Abstract
The infraclass Euthyneura (Mollusca, Heterobranchia) exhibits significant diversity in morphology, size, life habits, and color. Several species are important for research in evolution, ecology, chemistry, and pharmacology. Despite Colombia’s expansive Pacific and Caribbean coasts, which host ecosystems such as rocky shores and coral reefs, key habitats for sea slugs and sea hares, the biodiversity of Euthyneura remains largely understudied. This study aims to expand the inventory of Euthyneura diversity in intertidal and shallow subtidal rocky and coral reef environments in Colombia’s Pacific (Uramba Bahía Málaga National Natural Park) and Caribbean (Seaflower Biosphere Reserve) areas. Rapid biodiversity assessments using snorkeling and errant scuba diving at depths of 1–40 m resulted in the documentation of 31 species (14 in Caribbean coral reefs and 17 in Pacific intertidal and shallow subtidal rocky shores and reefs). Eleven species were new records. The family Aplysiidae was the richest with five species, followed by Facelinidae with four, and Aeolidiidae, Discodorididae, Chromodorididae, and Plakobranchidae with three each. Given the limited sampling effort (~40 h in the Caribbean and ~20 h in the Pacific) and the substantial new data collected, it is evident that there is still much to learn about this group in these areas. Increased efforts, combined with detailed morphological and molecular techniques, will enhance our understanding and documentation of Euthyneura diversity in Colombia.
1. Introduction
Mollusks are a major component of biodiversity and play crucial roles in ecosystem functioning [1]. They contribute to the circulation of matter and energy at all trophic levels by acting as primary consumers (grazers and filter-feeders), being preyed upon by many species (including human beings), functioning as key predators, and aiding in the decomposition of organic matter [2,3]. Among mollusks, sea slugs and sea hares (Gastropoda: Heterobranchia: Euthyneura) are perhaps the most morphologically and ecologically diverse groups [4] encompassing marine, freshwater, and terrestrial species [5].
Many species within this group have been extensively used in several lines of research, including phylogeny and evolution [6], ecology [7], and chemistry and pharmaceutical applications [8]. However, their evolutionary history remains controversial [9], prompting numerous phylogenetic [10,11,12] and morphological [13,14,15] studies aimed at clarifying phylogenetic relationships and disentangling species complexes.
Despite the strong reputation of mollusks as targets for various types of studies, including inventories, it is noteworthy that these are often incomplete. When implemented, they frequently reveal new geographic records (e.g., [16,17,18]) or new species to science (e.g., [19,20,21]). This is especially true in developing countries, where much effort is still needed to understand the true magnitude of biological diversity [22], particularly regarding marine invertebrates.
Although often colorful, sea slugs can be cryptic, rare, and difficult to find and document. This raises the question of how comprehensive the inventory of these animals is in poorly studied but potentially diverse regions, such as Colombia, a country with extensive shorelines in both the Pacific and the Caribbean, regions considered biodiversity hotspots [23].
The Great Caribbean (GC) is a region with highly biodiverse ecosystems, such as coral reefs, sea grasses, and mangroves, that host a myriad of species [24,25]. Valdés et al. [26] reported 310 species of “opisthobranchs” in the Tropical Northwestern Atlantic; Goodheart et al. [27] reported 82 species of heterobranch sea slugs in Bocas del Toro (Panamá); Camacho-García et al. [28] reported 70 species for the Caribbean coast of Costa Rica; and recently, Vital et al. [29] reported 31 species of heterobranch sea slugs for the Gulf of Mexico and the Mexican Caribbean Sea. The Tropical Eastern Pacific (TEP), on the other hand, has fewer coral reef ecosystems but hosts more rocky ecosystems, many of which experience large tidal variations. However, it has received less attention in the study of this group of heterobranchs. Behrens and Hermosillo [30] reported 314 species in the Eastern Pacific from Alaska to Central America, of which 238 are found in the TEP, and Camacho-Garcia et al. [28] reported 163 species.
In Colombia, the study of sea slugs and sea hares has been scarce and patchy. For the Caribbean, Ardila et al. [31] reported 83 species, a figure later updated to 105 [32]. Further research has significantly enhanced our understanding of the group’s regional biodiversity (e.g., [33,34]). As a result, 132 sea slug species are now recorded in the Colombian Caribbean, representing approximately 43% of the species reported for the Greater Caribbean. On the Pacific side, Ardila et al. [31] reported 32 species, but this number was recently updated to 97 species of Euthyneura [17]. Consequently, the number of Heterobranchia species in the Colombian Pacific ranges from about 41% (using [30]) to 60% (using [28]) of the species depending on the source of comparison. Although these figures are significant, we anticipate that additional species will be discovered in these under-studied yet potentially highly biodiverse localities.
Hence, this study aims to contribute to the Colombian inventory of heterobranch sea slugs by providing new information from two important, yet distant and unrelated localities: the Seaflower Biosphere Reserve in the Caribbean and the National Natural Park Uramba Bahía Málaga in the Pacific.
2. Materials and Methods
2.1. Study Area
2.1.1. Courtown Cay—Seaflower Biosphere Reserve
The islands of San Andrés, Providencia, and Santa Catalina, the Albuquerque, Bolívar (East Southeast Cay or Courtown Cay), Quitasueño, Roncador, Serranilla, and Serrana cays, and the Alicia and Nuevo banks, and their surrounding waters, were declared as the Seaflower Biosphere Reserve (SFBR) by UNESCO in the year 2000. Covering a total of 350,000 km2, this reserve is located 700 km off the northwest coast of Colombia. It hosts two of the largest coral reefs of the Colombian Caribbean, situated around the largest and most populated islands: San Andrés and Providencia [35].
