Sand Prawns Mitigate the Impact of Prolonged Drought on the Biology of a Temporary Open/Closed Estuary
Abstract
1. Introduction
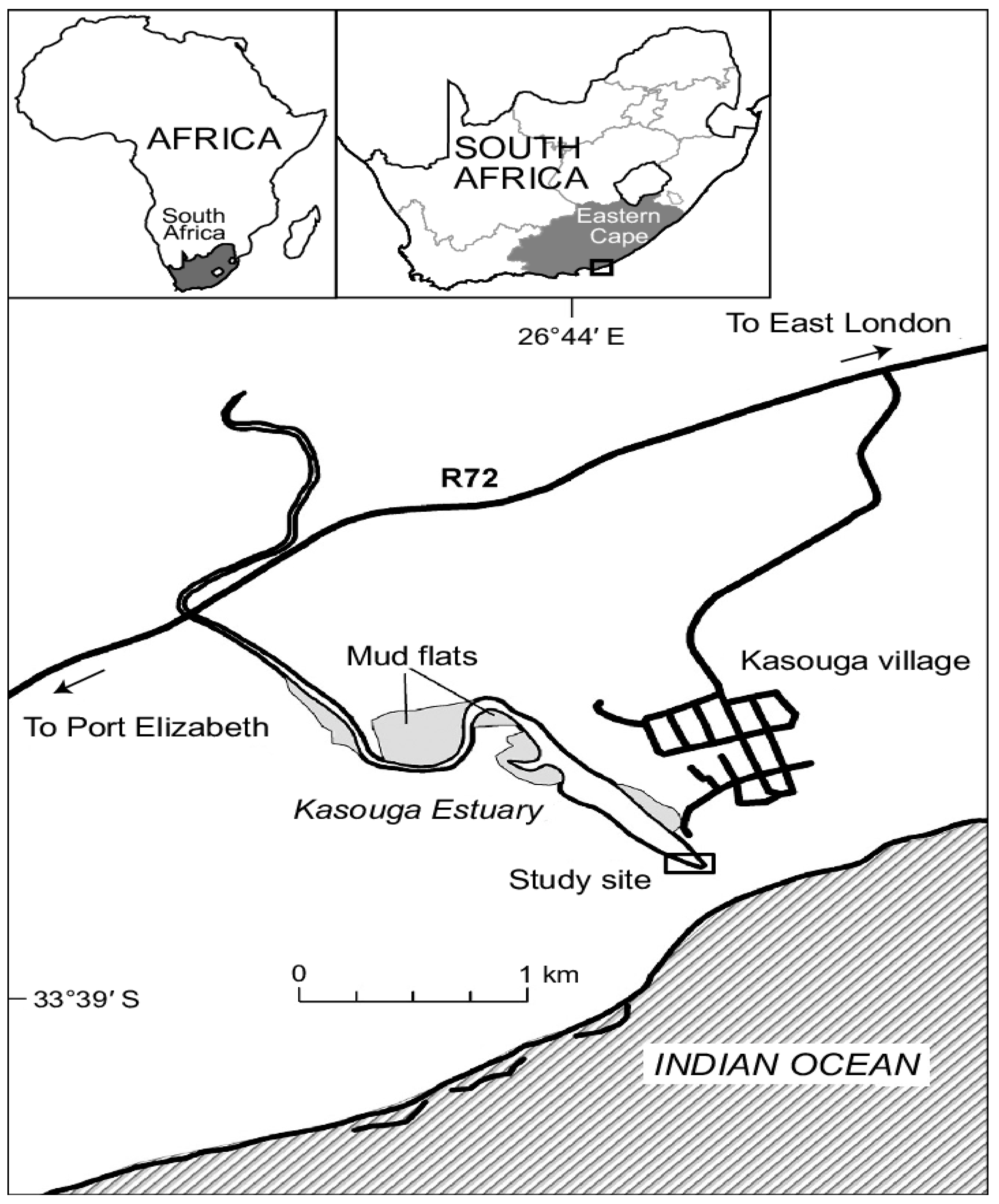
2. Study Site
3. Materials and Methods
3.1. Bioturbation
3.2. Microphytobenthic Algal Concentrations
3.3. Macrobenthic Community Structure
3.4. Statistical Analysis
4. Results
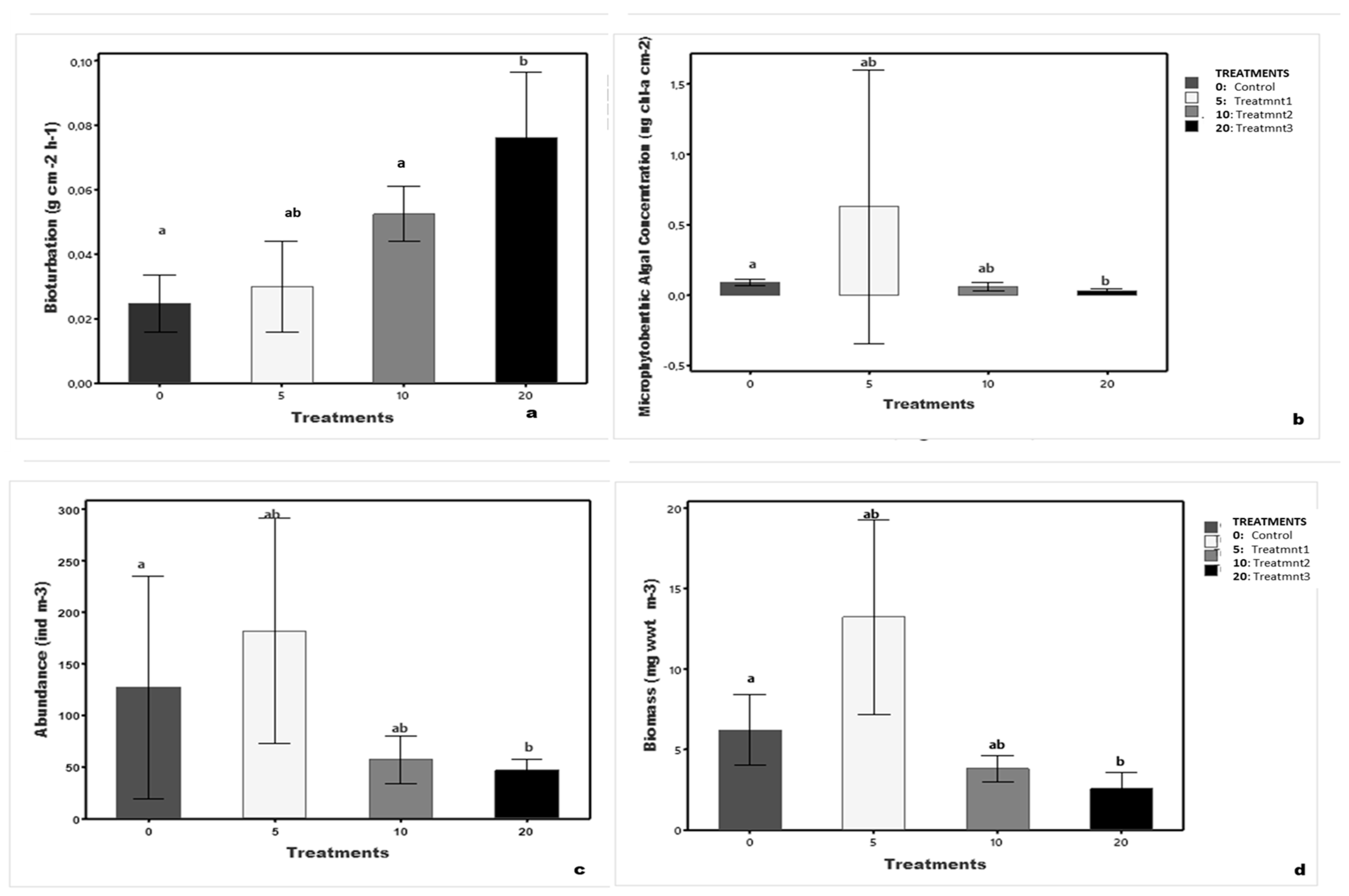
4.1. Bioturbation
4.2. Microphytobenthic Algal Concentration
4.3. Macrobenthic Abundance
4.4. Macrobenthic Biomass
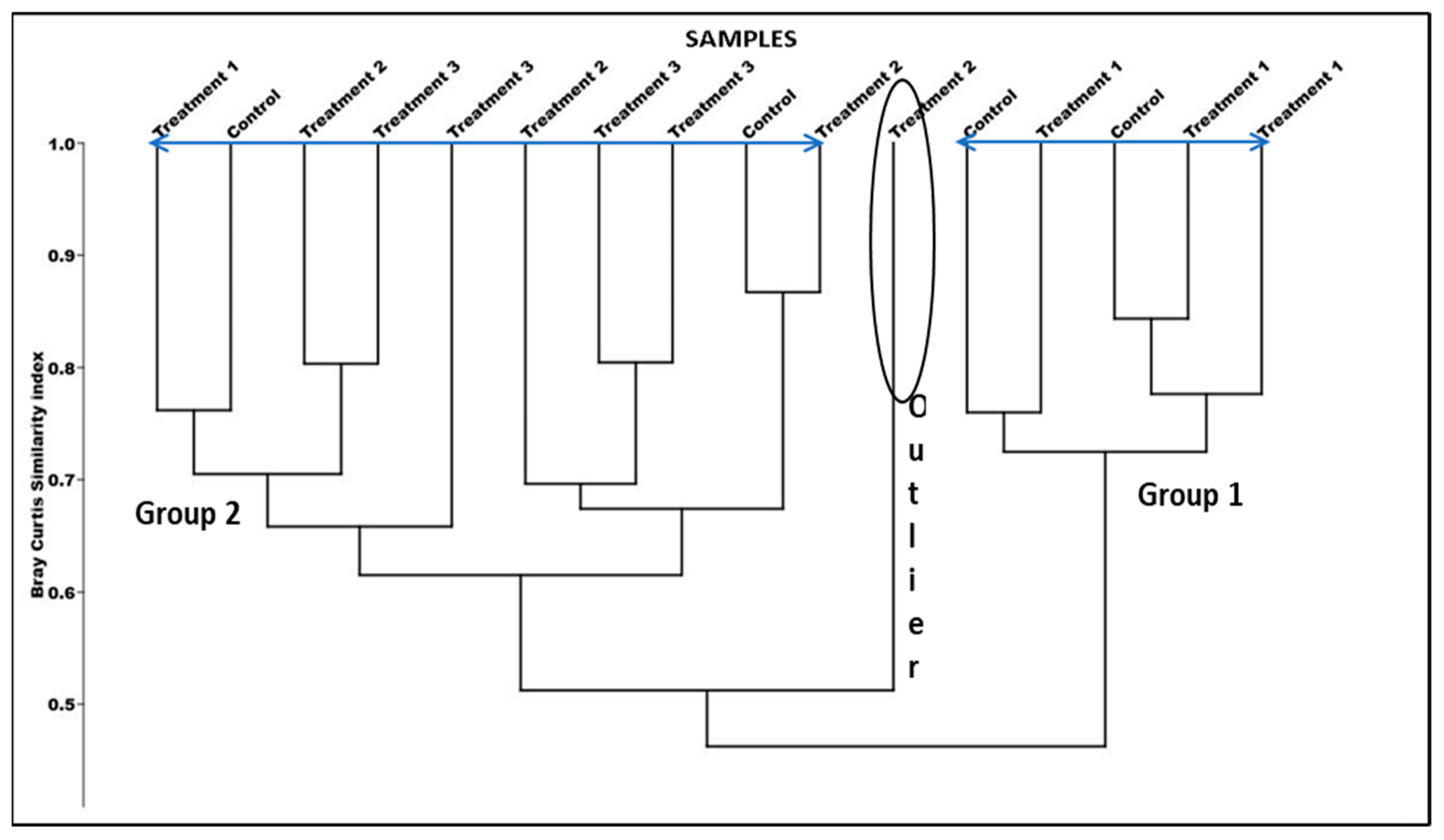
4.5. Bray–Curtis Cluster Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moyo, R. Ecology and Behaviour of Burrowing Prawns and Their Burrow Symbionts. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2014. [Google Scholar]
- Romero, G.; Gonçalves-Souza, T.; Curti, C.; Koricheva, J. Ecosystem engineering effects on species diversity across ecosystems: A Meta-analysis. Biol. Rev. 2014, 90, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.; Branch, G.M. Ecosystem