Aromatic Profiling and Bioactive Potentials of Thai Edible Flowers from the Curcuma spp. (Zingiberaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Analysis of the Chemical Composition of Aroma Compounds
2.3. Nutritional Composition
2.3.1. Proximate Composition
2.3.2. Crude Fiber
2.3.3. Elements
2.3.4. Free Fatty Acid Compositions
2.4. Phytochemical Analyses
2.4.1. Total Phenolic, Flavonoid Content
2.4.2. Antioxidant Activities
2.5. Statistical Analysis
3. Results and Discussion
3.1. Plant Utilization
3.2. Aroma Composition and Profiling
3.3. Nutritional Composition
3.3.1. Proximate and Mineral Composition
3.3.2. Free Fatty Acids
3.4. Phytochemical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knez, M.; Ranić, M.; Gurinović, M. Underutilized plants increase biodiversity, improve food and nutrition security, reduce malnutrition, and enhance human health and well-being. Let’s put them back on the plate! Nutr. Rev. 2024, 82, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Peduruhewa, P.; Jayathunge, K.; Liyanage, R. Potential of underutilized wild edible plants as the food for the future—A review. J. Food Secur. 2021, 9, 136–147. [Google Scholar]
- Sommano, S.R.; Tangpao, T. Chapter 4—Aromatic profile of rhizomes from the ginger family used in food. In Aromatic Herbs in Food; Galanakis, C.M., Ed.; Elsevier Inc.: London, UK, 2021; pp. 123–165. [Google Scholar]
- Rajkumari, S.; Sanatombi, K. Nutritional value, phytochemical composition, and biological activities of edible Curcuma species: A review. Int. J. Food Prop. 2017, 20, S2668–S2687. [Google Scholar] [CrossRef]
- Sirirugsa, P.; Larsen, K.; Maknoi, C. The genus Curcuma L. (Zingiberaceae): Distribution and classification with reference to species diversity in Thailand. Gard. Bull. Singap. 2007, 59, 203–220. [Google Scholar]
- Dutta, B. Study of secondary metabolite constituents and curcumin contents of six different species of genus Curcuma. J. Med. Plants Stud. 2015, 3, 116–119. [Google Scholar]
- Rachkeeree, A.; Kantadoung, K.; Suksathan, R.; Puangpradab, R.; Page, P.A.; Sommano, S.R. Nutritional compositions and phytochemical properties of the edible flowers from selected Zingiberaceae found in Thailand. Front. Nutr. 2018, 5, 3. [Google Scholar] [CrossRef]
- Sommano, S.R.; Suksathan, R.; Wongnak, M.; Chatkaewnapanon, Y. Edible Flowers in Thailand Rainy Season; RPP ALL Co., Ltd.: Chiang Mai, Thailand, 2017. [Google Scholar]
- Abdullah, A.T.M.; Rahman, M.M.; Sharif, M.; Khan, T.A.; Islam, S.N.; Karim, K.M.R. Edible flowers efficiency to boost the thermal oxidation stability of soybean oil: Polyphenolic and antioxidant insights. Future Foods 2024, 9, 100338. [Google Scholar] [CrossRef]
- Tangpao, T.; Chung, H.H.; Sommano, S.R. Aromatic profiles of essential oils from five commonly used Thai basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef]
