Integrated Taxonomic Analysis of Biomphalaria (Hygrophila: Planorbidae) from the Brazilian Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Approval from the Ethics Committee
2.2. Sample Collection Area
2.3. Embryonic Cycle
2.4. Morphological Identification
2.5. Molecular Analysis
3. Results
3.1. Aspects of the Embryonic Cycle
3.2. Morphological Aspects
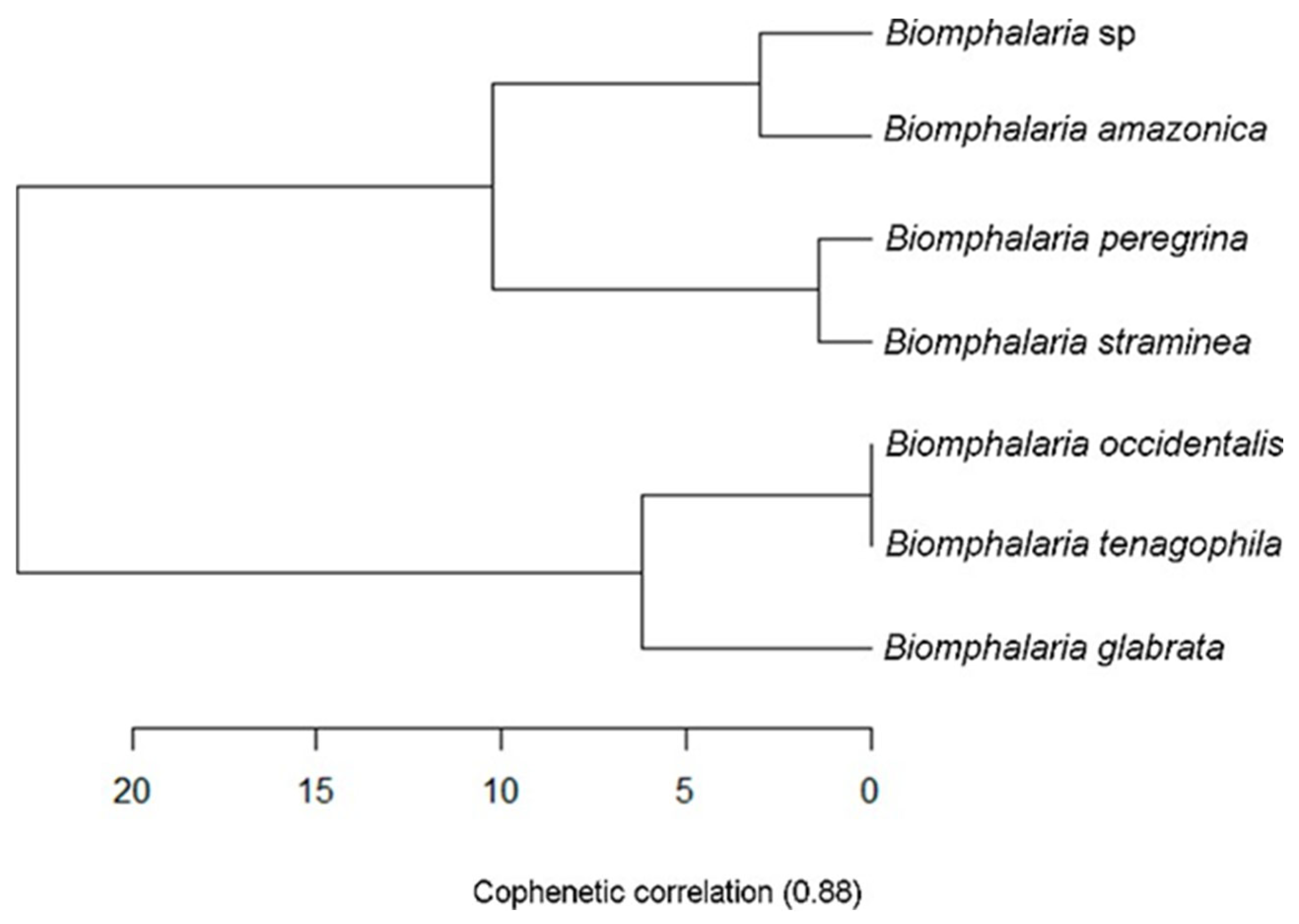
3.3. Molecular Biology
4. Discussion
- Blastula: 10 to 23 h after fertilization;
- Gastrula: 24 h after fertilization of the ovule, the beginning of growth, differentiation, and cell movement occurs;
- Trochophore: 40 to 65 h after fertilization of the ovule, the embryo becomes elongated, kidney-shaped, with slow movements initially, subsequently becoming more rapid.