The climatic conditions of the SFBR are characteristic of the region, with an average annual air temperature of 27.4 °C and annual precipitation around 1900 mm, as measured on San Andrés Island. The reserve may experience seasonal storms that occur mainly in the second half of the year. The average sea surface temperature is 27.5 °C, with monthly averages of 26.8 °C between February and March (dry season) and 30.2 °C between September and October (rainy season). The surface salinity ranges between 34.0 and 36.3. Tides in atolls are mixed with a strong diurnal component, which ranges between 0.3 and 0.6 m at San Andrés Island [36]. Cayo Bolívar (12.4201° N–81.4768° W, referred to as Courtown Cay or CC hereafter—Figure 1A), currently restricted to tourism, is located approximately 28 km southeast of San Andrés Island and consists of two small islands connected by a sandy strip.
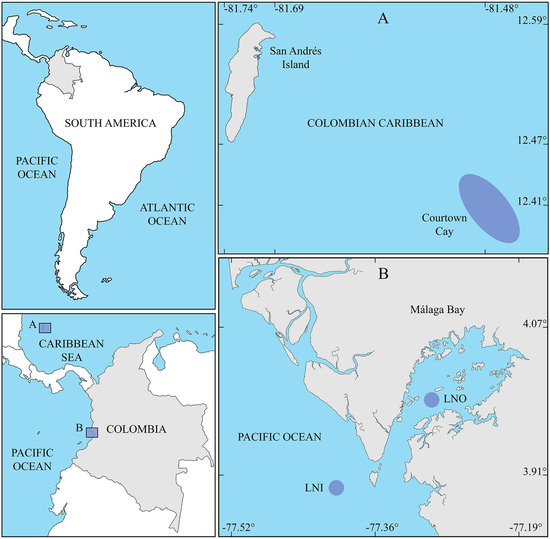
Figure 1.
Colombia in South America (upper left panel) and the sites of the two sampling locations (A,B) within the country (lower left panel): (A) Courtown Cay in the Colombian Caribbean and (B) Málaga Bay in the Colombian Pacific. Only the relevant coordinates are shown for the Caribbean site. Sampling sites are represented by purple ovals. LNO—Los Negros and LNI—Los Negritos.
2.1.2. Los Negros and Los Negritos National Natural Park Uramba Bahía Málaga
The bay, which hosts an archipelago, is situated along the central Colombian Pacific coast. Like most of the Pacific coast of Colombia, the area is sparsely populated. The bay covers approximately 136 km2 with a complex geography that includes several biotopes along its coastline, including small islands and islets, headlands, caves, pocket sandy beaches, rocky beaches, cliffs, mangroves, muddy flats, and intertidal rocky reefs [37]. Two of the largest rocky reefs: Los Negros (LNO) (3.9916° N–77.2952° W) and Los Negritos (LNI) (3.8966° N–77.4040° W), were selected as sampling sites (Figure 1B). The former is located inside the bay, while the latter is outside it.
The tides are semi-diurnal with a range of approximately 4.5 m during spring tides. The region experiences two periods of high rainfall: from April to June and from September to November, and two periods of low rainfall: from January to March and from July to August [38]. Rainfall in the region is high, ranging between 5000 and 7000 mm annually [39]. Air temperatures range from 18 to 25 °C, while the sea surface temperature averages 27 °C. The bay’s surface salinity, influenced by tides and rainfall, ranges from 1.3 to 30.0 [39].
2.2. Field Work
2.2.1. Sampling Methods
Field sampling was conducted under permits number 006/2022 and 601/2022 for the Pacific and Caribbean regions, respectively. These permits were granted by the National Natural Parks of Colombia (for the Pacific) and Coralina (for the Caribbean). The Ethical Committee of the Faculty of Natural and Exact Sciences of Universidad del Valle approved the collection methods for this research.
Two independent but complementary methods were employed for sampling:
- Rapid Biodiversity Assessment (RBA). This is a variation of the rapid assessment program methodology [40] focusing on specific taxa. It involves an intensive and detailed search for organisms, with particular attention to inspecting cracks, loose substrates, and potential food sources (e.g., sponges, algae, cnidarians) for the group of interest (sea slugs).
- Artificial Hard Substrates (AHS). This indirect method is a modification of the Autonomous Reef Monitoring Structures (ARMS). For this method, plastic baskets of approximately 30 × 20 × 15 cm were filled with coral rubble and fragmented clam shells. The baskets were covered with plastic mesh and secured to the substrate with cord. The AHS mimics hard environments with interstices that are colonized by benthic marine organisms. These AHS units were deployed in the intertidal and shallow subtidal zones (maximum depth of 4 m during low tide) and left in place for 4 months.
2.2.2. Sampling at Courtown Cay
Samplings were carried out between the 8th and the 20th of September 2022. Two divers performed the RBA independently, each with a sampling effort of 40 h. This methodology was implemented around the Cay by snorkeling at depths up to 6 m and SCUBA diving at depths ranging from approximately 5 to 40 m in coral reefs, coral rubble, sandbanks, and seagrass areas. Although it is known that many sea slug species exhibit nocturnal habits, night diving was not permitted for safety reasons. However, two short night snorkeling sessions were conducted in the shallow area (maximum depth of 2 m) around the Cay. Given that a second visit to Courtown Cay was not planned, the Artificial Hard Substrates (AHS) methodology was not implemented at this site.
2.2.3. Sampling at Los Negros and Los Negritos
Two sampling events were conducted: the first in July and the second in December 2022. The methods described above were implemented in this area. RBA was carried out in both the intertidal and shallow subtidal zones (with a maximum depth of approximately 3 m) using scuba diving. Intertidal sampling occurred during low tide, while subtidal sampling was performed during slack or flood tide. Each sampling session lasted up to three hours and was conducted by two people, with a total effort of 20 h per person. Additionally, a total of 24 AHS were retrieved after four months (six per site and depth) and examined in the field for the presence of sea slugs.
