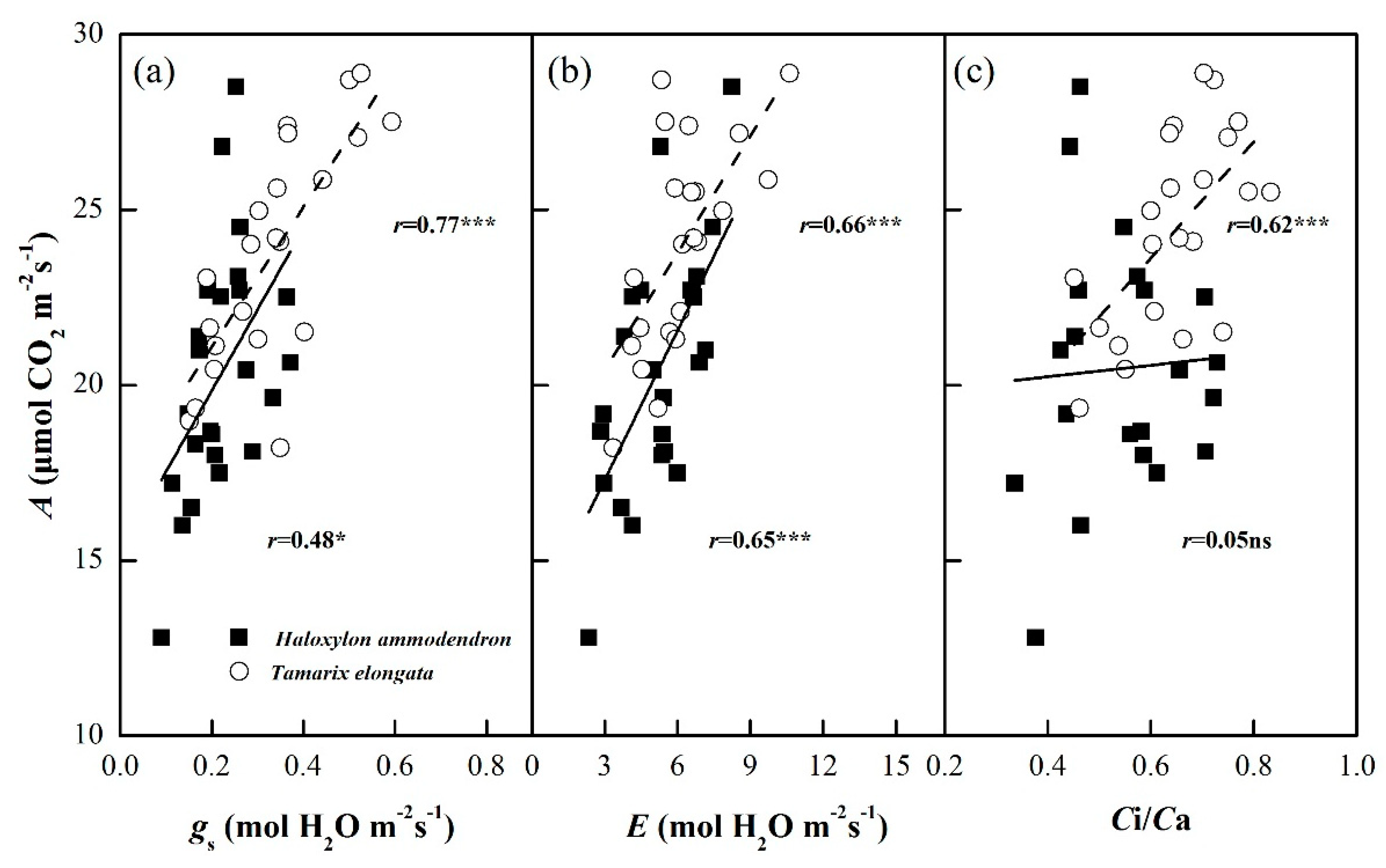
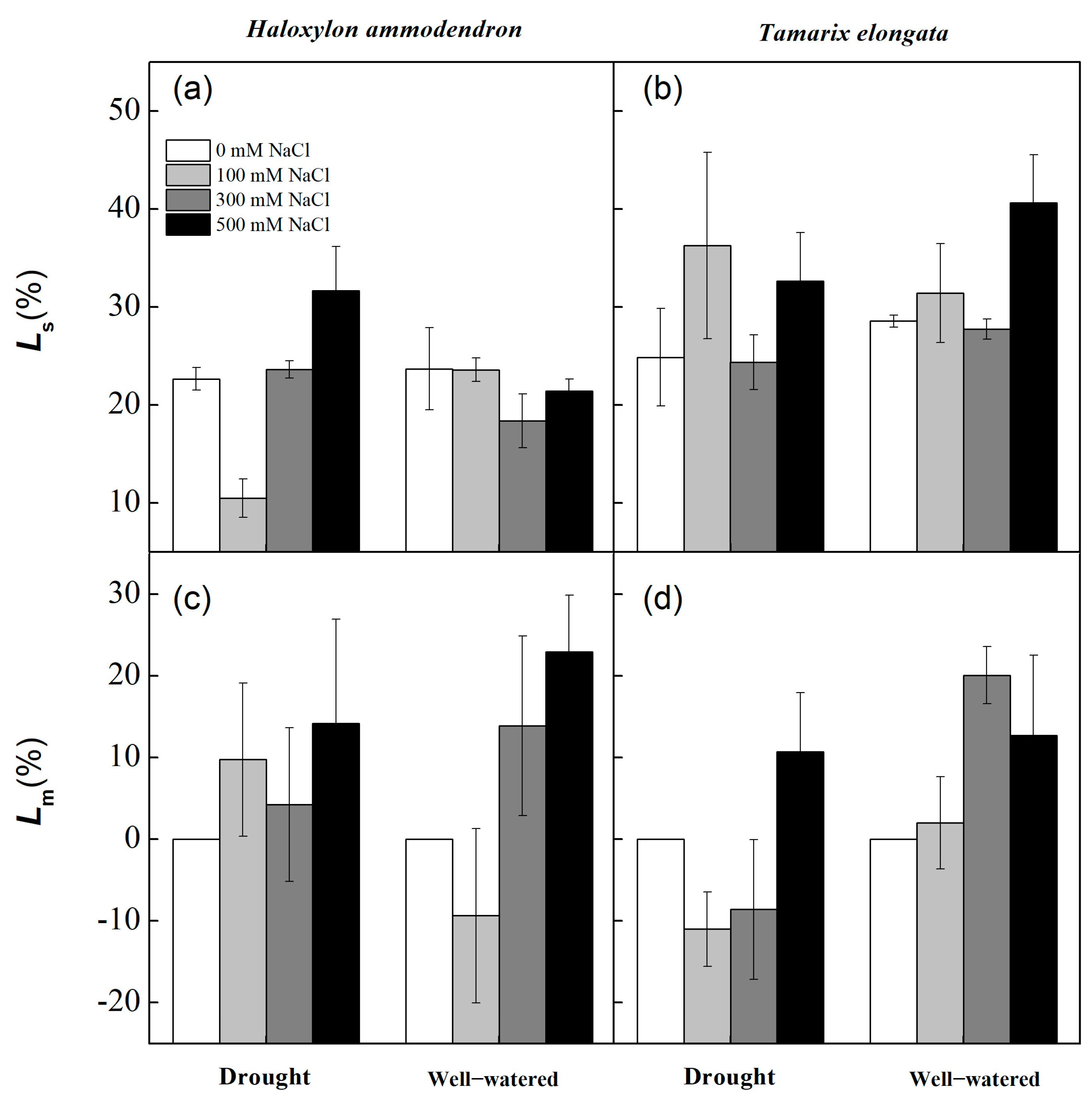
3.4. Leaf Photosynthetic Traits
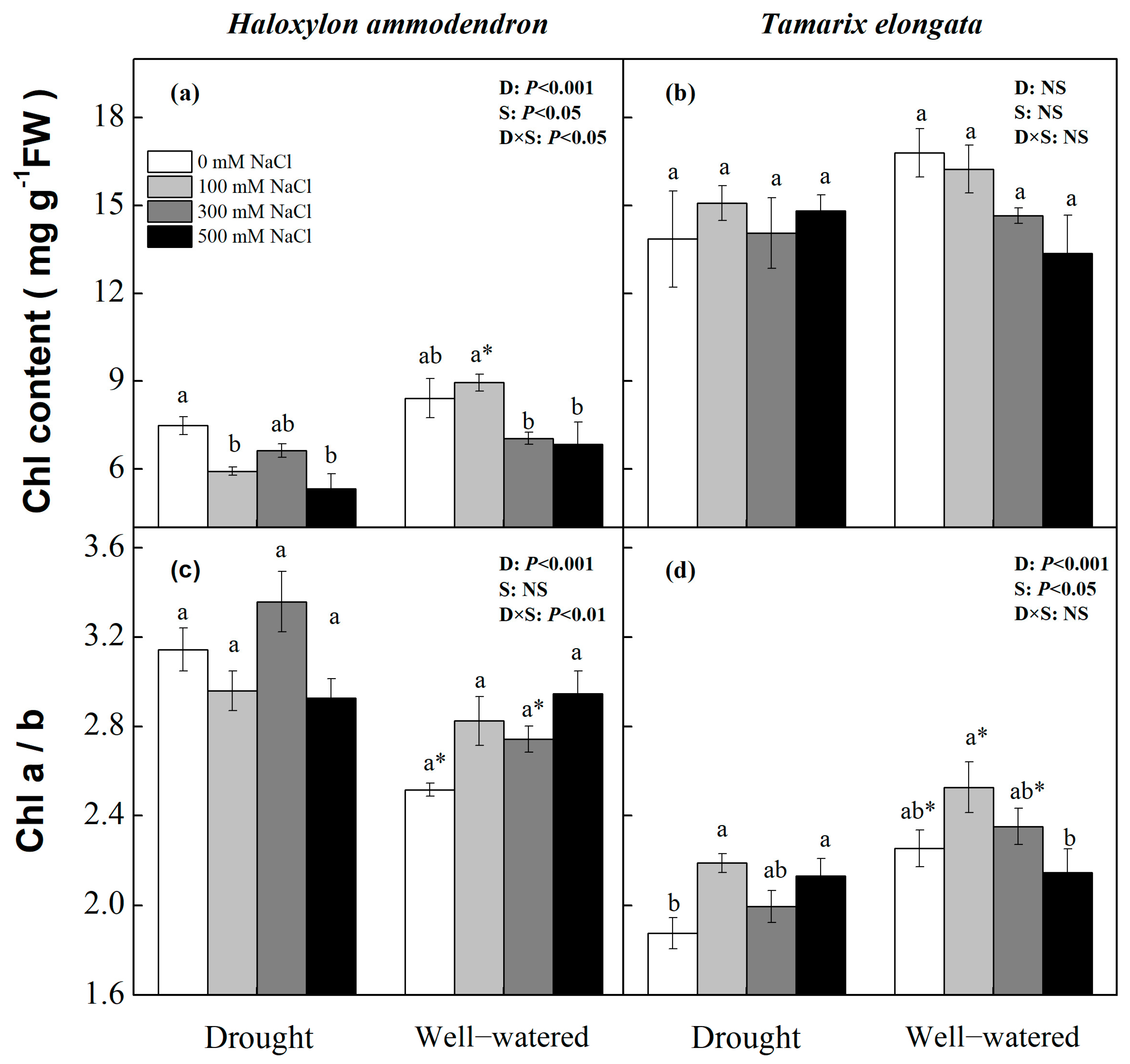
3.5. Chlorophyll Contents
4. Discussion
4.1. Plant Growth Under Salinity and Drought Stress
4.2. Water Relations Under Salinity and Drought Stress
4.3. Photosynthetic Traits Under Salinity and Drought Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ls | Stomatal mesophyll limitation |
| Lm | Mesophyll limitation |
| VSWC | Volumetric soil water content |
| EC | Electrical conductivity |
| Ψpd | Predawn water potentials |
| Ψmd | Midday water potentials |
| LSP | Light saturation point |
| LCP | Light compensation point |
| AQY | Apparent quantum yield |
| Amax | Maximum net assimilation rate |
| A | Net assimilation rate |
| gs | Stomatal conductance |
| E | Transpiration rate |
| Ci/Ca | The ratio of intercellular CO2 to atmospheric CO2 |
| Chl | Chlorophyll content |
References
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- FAO. FAO Launches First Major Global Assessment of Salt-Affected Soils in 50 Years; Rigillo, N., Ed.; FAO: Roma, Italy, 2024. [Google Scholar]
- Zhang, H.; Wang, Y.; Liu, L.; Zhou, J.; Wan, Q.; Chen, J.; Cao, Y.; Zhang, L.; Feng, F.; Ning, Q.J.A. Bibliometric Analysis of Contemporary Research on the Amelioration of Saline Soils. Agronomy 2024, 14, 2935. [Google Scholar] [CrossRef]
- Ma, P.; Li, J.; Sun, G.; Zhu, J. Comparative transcriptome analysis reveals the adaptive mechanisms of halophyte Suaeda dendroides encountering high saline environment. Front. Plant Sci. 2024, 15, 1283912. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Glenn, E.P.; Nelson, S.G.; Ambrose, B.; Martinez, R.; Soliz, D.; Pabendinskas, V.; Hultine, K. Comparison of salinity tolerance of three Atriplex spp. in well-watered and drying soils. Environ. Exp. Bot. 2012, 83, 62–72. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Chahal, S.; Verma, K.; Chauhan, K.; Kumar, R.; Mann, A. Morpho-physiological studies of sandalwood-host interaction under individual and interactive water and salt stress. Biol. Futur. 2024, 75, 497–506. [Google Scholar] [CrossRef]