- Veliger: About 120 h after fertilization of the ovule; the shell becomes dislocated and twisted to the right side of the animal and the eyes become visible, as well as the foot and mouth;
- Hippo: The veliger is more developed, and the tentacles, eyes, and foot become clearly visible and defined, along with the curving form of the main axis and nearly complete covering of the body by the shell. The mollusk then hatches at about 144 h (between 6 and 9 days) after fertilization of the ovule
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Biomphalaria amazonica PV252083 | Biomphalaria amazonica MZ778865 | Biomphalaria kuhniana MZ778865 | Biomphalaria kuhniana MZ778925 | Biomphalaria straminea MZ778902 | Biomphalaria straminea MZ778901 | Biomphalaria straminea MZ778899 | Biomphalaria glabrata MZ778984 | Biomphalaria glabrata MZ778983 | Biomphalaria glabrata DQ084824 | Biomphalaria tenagophila MF380482 | Biomphalaria tenagophila MF380481 | Biomphalaria tenagophila MF380479 | Biomphalaria tenagophila MF380477 | Biomphalaria choanomphala DQ084828 | Biomphalaria choanomphala HM768906 | Biomphalaria choanomphala HM768905 | Biomphalaria sudanica DQ084844 | Biomphalaria camerunensis DQ084827 | Biomphalaria angulosa DQ084826 | Biomphalaria pfeifferi MG780212 | Biomphalaria pfeifferi MG780211 | Biomphalaria pfeifferi MG780210 | Biomphalaria pfeifferi MG780209 | Biomphalaria straminea MF179836 | Biomphalaria straminea MF179835 | Biomphalaria straminea MF179834 | Biomphalaria straminea MF179833 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. amazonica PV252083 | ||||||||||||||||||||||||||||
| B. amazonica MZ778865 | 0.000 | |||||||||||||||||||||||||||
| B. kuhniana MZ778865 | 0.921 | 0.921 | ||||||||||||||||||||||||||
| B kuhniana MZ778925 | 0.005 | 0.005 | 0.921 | |||||||||||||||||||||||||
| B straminea MZ778902 | 0.009 | 0.009 | 0.921 | 0.005 | ||||||||||||||||||||||||
| B straminea MZ778901 | 0.005 | 0.005 | 0.921 | 0.009 | 0.005 | |||||||||||||||||||||||
| B straminea MZ778899 | 0.000 | 0.000 | 0.921 | 0.005 | 0.009 | 0.005 | ||||||||||||||||||||||
| B glabrata MZ778984 | 0.009 | 0.009 | 0.924 | 0.014 | 0.019 | 0.014 | 0.009 | |||||||||||||||||||||
| B glabrata MZ778983 | 0.009 | 0.009 | 0.921 | 0.014 | 0.018 | 0.014 | 0.009 | 0.000 | ||||||||||||||||||||
| B glabrata DQ084824 | 0.885 | 0.885 | 0.911 | 0.885 | 0.885 | 0.885 | 0.885 | 0.877 | 0.880 | |||||||||||||||||||
| B tenagophila MF380482 | 0.912 | 0.912 | 0.925 | 0.912 | 0.912 | 0.912 | 0.912 | 0.910 | 0.912 | 0.926 | ||||||||||||||||||
| B tenagophila MF380481 | 0.911 | 0.911 | 0.925 | 0.911 | 0.911 | 0.911 | 0.911 | 0.910 | 0.911 | 0.930 | 0.000 | |||||||||||||||||
| B tenagophila MF380479 | 0.911 | 0.911 | 0.925 | 0.911 | 0.911 | 0.911 | 0.911 | 0.910 | 0.911 | 0.930 | 0.000 | 0.000 | ||||||||||||||||
