Fossil Samaras of Acer in the Lower Miocene of Central Inner Mongolia, China, and Their Phytogeographical Implications
Abstract
1. Introduction
2. Materials and Methods
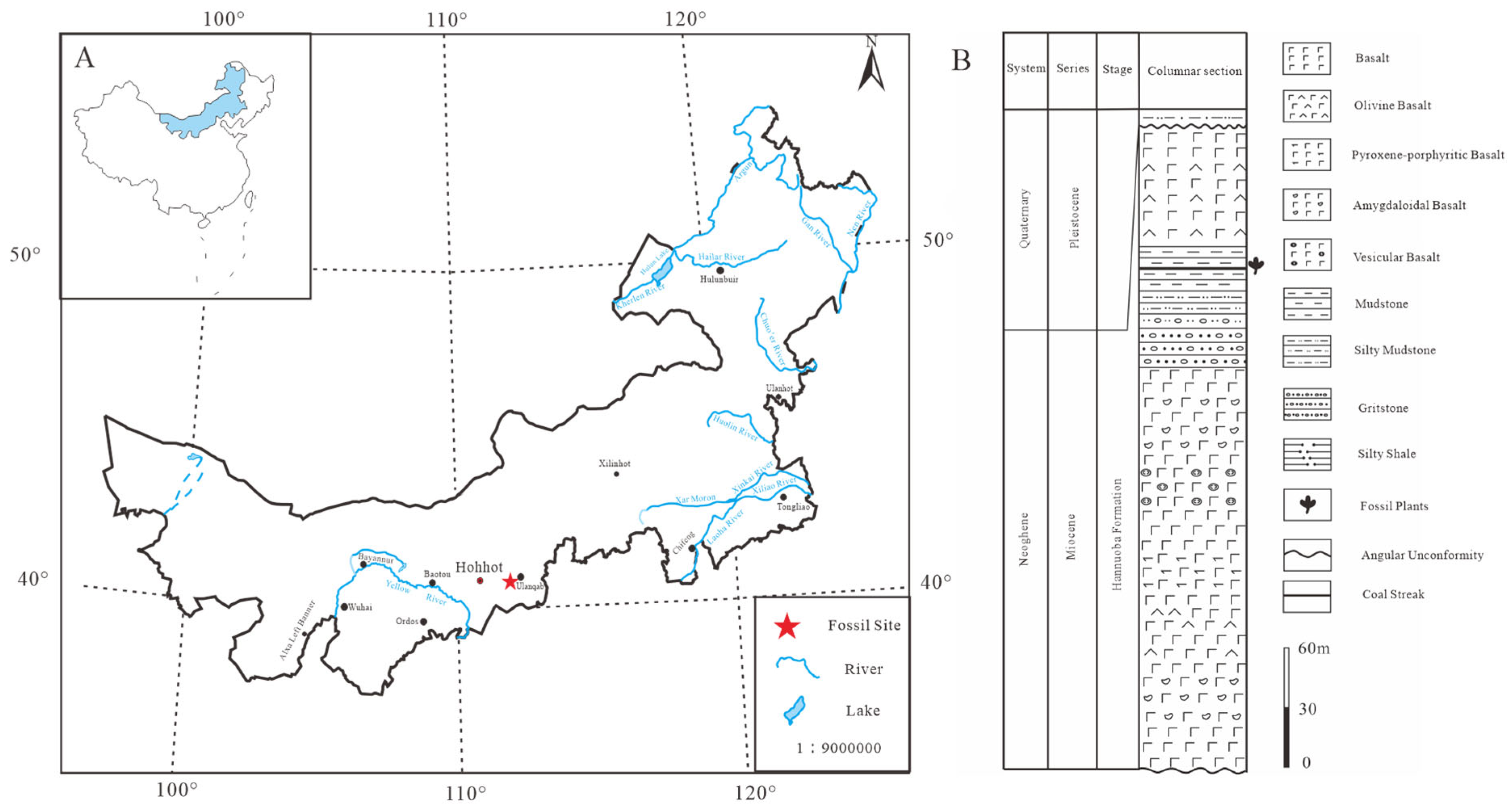
2.1. Research Materials
2.2. Processing of Fossil Specimens
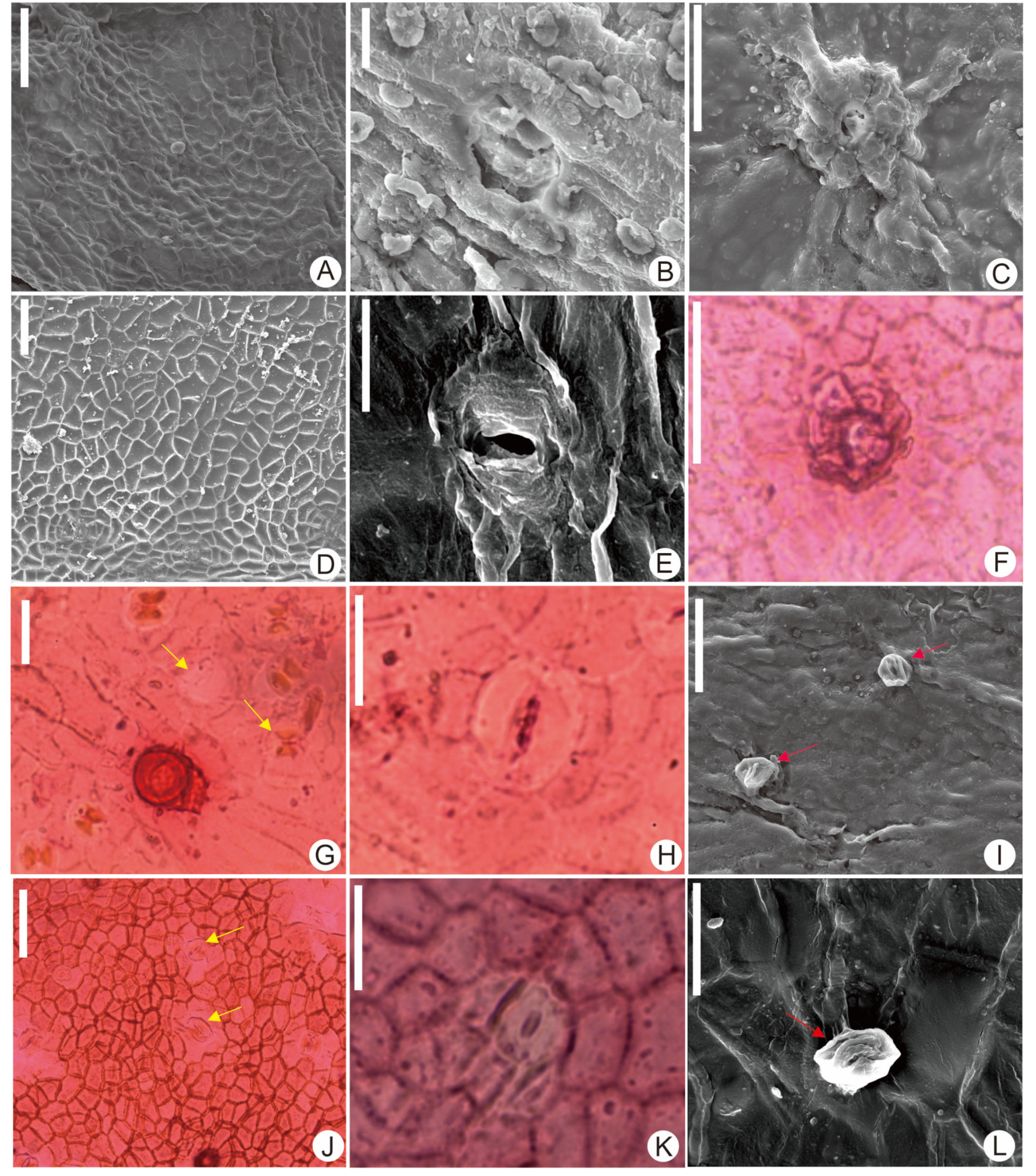
2.3. Processing of Living Specimens
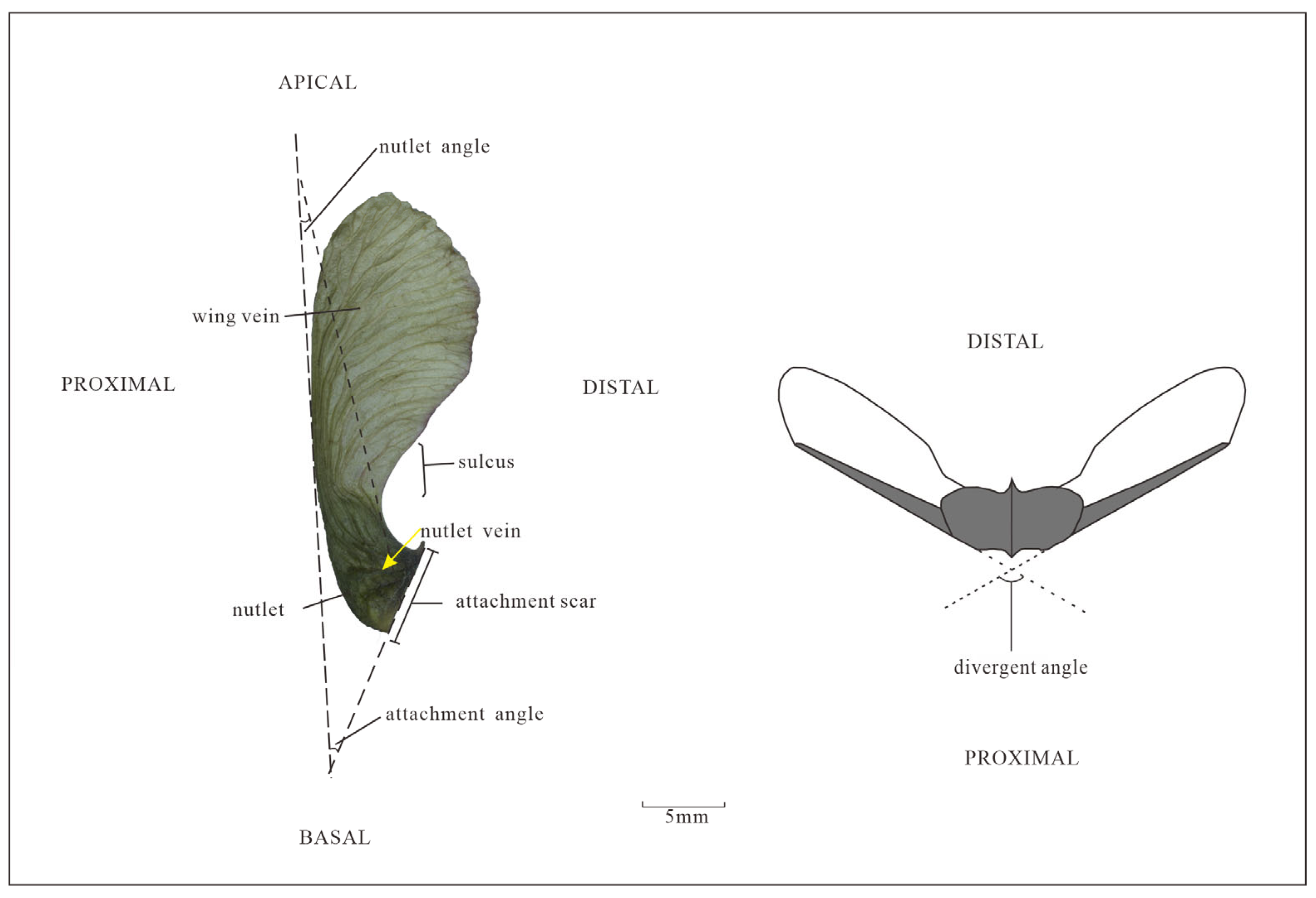
2.4. Terms Describing the Morphological Characteristics of Acer Samaras
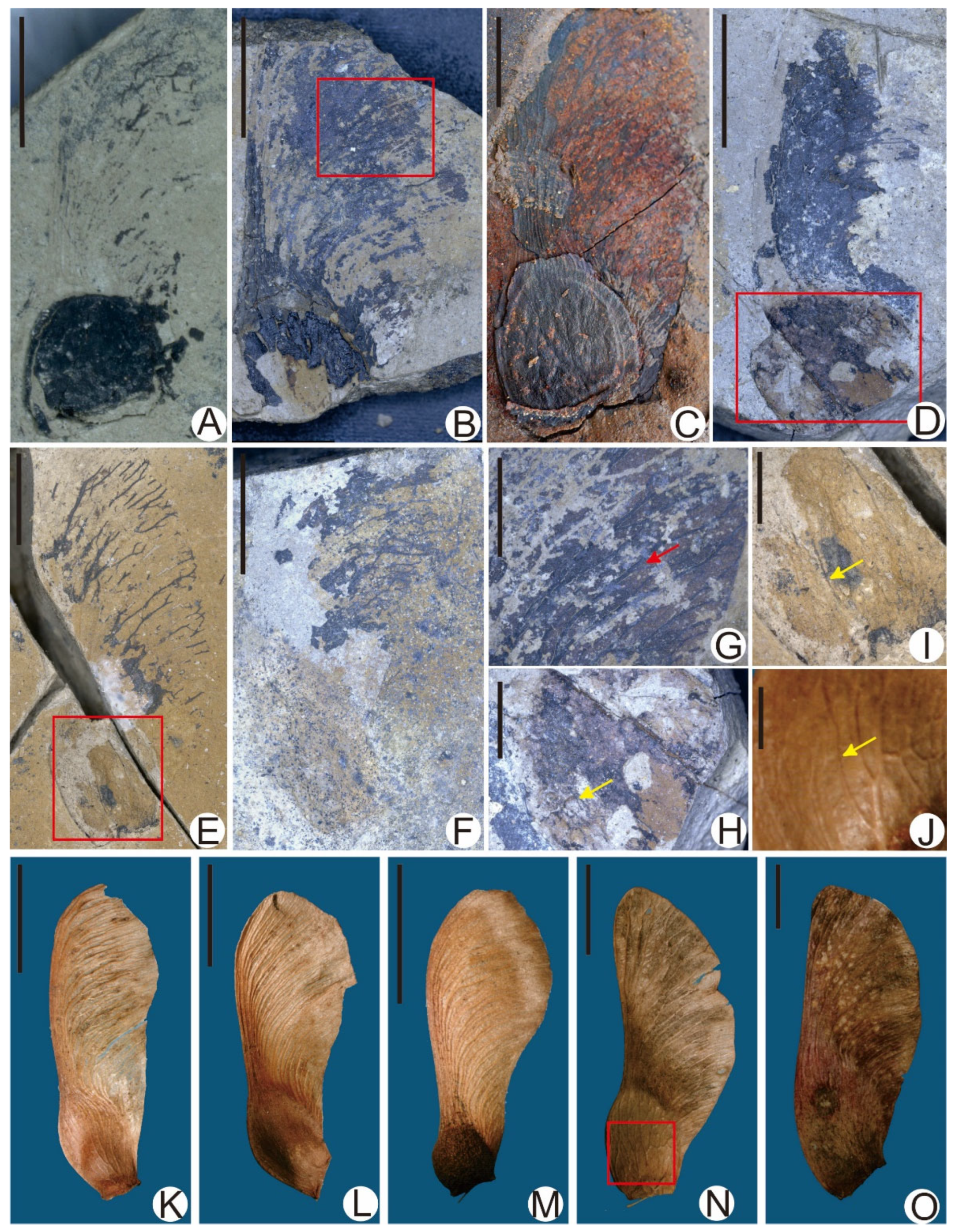
3. Results
| Species | GL * (cm) | GW * (cm) | NL * (cm) | NW * (cm) | NOS * | AA * | NA * | NI * | WR * | Sulus | ASL * (cm) | Age | Locality | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acer subginnala | 3 | 1.2 | 1.3 | 0.55 | Elliptical | 11–15° | 8–12° | - | Slightly straight | Auxetic | 0.6–0.7 | Miocene | Qinghai, China | [43] |
| Acer pseudoginnala | 4.2 | 1.2 | 0.9 | 0.6 | Elliptical | 15° | - | - | Straight | Auxetic | 0.7 | Miocene | Fukushima and Hokkaido, Japan | [46] |
| Acer ashwilli | 2.3–3.4 | 0.6–1.0 | 0.5–0.9 | 0.3–0.6 | Elliptical | 20–30° | 30–45° | Moderately inflated | Convex | Auxetic | - | Early Eocene | Oregon, U.S.A. | [7] |
| Acer hillsi | >2.5 | 1 | 0.5 | 0.5 | Suborbicular | 40° | 20° | - | Straight | Auxetic | - | Middle Eocene | Washington, U.S.A. | [7] |
| Acer browni | 3.3–4 | 1–1.2 | 0.8–1.0 | 0.7–0.8 | Elliptical to orbiculate | 25–30° | 40° | Flattened to strongly inflated | Convex | Auxetic | 0.6–0.7 | Miocene | Oregon, Washington, U.S.A. and Queen Charlotte Island, Canada | [7] |
| Acer busamarum | 4.7–10 | 1.1–2.8 | 0.7–2 | 0.6–1.5 | Ovate | 20–40° | 45–60° | Strongly inflated | Straight | Auxetic | 0.9–2.2 | Miocene | Colombia, U.S.A. | [47] |
| Acer schorni | 1.6–2.8 | 0.5–1 | 0.6–0.8 | 0.4–0.7 | Suborbicular | 10–30° | 25–80° | Strongly inflated | Straight to convex | Auxetic | 0.2–0.5 | Miocene | Oregon, Idaho, Nevada, U.S.A. | [7] |
| Acer tigilense | 1.7–2.7 | 0.5–0.7 | 0.5–0.7 | 0.3–0.5 | Elliptical | 20–30° | 20–30° | Slightly inflated | Convex | Auxetic | 0.3–0.5 | Miocene | Colombia, U.S.A. | [7] |
| Acer whitebirdense | 2.5–6.7 | 0.6–1.5 | 1–2.7 | 0.5–1.6 | Oblate | 20–55° | 10–40° | Moderately inflated | Convex | Auxetic | 0.2–0.7 | Miocene | Colombia, U.S.A. | [7] |
| Acer knolli | 2.8–4.2 | 0.6–1.2 | 1–1.7 | 0.3–0.7 | Lanceolate | 40–45° | 10–15° | Moderately inflated | Straight | Auxetic | 0.4–0.8 | Middle Miocene | Washington, U.S.A. | [7] |