engineers: Interactions between eelgrass Zostera capensis and the sandprawn Callianassa kraussi and their indirect effects on the mudprawn Upogebia africana. J. Exp. Mar. Biol. Ecol. 2006, 338, 253–270. [Google Scholar] [CrossRef]
- Meysman, F.J.R.; Middelburg, J.J.; Heip, C.H.R. Bioturbation: A fresh look at Darwin’s last idea. Trends Ecol. Evol. 2006, 21, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Henninger, T.O.; Froneman, P.W. Role of the sand prawn Callichirus kraussi as an ecosystem engineer in a South African temporarily open/closed estuary. Afr. J. Aquat. Sci. 2013, 38, 101–107. [Google Scholar] [CrossRef]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.O.; Banta, G.T. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef]
- Richter, R. Fluidal-texture in Sediment-Gesteinen und ober Sedifluktion überhaupt. Notizbl. Hess. L. Amt. Bodenforsch. 1952, 3, 67–81. [Google Scholar]
- Dauwe, B.; Herman PM, J.; Heip, C.H.R. Community structure and bioturbation potential of macrofauna at four North Sea stations with contrasting food supply. Mar. Ecol. Prog. Ser. 1998, 173, 67–83. [Google Scholar] [CrossRef]
- Wilkinson, M.T.; Richards, P.J.; Humphreys, G.S. Breaking ground: Pedological, geological, and ecological implications of soil bioturbation. Earth-Sci. Rev. 2009, 97, 257–272. [Google Scholar] [CrossRef]
- Vasquez-Cardenas, D.; Quintana, C.O.; Meysman, F.J.R.; Kristensen, E.; Henricus, T.S.; Boschker, H.T.S. Species-specific effects of two bioturbating polychaetes on sediment chemoautotrophic bacteria. Mar. Ecol. Prog. Ser. 2016, 549, 55–68. [Google Scholar] [CrossRef]
- Krantzberg, G. The influence of bioturbation on physical, chemical and biological parameters in aquatic environments: A review. Environ. Pollut. (Ser. A) 1985, 39, 99–122. [Google Scholar] [CrossRef]
- Biles, C.; Paterson, D.; Ford, R.; Solan, M.; Raffaelli, D. Bioturbation, ecosystem functioning and community structure. Hydrol. Earth Syst. Sci. 2002, 6, 999–1005. [Google Scholar] [CrossRef]
- Pillay, D.; Branch, G.M.; Forbes, A.T. Experimental evidence for the effects of the thalassinidean sand prawn Callianassa kraussi on macrobenthic communities. Mar. Biol. 2007, 152, 611–618. [Google Scholar] [CrossRef]
- Pillay, D.; Branch, G.M.; Forbes, A.T. Effects of Callianassa kraussi on microbial biofilms and recruitment of macrofauna: A novel hypothesis for adult-juvenile interactions. Mar. Ecol. Prog. Ser. 2007, 347, 1–14. [Google Scholar] [CrossRef]
- Hanekom, N.; Russell, I. Temporal changes in the macrobenthos of sand prawn (Callichirus kraussi) beds in Swartvlei Estuary. S. Afr. Zool. 2015, 50, 41–51. [Google Scholar] [CrossRef]
- Whitfield, A.K. Biology and Ecology of Fishes in Southern African Estuaries; J.L.B. Smith Institute of Ichthyology: Grahamstown, South Africa, 1998; p. 223. [Google Scholar]
- Whitfield, A.K. Fishes of Southern African Estuaries: From Species to Systems. 2022. Available online: http://hdl.handle.net/10962/97933 (accessed on 13 June 2023).