| B tenagophila MF380477 | 0.912 | 0.912 | 0.925 | 0.912 | 0.912 | 0.912 | 0.912 | 0.910 | 0.912 | 0.926 | 0.000 | 0.000 | 0.000 | |||||||||||||||
| B choanomphala DQ084828 | 0.888 | 0.888 | 0.935 | 0.888 | 0.884 | 0.884 | 0.888 | 0.882 | 0.884 | 0.898 | 0.916 | 0.916 | 0.916 | 0.916 | ||||||||||||||
| B choanomphala HM768906 | 0.890 | 0.890 | 0.911 | 0.890 | 0.890 | 0.890 | 0.890 | 0.882 | 0.885 | 0.018 | 0.926 | 0.930 | 0.930 | 0.926 | 0.898 | |||||||||||||
| B choanomphala HM768905 | 0.890 | 0.890 | 0.911 | 0.890 | 0.890 | 0.890 | 0.890 | 0.882 | 0.885 | 0.014 | 0.922 | 0.925 | 0.925 | 0.922 | 0.898 | 0.005 | ||||||||||||
| B sudanica DQ084844 | 0.014 | 0.014 | 0.925 | 0.019 | 0.023 | 0.019 | 0.014 | 0.014 | 0.014 | 0.884 | 0.916 | 0.916 | 0.916 | 0.916 | 0.888 | 0.888 | 0.888 | |||||||||||
| B camerunensis DQ084827 | 0.005 | 0.005 | 0.921 | 0.009 | 0.014 | 0.009 | 0.005 | 0.005 | 0.005 | 0.884 | 0.912 | 0.911 | 0.911 | 0.912 | 0.888 | 0.889 | 0.889 | 0.009 | ||||||||||
| B angulosa DQ084826 | 0.890 | 0.890 | 0.935 | 0.890 | 0.885 | 0.885 | 0.890 | 0.882 | 0.885 | 0.894 | 0.917 | 0.916 | 0.916 | 0.917 | 0.000 | 0.894 | 0.894 | 0.888 | 0.889 | |||||||||
| B pfeifferi MG780212 | 0.905 | 0.905 | 0.880 | 0.905 | 0.910 | 0.910 | 0.905 | 0.905 | 0.905 | 0.915 | 0.940 | 0.940 | 0.940 | 0.940 | 0.930 | 0.915 | 0.915 | 0.905 | 0.905 | 0.930 | ||||||||
| B pfeifferi MG780211 | 0.895 | 0.895 | 0.885 | 0.900 | 0.905 | 0.900 | 0.895 | 0.895 | 0.895 | 0.910 | 0.935 | 0.935 | 0.935 | 0.935 | 0.920 | 0.910 | 0.910 | 0.895 | 0.895 | 0.920 | 0.090 | |||||||
| B pfeifferi MG780210 | 0.905 | 0.905 | 0.880 | 0.905 | 0.910 | 0.910 | 0.905 | 0.905 | 0.905 | 0.915 | 0.940 | 0.940 | 0.940 | 0.940 | 0.930 | 0.915 | 0.915 | 0.905 | 0.905 | 0.930 | 0.000 | 0.090 | ||||||
| B pfeifferi MG780209 | 0.910 | 0.910 | 0.875 | 0.910 | 0.915 | 0.915 | 0.910 | 0.910 | 0.910 | 0.920 | 0.950 | 0.950 | 0.950 | 0.950 | 0.940 | 0.920 | 0.920 | 0.910 | 0.910 | 0.940 | 0.070 | 0.120 | 0.070 | |||||
| B straminea MF179836 | 0.908 | 0.908 | 0.883 | 0.908 | 0.913 | 0.913 | 0.908 | 0.906 | 0.908 | 0.917 | 0.942 | 0.941 | 0.941 | 0.942 | 0.927 | 0.917 | 0.917 | 0.908 | 0.908 | 0.927 | 0.005 | 0.091 | 0.005 | 0.076 | ||||
| B straminea MF179835 | 0.908 | 0.908 | 0.883 | 0.908 | 0.913 | 0.913 | 0.908 | 0.906 | 0.908 | 0.917 | 0.942 | 0.941 | 0.941 | 0.942 | 0.927 | 0.917 | 0.917 | 0.908 | 0.908 | 0.927 | 0.005 | 0.091 | 0.005 | 0.076 | 0.000 | |||
| B straminea MF179834 | 0.908 | 0.908 | 0.883 | 0.908 | 0.913 | 0.913 | 0.908 | 0.906 | 0.908 | 0.917 | 0.942 | 0.941 | 0.941 | 0.942 | 0.927 | 0.917 | 0.917 | 0.908 | 0.908 | 0.927 | 0.005 | 0.091 | 0.005 | 0.076 | 0.000 | 0.000 | ||
| B straminea MF179833 | 0.908 | 0.908 | 0.883 | 0.908 | 0.913 | 0.913 | 0.908 | 0.906 | 0.908 | 0.917 | 0.942 | 0.941 | 0.941 | 0.942 | 0.927 | 0.917 | 0.917 | 0.908 | 0.908 | 0.927 | 0.005 | 0.091 | 0.005 | 0.076 | 0.000 | 0.000 | 0.000 |