| Acer eomedianum | 3.2 | 0.8–0.9 | 0.7 | 0.5–0.6 | Elliptical | 20–35° | 15–20° | Moderately inflated | Straight | Auxetic | 0.6 | Eocene | Nevada, Montana, U.S.A. | [7] |
| Acer niklasi | 3–3.6 | 1.0–1.3 | 0.7–1.0 | 0.5–0.7 | Elliptical | 35–40° | 10° | Moderately inflated | Convex | Auxetic | 0.3–0.4 | Miocene | Idaho, Washington, U.S.A. | [7] |
| Acer glabroides | 3.4 | 1 | 0.7 | 0.6 | Triangular | 40° | 20° | Moderately inflated | Straight | Auxetic | 0.6 | Early Eocene | Oregon, U.S.A. | [7] |
| Acer hueberi | 2.6–3.1 | 0.8–1.2 | 0.6–0.7 | 0.6–0.7 | Hemicycle | 35–70° | 35–50° | Flattened | Slightly convex | Obsolete | 0.4–0.5 | Eocene | Montana, U.S.A. | [7] |
| Acer oligomedianum | 2.1–3.7 | 0.5–0.9 | 0.7–1 | 0.5–0.7 | Elliptical | 25–40° | 10–30° | - | Straight to convex | Obsolete | 0.5–0.7 | Oligocene | Oregon, U.S.A. | [7] |
| Acer medianum | 4–5 | 0.9–1.6 | 1.2–1.5 | 0.7–1.2 | Elliptical | 25–30° | 10–35° | Moderately inflated | Straight to convex | Obsolete | 0.7–1 | Late Miocene | Columbia, Nevada and Idaho, U.S.A. | [7] |
| Acer protomiyabei | 1.8–3.5 | 0.7–1.1 | 0.7–1.2 | 0.7–1.2 | Orbiculate | 90–135° | - | Strongly inflated | Sinuous | Obsolete | 0.6–1 | Miocene | North Korea | [45] |
| Acer palaeoplatanoides | 2.4–2.7 | 0.7–1.4 | 0.6–1 | 0.5–0.9 | Suborbicular | 60–90° | - | Flattened | Straight to sinuous | Obsolete | 0.5–0.9 | Miocene | North Korea | [45] |
| Acer subpictum | 2.7–5 | 0.7–1.2 | 0.4–1 | 0.3–0.5 | Ovate to oblong | 30–45° | 20–35° | - | Straight to sinuous | Obsolete | 0.2–0.4 | Miocene | Yunnan, China | [23] |
| Acer pretataricum sp. nov. | 2–4 | 0.42–1.2 | 0.55–0.9 | 0.43–0.8 | Ovate | 36–45° | 7–15° | Slightly inflated | Straight to slightly convex | Auxetic | 0.4–0.75 | Early Miocene | Inner Mongolia, China | This paper |
| cf. Acer mono | 2–2.5 | 0.75–1.2 | 0.65–0.9 | 0.5–0.7 | Elliptical | 40–80° | 7–12° | Flattened to slightly inflated | Slightly convex or sinuous | Obsolete | 0.35–0.55 | Early Miocene | Inner Mongolia, China | This paper |
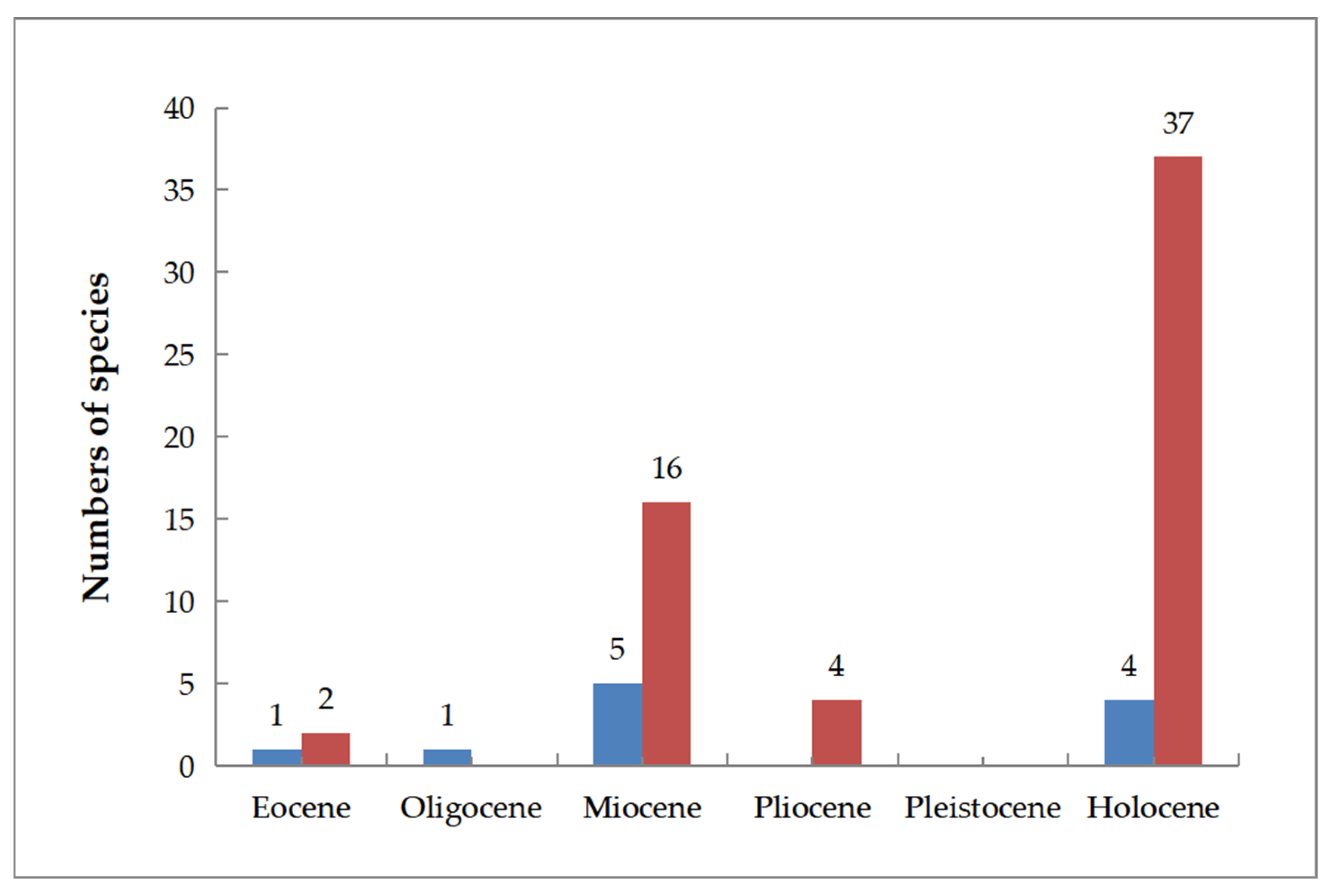
4. Discussion
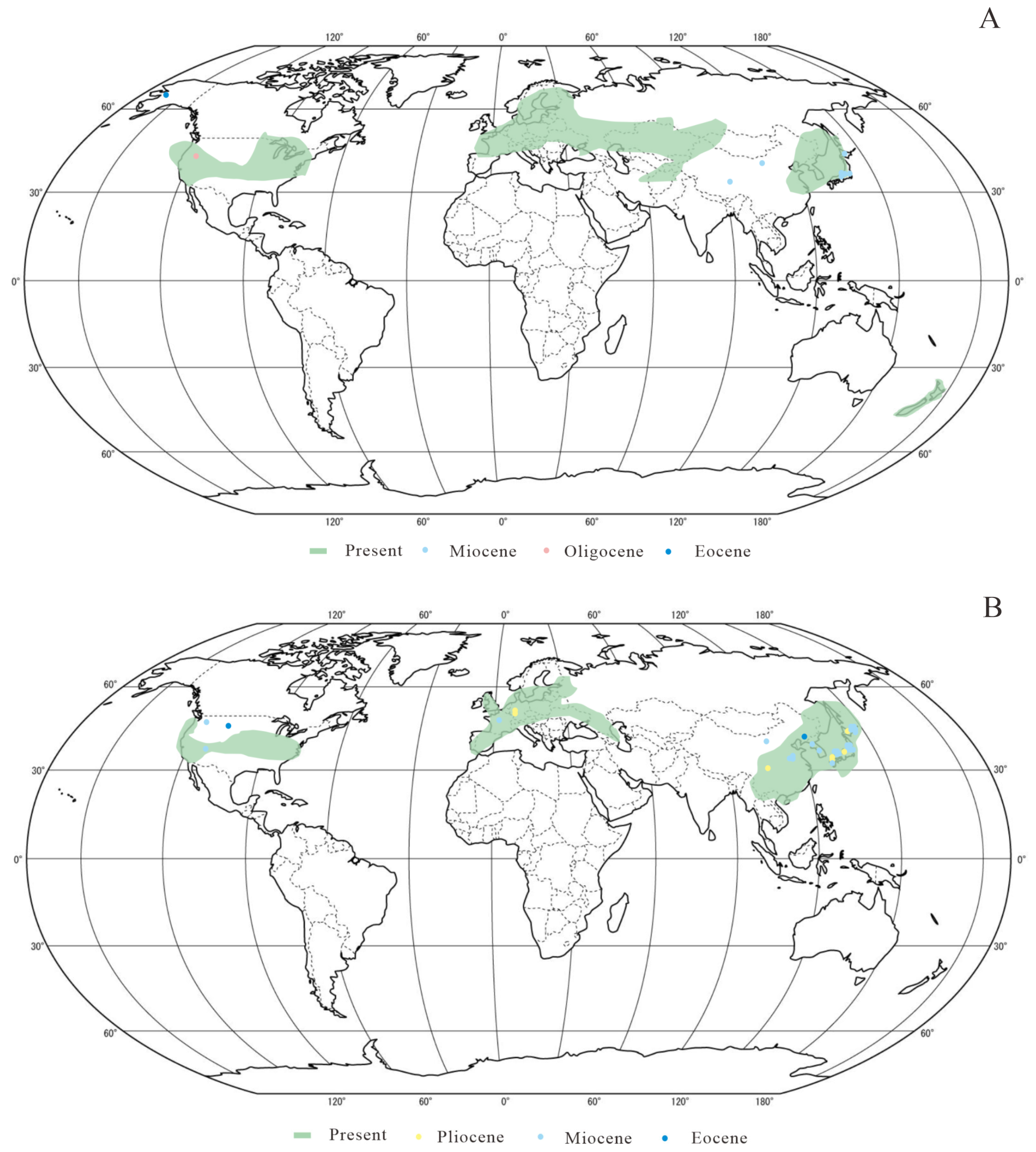
4.1. Paleogeographic Analysis of Sections Ginnala and Platanoidea
| Section | Species | Organ | Age | Locality | Reference |
|---|---|---|---|---|---|
| Section Ginnala | Acer tataricum L. | Leaf | Early Miocene | Inner Mongolia, China | [56] |
| Acer pretataricum Xiao | Samara | Early Miocene | Inner Mongolia, China | This paper | |
| Acer subginnala Guo | Samara | Miocene | Qinghai, China | [43] | |
