Ecological Role of the Clam Jukesena foveolata (Bivalve, Cyamiidae) Inferred by Digenean Parasites in Subantarctic Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. Parasites’ Morphological Study
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Phylogenetic Analysis
2.5. Histological Studies
3. Results
3.1. Family Monorchiidae Odhner, 1911
3.1.1. Description
3.1.2. Taxonomic Summary
3.1.3. Molecular Analysis
3.1.4. Histopathology
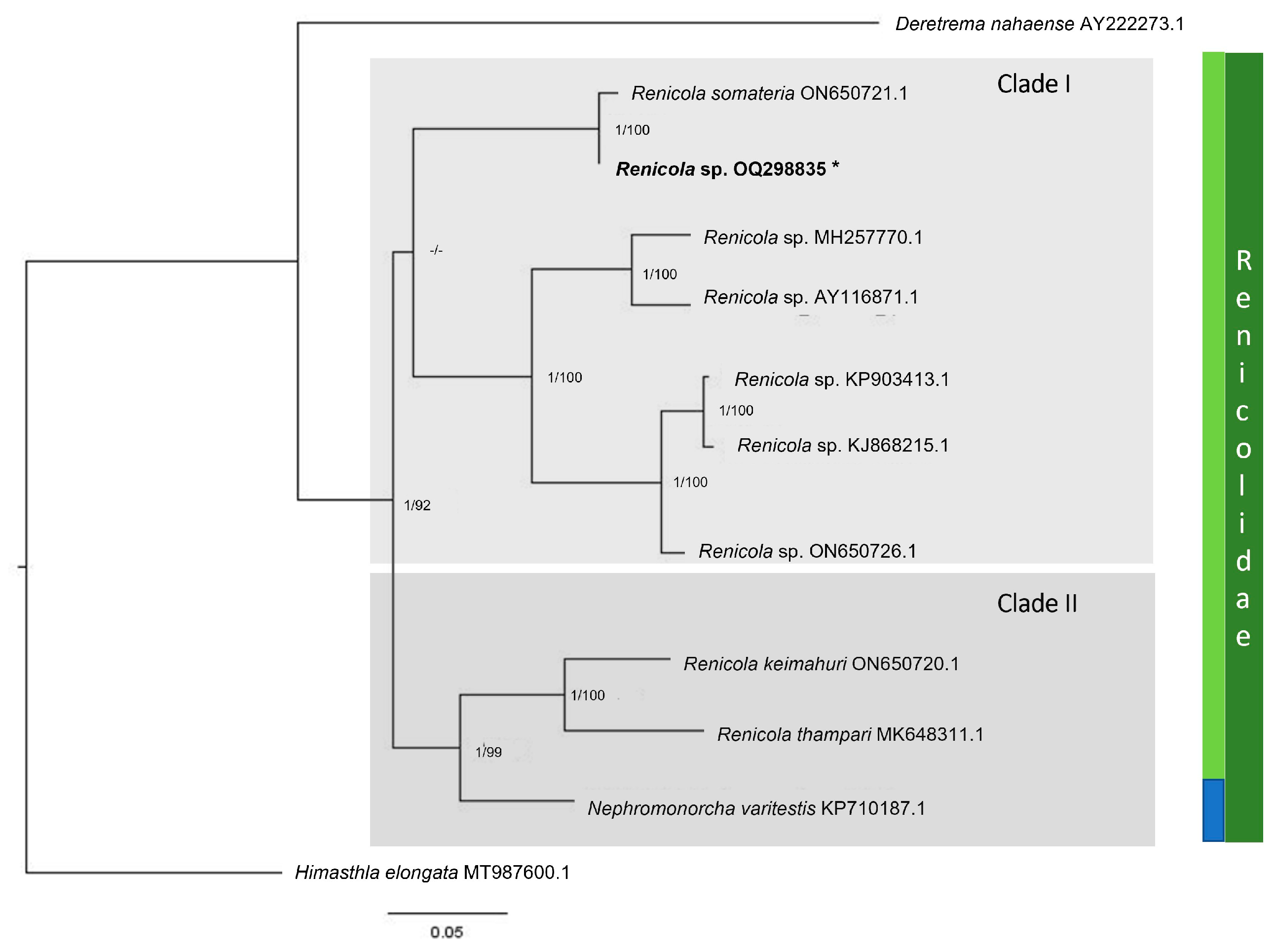
3.2. Renicolidae Dollfus, 1939
3.2.1. Description
3.2.2. Taxonomic Summary
3.2.3. Molecular Analysis
3.2.4. Histopathology
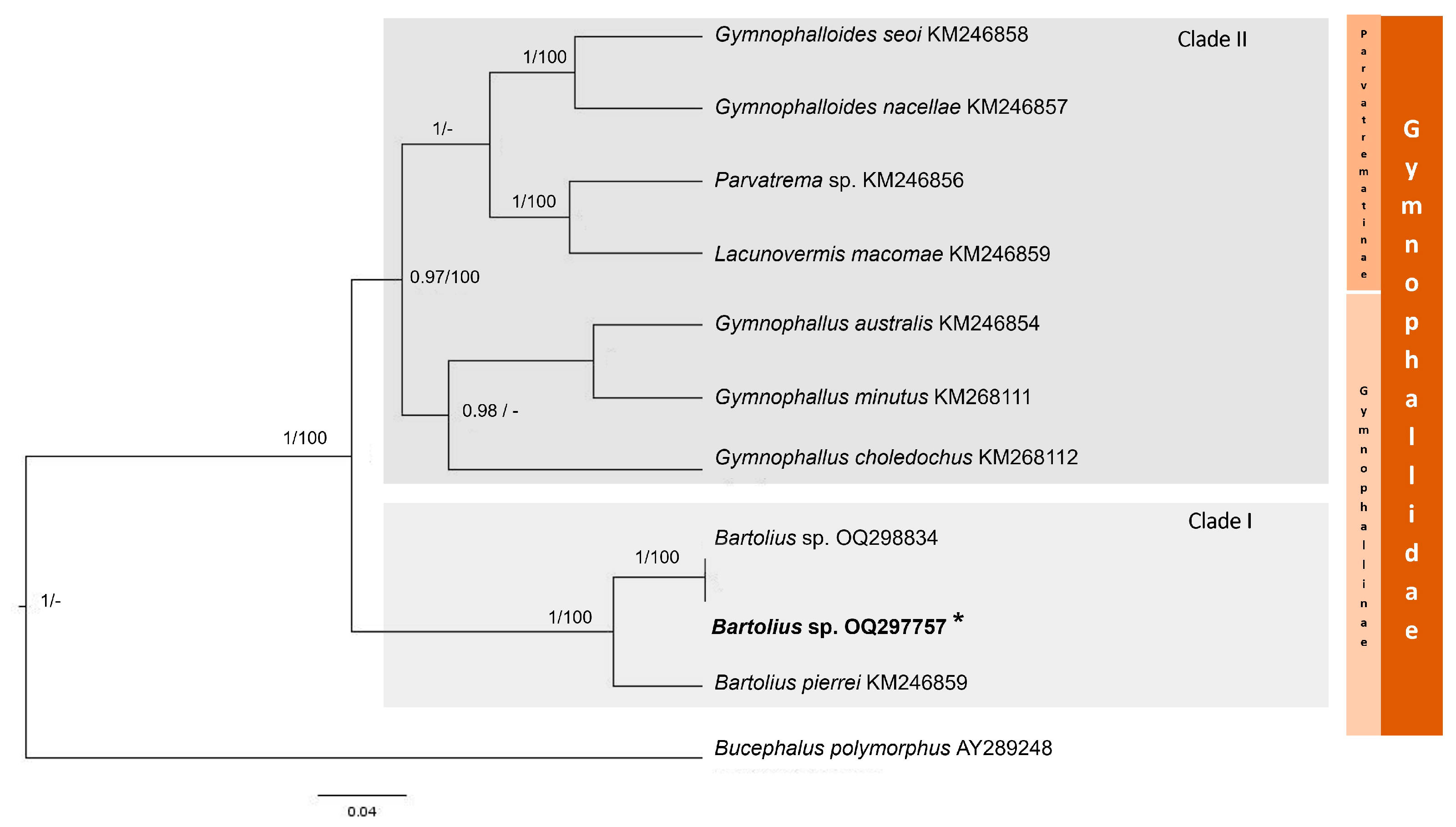
3.3. Family Gymnophallidae Odhner, 1905
3.3.1. Description
3.3.2. Taxonomic Summary
3.3.3. Molecular Analysis
3.3.4. Histopathology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ML | Maximum Likelihood |
| BI | Bayesian Inference |
| H&E | Hematoxylin and Eosin |
| MCMC | Markov Chain Monte Carlo |
References
- Kinne, O. Diseases of Marine Animals: General aspects, Protozoa to Gastropoda. In Diseases of Marine Animals; Kinne, O., Ed.; John and Wiley and Sons: Chichester, UK, 1980; Volume 1, p. 466. [Google Scholar]
- Cremonte, F. Enfermedades de moluscos bivalvos de interés comercial causadas por metazoos. In Enfermedades de Moluscos Bivalvos de Interés en Acuicultura; Figueras, A., Novoa, B., Eds.; Fundación Observatorio Español de Acuicultura: Madrid, Spain, 2011; ISBN 84-00-09288-0. [Google Scholar]
- Lauckner, G. Introduction, Bivalvia to Scaphopoda. In Diseases of Marine Animals; Kinne, O., Ed.; Biologische Anstalt Helgoland: Hamburg, Germany, 1983; Volume 2, pp. 477–977. [Google Scholar]
- Cremonte, F.; Gilardoni, C.; Pina, S.; Rodrigues, P.; Ituarte, C. Revision of the Family Gymnophallidae Odhner, 1905 (Digenea) Based on Morphological and Molecular Data. Parasitol. Int. 2015, 64, 202–210. [Google Scholar]
- Gilardoni, C.; Di Giorgio, G.; Bagnato, E.; Pina, S.; Rodrigues, P.; Cremonte, F. A Potential Zoonotic Parasite, the Digenean Gymnophalloides nacellae, on the Magellanic Coast in the Southwestern Atlantic Ocean: Its Life Cycle and Geographical Distribution. Pol. Biol. 2020, 43, 725–734. [Google Scholar] [CrossRef]