- Lu, Y.W.; Miao, X.L.; Song, Q.Y.; Peng, S.M.; Duan, B.L. Morphological and ecophysiological plasticity in dioecious plant Populus tomentosa under drought and alkaline stresses. Photosynthetica 2018, 56, 1353–1364. [Google Scholar] [CrossRef]
- He, F.-L.; Bao, A.-K.; Wang, S.-M.; Jin, H.-X. NaCl stimulates growth and alleviates drought stress in the salt-secreting xerophyte Reaumuria soongorica. Environ. Exp. Bot. 2019, 162, 433–443. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Yin, B.; Wang, Z.; Li, R.; Li, S. The Influence of Sodium Salt on Growth, Photosynthesis, Na+/K+ Homeostasis and Osmotic Adjustment of Atriplex canescens under Drought Stress. Agronomy 2023, 13, 2434. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Hasan, M.T.; Rahman, M.A.; Nuruzzaman, M.; Rahman, A.S.; Hasanuzzaman, M.; Haque, M.R.; Hossain, M.A.; Latef, A.A.H.A.; Murata, Y.; et al. Plant response to combined salinity and waterlogging stress: Current research progress and future prospects. Plant Stress 2023, 7, 100137. [Google Scholar] [CrossRef]
- Armas, C.; Padilla, F.M.; Pugnaire, F.I.; Jackson, R.B. Hydraulic lift and tolerance to salinity of semiarid species: Consequences for species interactions. Oecologia 2010, 162, 11–21. [Google Scholar]
- Leisner, C.P.; Cousins, A.B.; Offermann, S.; Okita, T.W.; Edwards, G.E. The effects of salinity on photosynthesis and growth of the single-cell C4 species Bienertia sinuspersici (Chenopodiaceae). Photosynth. Res. 2010, 106, 201–214. [Google Scholar] [PubMed]
- Mousavi, S.S.; Karami, A.; Maggi, F. Photosynthesis and chlorophyll fluorescence of Iranian licorice (Glycyrrhiza glabra L.) accessions under salinity stress. Front. Plant Sci. 2022, 13, 984944. [Google Scholar]