2.2.4. Sample Processing
All specimens found in both the Caribbean and Pacific regions were either collected or counted (for known species) and photographed while alive, either in the field or inside plastic containers filled with seawater. Each specimen was identified to the most specific taxonomic level possible, using specialized field guides for the Pacific [28,30] and the Caribbean [26,27], with additional support from specialists in the group. At least one specimen of each species was relaxed by gradually adding fresh water to the container to expose key identification structures such as rhinophores, gills, and tentacles, then fixed and preserved in 96% ethanol. These specimens were stored in plastic vials, properly labeled, and subsequently transported to the Reference Marine Biology Collection at Universidad del Valle. The specimens were also included in the Biodiversity Information System for Colombia (SiB-Colombia).
3. Results
3.1. General Taxonomic Composition
A total of 31 species of Euthyneurans (14 in the Caribbean and 17 in the Pacific) in 16 families were identified (Table 1). Of these, 11 species are new records for Colombia, with 3 from Courtown Cay and 8 from LNO and LNI. The order Nudibranchia represented approximately 58% of the species, while the remaining 42% were contributed by other taxa, including the order Aplysiida, the superorder Sacoglossa, the order Cephalaspidea, the superfamily Acteonoidea, and the order Pleurobranchida. The family Aplysiidae was the most species-rich, with five species, followed by Facelinidae with four species, and Aeolidiidae, Discodorididae, Chromodorididae, and Plakobranchidae, each with three species (Figure 2).

Table 1.
List of species and number of records at each locality. CC: Courtown Cay (Colombian Caribbean), LNO: Los Negros, LNI: Los Negritos (Colombian Pacific). New records are indicated by *.
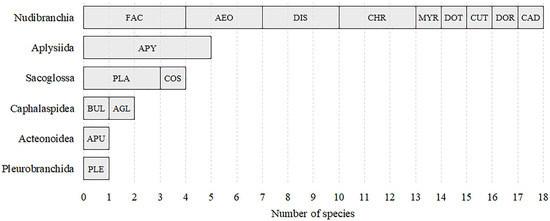
Figure 2.
Number of species per family within higher taxa, combined for all sampling localities on the Caribbean and Pacific coasts of Colombia. FAC = Facelinidae, AEO = Aeoliididae, DIS = Discodorididae, CHR = Chromodorididae, MYR = Myrrhinidae, DOT = Dotidae, CUT = Cuthonidae, DOR = Dorididae, CAD = Cadlinidae, APY = Aplysiidae, PLA = Plakobranchidae, COS = Costasiellidae, BUL = Bullidae, AGL = Aglajidae, APU = Aplustridae, PLE = Pleurobranchidae.
3.2. Species Comments
We briefly describe the main characteristics of the eleven species, which are either new records or potentially undescribed species.
3.2.1. Courtown Cay—Colombian Caribbean
A total of 14 species in 10 families were found. The richest families were Aplysiidae and Plakobranchidae with three species each (Figure 2). There were three new records for the Colombian Caribbean, one of which is perhaps an undescribed species of the genus Dondice. Three species, previously recorded in other localities of the Colombian Caribbean, are new records for the Cay. The most abundant species were Elysia crispata, Costasiella ocellifera, and Aplysia dactylomela, with 28, 20, and 7 specimens, respectively (Table 1).
- Superorder Nudipleura Wägele & Willan, 2000
Order Nudibranchia Cuvier, 1817
Family Myrrhinidae Bergh, 1905
Dondice sp. (Figure 3A)

Figure 3.
New records for the Colombian Caribbean. Seaflower Biosphere Reserve: (A). Dondice sp., (B). Costasiella ocellifera, and (C). Elysia patina. Scale bars: (A). 5 mm, (B,C). 2 mm.
Voucher: CRM-UV 2022-298 (1 specimen, approx. 1.5 cm in length)
Morphological Description. The body is elongated and whitish in color. The cerata are grouped in clumps of transverse rows on each side of the body. The inner cerata are larger than those on the sides, increasing in size from the head to the first third of the body and then decreasing toward the tail. They are pale white, with the extensions of the digestive system clearly visible. Rhinophores are annulated and retain the coloration of the body, but the tips are brilliant white. The anterior part of the head has three distinct orange stripes: one between the rhinophores and one on each side of the head. The central stripe is flanked by a white stripe that extends from the back of the head to the base of the mustache-like oral tentacles, where it becomes wider. The tentacles are smooth and approximately three times longer than the rhinophores.
Taxonomic Remarks. This species resembles D. occidentalis, but García-Méndez et al. [41] state that it is part of a species complex. Additionally, the distribution and coloration patterns of the lines on the head and notum, as well as the cerata, rhinophores, and tentacles of our specimen, do not correspond to any of the morphotypes described in their paper. Nonetheless, the description of these morphotypes suggests high variability, making it difficult to determine whether this represents an undescribed species within the complex.
- Superorder Sacoglossa
Family Costasiellidae K. B. Clark, 1984
Costasiella ocellifera (Simroth, 1895) (Figure 3B)
Voucher: CRM-UV 2022-310 (7 specimens, average length 0.3 cm)
Morphological Description. The body is whitish and translucent, with the notum covered in numerous brown dots. A yellow stripe extends from the anterior region of the head to behind the rhinophores, encircling a brown ocellus with a bluish-white center. The rhinophores are smooth and elongated with brown dots interrupted by two bands of white dots, one near the middle and the other at the tip. The eyes are located between the rhinophores, very close together, with the yellow stripe running between them. There are no dots around the eyes. The cerata are numerous and thick, predominantly dark green, ending in a thin tip banded with yellow, white, and black and may have one or two light blue dots near the apex.
Distribution and Ecological Notes. This species was collected only at one site, associated with fleshy algae, presumably feeding on it. This species is reported from the Bahamas, the Dominican Republic, the United States Virgin Islands, Belize, Jamaica, and Barbados [42]. This is the first record of this species in the country.