- Gama, P.T.; Adams, J.B.; Schael, D.M.; Skinner, T. Phytoplankton Chlorophyll a Concentration and Community Structure of Two Temporarily Open/Closed Estuaries; WRC Report No. 1255/1/05; Department of Botany, Nelson Mandela Metropolitan University: Port Elizabeth, South Africa, 2005; ISBN 1-77005-326-3. [Google Scholar]
- Whitfield, A.K.; Bate, G.C. (Eds.) A Review of Information on Temporary Open/Closed Estuaries in the Warm and Cool Temperate Biogeographic Region of South Africa, with Particular Emphasis on the Influence of River Flow on These Systems; WRC Report 1581/1/07; Water Research Commission: Pretoria, South Africa, 2007. [Google Scholar]
- Whitfield, A.K.; Adams, J.B.; Bate, G.C.; Bezuidenhout, K.; Bornman, T.G.; Cowley, P.D.; Froneman, P.W.; Gama, P.T.; James, N.C.; Mackenzie, B.; et al. A multidisciplinary study of a small, temporarily open/closed South African estuary, with particular emphasis on the influence of mouth state on the ecology of the system. Afr. J. Mar. Sci. 2008, 30, 453–473. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Bate, G.C.; Adams, J.B.; Cowley, P.D.; Froneman, P.W.; Gama, P.T.; Strydom, N.A.; Taljaard, S.; Theron, A.K.; Turpie, J.K.; et al. A review of the ecology and management of temporarily open/closed estuaries in South Africa, with particular emphasis on river flow and mouth state as primary drivers of these systems. Afr. J. Mar. Sci. 2012, 34, 163–180. [Google Scholar] [CrossRef]
- Harrison, T. Physico-chemical characteristics of South African estuaries in relation to the zoogeography of the region. Estuar. Coast. Shelf Sci. 2004, 61, 73–87. [Google Scholar] [CrossRef]
- Harrison, T.D.; Whitfield, A.K. Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries. Estuar. Coast. Shelf Sci. 2006, 66, 335–345. [Google Scholar] [CrossRef]
- Teske, P.; Wooldridge, T. A comparison of the benthic faunas of permanently open and temporarily open/closed South African estuaries. Hydrobiologia 2001, 464, 227–243. [Google Scholar] [CrossRef]
- Teske, P.R.; Wooldridge, T.H. What limits the distribution of subtidal macrobethos in permanently open and temporarily open/closed South African estuaries? Salinity vs sediment particle size. Estuar. Coast. Shelf Sci. 2003, 85, 407–421. [Google Scholar]
- Branch, G.M.; Pringle, A. The impact of the sand prawn Callianassa kraussi Stebbing on sediment turnover and on bacteria, meiofauna, and benthic microflora. J. Exp. Mar. Biol. Ecol. 1987, 107, 219–235. [Google Scholar] [CrossRef]
- Humphreys, G.S.; Mitchell, P.B. A Preliminary Assessment of the Role of Bioturbation and Rain Wash on Sandstone Hillslopes in the Sydney Basin; Australian and New Zealand Geomorphology Group: Queensland, Australia, 1983; pp. 66–80. [Google Scholar]
- Njozela, C. The Role of the Sand Prawn, Callichirus kraussi, as an Ecosystem Engineer in a Temporarily Open/Closed Eastern Cape Estuary, South Africa. Master’s Thesis, Rhodes University, Makhanda, South Africa, 2012; p. 163. [Google Scholar]
- Wyness, A.J.; Fortune, I.; Blight, A.J.; Browne, P.; Hartley, M.; Holden, M.; Paterson, D.M. Ecosystem engineers drive differing microbial community composition in intertidal estuarine sediments. PLoS ONE 2012, 16, e0240952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pillay, D.; Branch, G.M. Bioengineering effects of burrowing thalassinidean shrimps on marine soft-bottom ecosystems. Oceanogr. Mar. Biol. Ann. Rev. 2011, 49, 137–192. [Google Scholar]
- Goedefroo, N.; Braeckman, U.; Hostens, K.; Vanaverbeke, J.; Moens, T.; De Backer, A. Understanding the impact of sand extraction on benthic ecosystem functioning: A combination of functional indices and biological trait analysis. Front. Mar. Sci. 2023, 10, 1268999. [Google Scholar] [CrossRef]
