Carbon Stock Estimation and Human Disturbance in Selected Urban Un-Conserved Forests in Entoto Mountain Forest, Addis Ababa, Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
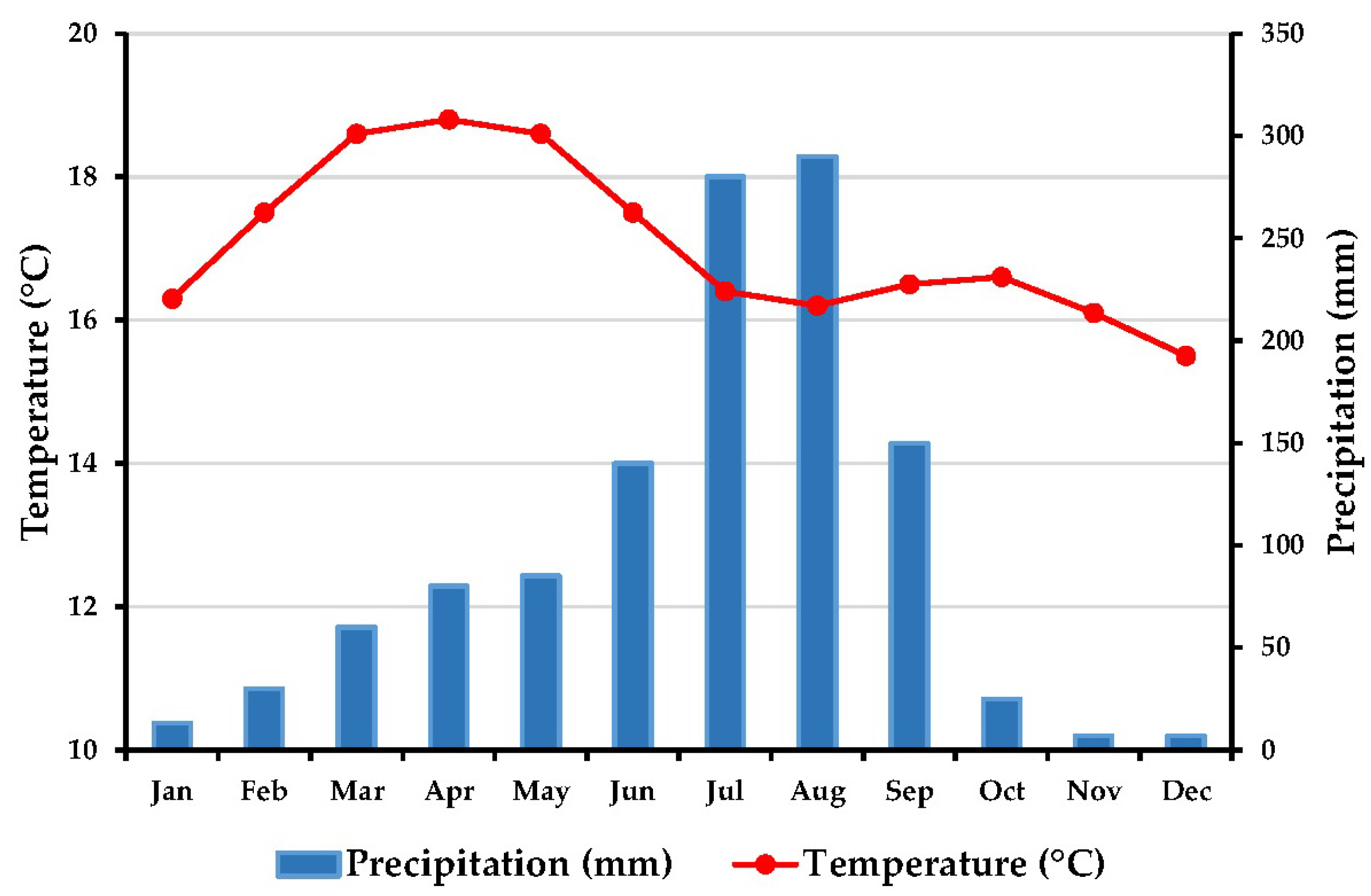
2.2. Climate of Study Area
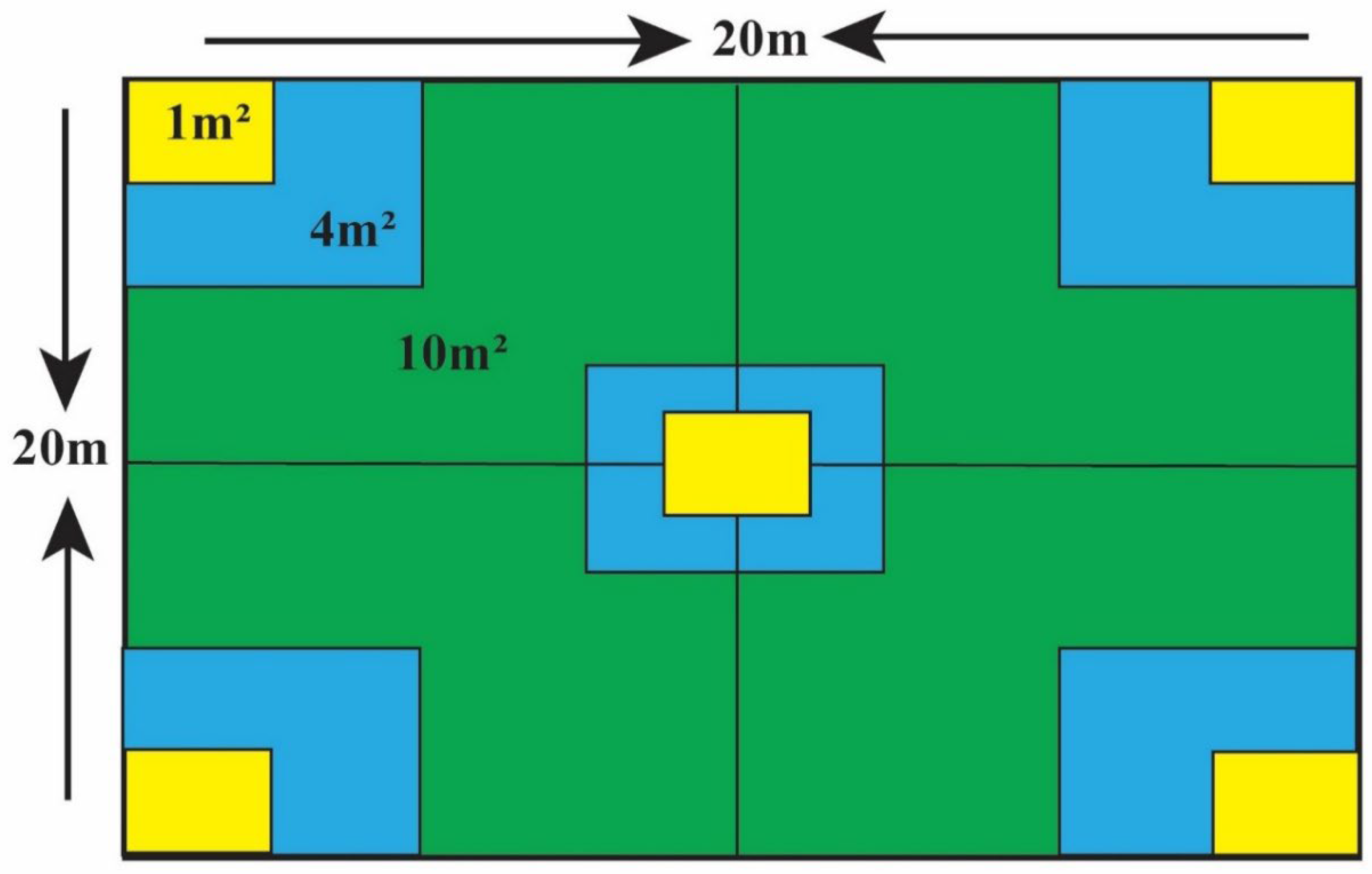
2.3. Sampling Design
2.4. Forest Structure Analysis
2.5. Woody Species Diversity Analysis
2.6. Regeneration Status Analysis
2.7. Field Carbon Stock Measurement
2.7.1. Aboveground Tree Biomass (AGB) and Belowground Biomass (BGB)
2.7.2. Litters Biomass (LB)
2.7.3. Soil Organic Carbon (SOC)
2.8. Estimation of Carbon Stocks in Different Carbon Pools
2.8.1. Aboveground Biomass Carbon Stock Estimation (CAGB)
2.8.2. Belowground Biomass Carbon Stock Estimation (CBGB)
2.8.3. Litter Biomass Carbon Stock Estimation (CL)
2.8.4. Soil Organic Carbon (SOC) Estimation
- BD = soil bulk density (g cm−3);
- d = the total depth at which the sample was taken (30 cm);
- %C = Carbon concentration (%),
2.8.5. Total Carbon Stock Estimation (TC)
2.9. Human Disturbance Index Estimation
2.10. Data Analysis
3. Results
3.1. The Forest Structures
3.1.1. Frequency
3.1.2. Density
3.1.3. Dominance and Important Value Index
3.2. Species Diversity and Evenness
3.3. Regeneration Status of Forest
3.4. Estimation of Biomass and Carbon Stocks in Different Pools
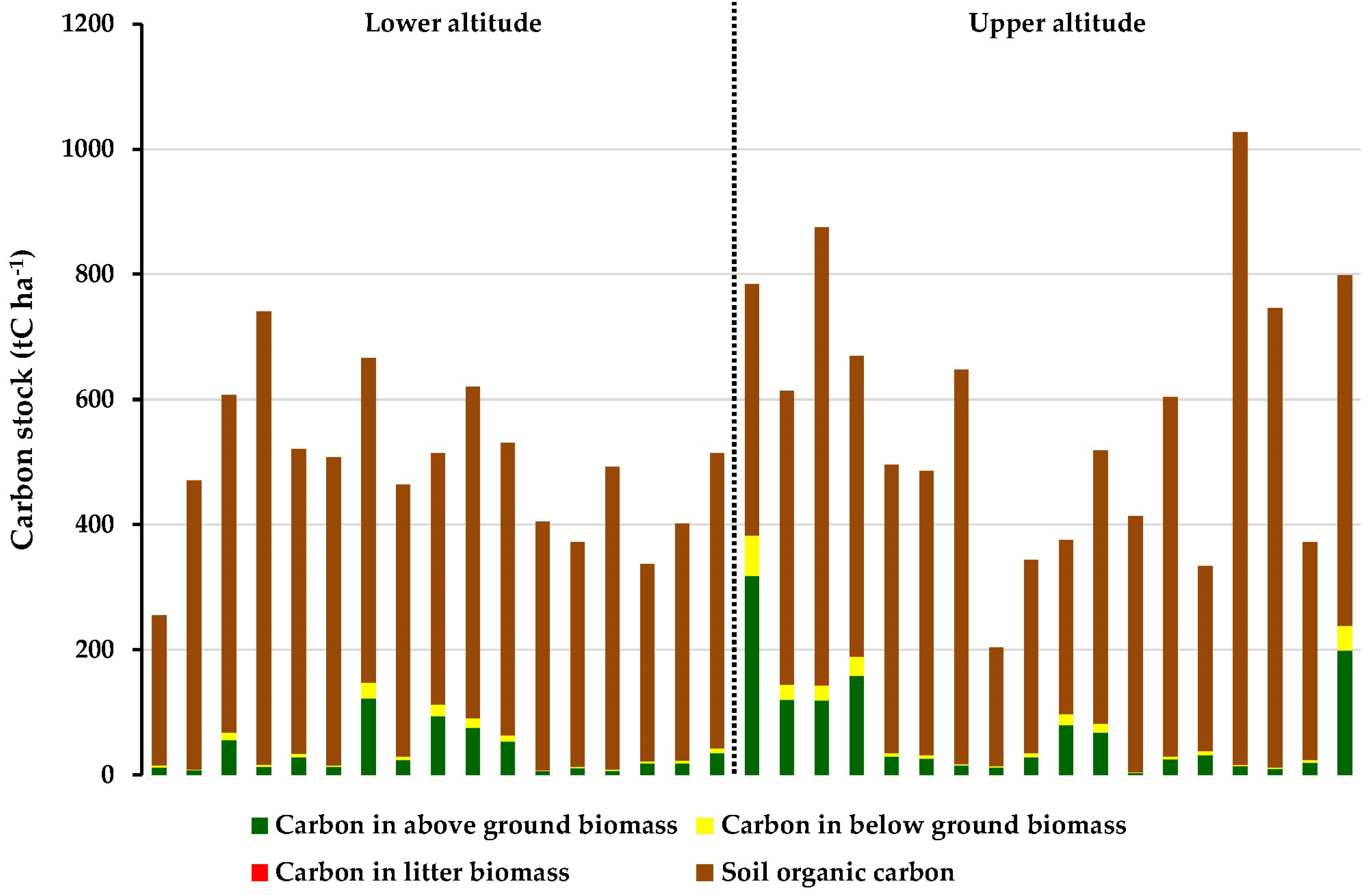
3.4.1. Total Carbon Stock
3.4.2. Carbon Stock Along Altitudinal Gradient
3.5. Human Disturbance Index
4. Discussion
4.1. Carbon Stock in Different Carbon Pools
4.2. The Effect of Forest Structure, Diversity, and Regeneration Pattern Status in Carbon Stock
4.3. The Effect of Altitude on Carbon Pools in Study Sites
4.4. The Effect of Human Disturbance in Forest Structures and Carbon Stock