| Acer pseudoginnala Tanai et Onoe | Samara | Miocene | Hokkaido, Japan | [46] | |
| Samara | Miocene | Fukushima, Japan | [44] | ||
| Acer prototataricum Tanai et Suzuki | Samara | Miocene | Hokkaido, Japan | [46] | |
| Leaf | Miocene | Hokkaido and Honshu, Japan | [49] | ||
| Acer ashwilli Wolfe et Tanai | Leaf and samara | Early Oligocene | Oregon, U.S.A. | [7] | |
| Acer sp. | Samara | Eocene | Alaska, U.S.A. | [7] | |
| Section Platanoidea | Acer cf. cappadocicum Gleditsch | Leaf and samara | Pliocene | Frankfurt, Germany | [57] |
| Acer cf. platanoides L. | Leaf and samara | Pliocene | Frankfurt, Germany | [57] | |
| Leaf | Miocene | France | [57] | ||
| Acer pictum Thunbg | Leaf | Pliocene | Nagasaki, Japan | [58] | |
| Acer subpictum Saporta | Leaf | Pliocene | Sichuan, China | [51] | |
| Leaf and samara | Early Miocene to Late Pliocene | Japan | [49] | ||
| Leaf and samara | Miocene | Shandong, China | [54] | ||
| Leaf and samara | Miocene | Hokkaido, Japan | [46] | ||
| Leaf and samara | Eocene | Liaoning, China | [54] | ||
| Acer scolliae MacGinitie | Leaf and samara | Early Miocene | Nevada, U.S.A. | [7] | |
| Late Miocene | Colombia, U.S.A. | [7] | |||
| Acer cf. mono Maxim | Samara | Early Miocene | Inner Mongolia, China | This paper | |
| Acer chiharae Huzioka et Nishida | Leaf | Miocene | Niigata, Japan | [59] | |
| Acer huziokae Tanai | Leaf | Miocene | Tottori, Japan | [27] | |
| Acer rotundatum Huzioka | Leaf and samara | Miocene | South Korea | [27] | |
| Acer shanwangense Tanai | Leaf | Miocene | Shandong, China | [27] | |
| Acer palaeoplatanoides Endo | Samara | Miocene | Fukushima, Japan | [27] | |
| Miocene | Hokkaido, Japan | [46] | |||
| Miocene | Japan | [49] | |||
| Samara | Miocene | North Korea | [45] | ||
| Acer Matsuii Tanai et Onoe | Samara | Miocene | Fukushima, Japan | [44] | |
| Acer sub Mayrii Tanai et Onoe | Samara | Miocene | Kyushu, Japan | [46] | |
| Acer protodistylum Endo | Samara | Miocene | Hokkaido, Japan | [46] | |
| Acer watarianum Takahashi et M. Suzuki | Tree | Miocene | Hokkaido, Japan | [60] | |
| Acer protojaponicum Tanai et Onoe | Leaf and samara | Miocene | Fukushima, Japan | [44] | |
| Acer florinii Hu et Chaney | Leaf | Miocene | Tottori, Japan | [61] | |
| Leaf | Miocene | Shandong, China | [54] | ||
| Acer integerrimum (Viviani) Massalongo | Leaf | Miocene | Tottori, Japan | [61] | |
| Acer hueberi Wolfe et Tanai | Samara | Late Eocene | Montana, U.S.A. | [7] |