- Emmerson, M.C.; Solan, M.; Emes, C.; Paterson, D.M.; Raffaelli, D. Consistent patterns and the idiosyncratic effects of biodiversity in marine systems. Nature 2001, 411, 73–77. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Summary for policymakers. In Climate Change. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Ziervogel, G.; New, M.; van Garderen, A.E.; Midgley, G.; Taylor, A.; Hamann, R.; Stuart-Hill, S.; Myers, J.; Warburton, M. Climate change impacts and adaptation in South Africa. Wiley Interdiscip. Rev. Clim. Chang. 2014, 5, 605–620. [Google Scholar] [CrossRef]
- Mahlalela, P.T.; Blamey, R.C.; Hart, N.C.G.; Reason, C.J.C. Drought in the Eastern Cape region of South Africa and trends in rainfall characteristics. Clim. Dyn. 2020, 55, 2743–2759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Froneman, P.W. The Ecology and Food Web Dynamics of South African Intermittently Open Estuaries. In Estuary; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Whitfield, A.K. Available scientific information on individual South African Estuarine systems. In Water Research Commission Report; No. 577/3/00; Water Research Commission: Pretoria, South Africa, 2000; p. 217. [Google Scholar]
- Wasserman, R.J.; Kramer, R.; Vink TJ FFroneman, P.W. Conspecific alarm cue sensitivity by the estuarine calanoid copepod Paracartia longipatella. Austral Ecol. 2014, 39, 732–738. [Google Scholar] [CrossRef]
- Froneman, P.W. Seasonal changes in selected physico-chemical and biological variables in the temporarily open/closed kasouga estuary, Eastern Cape, South Africa. Afr. J. Aquat. Sci. 2002, 27, 117–123. [Google Scholar] [CrossRef]
- Whitfield, A.K. A characterization of southern African estuarine systems. South. Afr. J. Aquat. Sci. 1992, 18, 89–103. [Google Scholar] [CrossRef]
- Froneman, P.W. Response of the biology to three different hydrological phases in the temporarily open/closed Kasouga estuary. Estuar. Coast. Shelf Sci. 2002, 55, 535–546. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51 Pt 2, 364–376. [Google Scholar] [CrossRef]
- Wooldridge, T.H.; McGwynne, L. The Estuarine Environment; Report No. C31; Institute of Coastal Research: Port Elizabeth, South Africa, 1996; p. 91. [Google Scholar]
- Froneman, P.W. In situ grazing rates of the copepods, Pseudodiaptomus hessei and Acartia longipatella in a temperate, temporarily open/closed estuary. S. Afr. J. Sci. 2004, 100, 577–583. [Google Scholar]
- Wasserman, R.J.; Froneman, P.W. Risk effects on copepods: Preliminary experimental evidence for the suppression of clutch size by predatory early life-history fish. J. Plankton Res. 2013, 35, 421–426. [Google Scholar] [CrossRef]
- Henninger, T.; Froneman, P.W.; Richoux, N.; Hodgson, A.N. Role of submerged macrohytes and a refuge and food source for the estuarine isopod Exospheroma hylocoetes. Estuar. Coast. Shelf Sci. 2009, 82, 285–293. [Google Scholar] [CrossRef]
- Ellis, J.; Cumming, V.; Hewitt, J.; Thrush, S.; Norkko, A. Determining effects of suspended sediment on condition of suspension bivalve (Atrina zelanica): Results from a survey, a laboratory experiment and field transplant experiment. J. Exp. Mar. Biol. Ecol. 2002, 267, 147–174. [Google Scholar] [CrossRef]
- Menge, B.; Berlow, E.; Blanchette, C.; Navarrete, S.; Yamada, S. The keystone species concept: Variation in interactionstrength in a rocky intertidal habitat. Ecol. Monogr. 1994, 64, 249–286. [Google Scholar] [CrossRef]
- Day, J.H. A Guide to Marine Life on South African Shores; Bakema, A.A., Ed.; University of Cape Town: Cape Town, South Africa, 1969; p. 247. [Google Scholar]
- Bouma, T.J.; Olenin, S.; Reise, K.; Ysebaert, T. Ecosystem engineering and biodiversity in coastal sediments: Posing hypotheses. Helgol. Mar. Res. 2009, 63, 95–106. [Google Scholar] [CrossRef]