- Centritto, M.; Loreto, F.; Chartzoulakis, K. The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ. 2003, 26, 585–594. [Google Scholar]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar]
- Bhattacharya, A. Effects of soil water deficit on carbon metabolism of plants: A review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 99–192. [Google Scholar]
- Cousins, A.B.; Mullendore, D.L.; Sonawane, B.V. Recent developments in mesophyll conductance in C3, C4, and crassulacean acid metabolism plants. Plant J. 2020, 101, 816–830. [Google Scholar] [CrossRef]
- Dorta-Santos, M.A.; Barriola, I.; Wassner, D.F.; Ploschuk, E.L. Photosynthesis, fluorescence and mesophyll conductance responses to increasing salinity levels in Jatropha curcas at early vegetative stages. J. Agron. Crop Sci. 2020, 206, 52–63. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Galmés, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar]
- Zait, Y.; Shtein, I.; Schwartz, A. Long-term acclimation to drought, salinity and temperature in the thermophilic tree Ziziphus spina-christi: Revealing different tradeoffs between mesophyll and stomatal conductance. Tree Physiol. 2019, 39, 701–716. [Google Scholar] [CrossRef]
- Hussain, T.; Koyro, H.-W.; Zhang, W.; Liu, X.; Gul, B.; Liu, X. Low salinity improves photosynthetic performance in Panicum antidotale under drought stress. Front. Plant Sci. 2020, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar]
- Bromham, L.; Bennett, T.H. Salt tolerance evolves more frequently in C4 grass lineages. J. Evol. Biol. 2014, 27, 653–659. [Google Scholar] [PubMed]
- Rudov, A.; Mashkour, M.; Djamali, M.; Akhani, H. A review of C4 plants in southwest Asia: An ecological, geographical and taxonomical analysis of a region with high diversity of C4 eudicots. Front. Plant Sci. 2020, 11, 546518. [Google Scholar] [CrossRef] [PubMed]
