Abstract
This work assessed the sexual maturity of breeding females and males of black sea turtles (Chelonia mydas agassizii) from the population in Michoacan, Mexico. This study also provides the first report of the age at sexual maturity for male black sea turtles in the eastern Pacific. Using information on juvenile growth rate, length, and age at recruitment of juveniles in the developmental habitats in Baja California (Magdalena Bay), sexual maturity was estimated from the minimum and average standard carapace lengths (SCL) of nesting females (n = 1500) on Colola Beach and males (n = 132) captured at sea using the “swim up” technique. Differential sexual maturity was found in females and males. The minimum age at sexual maturity for males was 23.0 years at a minimum size of 61.1 cm SCL and the maximum age at sexual maturity was 32.5 years at a maximum size of 76.6 cm SCL. The minimum age of sexual maturity for nesting females was 24.9 years at a minimum size of 64.2 cm SCL, while the age of sexual maturity for maximum size was 42.9 years at a 93.4 cm SCL. Differences in the age at sexual maturity influence reproductive behavior and female carapace shape, impacting mating success.
1. Introduction
The age of sexual maturity is one of the most intriguing aspects of the life history of sea turtles. Sexual maturity (SM) is the stage in an organism’s life when it can reproduce. In animals, this usually means the ability to produce viable gametes (sperm or eggs) and engage in mating behaviors. The age or size at which sexual maturity is reached varies significantly from species to species. It can be influenced by a variety of factors, such as genetics, environment, migrations, and life history strategies [1,2,3,4,5]. The most common maturation norm is one in which, when growth is rapid, organisms mature early and reach a large size, whereas when growth is slow, sexual maturity is delayed and a smaller size is reached at sexual maturity [6,7]. These adaptations, either for egg laying or live birth, have evolved to ensure reproductive success in harsh marine and terrestrial environments [8].
In sea turtles, estimating the age at sexual maturity is challenging because the age of living sea turtles cannot be determined, and sea turtles make extensive movements during a long period of immaturity [9,10]. An indirect method to estimate sexual maturity consists of determining the growth rate in a specific period and the minimum nesting size of a turtle population; with this information, it is possible to estimate sexual maturity [11].
Most estimates of SM in sea turtles are calculated as the time taken to grow from hatchling to reaching SM and are often based on somatic growth models [12,13,14,15]. A limitation of this approach is that it requires the designation of length at sexual maturity (LengthSM), but LengthSM in turtles appears to be highly variable. A significant variation in female body size is characteristic of sea turtle nesting aggregations [16]. These estimates are of high importance and are scarce for most sea turtle populations.
Mark–recapture and intensive nesting area monitoring provide valuable data on resource allocation for growth versus reproduction in sea turtles at sexual maturity [15,17]. These methods are crucial for assessing species status and understanding population dynamics for effective conservation and management.
In sea turtles, the age of sexual maturity varies both among individuals of the same population and individuals of different populations. The age of sexual maturity in Chelonia has been estimated in different populations of green turtle (Chelonia mydas): more than 30 years for the Australian population [18], between 25 and 30 years in Florida [19], from 40 to 50 years in Hawaii [20], from 12 to 26 years in Costa Rica, and at least 36 years in Galapagos [21,22]. On the other hand, for Kemp’s ridley turtles (Lepidochelys kempii), it was determined that they reach sexual maturity between 5 and 12 years [16], loggerhead turtles (Caretta caretta) can reach maturity at around 23–29 years [23], leatherback turtles (Dermochelys coriacea) may take 13–15 years to reach sexual maturity [24]; in hawksbill turtles (Eretmochelys imbricata), estimates show that they would be sexually mature in the range of 49–52 years [25], and for flatback turtles (Natator depressus), the estimated age at sexual maturity ranges from 12 to 23 years [26].
According to the growth rate reported in adult green sea turtles in the Galapagos Islands [21], the age of sexual maturity for females nesting in Michoacan, Mexico, was estimated to be 47 years, confirming late sexual maturity in black sea turtles [11].
This paper analyzed a data set that allowed us to generate updated information on the age of sexual maturity of black sea turtles (C. m. agassizii) from growth rate information in juvenile turtles.