- Marenco, K.; Bottjer, D. 8 Ecosystem engineering in the fossil record: Early examples from the Cambrian Period. Theoretical. Ecol. Ser. 2007, 4, 163–184. [Google Scholar] [CrossRef]
- Berke, S. Functional Groups of Ecosystem Engineers: A Proposed Classification with Comments on Current Issues. Integr. Comp. Biol. 2010, 50, 147–157. [Google Scholar] [CrossRef]
- Ellison, A.M. Foundation species, non-trophic interactions, and the value of being common. iScience 2019, 13, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.M.; Valente, N.F.; Pacheco, E.O.; Ganci, C.L.; Mathew, A.M.; Adriano, S.P.; Diogo, B. How Does the Landscape Affect Metacommunity Structure? A Quantitative Review for Lentic Environments. Curr. Landsc. Ecol. 2020, 5, 68–75. [Google Scholar] [CrossRef]
- Nozias, C.; Persinotto, R.; Mundree, S. Annual cycle of microalgal biomass in a South African temporarily open estuary: Nutrient versus light limitation. Mar. Ecol. Prog. Ser. 2001, 223, 39–48. [Google Scholar] [CrossRef]
- Perissinotto, R.; Iyer, K.; Nozais, C. Response of microphytobenthos to flow and trophic variation in two South African temporarily open/closed estuaries. Bot. Mar. 2006, 49, 10–22. [Google Scholar] [CrossRef]
- Scharler, U.M.; Lechman, K.; Radebe, T.; Jerling, H.L. Effects of prolonged mouth closure in a temporarily open/closed estuary: A summary of the responses of invertebrate communities in the uMdloti Estuary, South Africa. Afr. J. Aquat. Sci. 2020, 45, 121–130. [Google Scholar] [CrossRef]
- Pillay, D. Expanding the envelope: Linking invertebrate bioturbators with micro-evolutionary change. Mar. Ecol. Prog. Ser. 2010, 409, 301–303. [Google Scholar] [CrossRef]
- Queiros, A.M.; Stephens, N.; Cook, R.; Ravaglioli, C.; Nunes, J.; Dashfield, S.; Harris, C.; Tilstone, G.H.; Fishwick, J.; Braeckman, U.; et al. Can benthic community structure be used to predict the process of bioturbation in real ecosystems? Prog. Oceanogr. 2015, 137, 559–569. [Google Scholar] [CrossRef]
- Pillay, D.; Branch, G.M.; Dawson, J.; Henry, D. Contrasting effects of ecosystem engineering by cordgrass Spartima maritima and the sandprawn Callianassa kraussi in a marine-dominated lagoon. Estuar. Coast. Shelf Sci. 2011, 91, 169–176. [Google Scholar] [CrossRef]
- Venter, O.; Pillay, D.; Prayag, K. Water filtration by burrowing sandprawns provides novel insights on endobenthic engineering and solutions for eutrophication. Sci. Rep. 2020, 10, 1913. [Google Scholar] [CrossRef]
| Species Names | Average Dissimilarity | Contribution % | Cumulative % | Mean Abundance Group 1 | Mean Abundance Group 2 |
|---|---|---|---|---|---|
| Nassarius kraussianus | 12.6 | 27.7 | 27.7 | 35 | 13.67 |
| Pontegekloides laticeps | 9.069 | 19.94 | 47.64 | 45.5 | 25.33 |
| Exosphaeroma esturium | 8.475 | 18.63 | 66.27 | 28 | 20.67 |
| Grandiriella lignorum | 7.876 | 17.32 | 83.59 | 30.67 | 19.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yekani, C.; Froneman, W.P. Sand Prawns Mitigate the Impact of Prolonged Drought on the Biology of a Temporary Open/Closed Estuary. Diversity 2025, 17, 223. https://doi.org/10.3390/d17040223
Yekani C, Froneman WP. Sand Prawns Mitigate the Impact of Prolonged Drought on the Biology of a Temporary Open/Closed Estuary. Diversity. 2025; 17(4):223. https://doi.org/10.3390/d17040223
Chicago/Turabian StyleYekani, Celiwe, and William Pierre Froneman. 2025. "Sand Prawns Mitigate the Impact of Prolonged Drought on the Biology of a Temporary Open/Closed Estuary" Diversity 17, no. 4: 223. https://doi.org/10.3390/d17040223
APA StyleYekani, C., & Froneman, W. P. (2025). Sand Prawns Mitigate the Impact of Prolonged Drought on the Biology of a Temporary Open/Closed Estuary. Diversity, 17(4), 223. https://doi.org/10.3390/d17040223