- Adlard, R.D.; Barker, S.C.; Blair, D.; Cribb, T.H. Comparison of the Second Internal Transcribed Spacer (Ribosomal DNA) from Populations and Species of Fasciolidae (Digenea). Int. J. Parasitol. 1993, 23, 423–425. [Google Scholar] [CrossRef]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and Classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, C.; Etchegoin, J.; Cribb, T.; Pina, S.; Rodrigues, P.; Diez, M.E.; Cremonte, F. Cryptic Speciation of the Zoogonid Digenean Diphterostomum flavum n. sp. Demonstrated by Morphological and Molecular Data. Parasite 2020, 27, 44. [Google Scholar]
- Zelaya, D.G.; Güller, M.; Ituarte, C. Filling a Blank in Bivalve Taxonomy: An Integrative Analysis of Cyamioidea (Mollusca: Bivalvia). Zool. J. Linn. Soc. 2019, 190, 558–591. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar]
- Corpet, F. Multiple Sequence Alignment with Hierarchical Clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Molecular Evolution, Phylogenetics and Epidemiology. Available online: https://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 March 2023).
- Cremonte, F.; Figueras, A.; Burreson, E.M. A Histopathological Survey of Some Commercially Exploited Bivalve Molluscs in Northern Patagonia, Argentina. Aquaculture 2005, 249, 23–33. [Google Scholar] [CrossRef]
- Wee, N.Q.-X.; Cribb, T.H.; Cutmore, S.C. Four New Monorchiids from Marine Teleost Fishes of Moreton Bay and the Great Barrier Reef, Australia, Including the Proposal of a New Genus. Parasitol. Int. 2022, 89, 102566. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Solovyeva, A.I.; Blakeslee, A.M.; Skírnisson, K. Overview of renicolid digeneans (Digenea, Renicolidae) from marine gulls of northern Holarctic with remarks on their species statuses, Phylogeny and Phylogeography. Parasitology 2023, 150, 55–77. [Google Scholar] [CrossRef]
- Bowers, E.A.; James, B.L. Studies on the Morphology, Ecology and life-cycle of Meiogytnnophallus minutus (Cobbold, 1859) comb. nov. (Trematoda: Gymnophallidae). Parasitology 1967, 57, 281–300. [Google Scholar] [CrossRef]
- Ituarte, C.F.; Cremonte, F.; Deferrari, G. Mantle-Shell Complex Reactions Elicited by Digenean Metacercariae in Gaimardia trapesina (Bivalvia: Gaimardiidae) from the Southwestern Atlantic Ocean and Magellan Strait. Dis. Aquat. Org. 2001, 48, 47–56. [Google Scholar] [CrossRef]
- Cable, R.M. Marine cercariae of Puerto Rico. Sci. Surv. Porto Rico Virgin Islands 1956, 16, 491–577. [Google Scholar]
- Schell, S.C. Handbook of Trematodes of North America North of Mexico; University Press of Idaho: Montreal, QC, Canada, 1985; 263p. [Google Scholar]