4.2. Dispersal Pathway Comparison Between Acer Section Ginnala and Section Platanoidea
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, T.Z.; Chen, Y.S.; de Jong, P.C.; Oterdoom, H.J.; Chang, C.S. Aceraceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science: Beijing, China, 2008; Volume 11, pp. 515–553. [Google Scholar]
- Li, L.; Lin, L.-J.; Zhu, Z.-Y.; Ding, Y.-L.; Kuai, B.-K. Studies on the Taxonomy and Molecular Phylogeny of Acer in China. Acta Hortic. Sin. 2017, 44, 1535–1547. [Google Scholar]
- Huang, Y.J.; Zhu, H.; Chen, W.Y.; Zhou, Z.K. Intraspecific variation in samara morphology of Acer and its implication in fossil identification. Plant Divers. Resour. 2013, 35, 295–302. [Google Scholar] [CrossRef]
- Xu, T.Z. Geographical distribution of Aceraceae. Yunnan Bot. Res. 1996, 18, 43–50. [Google Scholar]
- Dong, P.-B.; Wang, R.-N.; Afzal, N.; Liu, M.-L.; Yue, M.; Liu, J.-N.; Tan, J.-L.; Li, Z.-H. Phylogenetic relationships and molecular evolution of woody forest tree family Aceraceae based on plastid phylogenomics and nuclear gene variations. Genomics. 2021, 113, 2365–2376. [Google Scholar] [CrossRef]
- Newberry, J.S. The Later Extinct Floras of North America; Geological Survey Monographs; U.S. Government Printing Office: Washington, DC, USA, 1898; Volume 35, pp. 1–295. [Google Scholar]
- Wolfe, J.A.; Tanai, T. Systematics, phylogeny, and distribution of Acer (maples) in the Cenozoic of western North America. J. Fac. Sci. Geol. Mineral. 1987, 22, 1–246. [Google Scholar]
- Crane, P.R.; Manchester, S.R.; Dilcher, D.L. A preliminary survey of fossil leaves and well-preserved reproductive structures from the Sentinel Butte Formation (Paleocene) near Almont, North Dakota. Field Geol. 1990, 20, 1–63. [Google Scholar]
- Budantsev, L.Y. The Fossil Flora of the Paleogene Climatic Optimum in North Eastern Asia. In Cenozoic Plants and Climates of the Arctic; Boulter, M.C., Fisher, H.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 297–313. [Google Scholar]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- LePage, B.A. A new species of Tsuga (Pinaceae) from the Middle Eocene of Axel Heiberg Island, Canada, and an assessment of the evolution and biogeographical history of the genus. Bot. J. Linn. Soc. 2003, 141, 257–296. [Google Scholar] [CrossRef]
- Qiu, Y.-X.; Fu, C.-X.; Comes, H.P. Plant molecular phylogeography in China and adjacent regions: Tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol. Phylogenetics Evol. 2011, 59, 225–244. [Google Scholar] [CrossRef]