References
- Pepe, M.S.; Caldeira, R.L.; Carvalho, O.d.S.; Muller, G.; Jannotti-Passos, L.K.; Rodrigues, A.P.; Amaral, H.L.; Berne, M.E.A. Biomphalaria molluscs (Gastropoda: Planorbidae) in Rio Grande do Sul, Brazil. Mem. Do Inst. Oswaldo Cruz 2009, 104, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Chitsulo, L.; Engels, D.; Montresor, A.; Savioli, L. The global status of schistosomiasis and its control. Acta Trop. 2000, 77, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; Dejong, R.J.; Snyder, S.D.; Mkoji, G.M.; Loker, E.S. Schistosoma mansoni and Biomphalaria: Past history and future trends. Parasitology 2001, 123, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, T.M.; Jannotti-Passos, L.K.; Carvalho, O.d.S.; Grijalva, M.J.; Baús, E.G.; Caldeira, R.L. Hybridism between Biomphalaria cousini and Biomphalaria amazonica and its susceptibility to Schistosoma mansoni. Mem. Inst. Oswaldo Cruz 2011, 106, 851–855. [Google Scholar] [CrossRef]
- Simões, L.F.; Bastos, L.D.B.; Camargo, E.A.F.; Neves, M.F.; Linhares, A.X.; Magalhães, L.A.; Zanotti-Magalhães, E.M. Host-parasite relationship between Biomphalaria amazonica (Paraense, 1966) and Schistosoma mansoni (Sambon, 1907). Braz. J. Biol. 2016, 77, 340–346. [Google Scholar] [CrossRef]
- Velásquez, L.E.; Caldeira, R.L.; Estrada, V.; Carvalho, O.S. Morphological and polymerase chain reaction-restriction fragment lenght polymorphism characterization of Biomphalaria kuhniana and Biomphalaria amazonica from Colombia. Mem. Inst. Oswaldo Cruz 2002, 97, 997–1004. [Google Scholar] [CrossRef]
- Paraense, W.L. Biomphalaria Amazonica and B. cousini, two new species of neotropical planorbid molluscs. Rev. Bras. Biol. 1966, 26, 115–126. [Google Scholar] [PubMed]
- Paraense, W.L. Estado atual da sistemática dos planorbídeos brasileiros. Arch. Mus. Nac. 1975, 55, 105–128. [Google Scholar]
- Ohlweiler, F.P.; Rossignoli, T.d.J.; Palasio, R.G.S.; Tuan, R. Taxonomic diversity of Biomphalaria (Planorbidae) in São Paulo state, Brazil. Biota Neotropica 2020, 20, e20200975. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar] [CrossRef]
- Bueno-Silva, M. Genética molecular e sistemática animal: Um breve histórico, contribuições e desafios. Estud. de Biol. 2012, 34, 157. [Google Scholar] [CrossRef]
- Kawano, T.; Nakano, E.; Watanabe, L.C. Estudo do desenvolvimento embrionário de Biomphalaria glabrata (Mollusca, Planorbidae) e suas aplicações. In Schistosoma Mansoni & Esquistossomose: Uma Visão Multidisciplinary; Fiocruz Editora: Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Barbosa, F.S. Tópicos em Malacologia Médica; Fiocruz Editora: Rio de Janeiro, Brazil, 1995; Available online: https://books.scielo.org/id/npy7z (accessed on 20 June 2023).