- Family Plakobranchidae Gray, 1840
Elysia patina Ev. Marcus, 1980 (Figure 3C)
Voucher: CRM-UV 2022-305 (1 specimen, approx. 0.3 cm in length)
Morphological Description. The body is predominantly white and covered with small papillae, with a light green hue toward the foot and the center of the body. The parapodia, notum, and head are covered with red dots. The rhinophores are coiled with a translucent band in the middle region. The parapodia is oval when extended and the pericardium is visible just behind the head. The head has two reddish stripes, one on each side, originating behind the rhinophores and passing through the eyes.
Distribution Notes. This species is distributed between Florida (United States) to Puerto Morelos (México) [29,43]; this is the first record for the country and extends the distribution range of the species more than 1000 km southward.
3.2.2. Los Negros and Los Negritos—Pacific Coast
Of the two rocky reefs, LNI with 13 species in total was the site with the highest richness, as compared to LNO, where only six species were recorded. There were two species in common between these two sites: Tayuva lilacina and Felimida sphoni.
- Superorder Nudipleura Wägele & Willan, 2000
Order Nudibranchia Cuvier, 1817
Family Aeolidiidae Gray, 1827
Anteaeolidiella ireneae Carmona, Bhave, Salunkhe, Pola, Gosliner & Cervera, 2014 (Figure 4A)

Figure 4.
New records for the Colombian Pacific. National Natural Park Uramba Bahía Málaga: (A). Anteaeolidiella ireneae, (B). Phidiana sp., (C). Emarcusia cf. morroensis, (D). Doto sp., (E). Cuthona sp., (F). Taringa cf. aivica, (G). Tyrinna evelinae, (H). Cadlina sp. Scale bars: (A,B,D,E). 2 mm, (C,F,H). 1 mm, (G). 5 mm.
Voucher: CRM-UV 2022-121 (1 specimen, approx. 0.5 cm in length)
Voucher: CRM-UV 2023-006 (5 specimens, average length 0.4 cm)
Morphological Description. The body is white with a pair of orange stripes in front of the rhinophores and a pair of crisscrossing orange stripes extending along the notum. The rhinophores and tentacles are smooth and orange from the base to the mid region, ending in a white tip.
Distribution Notes. This species was found during both visits to the LNI reef in intertidal pools, and all observed specimens were collected. This is the first report for the Colombian Pacific, extending the known distribution from Clipperton Island and the Pacific coast of Mexico, Costa Rica, and Panama [44] by approximately 480 km southward.
- Family Facelinidae Gray, 1850
Phidiana sp. (Figure 4B)
Voucher: CRM-UV 2023-013 (5 specimens, average length 0.2 cm)
Morphological Description. The body is translucent and elongated, with a line extending from its posterior end to the rhinophores, where it bifurcates and extends on each side above the oral tentacles. The rhinophores are annulate, and the tentacles are smooth; both structures are orange from the base to the middle region and white from there to the tips. The cerata are arranged in two parallel rows along the sides of the notum, with an orange core. The larger and thicker cerata have a white sub-apical band.
Taxonomic Remarks. All observed specimens were collected. Morphologically, this species resembles Phidiana lynceus; however, given that this species is distributed in the Caribbean and that the species reported for the Pacific coast are P. lascruscensis and P. hiltoni, this record will remain at the genus level until further analysis can be performed.
Voucher: CRM-UV 2022-120 (1 specimen, approx. 0.3 cm in length)
Voucher: CRM-UV 2023-009 (1 specimen, approx. 0.3 cm in length)
Morphological Description. Translucent body with multiple small, opaque white spots scattered on the body giving it a milky appearance. There are two dash-like orange spots in the middle of the notum, one ahead and the other behind the rhinophores. Additionally, there are two clusters of small orange spots located on each side near the oral tentacles. The rhinophores are smooth and, like the tentacles, have white tips. The cerata are thin at the base and thicken toward the middle. They have black and brown bands with white tips.
Taxonomic and Distributional Remarks. This species was found during both field trips to LNI, and the two specimens found were collected. They resemble E. morroensis morphologically; however, our specimens have fewer cerata, which is why we retain ‘cf.’ in their identification. Nevertheless, this is a monospecific genus, so the probability of it being the species is high. This constitutes a new record for Colombia and extends its distribution range by hundreds of kilometers southward, as E. morroensis has been reported as far south as San Diego, California, USA [30].
- Family Dotidae Gray, 1853Doto sp. (Figure 4D)
Voucher: CRM-UV 2023-118 (1 specimen, approx. 0.4 cm in length)
Voucher: CRM-UV 2023-008 (3 specimens, average length 0.2 cm)
Morphological Description. The body is yellowish with brown spots on the notum. The foot is translucent. The rhinophores are smooth, translucent, with white dots that increase in density toward the tips and are inserted in a bell-shaped sheath. The cerata are dark orange appendages with gray-violaceous tubercles that resemble grapes.
Distribution and Taxonomic Notes. This species was found in the most exposed shallow subtidal zone during both field trips to LNI. No members of this genus have been previously reported in the country. Considering that the characteristics of these specimens do not match any known species in the genus, this may be an undescribed species, although further research is needed. All observed specimens were collected.
- Family Cuthonidae Odhner, 1934
Cuthona sp. (Figure 4E)
Voucher: CRM-UV 2023-007 (3 specimens, average length 0.2 cm)
Morphological Description. The body is translucent with opaque white spots and two parallel bright orange stripes on the anterior part, running from the back of the rhinophores to the base of the tentacles, interrupted at the level of the eyes. The tentacles and rhinophores are simple, smooth, and have whitish tips. The cerata, which are easily detached when the animal is disturbed, are dark brown near the base and center, with orange dots of varying sizes. The cerata become lighter in color toward the apex, ending in white tips.
Taxonomic and Ecological Notes. This species was found in the intertidal pools of LNI. Given that the characteristics do not match those of any other species in the genus, this may represent an undescribed species.