- Wongkaew, M.; Sangta, J.; Chansakaow, S.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R. Volatile profiles from over-ripe purée of Thai mango varieties and their physiochemical properties during heat processing. PLoS ONE 2021, 16, e0248657. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (poly) phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Chapter 15 Therapeutic and medicinal uses of terpenes. In Medicinal Plants: From Farm to Pharmacy, 1st ed.; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The cannabis terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Lock, J.M.; Maas, H.; Maas, P.J.M. Zingiberaceae. In Flowering Plants Monocotyledons: Alismatanae and Commelinanae (except Gramineae); Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 474–495. [Google Scholar]
- Charoimek, N.; Sunanta, P.; Tangpao, T.; Suksathan, R.; Chanmahasathien, W.; Sirilun, S.; Hua, K.-F.; Chung, H.H.; Sommano, S.R.; Junmahasathien, T. Pharmaceutical potential evaluation of Damask rose by-products from volatile oil extraction. Plants 2024, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Suksathan, R.; Rachkeeree, A.; Puangpradab, R.; Kantadoung, K.; Sommano, S.R. Phytochemical and nutritional compositions and antioxidants properties of wild edible flowers as sources of new tea formulations. NFS J. 2021, 24, 15–25. [Google Scholar]
- Thiex, N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. J. AOAC Int. 2019, 92, 61–73. [Google Scholar] [CrossRef]
- The Food Drug Administration (FDA). Food labeling: Revision of the nutrition and supplement facts label. Fed. Regist. 2016, 81, 33742–33999. [Google Scholar]
- Xiao, J.H.; Xiao, D.M.; Sun, Z.H.; Xiong, Q.; Liang, Z.Q.; Zhong, J.J. Chemical compositions and antimicrobial property of three edible and medicinal Cordyceps Species. J. Food Agric. Environ. 2009, 7, 91–100. [Google Scholar]
- Folch, J.; Lee, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1975, 226, 497–509. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar]
- Ao, C.; Deba, F.; Tako, M.; Tawata, S. Biological activity and composition of extract from aerial root of Ficus microcarpa L. fil. Int. J. Food Sci. Technol. 2009, 44, 349–358. [Google Scholar]
- Settharaksa, S.; Madaka, F.; Sueree, L.; Kittiwisut, S.; Sakunpak, A.; Moton, C.; Charoenchai, L. Effect of solvent types on phenolic, flavonoid contents and antioxidant activities of Syzygium gratum (Wight) SN. Int. J. Pharm. Pharm. Sci. 2014, 6, 114–116. [Google Scholar]
- Yen, G.C.; Hsieh, C.L. Antioxidant effects of dopamine and related compounds. Biosci. Biotechnol. Biochem. 1997, 61, 1646–1649. [Google Scholar] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [PubMed]
- Nopporncharoenkul, N.; Jenjittikul, T.; Chuenboonngarm, N.; Anamthawat-Jónsson, K.; Umpunjun, P. Cytogenetic verification of Curcuma candida (Zingiberaceae) from Thailand and Myanmar. Thai For. Bull. (Bot.) 2020, 48, 7–17. [Google Scholar]
- Jenjittikul, T.; Larsen, K. Kaempferia candida Wall.(Zingiberaceae), a new record for Thailand. Thai For. Bull. (Bot.) 2000, 28, 45–49. [Google Scholar]