2. Materials and Methods
This study was conducted at Colola Beach, Mexico; the main nesting and reproduction area for black sea turtles (C. m. agassizii) in the eastern Pacific Ocean (18°18′38″ N, 103°25′50″ W); it is an unprotected open beach 4.8 km long and with an average width of 150 m that runs in an east–west direction (Figure 1).
Figure 1.
Nesting and reproduction area of the black sea turtle (C. m. agassizii) in Colola, Mexico.
To determine the age at sexual maturity in black turtles (C. m. agassizii) from the Michoacán population in Mexico, we measured straight carapace length (SCL) and curved carapace length (CCL) from 1500 nesting females after nesting, obtained in the period of 1985–2000 (100 females per season) and 132 males captured at sea in front of Colola Beach during the period of 1995–2023. A vernier caliper (0–150 cm scale) was used for SCL, while a flexible tape measure (0–130 cm scale) was utilized for CCL. Measurements were conducted from August to February, coinciding with the peak nesting and mating season.
Males were captured at sea in front of Colola Beach during the copulation and courtship process, which takes place near the shore of the beach (10–60 m). The males were captured by the “swim-up” technique, which consists of approaching the males and females during mating, utilizing a boat with an outboard motor at a distance of about 30 m; then, swimming stealthily towards them to capture the male. Once captured, the turtle was loaded onto the boat to record its morphometric measurements (Figure 2).

Figure 2.
Male black sea turtles (C. m. agassizii) captured by the “swim-up” technique.
The minimum size, maximum size, and average size of the nesting females and breeding males were obtained. To avoid duplicate sampling, turtles were marked with Inconel steel tags on the left posterior flipper. To determine the differences between male and female body size, Student’s t-test and Bonferroni’s multiple comparisons significance level correction test were conducted, and morphometric variables were analyzed using descriptive statistics with SYSTAT 12.0 software.
Differential Sexual Maturity
To estimate the sexual maturity of female and male black turtles, we used the juvenile growth rate (1.62 cm/year) in the developmental habitats (Magdalena Bay) in Baja California reported by Seminoff et al. [27] and Koch et al. [28]; the authors estimate that the recruitment of juvenile black turtles (C. m. agassizii) from the pelagic phase occurs at an average body size of 40 cm SCL at an age of 10 years [29].
The minimum and maximum age of sexual maturity was estimated by considering the minimum and maximum size of the straight carapace length (SCL) in adult black turtle (C. m. agassizii) breeding stock, considering the juvenile recruitment size reported in Baja California (40 cm SCL reached at 10 years of age) and an annual growth rate of 1.62 cm/year (assuming a similar growth rate between females and males).
The formula used to calculate the age at sexual maturity (ASM) of the sampled individuals was as follows:
where ASM is the age of sexual maturity. SCLmin is the straight length of the carapace measured in centimeters (minimum size and maximum size); 40 cm is the average size at which juveniles move into developmental habitats from the pelagic stage; 1.62 cm is the average growth rate reported for juveniles in developmental habitats. Ten years is the period of time spent in the pelagic phase (lost year) in which they reach a 40 cm SCL.
ASM = ((SCL cm − 40 cm)/1.62 cm) + 10
3. Results
We found significant differences between nesting females and breeding male black sea turtles (Table 1).

Table 1.
Measures of central tendency of morphometric variables of adult females and males of black sea turtles (measurements in cm).
The average length difference between female and male black turtles was found to be 10.4 cm for straight carapace length (SCL) and 10.6 cm for curved carapace length (CCL) (≅11 cm).
The size of nesting females and breeding males was statistically different (SCL) (Student’s t-test, t = −14.837, p = 0.000, and Bonferroni adjusted prob = 0.000) (Figure 3).
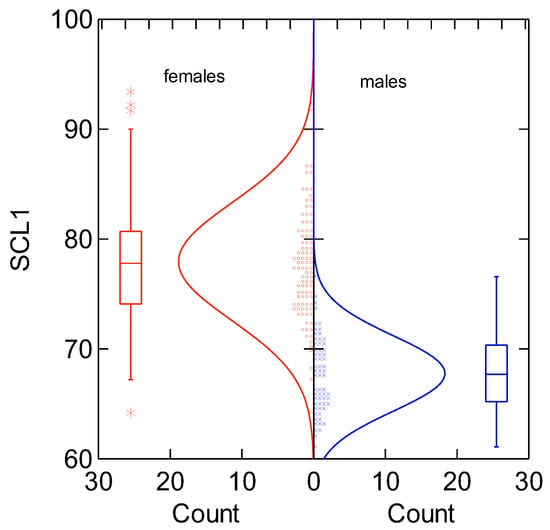
Figure 3.