- Cremonte, F.; Kroeck, M.A.; Martorelli, S.R. A New Monorchiid Cercaria (Digenea) Parasitising the Purple Clam Amiantis purpurata (Bivalvia: Veneridae) in the Southwest Atlantic Ocean, with Notes on Its Gonadal Effect. Folia Parasitol. 2001, 48, 217–223. [Google Scholar] [CrossRef]
- Gilardoni, C.; Carballo, M.C.; Cremonte, F. The Life Cycle and Geographical Distribution of the Monorchiid Proctotrema bartolii (Digenea) in the Clam Darina solenoides from the Patagonian Coast, Argentina. J. Helminthol. 2013, 87, 392–399. [Google Scholar] [PubMed]
- Bagnato, E.; Gilardoni, C.; Di Giorgio, G.; Cremonte, F. A Checklist of Marine Larval Trematodes (Digenea) in Molluscs from Argentina, Southwestern Atlantic Coast. Check List 2015, 11, 1706. [Google Scholar] [CrossRef]
- Madhavi, R. Family Monorchiidae Odhner, 1911. In Keys to the Trematoda; CABI: Wallingford, UK, 2008; Volume 3, pp. 145–175. [Google Scholar]
- Cremonte, F. Bartolius pierrei ng, n. sp. (Digenea: Gymnophallidae) from the Península Valdés, Argentina. Syst. Parasitol. 2001, 49, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, E.; Gilardoni, C.; Pina, S.; Rodrigues, P.; Cremonte, F. Redescription and Life Cycle of the Monorchiid Postmonorcheides maclovini Szidat, 1950 (Digenea) from the Southwestern Atlantic Ocean: Morphological and Molecular Data. Parasitol. Int. 2015, 65, 44–49. [Google Scholar] [CrossRef]
- Gilardoni, C.; Etchegoin, J.; Diaz, J.; Ituarte, C.; Cremonte, F. A Survey of Larval Digeneans in the Commonest Intertidal Snails from Northern Patagonian Coast, Argentina. Acta Parasitol. 2011, 56, 163–179. [Google Scholar] [CrossRef]
- Gilardoni, C.; Di Giorgio, G.; Bagnato, E.; Cremonte, F. Survey of trematodes in intertidal snails from Patagonia, Argentina: New larval forms and diversity assessment. J. Helminthol. 2019, 93, 342–351. [Google Scholar] [CrossRef]
- Gilardoni, C.; Lorenti, E.; Diaz, J.I.; Leonardi, S.; Cremonte, F. Parasitological Survey of Coastal Birds from the Magellanic Coast, Southwestern Atlantic Ocean. An. Acad. Bras. Ciências 2023, 95, e20201392. [Google Scholar] [CrossRef]
- Cremonte, F.; Vázquez, N.; Ituarte, C. The Development of Gymnophallus australis Szidat, 1962 (Digenea: Gymnophallidae) from the Patagonian Coast (Argentina) from Metacercaria to Adult, with an Amended Diagnosis of Gymnophallus Odhner, 1905. Syst. Parasitol. 2008, 69, 23–31. [Google Scholar]