- Rogers, D.C.; Damborenea, C.; Thorp, J. Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Ohlweiler, F.P. Manual de Gastrópodes Límnicos e Terrestres do Estado de São Paulo Associados às Helmintoses; Redes Editora: São Paulo, Brazil, 2010. [Google Scholar]
- dos Santos Carvalho, O.; Coelho, P.M.Z.; Lenzi, H.L. Schistosoma Mansoni & Esquistossomose: Uma Visão Multidisciplinar; Fiocruz Editora: Rio de Janeiro, Brazil, 2008; Available online: https://books.scielo.org/id/37vvw (accessed on 20 June 2023).
- Moszczynska, A.; Locke, S.A.; McLaughlin, J.D.; Marcogliese, D.J.; Crease, T.J. Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol. Ecol. Resour. 2009, 9, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- DeJong, R.J.; Morgan, J.A.T.; Paraense, W.L.; Pointier, J.-P.; Amarista, M.; Ayeh-Kumi, P.F.K.; Babiker, A.; Barbosa, C.S.; Brémond, P.; Canese, A.P.; et al. Evolutionary Relationships and Biogeography of Biomphalaria (Gastropoda: Planorbidae) with Implications Regarding Its Role as Host of the Human Bloodfluke, Schistosoma mansoni. Mol. Biol. Evol. 2001, 18, 2225–2239. [Google Scholar] [CrossRef]
- Jørgensen, A.; Kristensen, T.K.; Stothard, J.R. Phylogeny and biogeography of African Biomphalaria (Gastropoda: Planorbidae), with emphasis on endemic species of the great East African lakes. Zool. J. Linn. Soc. 2007, 151, 337–349. [Google Scholar] [CrossRef]
- Camey, T.; Verdonk, N.H. The Early Development of the Snail Biomphalaria glabrata (Say) and the Origin of the Head Organs. Neth. J. Zool. 1969, 20, 93–121. [Google Scholar] [CrossRef]
- Paraense, W.L.; Carvalho, O.S.; Coelho, P.M.Z.; Lenzi, H.L. Histórico do gênero Biomphalaria, morfologia e sistemática morfológica. In Schistosoma Mansoni: Uma Visão Multidisciplinar; Carvalho, O.S., Coelho, P.M.Z., Lenzi, H.L., Eds.; Editora Fiocruz: Rio de Janeiro, Brazil, 2008; pp. 285–308. [Google Scholar]
- David, N.F.; Cantanhede, S.P.D.; Monroe, N.B.; Pereira, L.P.L.A.; Silva-Souza, N.; Abreu-Silva, A.L.; Tchaicka, L. Spatial distribution and seasonality of Biomphalaria spp. in São Luís (Maranhão, Brazil). Parasitol. Res. 2018, 117, 1495–1502. [Google Scholar] [CrossRef]
- Paraense, W.L. Biomphalaria kuhniana (Clessin, 1883), planorbid mollusc from South America. Mem. Inst. Oswaldo Cruz 1988, 83, 1–12. [Google Scholar] [CrossRef]
- Species Link Network. Available online: https://specieslink.net/search/ (accessed on 10 January 2024).