- Inta, A.; Trisonthi, C.; Pongamornkul, W.; Panyadee, P. Ethnobotany of Zingiberaceae in Mae Hong Son, Northern Thailand. Biodiversitas J. Biol. Divers. 2023, 24, 2114–2124. [Google Scholar]
- Saisor, N.; Prathepha, P.; Saensouk, S. Ethnobotanical study and utilization of plants in Khok Nhong Phok forest, Kosum Phisai district, northeastern Thailand. Biodiversitas J. Biol. Divers. 2021, 22, 4336–4348. [Google Scholar]
- Ragsasilp, A.; Saensouk, P.; Saensouk, S. Ginger family from Bueng Kan Province, Thailand: Diversity, conservation status, and traditional uses. Biodiversitas J. Biol. Divers. 2022, 23, 2739–2752. [Google Scholar]
- Saensouk, S.; Saensouk, P.; Pasorn, P.; Chantaranothai, P. Diversity and uses of Zingiberaceae in Nam Nao National Park, Chaiyaphum and Phetchabun provinces, Thailand, with a new record for Thailand. Agric. Nat. Resour. 2016, 50, 445–453. [Google Scholar]
- Cruz-Garcia, G.S.; Price, L.L. Ethnobotanical investigation of ‘wild’ food plants used by rice farmers in Kalasin, Northeast Thailand. J. Ethnobiol. Ethnomed. 2011, 7, 33. [Google Scholar]
- Perry, L.M.; Methzger, J. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; MIT Press: London, UK, 1980; pp. 1–632. [Google Scholar]
- Song, C.; Tian, J.; Xie, D.; Lin, S.; Yang, Y.; Zhang, X.; Liao, X.; Wu, Z. Metabolomic and transcriptomic analyses provide insight into the variation of floral scent and molecular regulation in different cultivars and flower development of Curcuma alismatifolia. Hortic. Res. 2025, 12, uhae348. [Google Scholar]
- Peng, W.; Li, P.; Ling, R.; Wang, Z.; Feng, X.; Liu, J.; Yang, Q.; Yan, J. Diversity of Volatile Compounds in Ten Varieties of Zingiberaceae. Molecules 2022, 27, 565. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, L.; Huang, D.; Wu, G.; Li, X.; Yue, Y.; Yu, Y.; Yu, R.; Fan, Y. Identification and functional analysis of floral terpene synthase genes in Curcuma alismatifolia. Planta 2024, 260, 26. [Google Scholar] [CrossRef] [PubMed]
- Chane-Ming, J.; Vera, R.; Chalchat, J.-C.; Cabassu, P. Chemical Composition of Essential Oils from Rhizomes, Leaves and Flowers of Curcuma longa L. from Reunion Island. J. Essent. Oil Res. 2002, 14, 249–251. [Google Scholar] [CrossRef]
- Tran-Trung, H.; Dau, X.D.; Nguyen, T.C.; Nguyen-Thi-Thu, H.; Nguyen-Ngoc, H.; Nguyen, T.G.A.; Hoang, V.T.; Nguyen, D.K.; Nguyen, D.D.; Tran Van, C. Phytochemical analysis of the essential oils from the rhizomes of three Vietnamese Curcuma species and their antimicrobial activity. Nat. Prod. Commun. 2023, 18, 1–8. [Google Scholar] [CrossRef]
- Cuong, N.M.; Ha, V.T.; Khanh, P.N.; Van, D.T.; Cuong, T.D.; Huong, T.T.; Thuy, D.T.T.; Nhan, N.T.; Hanh, N.P.; Toan, T.Q. Chemical compositions and antimicrobial activity of essential oil from the rhizomes of Curcuma singularis growing in Vietnam. Am. J. Essent. Oils Nat. Prod. 2017, 5, 20–25. [Google Scholar]
- Kumari, P.; Bhargava, B. Phytochemicals from edible flowers: Opening a new arena for healthy lifestyle. J. Funct. Foods 2021, 78, 104375. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. The Unexplored Potential of Edible Flowers Lipids. Agriculture 2018, 8, 146. [Google Scholar] [CrossRef]