- Family Discodorididae Bergh, 1891
Voucher: CRM-UV 2022-117 (1 specimen, approx. 0.2 cm in length)
Morphological Description. The body is oval and yellow, with brownish spots on the notum. The mantle margin is white. The surface of the notum exhibits caryophyllidia and is covered with polyp-shaped papillae, each having a crown of spicules surrounding its central portion. The rhinophores and gill feathers are spiral-lamellated, yellow in color with white tips.
Distribution Notes. This specimen was found in the intertidal zone of LNI, associated with an unidentified sponge of similar coloration. This is the first report for Colombia and extends the previously known range, from California to the Pacific coast of Panama [30], even further southward.
- Family Chromodorididae Bergh, 1891
Tyrinna evelinae (Er. Marcus, 1958) (Figure 4G)
Voucher: CRM-UV 2023-002 (1 specimen, approx. 1.7 cm in length)
Morphological Description. The body is milky white and slightly translucent. The edge of the mantle is irregular, with creamy pink dots that appear embedded in the tissue. Multiple small orange dots are scattered across the surface of the notum. Both the mantle and foot have numerous white dots. The rhinophores are lamellated and light yellow, with a slightly whiter central area resembling a longitudinal line. The gill feathers are slightly yellower than the rhinophores.
Distribution Notes. This species was found in the shallow subtidal zone attached to a rock in LNO and represents a new record for the Colombian Pacific coast. Previous reports for the Eastern Pacific include Bahía Vizcaíno, Baja California, the Gulf of California, Costa Rica, Panama, and Peru (Behrens and Hermosillo, 2005), as well as Brazil, the Dominican Republic, Jamaica, Puerto Rico, and Venezuela in the Caribbean [45].
- Family Cadlinidae Bergh, 1891
Cadlina sp. (Figure 4H)
Voucher: CRM-UV 2023-003 (1 specimen, approx. 0.2 cm in length)
Morphological Description. The body is opaque white with dorsolateral yellow dots. Caryophyllidia are evident on the notum. The rhinophores and gills are the same color as the body, with the rhinophores being lamellated and showing a brownish spot in the inner middle part.
Taxonomic and Distribution Notes. This species was found in the low intertidal zone attached to a rock in LNO and resembles the species described by Behrens and Hermosillo [30] as Cadlina sp. 1 from the Pacific coast of Mexico. This finding extends its known distribution (Isla Isabel, Islas Revillagigedo, and Bahía de Banderas in Mexico) approximately 3500 km southward in the Pacific.
4. Discussion
Although some regions of the Tropical Eastern Pacific (TEP) have received considerable attention in the study of heterobranchs [46], others, such as the Pacific coast of Colombia, have been neglected due to various factors, including accessibility restrictions, limited financial support—common in many developing countries [22]—and biases toward taxonomic groups of direct economic significance to local communities [37]. In contrast, the Greater Caribbean—and particularly the Colombian Caribbean—has received more attention, likely due to greater sampling efforts and easier accessibility to coastal environments. This has led to a better understanding of the biodiversity of this group, as reflected in several publications (e.g., [31,32,33,34]).
Our results highlight this disparity: we found 1.2 times more species in the Pacific than in the Caribbean. This figure increases to 2.7 times when considering only new records. Many of the Pacific records fill existing gaps or extend species’ known distributions by hundreds—sometimes thousands—of kilometers southward, further enhancing our understanding of the region’s biodiversity. In contrast, the Caribbean findings primarily fill gaps, with one of the three new records for Colombia (Elysia patina) likely extending the species’ distribution range.
In general, species richness was not high. It has been suggested that finding heterobranchs in the Caribbean is challenging, with their abundance typically lower than in other tropical regions of the world [27]. During our sampling campaign, with an effort of 40 h per researcher, we found only about 4.5% of the potential species from the Caribbean [26], corroborating this observation. Of the 14 species recorded, only two (E. crispata and A. dactylomela) were relatively common. Another species (C. ocellifera) was abundant but found only once, suggesting it is rare.
The proportion of species found in the Pacific, with 20 h per researcher, represented approximately 7.1% of the potential species for the TEP [30], also a low value. Although information on this group in the Colombian Pacific was recently updated [17], much remains to be discovered. Remarkably, two small localities yielded eight new records for the country. Heterobranch species in the Pacific are also difficult to find and are generally uncommon, with exceptions like Dolabrifera nicaraguana (included in this study) and Elysia diomedea, which are seasonally abundant. Additional sampling methods, such as collecting food items (as recommended by [28,30]) and performing nocturnal surveys [47], could potentially increase species detection in both the Caribbean and Pacific regions of Colombia.
Our primary goal is to contribute to the understanding of euthyneuran biodiversity rather than compare sampling methods or environmental conditions. However, it is worth noting key differences between the two regions that may have influenced our results:
- Tidal conditions: The Pacific coast experiences large tidal ranges, providing a wide intertidal zone for thorough substrate inspection without scuba gear. In contrast, the Caribbean has small tidal ranges, requiring underwater surveys via snorkeling or scuba diving.
- Underwater visibility: Visibility is generally higher in the Caribbean, facilitating the search for sea slugs compared to the poor visibility in the Pacific.
- Ecosystem differences: The Caribbean ecosystems visited were predominantly coral reefs, while those in the Pacific were the intertidal and shallow subtidal of rocky reefs. Both ecosystems offer suitable substrates for sea slugs, but their structural differences affect search methods. In coral reefs, loose substrates suitable for turning and inspecting are rare, and detaching fixed substrates is discouraged, limiting searches to the top and side surfaces. This leaves important underside microhabitats unexplored. In contrast, in the rocky reefs of the Pacific, loose boulders and rocks that can be lifted and carefully examined are relatively common. This allows for more thorough searches and access to a wider range of microhabitats.