Differences in straight carapace length (SCL) between female and male black sea turtles. Boxes show the average straight carapace length and standard deviation.
Differential Sexual Maturity
Considering a constant and similar growth rate between females and males, males reach sexual maturity at a minimum size of 61.1 cm SCL at approximately 23 years, and it takes 32.5 years to reach sexual maturity at maximum size (76.6 cm SCL). On the other hand, using the estimated growth rate for black turtles in breeding adults of 0.55 cm/year [15], males that reach sexual maturity at minimum size (61.1 cm SCL) would need an additional 11.6 years to reach the average size of males (67.5 cm SCL).
Females reach sexual maturity at a minimum size of 64.2 cm (SCL) at around 24.9 years of age and would require 42.9 years to reach sexual maturity at maximum size (93.5 cm SCL). Considering an adult growth rate (0.55 cm/year), females that reach sexual maturity at minimum size (64.2 cm SCL) would need an additional 24.9 years to reach the average size (77.9 cm SCL); however, we believe that the size of adult female and male black turtles is determined more by the existence of different sizes at which they reach sexual maturity than by the growth rate after reaching sexual maturity (0.55 cm/year). Considering that 70% of female black turtles are grouped in a size range between 71 and 80 cm SCL, we estimate that the majority (70%) of nesting female black turtles nesting in Colola reach sexual maturity between 29.1 and 34.6 years; on the other hand, 73% of nesting males are in a size range between 61 and 70 cm SCL, and these would mature between 23.0 and 28.5 years. This disparity at which females and males mature has significant implications for carapace morphology and adult reproductive behavior.
The heart-shaped carapace with a distinctive narrowing at the back of the female black sea turtle would allow copulation with males 11 cm smaller on average.
In total, ±300 h of observations of copulation and courtship interactions between breeding adult black turtles show that male rear flipper nails attach to the female carapace between marginal scutes 10 and 11, on which it is possible to observe the scars caused by the males (Figure 4).

Figure 4.
Narrowing of the carapace in female black sea turtles (C. m. agassizii) between marginal scute 10 and 11.
4. Discussion
Different ages of sexual maturity have been reported in sea turtles [11,15,16,18,19,20,21,22,23,24,25,26]. For the eastern Pacific population of green turtles, the age at sexual maturity was estimated to be between 45 and 50 years [11]. This study estimates the minimum sexual maturity of female black turtles at 24.2 years, while 70% of females grouped in a size range between 71 and 80 cm would mature between 29.1 and 34 years, which is close to that reported by Green of 35 years for the Galapagos Islands population [21]. It also marks the first report on the age of sexual maturity for breeding males in the eastern Pacific black turtle population. The difference of ≅11 cm in carapace length (SCL and CCL) between females and males represents 6.7 years at a growth rate of 1.63 cm/year. The estimate of minimum age at sexual maturity for nesting females (24.9 years) almost coincides with the beginning of the recovery of the nesting female population at Colola from 2002, 22 years after conservation activities began with the start of the recruitment of hatchlings at sea from 1980 onwards.
Due to the polyandrous mating behavior exhibited by female black turtles, they select multiple males to mate with during the breeding season. Therefore, females control the occurrence of copulations; consequently, there is no intrasexual sexual selection. This means that there are no fights between males to establish dominance patterns [30]. For this reason, males benefit from reaching sexual maturity at an earlier age than females, maximizing their reproductive success by mating with as many females as possible. On the other hand, females maximize their reproductive success by producing as many eggs as possible, which is why females maximize their size to produce more eggs but mature later than males.
Males mature earlier than females and sacrifice their size in a kind of trade-off in their life history traits [15,31]; however, size differences between females and males have implications for mating performance. During copulation, males must hold on to the female’s carapace with the nails of the back and front flippers. However, because the males are smaller than the females, they would have mechanical complications to hold on to the females, and therefore, mating would not occur. The characteristic shape of the female’s carapace favors copulation with males smaller than themselves.