- Choudhury, D. Study on the nutrient composition of local variety of turmeric (Curcuma longa). J. Pharm. Innov. 2019, 8, 205–207. [Google Scholar]
- Mlcek, J.; Plaskova, A.; Jurikova, T.; Sochor, J.; Baron, M.; Ercisli, S. Chemical, nutritional and sensory characteristics of six ornamental edible flowers species. Foods 2021, 10, 2053. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Meller, E.; Wysocka, G. Mineral composition of some edible flowers. J. Elem. 2018, 23, 151–162. [Google Scholar] [CrossRef]
- Dowhan Hoag, L.; Dharmarajan, T. Calcium and phosphorus. In Geriatric Gastroenterology; Pitchumoni, C.S., Dharmarajan, T.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–29. [Google Scholar]
- Prasad, R.; Prasad, S.; Lal, R. Phosphorus in soil and plants in relation to human nutrition and health. In Soil Phosphorus, 1st ed.; Lal, R., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 65–80. [Google Scholar]
- Si, M.K.; Ali, A.; Vaidya, A.; Singh, S.; Deshmukh, K.; Bhagat, N.; Hanwat, P.; Sahu, N.; Bhagat, P.; Tilase, S. Quantitative analysis of essential metals (magnesium, calcium and iron) in various Indian vegetables and fruits. Int. J. Eng. Res. Technol. 2021, 10, 266–269. [Google Scholar]
- Suliburska, J.; Kaczmarek, K. Herbal infusions as a source of calcium, magnesium, iron, zinc and copper in human nutrition. Int. J. Food Sci. Nutr. 2012, 63, 194–198. [Google Scholar]
- Wei, S.; Liu, H.; Li, J.; Ren, T.; Xie, J. Metabolite variations of sugars, organic acids, fatty acids and amino acids in flower buds of Zingiber mioga Roscoe at different developmental stages. J. Food Compos. Anal. 2023, 116, 105050. [Google Scholar]
- Connor, W.E. α-Linolenic acid in health and disease. Am. J. Clin. 1999, 69, 827–828. [Google Scholar]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. 2014, 100, 443S–448S. [Google Scholar]
- Innis, S.M. Palmitic acid in early human development. Crit. Rev. Food Sci. Nutr. 2016, 56, 1952–1959. [Google Scholar]
- Rachkeeree, A.; Kantadoung, K.; Puangpradub, R.; Suksathan, R. Phytochemicals, Antioxidants and Anti-Tyrosinase Analyses of Selected Ginger Plants. Pharmacogn. J. 2020, 12, 872–883. [Google Scholar]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Rs. 2019, 10, 1567–1574. [Google Scholar]
- Panche, A.N.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar]
- Petrova, I.; Petkova, N.; Ivanov, I. Five edible flowers–valuable source of antioxidants in human nutrition. Int. J. Pharmacogn. Pharm. Res. 2016, 8, 604–610. [Google Scholar]
- Sandeep, I.S.; Kuanar, A.; Akbar, A.; Kar, B.; Das, S.; Mishra, A.; Sial, P.; Naik, P.K.; Nayak, S.; Mohanty, S. Agroclimatic zone based metabolic profiling of turmeric (Curcuma Longa L.) for phytochemical yield optimization. Ind. Crops Prod. 2016, 85, 229–240. [Google Scholar]