- Still, C.J.; Berry, J.A.; Collatz, G.J.; Defries, R.S. Global distribution of C3 and C4 vegetation: Carbon cycle implications. Glob. Biogeochem. Cycles 2003, 17, 6-1–6-14. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, L. Study on Organs Morphology and its Taxonomic Significance of Tamarix L. Arid Zone Res. 2000, 17, 40–45. [Google Scholar]
- Zhang, K.; Tian, C.Y.; Li, C.J.; Huang, P.Z.; Ma, Y.J.; Yin, C.H.; Zhao, Z.Y. Brief introduction to the halophytes in Fukang Halophytes Botanic Garden, Xinjiang. Arid Zone Res. 2010, 27, 474–479. [Google Scholar]
- Tiemuerbieke, B.; Min, X.J.; Zang, Y.X.; Xing, P.; Ma, J.Y.; Sun, W. Water use patterns of co-occurring C3 and C4 shrubs in the Gurbantonggut desert in northwestern China. Sci. Total Environ. 2018, 634, 341–354. [Google Scholar]
- Xu, H.; Li, Y. Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant Soil 2006, 285, 5–17. [Google Scholar]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Biol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Tezara, W.; Martinez, D.; Rengifo, E.; Herrera, A. Photosynthetic responses of the tropical spiny shrub Lycium nodosum (Solanaceae) to drought, soil salinity and saline spray. Ann. Bot. 2003, 92, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.F.; Fan, H.; Zhou, S.; Song, J. Study on the salt and drought tolerance of Suaeda salsa and Kalanchoe claigremontiana under iso-osmotic salt and water stress. Plant Sci. 2003, 165, 837–844. [Google Scholar]
- Sucre, B.; Suárez, N. Effect of salinity and PEG-induced water stress on water status, gas exchange, solute accumulation, and leaf growth in Ipomoea pes-caprae. Environ. Exp. Bot. 2011, 70, 192–203. [Google Scholar]
- Arndt, S.K.; Arampatsis, C.; Foetzki, A.; Li, X.Y.; Zeng, F.J.; Zhang, X.M. Contrasting patterns of leaf solute accumulation and salt adaptation in four phreatophytic desert plants in a hyperarid desert with saline groundwater. J. Arid Environ. 2004, 59, 259–270. [Google Scholar]