The black turtle population has addressed the mating challenge through the unique heart-shaped carapace of females, which narrows between the marginal shields 10 and 11. This shape allows smaller males to securely attach to females during copulation, preventing mechanical isolation. Consequently, the carapace shape of eastern Pacific female black turtles indicates their compatibility with smaller males. Larger females from other Pacific populations may be mechanically incompatible with these males due to differing carapace shapes. Other authors such as Kamezaki and Matsui [32] have reported morphometric differences in the skull with other populations of green turtles in their pantropical distribution, and geometric morphology analysis has shown significant differences in carapace measurements between juveniles of the yellow and dark morphotypes in the north Pacific [33]; these studies suggest an important morphometric differentiation between populations of green turtles (Chelonia mydas mydas) and eastern Pacific green turtles or black turtles, even from the juvenile stage.
To overcome this challenge, the black turtle population has adapted mating challenges through the unique heart-shaped carapace of females, which narrows between marginal shields 10 and 11 [34]. This design enables smaller males to securely attach during copulation, preventing mechanical isolation. Thus, the carapace shape of eastern Pacific female black turtles indicates their compatibility with smaller males, while larger females from other Pacific populations may be incompatible due to differing shapes [15]. Our observations of male reproductive behavior and feeding ecology indicate that breeding male black turtles are residents in the breeding area and do not make reproductive migrations like females. Female size is linked to larger clutch sizes and increased nesting success, thereby enhancing reproductive output. In contrast, males invest little energy in sperm production [35] and tend to reproduce earlier, prioritizing mating over size in a polyandrous system without intrasexual competition.
5. Conclusions
This paper highlights differential sexual maturity among breeding adults of the black sea turtle (C. m. agassizii) in Michoacan, Mexico, and this is the first report of male sexual maturity age for this species in the eastern Pacific. The distinct carapace shapes of adult males and females relate to the smaller size of males, enabling successful mating with larger females. The reduced size of the male population indicates potential isolation from other breeding females outside the eastern Pacific, which do not exhibit the typical female carapace shape; however, research is needed to determine the connectivity between green turtle populations in the Pacific Basin. The small size of females and males of the black turtle population in the eastern Pacific region may be the result of a particular adaptation process in a region where environmental stochasticity generated by the effects of the “El Niño” phenomenon has played a fundamental role.
Author Contributions
Conceptualization, C.D.-T., M.Á.R.-L. and C.B.-O.; methodology, C.D.-T. and C.B.-O.; software, C.D.-T., C.B.-O. and O.D.-D.; validation, C.D.-T., D.G.P.-I., O.D.-D. and M.Á.R.-L.; formal analysis, C.D.-T., M.Á.R.-L., A.T.-G. and C.B.-O.; investigation, C.D.-T., M.Á.R.-L., A.T.-G. and C.B.-O.; resources, C.D.-T.; data curation, C.D.-T., C.B.-O. and O.D.-D.; writing—original draft preparation, C.D.-T. and C.B.-O.; writing—review and editing, C.D.-T., M.Á.R.-L., C.B.-O., D.G.P.-I., O.D.-D. and F.Y.C.-S.; visualization, C.D.-T.; supervision, C.D.-T., M.Á.R.-L., D.G.P.-I., C.B.-O. and F.Y.C.-S.; project administration, C.D.-T. and C.B.-O.; funding acquisition, C.D.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U.S. Fish and Wildlife Service’s Sea Turtle Conservation Fund, grant number F19AP00496, and the Instituto Politécnico Nacional SIP-IPN: 20231066, 20242503.