5. Conclusions and Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. of Plots | Altitude | Stump | Road | Firewood Collection | Soil Excavation | Infra- Structure | Total Frequency of HDI | HDI | HDI% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2137 | √ | √ | 2 | 0.40 | 40.00 | |||
| 2 | 2341 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 3 | 2619 | √ | √ | √ | √ | 4 | 0.80 | 80.00 | |
| 4 | 2619 | √ | √ | 2 | 0.40 | 40.00 | |||
| 5 | 2629 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 6 | 2635 | √ | √ | 2 | 0.40 | 40.00 | |||
| 7 | 2641 | √ | 1 | 0.20 | 20.00 | ||||
| 8 | 2643 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 9 | 2644 | √ | √ | 2 | 0.40 | 40.00 | |||
| 10 | 2645 | √ | √ | 2 | 0.40 | 40.00 | |||
| 11 | 2652 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 12 | 2653 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 13 | 2677 | √ | √ | 2 | 0.40 | 40.00 | |||
| 14 | 2679 | √ | 1 | 0.20 | 20.00 | ||||
| 15 | 2679 | √ | √ | 2 | 0.40 | 40.00 | |||
| 16 | 2683 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 17 | 2686 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 18 | 2690 | √ | √ | 2 | 0.40 | 40.00 | |||
| 19 | 2693 | √ | √ | 2 | 0.40 | 40.00 | |||
| 20 | 2695 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 21 | 2699 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 22 | 2704 | √ | √ | √ | √ | 4 | 0.80 | 80.00 | |
| 23 | 2709 | √ | √ | 2 | 0.40 | 40.00 | |||
| 24 | 2710 | √ | 1 | 0.20 | 20.00 | ||||
| 25 | 2711 | √ | √ | √ | √ | 4 | 0.80 | 80.00 | |
| 26 | 2714 | √ | √ | 2 | 0.40 | 40.00 | |||
| 27 | 2716 | √ | √ | 2 | 0.40 | 40.00 | |||
| 28 | 2718 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 29 | 2720 | √ | 1 | 0.20 | 20.00 | ||||