- Kawazoe, U. Alguns aspectos da biologia de Biomphalaria glabrata (Say, 1818) e Biomphalaria tenagophila (D’Orbigny, 1835) (Pulmonata, planorbidae): II-Fecundidade e fertilidade. Rev. De Saúde Pública 1977, 11, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Valente, W.C.G.; Garcia, J.S.; Neto, J.d.M.R.; Schirato, G.V.; Faro, M.J. Avaliação da biologia reprodutiva de moluscos da espécie Biomphalaria glabrata (Say, 1818) expostos a diferentes tipos de alimentação e substratos. Biotemas 2022, 35, 1–14. [Google Scholar] [CrossRef]
- Bezerra, F.S.d.M.; Pinheiro, M.C.C.; Filho, J.D.d.S.; de Castro, I.M.N.; Caldeira, R.L.; Sousa, M.S.; Cavalcante, A.B.; Júnior, A.N.R. Identification of Biomphalaria sp and other freshwater snails in the large-scale water transposition project in the Northeast of Brazil. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e41. [Google Scholar] [CrossRef]
- GenBank Overview. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 10 January 2024).
- Carvalho, O.S.; Caldeira, R.L.; Simpson, A.J.G.; Vidigal, T.H.D.A. Genetic variability and molecular identification of Brazilian Biomphalaria species (Mollusca: Planorbidae). Parasitology 2001, 123, 197–209. [Google Scholar] [CrossRef]
- Miranda, G.S.; Rodrigues, J.G.M.; Lira, M.G.S.; Nogueira, R.A.; Gomes, G.C.C.; Miranda, B.S.; De Araújo, A.; Silva-Souza, N. Moluscos límnicos como hospedeiros de trematódeos digenéticos de uma região metropolitana da ilha do Maranhão, Brasil. Sci. Plena 2016, 12. [Google Scholar] [CrossRef]
- Carmo, A.M.; dos Amaral Sousa, K.; dos Santos Rodrigues, R.T.; Social, S. A cidade como extensão das águas: O processo de antropização e os impactos socioambientais no lago Mapiri em Santarém–PA. O direito à moradia nas cidades da Amazônia, 18. In Anais do III Seminário de Direito à Cidade em Santarém–PA; Universidade Federal do Oeste do Pará: Santarém, Brazil, 2022; Available online: https://www.ufopa.edu.br (accessed on 20 June 2023).






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza Barros, L.d.; Silva, A.C.; Santos, J.A.d.; Santos da Silva, A.M.; Ramos, A.T.; Batista, B.B.; Corrêa, L.L.; Couceiro, S.R.M. Integrated Taxonomic Analysis of Biomphalaria (Hygrophila: Planorbidae) from the Brazilian Amazon. Diversity 2025, 17, 227. https://doi.org/10.3390/d17040227
Souza Barros Ld, Silva AC, Santos JAd, Santos da Silva AM, Ramos AT, Batista BB, Corrêa LL, Couceiro SRM. Integrated Taxonomic Analysis of Biomphalaria (Hygrophila: Planorbidae) from the Brazilian Amazon. Diversity. 2025; 17(4):227. https://doi.org/10.3390/d17040227
Chicago/Turabian StyleSouza Barros, Larissa de, Anderson Costa Silva, Jéssica Aires dos Santos, Ayla Monique Santos da Silva, Andressa Teixeira Ramos, Bruno Braulino Batista, Lincoln Lima Corrêa, and Sheyla Regina Marques Couceiro. 2025. "Integrated Taxonomic Analysis of Biomphalaria (Hygrophila: Planorbidae) from the Brazilian Amazon" Diversity 17, no. 4: 227. https://doi.org/10.3390/d17040227
APA StyleSouza Barros, L. d., Silva, A. C., Santos, J. A. d., Santos da Silva, A. M., Ramos, A. T., Batista, B. B., Corrêa, L. L., & Couceiro, S. R. M. (2025). Integrated Taxonomic Analysis of Biomphalaria (Hygrophila: Planorbidae) from the Brazilian Amazon. Diversity, 17(4), 227. https://doi.org/10.3390/d17040227