- Albaqami, J.J.; Hamdi, H.; Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Kuttithodi, A.M.; Famurewa, A.C.; Pathrose, B. Chemical composition and biological activities of the leaf essential oils of Curcuma longa, Curcuma aromatica and Curcuma angustifolia. Antibiotics 2022, 11, 1547. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, I.S.; Das, S.; Nasim, N.; Mishra, A.; Acharya, L.; Joshi, R.K.; Nayak, S.; Mohanty, S. Differential expression of CURS gene during various growth stages, climatic condition and soil nutrients in turmeric (Curcuma longa): Towards site specific cultivation for high curcumin yield. Plant Physiol. Biochem. 2017, 118, 348–355. [Google Scholar] [PubMed]
- Sasikumar, B. Genetic resources of Curcuma: Diversity, characterization and utilization. Plant Genet. Resour. 2005, 3, 230–251. [Google Scholar]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Alam, M.A.; Roy, S.; Rahman, M.A.; Islam, M.R.; Rahman, M.M.; Obaidullah, A.J.; Farid, M.N.; Rahman, M.M.; Islam, M.R.; Mozumder, S.N. Study on the genetic variability and adaptability of turmeric (Curcuma longa L.) genotypes for development of desirable cultivars. PLoS ONE 2024, 19, e0297202. [Google Scholar]
- Anandaraj, M.; Prasath, D.; Kandiannan, K.; Zachariah, T.J.; Srinivasan, V.; Jha, A.; Singh, B.; Singh, A.; Pandey, V.; Singh, S. Genotype by environment interaction effects on yield and curcumin in turmeric (Curcuma longa L.). Ind. Crops Prod. 2014, 53, 358–364. [Google Scholar]
- Dudekula, M.V.; Kandasamy, V.; Balaraman, S.S.; Selvamani, S.B.; Muthurajan, R.; Adhimoolam, K.; Manoharan, B.; Natesan, S. Unlocking the genetic diversity of Indian turmeric (Curcuma longa L.) germplasm based on rhizome yield traits and curcuminoids. Front. Plant Sci. 2022, 13, 1036592. [Google Scholar]
- Alam, M.A.; Rahman, M.; Ahmed, S.; Jahan, N.; Khan, M.A.-A.; Islam, M.R.; Alsuhaibani, A.M.; Gaber, A.; Hossain, A. Genetic variation and genotype by environment interaction for agronomic traits in maize (Zea mays L.) hybrids. Plants 2022, 11, 1522. [Google Scholar] [CrossRef]
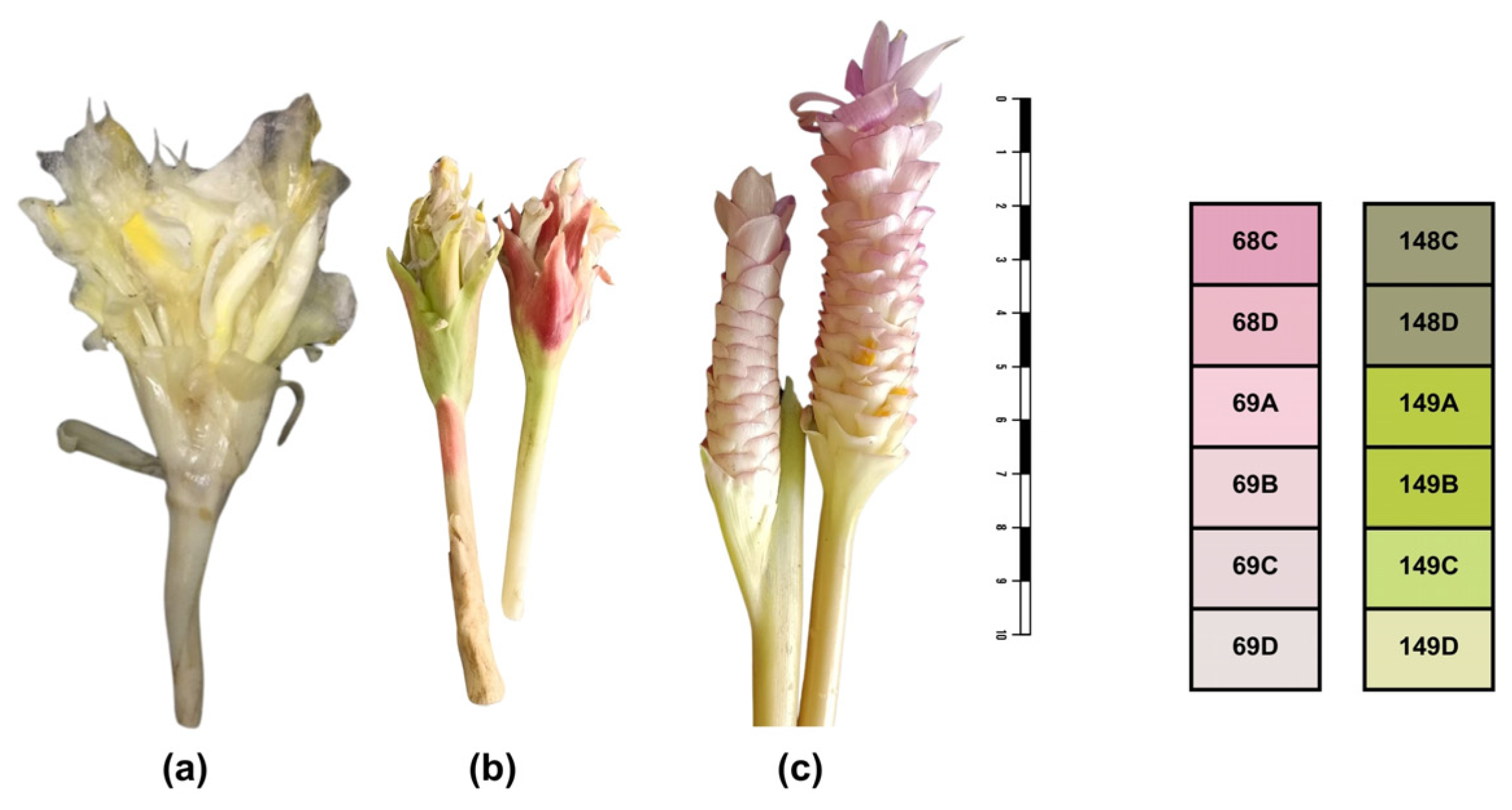

| No | Collection Numbers | Local Name; Scientific Name | Plant Parts Used | Utilization |
|---|---|---|---|---|
| 1 | LC014 | Dok din, Curcuma candida | Inflorescences | Young inflorescences are steamed and consumed as a vegetable, commonly paired with chili, stir-fried, or incorporated into spicy soups. |
| 2 | LC012 | Krachiao khao, Curcuma singularis | Inflorescences | Young inflorescences are steamed and consumed as a vegetable |
| 3 | LC017 | Queen lily ginger, Curcuma petiolata | Inflorescences | Young inflorescences are occasionally steamed, consumed as a vegetable and seasonal food source |
| Nutritional Parameters | Curcuma candida | Curcuma singularis | Curcuma petiolata |
|---|---|---|---|
| Proximate composition (g/100 g DW) | |||
| Moisture content | 14.62 ± 0.69 b | 17.81 ± 0.88 a | 12.83 ± 0.66 c |
| Ash | 17.04 ± 0.12 b | 14.68 ± 0.16 c | 18.27 ± 0.19 a |
| Fat | 5.60 ± 0.07 b | 5.36 ± 0.39 c | 6.72 ± 0.01 a |
| Carbohydrate | 81.68 ± 0.61 b | 77.23 ± 0.06 c | 83.47 ± 0.33 a |
| Fiber | 19.64 ± 0.26 | 19.19 ± 1.14 | 19.49 ± 0.23 |