| 30 | 2722 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 31 | 2724 | √ | √ | 2 | 0.40 | 40.00 | |||
| 32 | 2725 | √ | √ | 2 | 0.40 | 40.00 | |||
| 33 | 2735 | √ | √ | √ | 3 | 0.60 | 60.00 | ||
| 34 | 2735 | √ | √ | 2 | 0.40 | 40.00 | |||
| 35 | 2739 | √ | √ | 2 | 0.40 | 40.00 | |||
| Total | - | 25 | 19 | 33 | 4 | 3 | 84 | 0.48 | 48.00 |
References
- Nowak, D.J.; Greenfield, E.J. The increase of impervious cover and decrease of tree cover within urban areas globally (2012–2017). Urban For. Urban Green. 2020, 49, 126638. [Google Scholar]
- FAO. The State of the World’s Forests 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Joshi, V.C.; Negi, V.S.; Bisht, D.; Sundriyal, R.C.; Arya, D. Tree biomass and carbon stock assessment of subtropical and temperate forests in the Central Himalaya, India. Trees For. People 2021, 6, 100147. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [PubMed]
- Negassa, M.D.; Mallie, D.T.; Gemeda, D.O. Forest cover change detection using Geographic Information Systems and remote sensing techniques: A spatio-temporal study on Komto Protected forest priority area, East Wollega Zone, Ethiopia. Environ. Syst. Res. 2020, 9, 1. [Google Scholar]
- Abebe, M.T.; Megento, T.L. The city of addis ababa from ‘forest city’ to ‘urban heat island’: Assessment of urban green space dynamics. J. Urban Environ. Eng. 2016, 10, 254–262. [Google Scholar]
- Bisht, S.; Bargali, S.S.; Bargali, K.; Rawat, G.S.; Rawat, Y.S.; Fartyal, A. Influence of Anthropogenic Activities on Forest Carbon Stocks—A Case Study from Gori Valley, Western Himalaya. Sustainability 2022, 14, 16918. [Google Scholar] [CrossRef]