- Xu, T.-Z. The systematic evolution and distribution of the genus Acer. Acta Bot. Yunnanica 1998, 20, 383–393. (In Chinese) [Google Scholar]
- Pax, F. Aceraceae. In Das Pflanzenreich, 4th ed.; Engler, H.G.A., Ed.; W. Engelmann: Leipig, Germany, 1902. [Google Scholar]
- Pojarkova, A.Z. Botanico-geographical survey of the maples in USSR, in cconnection with the history of the whole genus Acer L. Acta Inst. Bot. Acad. Sci. USSR 1933, 1, 224–374. [Google Scholar]
- Gao, J.; Liao, P.-C.; Huang, B.H.; Yu, T.; Zhang, Y.Y.; Li, J.Q. Historical biogeography of Acer L. (Sapindaceae): Genetic evidence for Out-of-Asia hypothesis with multiple dispersals to North America and Europe. Sci. Rep. 2020, 10, 21178. [Google Scholar] [CrossRef]
- Areces-Berazain, F.; Hinsinger, D.D.; Strijk, J.S. Genome-wide supermatrix analyses of maples (Acer, Sapindaceae) reveal recurring inter-continental migration, mass extinction, and rapid lineage divergence. Genomics 2021, 113, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-F. Phylogeny and Historical Biogeography of Acer. Ph.D. Thesis, University of Missouri-St. Louis, St. Louis, MO, USA, 2002. [Google Scholar]
- Kvacek, Z. Connecting Links Between the Arctic Paleocene and European Tertiary Floras. In Cenozoic Plants and Climates of the Arctic; Boulter, M.C., Fisher, H.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 251–266. [Google Scholar]
- Boulter, M.C.; Benfield, J.N.; Fisher, H.C.; Gee, D.A.; Lhotak, M. The evolution and global migration of the Aceraceae. Philosophical Trans. R. Soc. London. Ser. B Biol. Sci. 1996, 351, 589–603. [Google Scholar]
- Wheeler, E.A.; Manchester, S.R. A diverse assemblage of Late Eocene woods from Oregon, western USA. Foss. Impr. 2022, 77, 299–329. [Google Scholar] [CrossRef]
- Buechler, W.K.; Dunn, M.T.; Rember, W.C. Late Miocene Pickett Creek Flora of Owyhee County, Idaho. Contrib. Mus. Paleontol. 2007, 31, 305–362. [Google Scholar]
- Wang, Y.-F.; Shao, Y.; Li, B.-K.; Liu, K.-N.; Xie, S.-P. Acer leaves and samaras from the late Miocene of Lincang, Yunnan Province. Geol. J. China Univ. 2015, 21, 105–116. [Google Scholar]
- Chen, Y.-F.; Wong, W.O.; Hu, Q.; Liufu, Y.-Q.; Xie, Z.-M. A new fossil-species of Acer (Sapindaceae) from the Ningming Basin in Guangxi, South China. Phytotaxa 2017, 298, 158–164. [Google Scholar] [CrossRef]