- Ueda, A.; Kanechi, M.; Uno, Y.; Inagaki, N. Photosynthetic limitations of a halophyte sea aster (Aster tripolium L.) under water stress and NaCl stress. J. Plant Res. 2003, 116, 63–68. [Google Scholar]
- Song, J.; Feng, G.; Tian, C.Y.; Zhang, F.S. Osmotic adjustment traits of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum in field or controlled conditions. Plant Sci. 2006, 170, 113–119. [Google Scholar]
- Carter, J.M.; Nippert, J.B. Leaf-level physiological responses of Tamarix ramosissima to increasing salinity. J. Arid Environ. 2012, 77, 17–24. [Google Scholar]
- Geissler, N.; Hussin, S.; Koyro, H.W. Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L. Environ. Exp. Bot. 2009, 65, 220–231. [Google Scholar]
- Yang, F.; Lv, G.; Qie, Y. Hydraulic characteristics and carbon metabolism of Haloxylon ammodendron under different water–salt content. Aob Plants 2022, 14, plac042. [Google Scholar]
- Shabala, S.; Bose, J.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar]
- Colak Esetlili, B.; Yildiz Aktas, L.; Esetlili, M.T.; Oztekin, T.; Kılıc, C.C.; Kurucu, Y. Salinity Tolerance Mechanism of Crithmum maritimum L.: Implications for Sustainable Agriculture in Saline Soils. Sustainability 2024, 16, 8165. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Raman, A.; Hodgkins, D.; Mitchell, D.; Nicol, H. Physiological response and ion accumulation in two grasses, one legume, and one saltbush under soil water and salinity stress. Ecohydrology 2015, 8, 1547–1559. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, D.; Li, W.; Liu, Y.; Li, E.; Li, X.; Yang, G.; He, X. Variations in physiological and biochemical characteristics of Kalidium foliatum leaves and roots in two saline habitats in desert region. Forests 2024, 15, 148. [Google Scholar] [CrossRef]