- Deribew, K.T. Spatiotemporal analysis of urban growth on forest and agricultural land using geospatial techniques and Shannon entropy method in the satellite town of Ethiopia, the western fringe of Addis Ababa city. Ecol. Process. 2020, 9, 46. [Google Scholar]
- Gebeyehu, G.; Soromessa, T.; Bekele, T.; Teketay, D. Carbon stocks and factors affecting their storage in dry Afromontane forests of Awi Zone, northwestern Ethiopia. J. Ecol. Environ. 2019, 43, 7. [Google Scholar]
- Ryan, C.M.; Williams, M.; Grace, J. Above- and belowground carbon stocks in a miombo woodland landscape of mozambique. Biotropica 2011, 43, 423–432. [Google Scholar]
- Solomon, N.; Birhane, E.; Tadesse, T.; Treydte, A.C.; Meles, K. Carbon stocks and sequestration potential of dry forests under community management in Tigray, Ethiopia. Ecol. Process. 2017, 6, 20. [Google Scholar]
- Nowak, D.J.; Hoehn, R.E.; Bodine, A.R.; Greenfield, E.J.; O’Neil-Dunne, J. Urban forest structure, ecosystem services and change in Syracuse, NY. Urban Ecosyst. 2016, 19, 1455–1477. [Google Scholar]
- Davies, Z.G.; Edmondson, J.L.; Heinemeyer, A.; Leake, J.R.; Gaston, K.J. Mapping an urban ecosystem service: Quantifying above-ground carbon storage at a city-wide scale. J. Appl. Ecol. 2011, 48, 1125–1134. [Google Scholar]
- Benti, T.R.; Zelalam Gasisa, S. An Assessment of Urban and Per-Urban Household’s Attitude to Participate in Urban Forest Conservation Practice in Assosa District, Western Ethiopia. Int. J. Clim. Stud. 2023, 2. Available online: www.iprjb.org (accessed on 20 January 2024).
- Meseret, H.; Eyasu, E. Role of Urban Forests for Carbon Emission Reduction in Addis Ababa:A review. Civ. Environ. Res. 2022, 12, 316. [Google Scholar]