- Jeong, E.K.; Kim, K.; Suzuki, M.; Kim, J.W. Fossil woods from the Lower Coal-bearing Formation of the Janggi Group (Early Miocene) in the Pohang Basin, Korea. Rev. Palaeobot. Palynol. 2009, 153, 124–138. [Google Scholar] [CrossRef]
- Jin, J.H. Two Eocene fossil fruits from the Changchang basin of Hainan Island, China. Rev. Palaeobot. Palynol. 2009, 153, 150–152. [Google Scholar] [CrossRef]
- Tanai, T. Revisions of Tertiary Acer from East Asia. J. Fac. Sci. Geol. Mineral. 1983, 20, 291–390. [Google Scholar]
- Mai, D.H. Die Endokarpien bei der Gattung Acer L. (Aceraceae)-Eine biosystematische Studie. Gleditschia 1984, 11, 17–46. [Google Scholar]
- Wang, Z.-E.; Cao, R.; Ding, H.; Huang, Y.-T.; Song, Z.-H.; Ding, S.-T.; Wu, J.-Y. Fossil samaras of Acer L. (Sapindaceae) from the Upper Pliocene of western Yunnan, southwestern China. Palaeobiodiversity Palaeoenvironments 2023, 103, 695–710. [Google Scholar] [CrossRef]
- Wang, H.-F.; Dai, T.-M.; Fan, S.-K.; Yang, X.-C. K-Ar daeing of Hannuoba basalts at Zhangjiakou. Geochimica 1985, 3, 206–215. (In Chinese) [Google Scholar]
- Zhang, Y.-S. Origin discussion of Cenozoic Hannuoba basalts in Shanxi Province. Geol. Surv. China 2018, 6, 58–67. [Google Scholar] [CrossRef]
- Inner Mongolia Autonomous Region Bureau of Geology and Mineral Resources. Regional Geology of Inner Mongolia Autonomous Region; Geology Press: Beijing, China, 1991; pp. 1–725. (In Chinese) [Google Scholar]
- Li, C.-K. A Tertiary Beaver from Changpei, Hopei Province. Vertebr. Palasiat. 1962, 1, 72–79. [Google Scholar]
- Guo, C.-Q.; Li, Y.; Wu, P.-C.; Yao, J.-X. The phytogeographic significance of Early Miocene mosses from Weichang area, Hebei Province. Geol. Bull. China 2016, 35, 1976–1984. [Google Scholar]
- Li, D.-m.; Li, Q. Serial K-Ar Dating along the Volcanic Section in Zuoyun, Shanxi Province. Acta Geosci. Sin. 2003, 24, 559–562. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Han, B.-F. K-Ar Chronology and Geochemistry of Jining Cenozoic basaits, Inner Mongolia, and geodynamic Implications. Acta Petrol. Sin. 2006, 22, 1597–1607. (In Chinese) [Google Scholar]
- Liang, J.-Q.; Leng, Q.; Höfig, D.F.; Niu, G.; Wang, L.; Royer, D.L.; Burke, K.; Xiao, L.; Zhang, Y.-G.; Yang, H. Constraining conifer physiological parameters in leaf gas-exchange models for ancient CO2 reconstruction. Glob. Planet. Change 2022, 209, 103737. [Google Scholar] [CrossRef]
- Xu, T.Z. Samara shape of Aceraceae and its implications in systematics and evolution. Guangxi Zhiwu 1996, 16, 109–122. (In Chinese) [Google Scholar]