- Xu, G.; Huang, T.F.; Zhang, X.L.; Duan, B.L. Significance of mesophyll conductance for photosynthetic capacity and water-use efficiency in response to alkaline stress in Populus cathayana seedlings. Photosynthetica 2013, 51, 438–444. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non-stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Lu, C.; Qiu, N.; Wang, B.; Zhang, J. Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J. Exp. Bot. 2003, 54, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Płażek, A.; Dubert, F.; Kościelniak, J.; Tatrzańska, M.; Maciejewski, M.; Gondek, K.; Żurek, G. Tolerance of Miscanthus × giganteus to salinity depends on initial weight of rhizomes as well as high accumulation of potassium and proline in leaves. Ind. Crops Prod. 2014, 52, 278–285. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Cao, X.; Jia, J.B.; Zhang, C.; Li, H.; Liu, T.X.; Jiang, X.N.; Polle, A.; Peng, C.H.; Luo, Z.B. Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol. Plant. 2014, 151, 480–494. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Ogrady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef] [PubMed]



| Species | Water Treatment | NaCl Treatments (mM) | Chl a | Chl b | LWC (%) | Fv/Fm |
|---|---|---|---|---|---|---|
| H. ammodendron | Drought | 0 | 5.67 ± 0.22 a | 1.81 ± 0.10 a | 69.78 ± 2.55 b | 0.806 ± 0.004 a |
| Drought | 100 | 4.42 ± 0.11 bc | 1.50 ± 0.05 b | 80.70 ± 2.07 a | 0.791 ± 0.010 a | |
| Drought | 300 | 5.11 ± 0.21 ab | 1.52 ± 0.05 b | 77.66 ± 1.33 a | 0.804 ± 0.007 a | |
| Drought | 500 | 3.98 ± 0.41 c | 1.35 ± 0.10 b | 77.79 ± 0.33 a | 0.807 ± 0.001 a | |
| Well-watered | 0 | 6.02 ± 0.47 AB | 2.40 ± 0.21 A | 78.29 ± 0.70 A | 0.800 ± 0.004 A | |
| Well-watered | 100 | 6.60 ± 0.19 A | 2.35 ± 0.12 A | 78.59 ± 1.10 A | 0.785 ± 0.005 A | |
| Well-watered | 300 | 5.16 ± 0.18 B | 1.88 ± 0.03 B | 78.90 ± 1.21 A | 0.797 ± 0.009 A | |
| Well-watered | 500 | 5.11 ± 0.60 B | 1.73 ± 0.17 B | 79.58 ± 1.42 A | 0.801 ± 0.003 A | |