- Alemu, Z.A.; Dioha, M.O. Climate change and trend analysis of temperature: The case of Addis Ababa, Ethiopia. Environ. Syst. Res. 2020, 9, 27. [Google Scholar]
- Taylor, D.; Kent, M.; Coker, P. Vegetation description and analysis: A practical approach. Geogr. J. 1993, 159, 237. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Pandey, S.; Shukla, R. Plant Diversity and Community Patterns Along the Disturbance Gradient in Plantation Forests of Sal (Shorea robusta Gaertn.). Curr. Sci. 1999, 77, 814–818. [Google Scholar]
- Pearson, T.R.H.; Brown, S.L.; Birdsey, R.A. United States Department of Agriculture Measurement Guidelines for the Seques-tration of Forest Carbon. 2007. Available online: http://www.nrs.fs.fed.us/ (accessed on 23 January 2024).
- Subedi, B.P.; Pandey, S.S.; Pandey, A.; Rana, E.B.; Bhattarai, S.; Banskota, T.R.; Charmakar, S.; Tamrakar, R. Forest Carbon Stock Measurement: Guidelines for Measuring Carbon Stocks in Community-Managed Forests. 2010. Available online: https://ansab.org.np/wp-content/uploads/2010/08/forest-carbon-measurement-guideline.pdf (accessed on 18 December 2023).
- Ebasan, M.S.; Aranico, E.C.; Tampus, A.D.; Amparado, R.F. Carbon stock assessment of three different forest covers in Panaon, Misamis Occidental, Philippines. J. Bio. Env. Sci. 2016, 8, 252–264. Available online: http://www.innspub.net (accessed on 23 January 2024).
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to es-timate the aboveground biomass of tropical trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar]
- EFRL. ETHIOPIA’S FOREST REFERENCE LEVEL SUBMISSION TO THE UNFCCC. March 2017. Available online: https://redd.unfccc.int/media/ethiopia_frel_3.2_final_modified_submission.pdf (accessed on 15 December 2023).