Institutional Review Board Statement
This study was approved by Dirección General de Vida Silvestre (DGVS) of the Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT), Oficio No. SPARN/DGVS/07190-24. 24 June 2024.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the turtle camp “Colola: Capital Mundial de la Tortuga Negra A.C.” for their support in carrying out the fieldwork and for their efforts to continue protecting the black sea turtle. We would also like to thank the U.S. Fish and Wildlife Service for funding to carry out conservation and research activities, and to express our gratitude to the IPN and CONAHCYT for their support and for granting the necessary permissions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bernardo, J. Determinants of maturation in animals. Trends Ecol. Evol. 1993, 8, 166–173. [Google Scholar] [CrossRef]
- Van Buskirk, J.; Crowder, L.B. Life-history variation in marine turtles. Copeia 1994, 1994, 66–81. [Google Scholar] [CrossRef]
- Davenport, J. Temperature and the life-history strategies of sea turtles. J. Therm. Biol. 1997, 22, 479–488. [Google Scholar] [CrossRef]
- Wapstra, E.; Swain, R. Geographic and annual variation in life-history traits in a temperate zone Australian skink. J. Herpetol. 2001, 35, 194–203. [Google Scholar] [CrossRef]
- Hartnoll, R.G. Growth, Sexual Maturity and Reproductive Output. In Crustacean Issues 3; Routledge: Abingdon, UK, 2017; pp. 101–128. [Google Scholar]
- Stearns, S.C.; Koella, J.C. The evolution of phenotypic plasticity in life-history traits: Predictions of reaction norms for age and size at maturity. Evolution 1986, 40, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S.C. Life history evolution: Successes, limitations, and prospects. Naturwissenschaften 2000, 87, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.W.; Morris, Y.A. The comparative endocrinology of sea turtles. Copeia 1985, 1985, 723–735. [Google Scholar] [CrossRef]
- Bjorndal, K.A.; Bowen, B.W.; Chaloupka, M.; Crowder, L.B.; Heppell, S.S.; Jones, C.M.; Lutcavage, M.E.; Policansky, D.; Solow, A.R.; Witherington, B.E. From crisis to opportunity: Better science needed for restoration in the Gulf of Mexico. Science 2011, 331, 537–538. [Google Scholar] [CrossRef]
- Wildermann, N.E.; Gredzens, C.; Avens, L.; Barrios-Garrido, H.A.; Bell, I.; Blumenthal, J.; Bolten, A.B.; McNeill, J.B.; Casale, P.; Di Domenico, M.; et al. Informing research priorities for immature sea turtles through expert elicitation. Endanger. Species Res. 2018, 37, 55–76. [Google Scholar] [CrossRef]
- Alvarado, J.; Figueroa, A. Madurez sexual tardía de las tortugas marinas. Cienc. Desarro. 1989, 89, 59–63. [Google Scholar]
- Scott, R.; Marsh, R.; Hays, G.C. Life in the really slow lane: Loggerhead sea turtles mature late relative to other reptiles. Funct. Ecol. 2012, 26, 227–235. [Google Scholar] [CrossRef]
- Avens, L.; Snover, M.L. Age and age estimation in sea turtles. In The Biology of Sea Turtles; Wyneken, J., Lohmann, K.J., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 3, pp. 97–133. [Google Scholar]
- Bjorndal, K.A.; Parsons, J.; Mustin, W.; Bolten, A.B. Threshold to maturity in a long-lived reptile: Interactions of age, size, and growth. Mar. Biol. 2013, 160, 607–616. [Google Scholar] [CrossRef]
- Cutzi, B.-O.; Angel, R.-L.M.; Hervey, R.-G.; Carlos, D.-T. Black Sea Turtle (Chelonia mydas agassizii) Life History in the Sanctuary of Colola Beach, Michoacan, Mexico. Animals 2023, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Bjorndal, K.A.; Parsons, J.; Mustin, W.; Bolten, A.B. Variation in age and size at sexual maturity in Kemp’s ridley sea turtles. Endanger. Species Res. 2014, 25, 57–67. [Google Scholar] [CrossRef]
- Omeyer, L.C.; Godley, B.J.; Broderick, A.C. Growth rates of adult sea turtles. Endanger. Species Res. 2017, 34, 357–371. [Google Scholar] [CrossRef]
- Limpus, C.J.; David, G.W. The Growth of Immature Green Turtles (Chelonia mydas) under Natural Conditions. Herpetologica 1980, 36, 162–165. [Google Scholar]
- Mendonça, M.T. Comparative Growth Rates of Wild Immature Chelonia mydas and Caretta caretta in Florida. J. Herpetol. 1981, 15, 447–451. [Google Scholar] [CrossRef]
- Zug, G.R.; Balazs, G.H. Skeletochronological age estimates for Hawaiian green turtles. Mar. Turt. Newsl. 1985, 33, 9–10. [Google Scholar]
- Green, D. Growth rates of wild immature green turtles in the Galapagos Islands, Ecuador. J. Herpetol. 1993, 27, 338–341. [Google Scholar] [CrossRef]
- Hirth, H.F. Synopsis of the Biological Data on the Green Turtle Chelonia mydas (Linnaeus 1758); U.S. Fish and Wildlife Service: Washington, DC, USA, 1997.