- Sampling methods and effort: In the Caribbean, only underwater RBAs were conducted, while in the Pacific, both RBAs and AHSs were performed, both intertidally and underwater. The sampling effort per researcher in the Caribbean (40 h) doubled that in the Pacific (20 h).
We believe the heterobranch checklist for Colombia remains incomplete and that continued research will yield significant findings, potentially including new undescribed species. With this study, the known heterobranch richness in the Colombian Caribbean now stands at 131 species, compared to 111 in the Pacific. This gap could narrow with exploration of the northern Colombian Pacific, a region with significant potential due to its rocky shores and reefs.
Finally, given that many records in this study are based on single specimens, further sampling and molecular analyses are necessary to confirm identifications. Additionally, tailored approaches that account for environmental differences between the Caribbean and Pacific are crucial to advancing our understanding of sea slug biodiversity in Colombia.
Author Contributions
Conceptualization: E.L.-C. and D.V.G.-S.; Methodology and Fieldwork: E.L.-C. and D.V.G.-S.; Photography: E.L.-C.; Plates: D.V.G.-S.; Species descriptions: E.L.-C. and D.V.G.-S.; Checklist: E.L.-C. and D.V.G.-S.; Writing: E.L.-C., D.V.G.-S. and J.R.C.-K.; Review and Editing: all authors; Supervision: E.L.-C.; Funding Acquisition: E.L.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Universidad del Valle (CI71301) and Comisión Colombiana del Océano. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Collections in the Pacific were conducted under Permit No. 006/2022 issued by the National Natural Parks of Colombia, while those in the Caribbean were carried out under Permit No. 601/2022 issued by Coralina.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Universidad del Valle (Act No. 6, Internal Call 131-2021, issued on 10/02/2022).
Data Availability Statement
All data are provided in the manuscript.
Acknowledgments
We want to express our deepest gratitude to J. Acero for her support during the Courtown Cay Expedition and to W. Aguirre for transporting and helping us during our stays at Bahía Málaga. We also want to thank A. Valdés for his comments on species identification. Finally, we thank Colombian Army for supporting field activities at both sampling locations. This is contribution number 26 of the Instituto de Ciencias del Mar y Limnología (INCIMAR), Universidad del Valle.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript: GC: Great Caribbean; TEP: Tropical Eastern Pacific; SFBR: Seaflower Biosphere Reserve; CC: Courtown Cay; LNO: Los Negros; LNI: Los Negritos; RBA: Rapid Biodiversity Assessment; AHS: Artificial Hard Substrates.
References
- Fortunato, H. Mollusks: Tools in Environmental and Climate Research. Am. Malacol. Bull. 2015, 33, 310–324. [Google Scholar] [CrossRef]
- Floyd, M.; Mizuyama, M.; Obuchi, M.; Sommer, B.; Miller, M.G.; Kawamura, I.; Kise, H.; Reimer, J.D.; Beger, M. Functional Diversity of Reef Molluscs along a Tropical-to-Temperate Gradient. Coral Reefs 2020, 39, 1361–1376. [Google Scholar] [CrossRef]
- Firth, L.B.; White, F.J.; Schofield, M.; Hanley, M.E.; Burrows, M.T.; Thompson, R.C.; Skov, M.W.; Evans, A.J.; Moore, P.J.; Hawkins, S.J. Facing the Future: The Importance of Substratum Features for Ecological Engineering of Artificial Habitats in the Rocky Intertidal. Mar. Freshw. Res. 2016, 67, 131–143. [Google Scholar] [CrossRef]
- Dinapoli, A.; Klussmann-Kolb, A. The Long Way to Diversity—Phylogeny and Evolution of the Heterobranchia (Mollusca: Gastropoda). Mol. Phylogenet Evol. 2010, 55, 60–76. [Google Scholar] [CrossRef]
- Oliva-Martín, A. Sistemática de Heterobranchia (Gastropoda) en el registro fósil de Cuba. Ciencias Tierra Espacio 2016, 17, 112–122. [Google Scholar]
- Ayyagari, V.S.; Sreerama, K. Molecular Phylogeny and Evolution of Pulmonata (Mollusca: Gastropoda) on the Basis of Mitochondrial (16S, COI) and Nuclear Markers (18S, 28S): An Overview. J. Genet. 2020, 99, 17. [Google Scholar] [CrossRef]
- Bertsch, H. Nudibranch Feeding Biogeography: Ecological Network Analysis of Inter- and Intra-Provincial Variations. Thalassas 2011, 27, 155–168. [Google Scholar]
- Gomes, N.G.M.; Fernandes, F.; Madureira-Carvalho, Á.; Valentão, P.; Lobo-da-Cunha, A.; Calado, G.; Andrade, P.B. Profiling of Heterobranchia Sea Slugs from Portuguese Coastal Waters as Producers of Anti-Cancer and Anti-Inflammatory Agents. Molecules 2018, 23, 1027. [Google Scholar] [CrossRef]
- Moles, J.; Giribet, G. A Polyvalent and Universal Tool for Genomic Studies in Gastropod Molluscs (Heterobranchia). Mol. Phylogenet Evol. 2021, 155, 106996. [Google Scholar] [CrossRef]