- Bastos, J.R.; Capellesso, E.S.; Vibrans, A.C.; Marques, M.C.M. Human impacts, habitat quantity and quality affect the dimensions of diversity and carbon stocks in subtropical forests: A landscape-based approach. J. Nat. Conserv. 2023, 73, 126383. [Google Scholar]
- Clapham, A.R.; Raunkiaer, C.; Gilbert-Carter, H.; Tansley, A.G.; Fausboll. The life forms of plants and statistical plant geography. J. Ecol. 1935, 23, 247. [Google Scholar]
- Abegaz, A.; Tamene, L.; Abera, W.; Yaekob, T.; Hailu, H.; Nyawira, S.S.; Da Silva, M.; Sommer, R. Soil organic carbon dynamics along chrono-sequence land-use systems in the highlands of Ethiopia. Agric. Ecosyst. Environ. 2020, 300, 106997. [Google Scholar]
- Woldegerima, T.; Yeshitela, K.; Lindley, S. Ecosystem Services Assessment of the Urban Forests of Addis Ababa, Ethiopia. Urban Ecosyst. 2017, 20, 683–699. [Google Scholar]
- Nega Bazezew, M. Above- and Below-Ground Reserved Carbon in Danaba Community Forest of Oromia Region, Ethiopia: Implications for CO2 Emission Balance. Am. J. Environ. Prot. 2015, 4, 75. [Google Scholar]
- Yohannes, H. Carbon Stock Analysis Along Altitudinal Gradient in Gedo Forest: Implications for Forest Management and Climate Change Mitigation. Am. J. Environ. Prot. 2015, 4, 237. [Google Scholar]
- Houghton, R.A. Aboveground forest biomass and the global carbon balance. Glob. Change Biol. 2005, 11, 945–958. [Google Scholar]
- Chinasho, A. Carbon Stock in Woody Plants of Humbo Forest and its Variation along Altitudinal Gradients: The Case of Humbo District, Wolaita Zone, Southern Ethiopia. Int. J. Environ. Prot. Policy 2015, 3, 97. [Google Scholar]
- Magurran, A.E. Measuring biological diversity. Curr. Biol. 2021, 31, R1174–R1177. [Google Scholar]
- Worku, A.; Teketay, D.; Lemenih, M.; Fetene, M. Diversity, regeneration status, and population structures of gum and resin producing woody species in borana, southern ethiopia. For. Trees Livelihoods 2012, 21, 85–96. [Google Scholar]
- Amlin, G.; Suratman, M.N.; Isa, N.N.M. Anthropogenic impacts on forest regeneration: Challenges and the way forward. In ISBEIA 2012—IEEE Symposium on Business, Engineering and Industrial Applications; IEEE: Piscataway, NJ, USA, 2012; pp. 158–162. [Google Scholar]
- Giri, A.; Katzensteiner, K. Anthropogenic disturbances on the regeneration of tree species in the mixed broadleaved forest of the himalayan region, Nepal. J. Nat. Hist. Mus. 2013, 27, 35–44. [Google Scholar]
- Salete Capellesso, E.; Cequinel, A.; Marques, R.; Luisa Sausen, T.; Bayer, C.; Marques, M.C.M. Co-benefits in biodiversity con-servation and carbon stock during forest regeneration in a preserved tropical landscape. For. Ecol. Manag. 2021, 492, 119222. [Google Scholar]
- Raich, J.W.; Schlesinger, W.H. The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 1992, 44, 81–99. [Google Scholar]
- Arunrat, N.; Pumijumnong, N.; Sereenonchai, S.; Chareonwong, U. Factors controlling soil organic carbon sequestration of highland agricultural areas in the mae chaem basin, Northern Thailand. Agronomy 2020, 10, 305. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Fang, J.; Piao, S.; Shen, H.; Zhao, S.; Peng, C. Altitudinal changes in carbon storage of temperate forests on Mt Changbai, Northeast China. J. Plant Res. 2010, 123, 439–452. [Google Scholar]
- Tadese, S.; Soromessa, T.; Aneseye, A.B.; Gebeyehu, G.; Noszczyk, T.; Kindu, M. The impact of land cover change on the carbon stock of moist afromontane forests in the Majang Forest Biosphere Reserve. Carbon Balance Manag. 2023, 18, 24. [Google Scholar]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Annu. Rev. Ecol. Evol. Syst. 2024, 8, 419–464. [Google Scholar] [CrossRef]
- Klein, L.J. Urban Forests for Carbon Sequestration and Heat Island Mitigation. 2022. Available online: https://elib.dlr.de/187233/1/Paper_2022_KDD_AI4GoodWorkshop-UrbanForests4CO2SequestrationAndHeatIslandMitigation.pdf (accessed on 18 December 2023).