- Casale, P.; Mazaris, A.D.; Freggi, D. Estimation of age at maturity of loggerhead sea turtles Caretta caretta in the Mediterranean using length-frequency data. Endang. Species Res. 2011, 13, 123–129. [Google Scholar] [CrossRef]
- Zug, G.R.; Parham, J.F. Age and growth in leatherback turtles, Dermochelys coriacea (Testudines: Dermochelyidae): A skeletochronological analysis. Chelonian Conserv. Biol. 1996, 2, 244–249. [Google Scholar]
- Sanchez, C.L.; Bunbury, N.; Mortimer, J.A.; A’bear, L.; Betts, M.; von Brandis, R.; Burt, A.J.; Cooke, L.; van de Crommenacker, J.; Currie, J.C.; et al. Growth rate and projected age at sexual maturity for immature hawksbill turtles and green turtles foraging in the remote marine protected area of Aldabra Atoll, Seychelles. Mar. Biol. 2023, 170, 49. [Google Scholar] [CrossRef]
- Turner Tomaszewicz, C.N.; Avens, L.; Seminoff, J.A.; Limpus, C.J.; FitzSimmons, N.N.; Guinea, M.L.; Pendoley, K.L.; Whittock, P.A.; Vitenbergs, A.; Whiting, S.D.; et al. Age-specific growth and maturity estimates for the flatback sea turtle (Natator depressus) by skeletochronology. PLoS ONE 2022, 17, e0271048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seminoff, J.A.; Resendiz, A.; Nichols, W.J.; Jones, T.T. Growth rates of wild green turtles (Chelonia mydas) at a temperate foraging area in the Gulf of California, Mexico. Copeia 2002, 2002, 610–617. [Google Scholar] [CrossRef]
- Koch, V.; Brooks, L.B.; Nichols, W.J. Population ecology of the green/black turtle (Chelonia mydas) in Bahía Magdalena, Mexico. Mar. Biol. 2007, 153, 35–46. [Google Scholar] [CrossRef]
- Nichols, W.J. Biology and Conservation of Sea Turtles in Baja California, Mexico. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 2003; 474p. [Google Scholar]
- Manderson, L. David Barash. “Sociobiology: The Whispering within” (Book Review). Aust. J. Anthropol. 1982, 13, 431. [Google Scholar]
- Benabib, M. Los Vertebrados y la Historia de la Vida. Ciencias, (007). Recuperado a Partir de 2009. Available online: https://www.revistas.unam.mx/index.php/cns/article/view/11310 (accessed on 22 January 2025).
- Kamezaki, N.; Matsui, M. Geographic Variation in Skull Morphology of the Green Turtle, Chelonia mydas, with a Taxonomic Discussion. J. Herpetol. 1995, 29, 51. [Google Scholar] [CrossRef]
- Okamoto, K.; Kamezaki, N. Morphological variation in Chelonia mydas (Linnaeus, 1758) from the coastal waters of Japan, with special reference to the turtles allied to Chelonia mydas agassizii Bocourt, 1868. Curr. Herpetol. 2014, 33, 46–56. [Google Scholar] [CrossRef]
- Pritchard, P.; Bacon, F.; Berry, A.; Carr, J.; Fletmeyer, R.; Gallagher, S.; Hopkins, R.; Lankford, R.; Marquez, L.; Ogren, W.; et al. Manual Sobre Técnicas de Investigación y Conservación de Las Tortugas Marinas; Center for Environmental Education: Washington, DC, USA, 1983; 134p. [Google Scholar]
- Hamann, M.; Limpus, C.J.; Owens, D.W.; Lutz, P.L.; Musick, J.A.; Wyneken, J. Reproductive Cycles of Males and Females. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Wyneken, J., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Volume II, p. 472. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).