- Diaz, J.I.; Cremonte, F.; Navone, G.T. Helminths of the kelp gull, Larus dominicanus, from the northern Patagonian coast. Parasitol. Res. 2011, 109, 1555–1562. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Ngo, H.D.; Van Ha, N.; Van Tang, N.; Ermolenko, A.V.; Beloded, A.Y. Morphometric and molecular data of the two digenean species Lasiotocus lizae Liu, 2002 (Monorchiidae) and Paucivitellosus vietnamensis sp. n. (Bivesiculidae) from Mullet Fish in Tonkin Bay, Vietnam. J. Helminthol. 2017, 91, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Jousson, O.; Bartoli, P.; Pawlowski, J. Cryptic Speciation among Intestinal Parasites (Trematoda: Digenea) Infecting Sympatric Host Fishes (Sparidae). J. Evol. Biol. 2000, 13, 778–785. [Google Scholar] [CrossRef]
- Bartoli, P.; Jousson, O.; Russell-Pinto, F. The Life Cycle of Monorchis parvus (Digenea: Monorchiidae) Demonstrated by Developmental and Molecular Data. J. Parasitol. 2000, 86, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Culurgioni, J.; Aceto, S.; Fichi, G.; Pretto, T.; Luise, D.; Gustinelli, A.; De Vico, G. Postmonorchis sp. inq. (Digenea: Monorchiidae) metacercariae Infecting Natural Beds of Wedge Clam Donax trunculus in Italy. Dis. Aquat. Org. 2013, 106, 163–172. [Google Scholar] [CrossRef]
- Searle, E.L.; Cutmore, S.C.; Cribb, T.H. Monorchiid trematodes of the painted sweetlips, Diagramma labiosum (Perciformes: Haemulidae), from the Southern Great Barrier Reef, Including a New Genus and Three New Species. Syst. Parasitol. 2014, 88, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Wee, N.-X.; Bray, R.A.; Cutmore, S.C. Monorchis lewisi n.sp. (Trematoda: Monorchiidae) from the Surf Bream, Acanthopagrus australis (Sparidae), in Moreton Bay, Australia. J. Helminthol. 2018, 92, 100–108. [Google Scholar] [CrossRef]
- Tkach, V.V.; Pawlowski, J.; Mariaux, J.; Swiderski, Z.; Littlewood, D.T.J.; Bray, R.A. Molecular Phylogeny of the Suborder Plagiorchiata and Its Position in the System of Digenea. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; CRC Press: London, UK, 2001; Chapter 17; pp. 186–193. [Google Scholar]
- Patitucci, K.F.; Kudlai, O.; Tkach, V.V. Nephromonorcha varitestis n. sp.(Digenea: Renicolidae) from the American White Pelican, Pelecanus Erythrorhynchos in North Dakota, USA. Comp. Parasitol. 2015, 82, 254–261. [Google Scholar]
- De León, G.P.-P.; Hernández-Mena, D. Testing the higher-level phylogenetic classification of digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the Age of the ‘next-generation’ Tree of Life. J. Helminthol. 2019, 93, 260–276. [Google Scholar] [CrossRef]