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar]



| Upper Altitude | ||
|---|---|---|
| Species Name | Raunkiaer Percentage Frequency Class | Percentage Distribution of Species |
| Eucalyptus globulus Labill | 3rd | 51.46% |
| Juniperus procera Hoechst. Ex Endl | 4th | 36.49% |
| Acacia decurrens Willd | 5th | 10.42% |
| Olea europaea L. subsp. cuspidate | - | 0.00% |
| Hagenia abyssinica J.F.Gmel | 5th | 1.01% |
| Acacia melanoxylon R. Br. | - | 0.00% |
| Schefflera abyssinica (Hochst. ex A. Rich.) Harms | - | 0.62% |
| Lower altitude | ||
| Eucalyptus globulus Labill | 5th | 18.32% |
| Juniperus procera Hoechst. Ex Endl | 4th | 27.47% |
| Acacia decurrens Willd | 3rd | 52.35% |
| Olea europaea L. subsp. cuspidate | 5th | 1.07% |
| Hagenia abyssinica J.F.Gmel | - | 0.20% |
| Acacia melanoxylon R. Br. | - | 0.59% |
| Schefflera abyssinica (Hochst. ex A. Rich.) Harms | - | 0.00% |
| DBH Class | Total Density of Species | Percentage DBH Size Distribution of Species |
|---|---|---|
| 4.5–10 cm | 50 | 11.99% |
| 10.1–20 cm | 255 | 61.15% |
| >20cm | 112 | 26.86% |
| Species Name | Total Basal Area (m² ha−1) by DBH Class (cm) | IVI Components (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| (4.5–10) | (10.1–20) | (>20) | RD | RF | RDo | IVI | Av. IVI | |
| Eucalyptus globulus Labill | 0.11 | 2.01 | 4.71 | 45.08 | 44.29 | 42.88 | 132.25 | 44.08 |
| Juniperus procera Hoechst. Ex Endl | 0.11 | 1.73 | 4.80 | 38.13 | 40.53 | 41.65 | 120.31 | 40.10 |
| Acacia decurrens Willd | 0.04 | 0.52 | 1.75 | 14.15 | 12.54 | 14.48 | 41.17 | 13.72 |
| Olea europaea L. subsp. cuspidate | 0.00 | 0.07 | 0.00 | 0.96 | 1.04 | 0.42 | 2.42 | 0.81 |
| Hagenia abyssinica J.F.Gmel | 0.00 | 0.05 | 0.00 | 0.72 | 0.71 | 0.34 | 1.77 | 0.59 |
| Acacia melanoxylon R. Br. | 0.01 | 0.02 | 0.00 | 0.72 | 0.57 | 0.20 | 1.49 | 0.50 |
| Schefflera abyssinica (Hochst. ex A. Rich.) Harms | 0.11 | 0.00 | 0.00 | 0.24 | 0.32 | 0.03 | 0.59 | 0.20 |
| Total | 0.38 | 4.40 | 11.26 | 100 | 100 | 100 | 300 | 100 |
| Species Name | Species Richness | Shannon–Wiener Diversity Index | Evenness Index |
|---|---|---|---|
| Eucalyptus globulus Labill | 27 | 1.792 | 0.544 |
| Juniperus procera Hoechst. Ex Endl. | 31 | 1.649 | 0.480 |
| Acacia decurrens Willd | 10 | 0.558 | 0.242 |
| Hagenia abyssinica J.F.Gmel | 2 | 0.040 | 0.058 |
| Olea europaea L. subsp. cuspidate | 1 | 0.045 | 0.000 |
| Acacia melanoxylon R. Br. | 1 | 0.035 | 0.000 |
| Schefflera abyssinica (Hochst. ex A. Rich.) Harms | 1 | 0.014 | 0.000 |
| Average total | 10.857 | 0.590 | 0.187 |
| Habit | Total Density of Plant Individuals | Percentage Distribution of Plant Habit |
|---|---|---|
| Seedlings | 113 | 16.84% |
| Saplings | 141 | 21.01% |
| Trees | 417 | 62.15% |
| n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Carbon in Aboveground Biomass | 35 | ||||||||||
| 2 | Carbon in Belowground Biomass | 35 | 10.00 ** | |||||||||
| 3 | Carbon in Litter Biomass | 35 | 0.07 | 0.08 | ||||||||
| 4 | Soil Organic Carbon | 35 | 0.03 | 0.02 | 0.09 | |||||||
| 5 | Total Carbon | 35 | 0.47 ** | 0.46 ** | 0.11 | 0.90 ** | ||||||