| T. elongata | Drought | 0 | 9.05 ± 1.13 a | 4.80 ± 0.53 a | 73.69 ± 1.31 a | 0.840 ± 0.003 a |
| Drought | 100 | 10.34 ± 0.37 a | 4.74 ± 0.23 a | 69.87 ± 1.18 ab | 0.845 ± 0.002 a | |
| Drought | 300 | 9.35 ± 0.74 a | 4.72 ± 0.48 a | 67.70 ± 2.02 b | 0.844 ± 0.003 a | |
| Drought | 500 | 10.07 ± 0.25 a | 4.76 ± 0.28 a | 60.17 ± 2.67 c | 0.845 ± 0.002 a | |
| Well-watered | 0 | 11.64 ± 0.67 A | 5.16 ± 0.19 A | 67.51 ± 0.69 A | 0.838 ± 0.004 A | |
| Well-watered | 100 | 11.63 ± 0.64 A | 4.61 ± 0.24 A | 67.20 ± 1.56 A | 0.840 ± 0.005 A | |
| Well-watered | 300 | 10.27 ± 0.20 AB | 4.38 ± 0.14 A | 65.25 ± 1.87 A | 0.843 ± 0.005 A | |
| Well-watered | 500 | 9.14 ± 1.02 B | 4.22 ± 0.30 A | 64.84 ± 1.42 A | 0.834 ± 0.006 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Ma, J.; Min, X.; Tong, Y. Effect of Drought and Salinity on Water Relations and Photosynthetic Responses of Tamarix elongata and Haloxylon ammodendron in Wutonggou Desert Tourist Area, Northwest China. Diversity 2025, 17, 235. https://doi.org/10.3390/d17040235
Tong Y, Ma J, Min X, Tong Y. Effect of Drought and Salinity on Water Relations and Photosynthetic Responses of Tamarix elongata and Haloxylon ammodendron in Wutonggou Desert Tourist Area, Northwest China. Diversity. 2025; 17(4):235. https://doi.org/10.3390/d17040235
Chicago/Turabian StyleTong, Yuping, Jianying Ma, Xiaojun Min, and Yuling Tong. 2025. "Effect of Drought and Salinity on Water Relations and Photosynthetic Responses of Tamarix elongata and Haloxylon ammodendron in Wutonggou Desert Tourist Area, Northwest China" Diversity 17, no. 4: 235. https://doi.org/10.3390/d17040235
APA StyleTong, Y., Ma, J., Min, X., & Tong, Y. (2025). Effect of Drought and Salinity on Water Relations and Photosynthetic Responses of Tamarix elongata and Haloxylon ammodendron in Wutonggou Desert Tourist Area, Northwest China. Diversity, 17(4), 235. https://doi.org/10.3390/d17040235