- Varney, R.M.; Brenzinger, B.; Malaquias, M.A.E.; Meyer, C.P.; Schrödl, M.; Kocot, K.M. Assessment of Mitochondrial Genomes for Heterobranch Gastropod Phylogenetics. BMC Ecol. Evol. 2021, 21, 6. [Google Scholar] [CrossRef]
- Valdés, Á. A Phylogenetic Analysis and Systematic Revision of the Cryptobranch Dorids (Mollusca, Nudibranchia, Anthobranchia). Zool. J. Linn. Soc. 2002, 136, 535–636. [Google Scholar] [CrossRef]
- Malaquias, A.M.E.; Reid, D.G. Systematic Revision of the Living Species of Bullidae (Mollusca: Gastropoda: Cephalaspidea), with a Molecular Phylogenetic Analysis. Zool. J. Linn. Soc. 2008, 153, 453–543. [Google Scholar] [CrossRef]
- Churchill, C.K.C.; Valdés, Á.; Foighil, D.Ó. Molecular and Morphological Systematics of Neustonic Nudibranchs (Mollusca:Gastropoda:Glaucidae: Glaucus), with Descriptions of Three New Cryptic Species. Invertebr. Syst. 2014, 28, 174–195. [Google Scholar] [CrossRef]
- Chaban, E.; Ekimova, I.; Lubin, P.; Nikitenko, E.; Schepetov, D. Bizarre Morphology Obscures Real Affiliation: An Integrative Study of Enigmatic Cephalaspid Philine denticulata from Arctic Waters Reveals Its Unique Phylogenetic Position. Diversity 2023, 15, 395. [Google Scholar] [CrossRef]
- Ekimova, I.A.; Antokhina, T.I.; Schepetov, D.M. Molecular Data and Updated Morphological Description of Flabellina rubrolineata (Nudibranchia: Flabellinidae) from the Red and Arabian Seas. Ruthenica 2020, 30, 183–194. [Google Scholar] [CrossRef]
- Andrimida, A. New Records of Nudibranchs and a Sacoglossan (Gastropoda: Heterobranchia) from Sempu Strait, Indonesia. Indo Pac. J. Ocean. Life 2022, 6, 1–9. [Google Scholar] [CrossRef]
- Londoño-Cruz, E. The Contribution of Heterobranchia (Mollusca: Gastropoda) to the Biodiversity of the Colombian Tropical Eastern Pacific. Mar. Biodivers. 2021, 51, 93. [Google Scholar] [CrossRef]
- Carmona, L.; Cervera, J.L.; Kumar, A.B.; Snehachandran, B.K. First Record of the Aeolid Anteaeolidiella fijensis (Nudibranchia, Aeolidiidae) from India. Mar. Biodivers. 2017, 47, 823–830. [Google Scholar] [CrossRef]
- Do, T.D.; Jung, D.W.; Kil, H.J.; Kim, C.B. A Report of a New Species and New Record of Cadlina (Nudibranchia, Cadlinidae) from South Korea. Zookeys 2020, 996, 1–18. [Google Scholar] [CrossRef]
- Bharate, M.; Oskars, T.R.; Narayana, S.; Ravinesh, R.; Kumar, A.B.; Malaquias, M.A.E. Description of a New Species of Haminoea (Gastropoda: Cephalaspidea) from India, with an Account of the Diversity of the Genus in the Indo-West Pacific. J. Nat. Hist. 2018, 52, 2437–2456. [Google Scholar] [CrossRef]
- Bertsch, H.; Valdés, Á.; Gosliner, T.M. A New Species of Tritoniid Nudibranch, the First Found Feeding on a Zoanthid Anthozoan, with a Preliminary Phylogeny of the Tritoniidae. Proc. Calif. Acad. Sci. Ser. 4 2009, 60, 431–446. [Google Scholar]
- Barber, P.H.; Ablan-Lagman, M.C.A.; Ambariyanto, A.; Berlinck, R.G.S.; Cahyani, D.; Crandall, E.D.; Ravago-Gotanco, R.; Juinio-Meñez, M.A.; Mahardika, I.G.N.; Shanker, K.; et al. Advancing Biodiversity Research in Developing Countries: The Need for Changing Paradigms. Bull. Mar. Sci. 2014, 90, 187–210. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Robles-Gil, P.; Hoffmann, M.; Pilgrim, J.D.; Brooks, T.B.; Mittermeier, C.G.; Lamoreux, J.L.; Fonseca, G.A.B. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; CEMEX: Mexico City, Mexico, 2004; ISBN 9686397779. [Google Scholar]
- Miloslavich, P.; Klein, E.; Díaz, J.M.; Hernández, C.E.; Bigatti, G.; Campos, L.; Artigas, F.; Castillo, J.; Penchaszadeh, P.E.; Neill, P.E.; et al. Marine Biodiversity in the Atlantic and Pacific Coasts of South America: Knowledge and Gaps. PLoS ONE 2011, 6, e14631. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Conservation: The Critical Role of Hotspots. In Biodiversity Hotspots. Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. ISBN 9783642209918. [Google Scholar]
- Valdés, Á.; Hamnn, J.; Behrens, D.W.; DuPont, A. Caribbean Sea Slugs—A Field Guide to the Opisthobranch Mollusks from the Tropical Northwestern Atlantic; Sea Challengers: Monterey, CA, USA, 2006; ISBN 9780970057426. [Google Scholar]
- Goodheart, J.A.; Ellingson, R.A.; Vital, X.G.; Filho, H.C.G.; McCarthy, J.B.; Medrano, S.M.; Bhave, V.J.; García-Méndez, K.; Jiménez, L.M.; López, G.; et al. Identification Guide to the Heterobranch Sea Slugs (Mollusca: Gastropoda) from Bocas Del Toro, Panama. Mar. Biodivers. Rec. 2016, 9, 56. [Google Scholar] [CrossRef]
- Camacho-Garcia, Y.; Gosliner, T.M.; Valdés, Á. Field Guide to the Sea Slugs of the Tropical Eastern Pacific; California Academy of Sciences: San Francisco, CA, USA, 2005; ISBN 0-940228-63-7. [Google Scholar]