| 6 | Important Value Index | 35 | 0.51 ** | 0.52 ** | −0.25 | 0.15 | 0.36 * | |||||
| 7 | Shannon–Wiener Woody Species Diversity | 35 | −0.23 | −0.23 | −0.05 | 0.13 | 0.02 | 0.01 | ||||
| 8 | Number of Trees | 35 | −0.23 | −0.23 | −0.02 | 0.13 | 0.01 | 0.01 | 0.91 ** | |||
| 9 | Number of Saplings | 35 | −0.01 | −0.01 | −0.39 * | −0.02 | −0.02 | 0.43 ** | −0.34 * | −0.36 * | ||
| 10 | Number of Seedlings | 35 | 0.25 | 0.24 | −0.21 | 0.11 | 0.2 | 0.46 ** | −0.12 | −0.18 | 0.34 * |
| n | 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|
| 1 | Shannon–Wiener woody species diversity | 35 | ||||
| 2 | Number of trees | 35 | 0.91 ** | |||
| 3 | Number of sapling | 35 | −0.34 * | −0.36 * | ||
| 4 | Number of seedling | 35 | −0.12 | −0.18 | 0.34 * |
| n | Mean | SD | F-Value | p-Value | |
|---|---|---|---|---|---|
| CAGB in lower altitude | 17 | 35.24 | 34.22 | 2.581 | 0.314 |
| CAGB in upper altitude | 18 | 71.19 | 84.08 | 17.687 | 0.055 * |
| CBGB in lower altitude | 17 | 7.04 | 6.85 | 2.565 | 0.316 |
| CBGB in upper altitude | 18 | 14.37 | 16.84 | 17.733 | 0.055 * |
| CLB in lower altitude | 17 | 0.09 | 0.02 | 0.961 | 0.621 |
| CLB in upper altitude | 18 | 0.09 | 0.04 | 0.361 | 0.905 |
| SOC in lower altitude | 17 | 452.67 | 106.22 | 0.679 | 0.737 |
| SOC in upper altitude | 18 | 486.98 | 197.94 | 0.176 | 0.986 |
| n | Mean | SD | F-Value | p-Value | |
|---|---|---|---|---|---|
| CAGB | 35 | 53.73 | 66.47 | 0.436 | 0.729 |
| CBGB | 35 | 10.81 | 13.33 | 0.460 | 0.712 |
| CLB | 35 | 0.09 | 0.03 | 0.553 | 0.650 |
| SOC | 35 | 470.32 | 156.75 | 0.559 | 0.646 |
| No. of T | 35 | 11.91 | 3.89 | 1.185 | 0.331 |
| No. of SA | 35 | 4.03 | 4.91 | 1.049 | 0.385 |
| No. of SE | 35 | 3.23 | 5.62 | 0.591 | 0.626 |
| IVI | 35 | 10.34 | 4.15 | 0.806 | 0.500 |
| H’ | 35 | 0.11 | 0.04 | 1.444 | 0.249 |
| Alt | 35 | 2660 | 113.83 | 0.112 | 0.953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomon, L.W.; Arunrat, N.; Phutthai, T.; Wisawapipat, W.; Sereenonchai, S.; Hatano, R. Carbon Stock Estimation and Human Disturbance in Selected Urban Un-Conserved Forests in Entoto Mountain Forest, Addis Ababa, Ethiopia. Diversity 2025, 17, 225. https://doi.org/10.3390/d17040225
Solomon LW, Arunrat N, Phutthai T, Wisawapipat W, Sereenonchai S, Hatano R. Carbon Stock Estimation and Human Disturbance in Selected Urban Un-Conserved Forests in Entoto Mountain Forest, Addis Ababa, Ethiopia. Diversity. 2025; 17(4):225. https://doi.org/10.3390/d17040225
Chicago/Turabian StyleSolomon, Lemlem Wondwossen, Noppol Arunrat, Thamarat Phutthai, Worachart Wisawapipat, Sukanya Sereenonchai, and Ryusuke Hatano. 2025. "Carbon Stock Estimation and Human Disturbance in Selected Urban Un-Conserved Forests in Entoto Mountain Forest, Addis Ababa, Ethiopia" Diversity 17, no. 4: 225. https://doi.org/10.3390/d17040225
APA StyleSolomon, L. W., Arunrat, N., Phutthai, T., Wisawapipat, W., Sereenonchai, S., & Hatano, R. (2025). Carbon Stock Estimation and Human Disturbance in Selected Urban Un-Conserved Forests in Entoto Mountain Forest, Addis Ababa, Ethiopia. Diversity, 17(4), 225. https://doi.org/10.3390/d17040225