- O’Dwyer, K.; Faltýnková, A.; Georgieva, S.; Kostadinova, A. An integrative taxonomic investigation of the diversity of digenean parasites infecting the intertidal snail Austrolittorina unifasciata Gray, 1826 (Gastropoda: Littorinidae) in Australia. Parasitol. Res. 2015, 114, 2381–2397. [Google Scholar] [CrossRef]
- Huston, D.C.; Cutmore, S.C.; Cribb, T.H. Molecular systematics of the digenean community parasitising the cerithiid gastropod Clypeomorus batillariaeformis Habe & Kusage on the Great Barrier Reef. Parasitol. Int. 2018, 67, 722–735. [Google Scholar]
- O’Dwyer, K.; Blasco-Costa, I.; Poulin, R.; Faltýnková, A. Four Marine Digenean Parasites of Austrolittorina spp. (Gastropoda: Littorinidae) in New Zealand: Morphological and Molecular Data. Syst. Parasitol. 2014, 89, 133–152. [Google Scholar] [PubMed]
- Cremonte, F.; Pina, S.; Gilardoni, C.; Rodrigues, P.; Chai, J.-Y.; Ituarte, C. A new species of Gymnophallid (Digenea) and an amended diagnosis of the Genus Gymnophalloides Fujita, 1925. J. Parasitol. 2013, 99, 85–92. [Google Scholar] [PubMed]
- Lee, S.-H.; Chai, J.-Y.; Seo, M.; Choi, M.-H.; Kim, D.-C.; Lee, S.-K. Two Cases of Gymnophalloides seoi Infection Accompanied by Diabetes Mellitus. Korean J. Parasitol. 1995, 33, 61–64. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trani, C.; Medina, C.D.; Cremonte, F.; Gilardoni, C. Ecological Role of the Clam Jukesena foveolata (Bivalve, Cyamiidae) Inferred by Digenean Parasites in Subantarctic Waters. Diversity 2025, 17, 233. https://doi.org/10.3390/d17040233
Trani C, Medina CD, Cremonte F, Gilardoni C. Ecological Role of the Clam Jukesena foveolata (Bivalve, Cyamiidae) Inferred by Digenean Parasites in Subantarctic Waters. Diversity. 2025; 17(4):233. https://doi.org/10.3390/d17040233
Chicago/Turabian StyleTrani, Cecilia, Cintia Debora Medina, Florencia Cremonte, and Carmen Gilardoni. 2025. "Ecological Role of the Clam Jukesena foveolata (Bivalve, Cyamiidae) Inferred by Digenean Parasites in Subantarctic Waters" Diversity 17, no. 4: 233. https://doi.org/10.3390/d17040233
APA StyleTrani, C., Medina, C. D., Cremonte, F., & Gilardoni, C. (2025). Ecological Role of the Clam Jukesena foveolata (Bivalve, Cyamiidae) Inferred by Digenean Parasites in Subantarctic Waters. Diversity, 17(4), 233. https://doi.org/10.3390/d17040233