- Vital, X.G.; Palomino-Alvarez, L.A.; Ortigosa, D.; Guerra-Castro, E.J.; Simões, N. Sea Slugs (Gastropoda: Heterobranchia) Associated with Autonomous Reef Monitoring Structures (ARMS) in Southern Gulf of Mexico and Mexican Caribbean Sea. J. Mar. Biol. Assoc. U. K. 2023, 103, e50. [Google Scholar] [CrossRef]
- Behrens, D.W.; Hermosillo, A. Eastern Pacific Nudibranchs: A Guide to the Opisthobranchs from Alaska to Central America; Sea Challengers: Monterey, CA, USA, 2005; ISBN 0-930118-36-7. [Google Scholar]
- Ardila, N.E.; Báez, D.P.; Valdés, Á. Babosas y Liebres de Mar (Mollusca: Gastropoda: Opisthobranchia) de Colombia. Biota Colomb. 2007, 8, 185–197. [Google Scholar]
- Fernández, M.F. Diversidad de Opistobranquios (Mollusca: Gastropoda) del mar Caribe Colombiano: Lista Actualizada y Perspectiva de Futuras Investigaciones. Bachelor’s Thesis, Universidad de Córdoba, Córdoba, Colombia, 2021. [Google Scholar]
- Carvajal-Florian, A.; Gracia, C.A. New Records and Status of Knowledge of Marine Heterobranchs (Mollusca: Gastropoda) in the Department of Atlántico, Colombian Caribbean. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2022, 46, 426–437. [Google Scholar] [CrossRef]
- Mejía Robledo, A.I. Babosas Marinas de La Reserva de La Biósfera Seaflower, Caribe Colombiano. Bachelor’s Thesis, Universidad de Antioquia, Antioquia, Colombia, 2022. [Google Scholar]
- Friedlander, A.; Sladek Nowlis, J.; Sanchez, J.A.; Appeldoorn, R.; Usseglio, P.; McCormick, C.; Bejarano, S.; Mitchell-Chui, A. Designing Effective Marine Protected Areas in Seaflower Biosphere Reserve, Colombia, Based on Biological and Sociological Information. Conserv. Biol. 2003, 17, 1769–1784. [Google Scholar] [CrossRef]
- Martínez-Clavijo, S.; López-Muñoz, P.; Cabarcas-Mier, A.; Payares-Varela, J.L.; Gutiérrez, J.; Quintero, J. Geomorphological Units and Distribution of Sedimentary Facies in the Alburquerque Key Island, Seaflower Biosphere Reserve, Colombian Caribbean. Bol. Geol. 2021, 43, 143–164. [Google Scholar] [CrossRef]
- Cantera Kintz, J.R.; Londoño-Cruz, E.; Mejía-Ladino, L.M.; Herrera-Orozco, L.; Satizabal, C.A.; Uribe-Castañeda, N. Environmental Issues of a Marine Protected Area in a Tectonic Estuary in the Tropical Eastern Pacific: Uramba (Malaga Bay Colombia): Context, Biodiversity, Threats and Challenges. J. Water Resour. Prot. 2013, 5, 1037–1047. [Google Scholar] [CrossRef]
- Espinal-García, P.; Giraldo, A.; Londoño-Mesa, M.; Mejía-Ladino, L.M. Variabilidad en la abundancia de larvas de crustáceos y poliquetos en Bahía Málaga, Pacífico colombiano (enero-junio de 2010). Boletín Investig. Mar. Costeras-INVEMAR 2012, 41, 355–373. [Google Scholar]
- Betancourt Portela, J.M.; Sánchez Díazgranados, J.G.; Mejía-Ladino, L.M.; Cantera Kintz, J.R. Calidad de Las Aguas Superficiales de Bahía Málaga, Pacífico Colombiano. Acta Biolo Colomb. 2011, 16, 175–192. [Google Scholar]
- Alonso, L.E.; Deichmann, J.L.; McKenna, S.A.; Naskrecki, P.; Richards, S.J. Still Counting...: Biodiversity Exploration for Conservation—The First 20 Years of the Rapid Assessment Program; Alonso, L.E., Deichmann, J.L., McKenna, S.A., Naskrecki, P., Richards, S.J., Eds.; Conservation International: Arlington, VA, USA, 2011; ISBN 9781934151440. [Google Scholar]
- García-Méndez, K.; Padula, V.; Valdés, Á. Integrative Systematics of the Genus Dondice Marcus, 1958 (Gastropoda, Nudibranchia, Myrrhinidae) in the Western Atlantic. Mar. Biodivers. 2022, 52, 42. [Google Scholar] [CrossRef]
- Espinoza, E.; DuPont, A.; Valdés, Á. Molecular Data Reveal an Undescribed Cryptic Species of Costasiella Pruvot-Fol, 1951 (Euthyneura: Sacoglossa: Limapontidae) in the Bahamas. Am. Malacol. Bull. 2014, 32, 173–182. [Google Scholar] [CrossRef]
- Krug, P.J.; Vendetti, J.E.; Valdés, Á. Molecular and Morphological Systematics of Elysia Risso, 1818 (Heterobranchia: Sacoglossa) from the Caribbean Region. Zootaxa 2016, 4148, 1–137. [Google Scholar] [CrossRef] [PubMed]
- Carmona, L.; Bhave, V.; Salunkhe, R.; Pola, M.; Gosliner, T.M.; Cervera, J.L. Systematic Review of Anteaeolidiella (Mollusca, Nudibranchia, Aeolidiidae) Based on Morphological and Molecular Data, with a Description of Three New Species. Zool. J. Linn. Soc. 2014, 171, 108–132. [Google Scholar] [CrossRef]
- De La Cruz-Francisco, V.; Ortigosa, D.; González-González, M. Primeros registros de babosas marinas (Gastropoda: Heterobranchia) del Sistema Arrecifal Tuxpan, México, con ampliaciones de ámbito de distribución. Biodivers. Nat. Hist. 2017, 3, 15–23. [Google Scholar]
- Bertsch, H. A History of Eastern Pacific Marine Heterobranch Research. Nautilus 2020, 134, 71–88. [Google Scholar]
- Salvador, X.; Fernández-Vilert, R.; Moles, J. Sea Slug Night Fever: 39 New Records of Elusive Heterobranchs in the Western Mediterranean (Mollusca: Gastropoda). J. Nat. Hist. 2022, 56, 265–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).