- Mirle, C.; Burnham, R.J. Identification of asymmetrically winged samaras from the Western Hemisphere. Brittonia 1999, 51, 1–14. [Google Scholar] [CrossRef]
- Anderson, W.R.; Gates, B. Barnebya, a New Genus of Malpighiaceae from Brazil. Brittonia 1981, 33, 275. [Google Scholar] [CrossRef]
- Gates, B. Banisteriopsis, Diplopterys (Malpighiaceae); New York Botanical Garden Press: New York, NY, USA, 1982. [Google Scholar]
- Tan, K.; Dong, S.-p.; Lu, T.; Zhang, Y.-J.; Xu, S.-T.; Ren, M.-X. Diversity and evolution of samara in angiosperm. Chin. J. Plant Ecol. 2018, 42, 806–817. [Google Scholar] [CrossRef]
- Guo, S.-x. Miocene flora of Zeku, Qinghai. Acta Palaeontol. Sin. 1980, 5, 406–411+441. (In Chinese) [Google Scholar]
- Tanai, T.; Onoe, T. A Miocene flora from the northern part of the Joban Coal Field, Japan. Bull. Geol. Surv. Jpn. 1959, 10, 261–286. [Google Scholar]
- Endo, S. On the fossil Acer from Japan, Korea and south Manchuria. Short Pap. Inst. Geol. Paleontol. 1950, 1, 11–17. [Google Scholar]
- Tanai, T.; Suzuki, N. Miocene Maples from Southwestern Hokkaido, Japan. Fac. Sci. Geol. Mineral. 1960, 10, 551–570. [Google Scholar]
- Knowlton, F.H. Fossil Flora of the John Day Basin, Oregon. U.S. Ceol. Sury. Bull. 1902, 204, 153. [Google Scholar]
- Jiang, Y.; Gao, M.; Meng, Y.; Wen, J.; Ge, X.-J.; Nie, Z.-L. The importance of the North Atlantic land bridges and eastern Asia in the post-Boreotropical biogeography of the Northern Hemisphere as revealed from the poison ivy genus (Toxicodendron, Anacardiaceae). Mol. Phylogenetics Evol. 2019, 139, 106561. [Google Scholar] [CrossRef]
- Tanai, T. Neogene Floral Change in Japan. Fac. Sci. Geol. Mineral. 1961, 11, 119–398. [Google Scholar]
- Mosbrugger, V.; Utescher, T.; Dilcher, D.L. Cenozoic continental climatic evolution of Central Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 14964–14969. [Google Scholar] [CrossRef]
- Tao, J.-R.; Zhou, Z.-K.; Liu, Y.-S. Development and Evolution of China’s Flora from the Late Cretaceous to the Cenozoic; Science Press: Beijing, China, 2000; pp. 49–290. [Google Scholar]
- Sanmartin, I.; Enghoff, H.; Ronquist, F. Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biol. J. Linn. Soc. 2001, 73, 345–390. [Google Scholar] [CrossRef]
- Ranatunga, D.; Hoare, B. Auckland Museum Botany Collection; Version 1.96; Auckland War Memorial Museum: Auckland, New Zealand, 2024; Occurrence Dataset; Available online: https://www.gbif.org/occurrence/2883314201 (accessed on 19 October 2024).