| Protein | 10.71 ± 0.30 c | 12.74 ± 0.19 b | 18.04 ± 1.18 a |
| Total energy | 333.26 ± 0.04 b | 318.40 ± 1.93 c | 342.83 ± 3.66 a |
| Macro-elements (g/100 g DW) | |||
| Phosphorus | 0.40 ± 0.00 a | 0.28 ± 0.04 c | 0.36 ± 0.01 b |
| Potassium | 3.06 ± 0.01 a | 2.60 ± 0.03 b | 1.82 ± 0.01 c |
| Sodium | 0.46 ± 0.01 b | 0.48 ± 0.01 a | 0.33 ± 0.00 c |
| Calcium | 2.49 ± 0.00 a | 1.01 ± 0.01 c | 1.30 ± 0.01 b |
| Micro-elements (g/100 g DW) | |||
| Iron | 0.47 ± 0.02 a | 0.29 ± 0.00 b | 0.22 ± 0.00 c |
| Magnesium | 0.59 ± 0.01 a | 0.36 ± 0.00 c | 0.41 ± 0.00 b |
| Free fatty Acid Composition (mg/100 g DW) | Curcuma candida | Curcuma petiolata | Curcuma singularis |
|---|---|---|---|
| Behenic acid | 1647.00 ± 106.07 | Nd. | Nd. |
| Linoleic acid | 30,323.50 ± 433.46 b | 45,356.00 ± 181.02 a | 34,953.50 ± 142.13 b |
| Oleic acid | 10,266.50 ± 258.09 a | 6851.50 ± 33.23 b | 13,217.50 ± 190.21 a |
| Palmitic acid | 42,943.00 ± 219.20 a | 31,097.50 ± 96.87 b | 32,978.50 ± 54.45 b |
| Stearic acid | 6151.00 ± 8.49 a | 3924.00 ± 2.83 c | 4446.50 ± 116.67 b |
| α-Linolenic acid | 8669.00 ± 55.15 c | 12,770.50 ± 248.19 b | 14,404.00 ± 15.56 a |
| Phytochemicals | Curcuma candida | Curcuma singularis | Curcuma petiolata |
|---|---|---|---|
| Total phenolics (mg GAE/g DW) | 0.06 ± 0.00 c | 0.26 ± 0.01 a | 0.23 ± 0.02 b |
| Total flavonoids (mg QE/g DW) | 0.09 ± 0.01 c | 0.11 ± 0.01 b | 0.20 ± 0.03 a |
| DPPH (mg TEAC/g DW) | 3.06 ± 0.01 c | 3.34 ± 0.08 b | 4.98 ± 0.02 a |
| DPPH (IC50 mg/g DW) | 10.01 ± 0.01 b | 10.32 ± 0.09 c | 8.80 ± 0.07 a |
| ABTS+ (mg TEAC/g DW) | 0.70 ± 0.02 c | 1.47 ± 0.03 b | 2.57 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommano, S.R.; Thomya, S.; Ai, P.N.; Khemacheewakul, J.; Sindhu, R.; Prasad, S.K.; Thongdang, P.; Rachkeeree, A.; Wongnak, M.; Panyadee, P.; et al. Aromatic Profiling and Bioactive Potentials of Thai Edible Flowers from the Curcuma spp. (Zingiberaceae). Diversity 2025, 17, 224. https://doi.org/10.3390/d17040224
Sommano SR, Thomya S, Ai PN, Khemacheewakul J, Sindhu R, Prasad SK, Thongdang P, Rachkeeree A, Wongnak M, Panyadee P, et al. Aromatic Profiling and Bioactive Potentials of Thai Edible Flowers from the Curcuma spp. (Zingiberaceae). Diversity. 2025; 17(4):224. https://doi.org/10.3390/d17040224
Chicago/Turabian StyleSommano, Sarana Rose, Sureerat Thomya, Pasin Norkum Ai, Julaluk Khemacheewakul, R. Sindhu, Shashanka K. Prasad, Pawenud Thongdang, Apinya Rachkeeree, Methee Wongnak, Prateep Panyadee, and et al. 2025. "Aromatic Profiling and Bioactive Potentials of Thai Edible Flowers from the Curcuma spp. (Zingiberaceae)" Diversity 17, no. 4: 224. https://doi.org/10.3390/d17040224
APA StyleSommano, S. R., Thomya, S., Ai, P. N., Khemacheewakul, J., Sindhu, R., Prasad, S. K., Thongdang, P., Rachkeeree, A., Wongnak, M., Panyadee, P., Puangpradab, R., & Suksathan, R. (2025). Aromatic Profiling and Bioactive Potentials of Thai Edible Flowers from the Curcuma spp. (Zingiberaceae). Diversity, 17(4), 224. https://doi.org/10.3390/d17040224