- Writing Group of Cenozoic Plants of China (WGCPC) (Ed.) Cenozoic Plants from China. In Fossil Plants of China; Science Press: Beijing, China, 1978; pp. 1–232. (In Chinese) [Google Scholar]
- Loidi, J.; Marcenò, C. The Temperate Deciduous Forests of the Northern Hemisphere. A review. Mediterr. Bot. 2022, 43, e75527. [Google Scholar] [CrossRef]
- Liang, J.-Q.; Xiao, L.; Li, X.-C.; Wang, X.; Wang, Q. The first discovery of the fossils of Acer from the Miocene epoch in Inner Mongolia. Geol. Rev. 2019, 65, 69–70. [Google Scholar]
- Ivan, G.; Johanna, K.E. The genus Acer from the lower/middle Pleistocene Sisian Formation, Syunik region, South Armenia. Rev. Palaeobot. Palynol. 2011, 165, 111–134. [Google Scholar]
- Nathorst, A.G. Contributions a la flore fossile du Japon. KgI. Svensk. Vel. Akad. Handt. 1883, 20, 3–92. [Google Scholar]
- Huzioka, K.; Nishida, S. The Seki flora of the island Sado. Sodo Mus. Publ. 1960, 3, 1–26. [Google Scholar]
- Takahashi, A.; Suzuki, M. Two new fossil woods of Acer and a new combination of Prunus from the Tertiary of Japan. Bot. Mag. Tokyo. 1988, 101, 473–481. [Google Scholar] [CrossRef]
- Tanai, T.; Ozaki, K. The genus Acer from the Upper Miocene in Tottori Prefecture, western Japan. J. Fac. Sci. Hokkaido Univ. 1977, 17, 575–606. [Google Scholar]



| Section | Species | GL * (cm) | GW * (cm) | DA * | NL * (cm) | NW * (cm) | NI * | NS * | Sulus | NV * |
|---|---|---|---|---|---|---|---|---|---|---|
| Acer | Acer pseudoplatanus L. | 4–10 | 0.5–1.2 | Variously | 1.5 | 0.5 | Moderately inflated | Spherical | Auxetic | Longitudinal |
| Arguta | Acer tetramerum Pax | 2–3.5 | 1–1.2 | Acute or nearly erect | 0.8 | 0.6 | Strongly inflated | Lanceolate | Auxetic | Ridged |
| Ginnala | Acer ginnala Maxim | 2–4 | 0.4–1 | Acute or nearly erect | 0.5–1 | 0.5 | Flattened to slightly inflated | Elliptical | Auxetic | Reticular |
| Goniocarpa | Acer monspessulanum L. | 2–2.5 | 0.7–1.1 | Acute | 0.6–0.9 | 0.6–0.8 | Strongly inflated | Orbiculate | Auxetic | Reticular |
| Integrifolia | Acer buergerianum Miq | 2–2.5 | 0.8–1.2 | Acute or nearly erect | 3.6–6 | 3.6–6 | Strongly inflated | Elliptical | Auxetic | Longitudinal |
| Macrantha | Acer davidii Frach. | 2.5–3 | 1–1.5 | Obtuse or nearly horizontal | 0.7–1.5 | 0.7–1.5 | Flattened to slightly inflated | Elliptical | Auxetic | Sub-parallel |
| Macrantha | Acer grosseri Pax | 2.5–2.9 | 0.5 | Acute or nearly erect | 0.7 | 0.4 | Flattened | Elliptical | Obsolete | Sub-parallel |
| Macrantha | Acer pensylvanicum L. | 1.8–2.7 | 0.8–1.2 | Acute | 0.5–1.1 | 0.35–0.6 | Flattened | Elliptical | Obsolete | Longitudinal |
| Macrantha | Acer rufinerve Sieb. et Zucc. | 2–3 | - | Obtuse | 0.4 | 0.4 | Strongly inflated | Spherical | Obsolete | Reticular |
| Platanoidea | Acer mono Maxim., 1856 | 2–2.5 | 0.5–1 | Acute or nearly horizontal | 0.5–1.3 | 0.5–0.8 | Flattened to slightly inflated | Elliptical | Obsolete | Sub-parallel |
| Spicata | Acer caudatum Wall | 2.5–2.8 | 0.7–0.9 | Acute or nearly erect | 0.8 | 0.6 | Moderately to strongly inflated | Elliptical | Auxetic | Reticular or sub-parallel |
| Acer pretataricum sp. nov. | 2–4 | 0.42–1.2 | Acute or nearly erect | 0.55–0.9 | 0.5–1 | Slightly inflated | Ovate | Auxetic | Reticular | |
| cf. Acer mono Maxim | 2–2.5 | 0.75–1.2 | Acute or nearly obtuse | 0.65–0.9 | 0.5–0.7 | Flattened to slightly inflated | Elliptical | Obsolete | Sub-parallel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Wu, Y.; Wang, X.; Wang, M.; Ji, D.; Liang, J.; Xiao, L. Fossil Samaras of Acer in the Lower Miocene of Central Inner Mongolia, China, and Their Phytogeographical Implications. Diversity 2025, 17, 218. https://doi.org/10.3390/d17030218
Dong H, Wu Y, Wang X, Wang M, Ji D, Liang J, Xiao L. Fossil Samaras of Acer in the Lower Miocene of Central Inner Mongolia, China, and Their Phytogeographical Implications. Diversity. 2025; 17(3):218. https://doi.org/10.3390/d17030218
Chicago/Turabian StyleDong, Han, Yong Wu, Xiaoyan Wang, Meiting Wang, Deshuang Ji, Jiwei Liang, and Liang Xiao. 2025. "Fossil Samaras of Acer in the Lower Miocene of Central Inner Mongolia, China, and Their Phytogeographical Implications" Diversity 17, no. 3: 218. https://doi.org/10.3390/d17030218
APA StyleDong, H., Wu, Y., Wang, X., Wang, M., Ji, D., Liang, J., & Xiao, L. (2025). Fossil Samaras of Acer in the Lower Miocene of Central Inner Mongolia, China, and Their Phytogeographical Implications. Diversity, 17(3), 218. https://doi.org/10.3390/d17030218