The Current State of Populations of Rhaponticum altaicum (Asteraceae) in the Northern and Central Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Population Studies
2.3. Ontogenetic Studies
2.4. Study of the Floristic Composition of Communities with Rh. altaicum
2.5. Data Analysis
3. Results
3.1. Floristic Composition of Plant Communities with Rh. altaicum
3.2. Ontogeny of Rh. altaicum
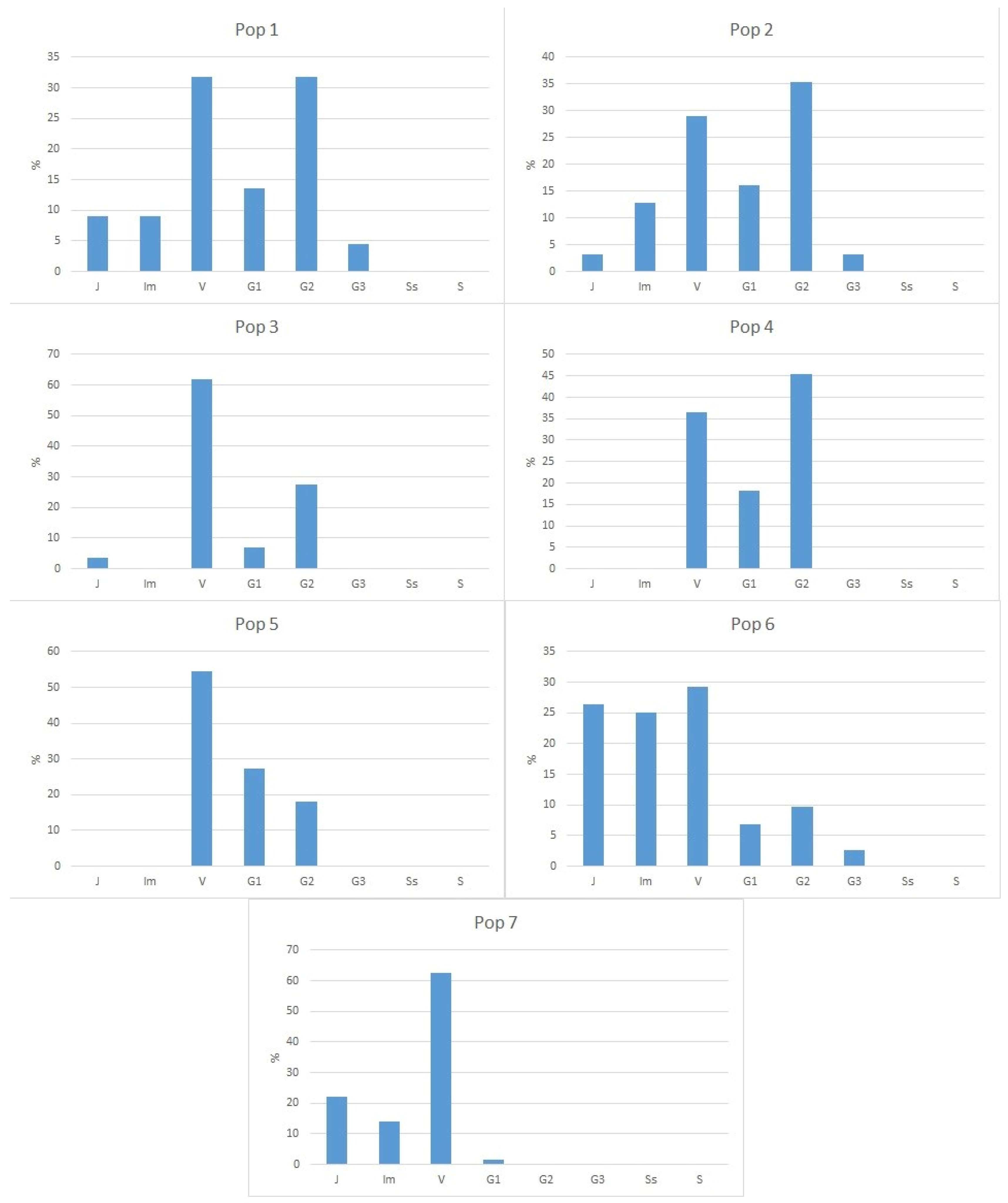
3.3. Age Structure of Populations
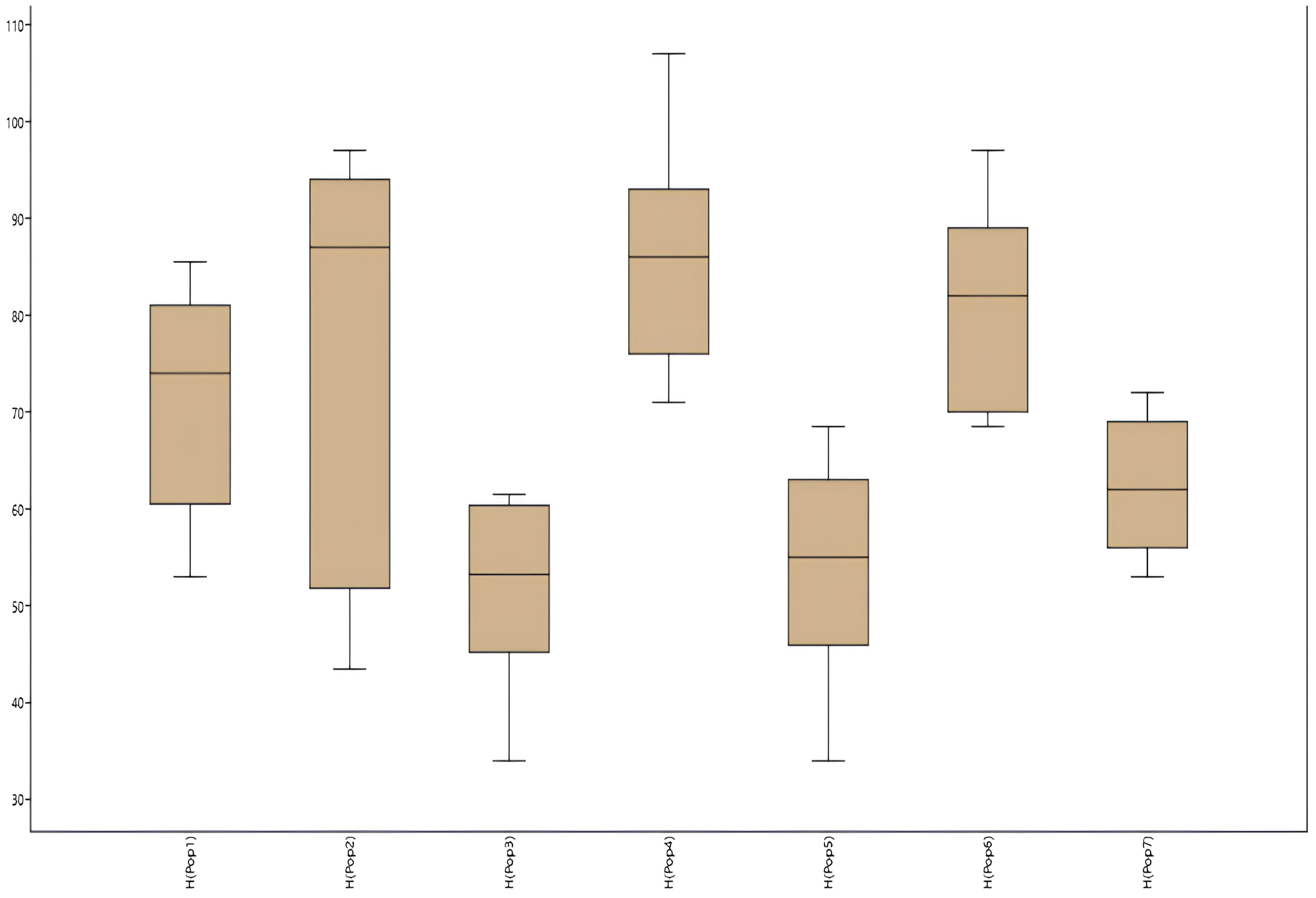
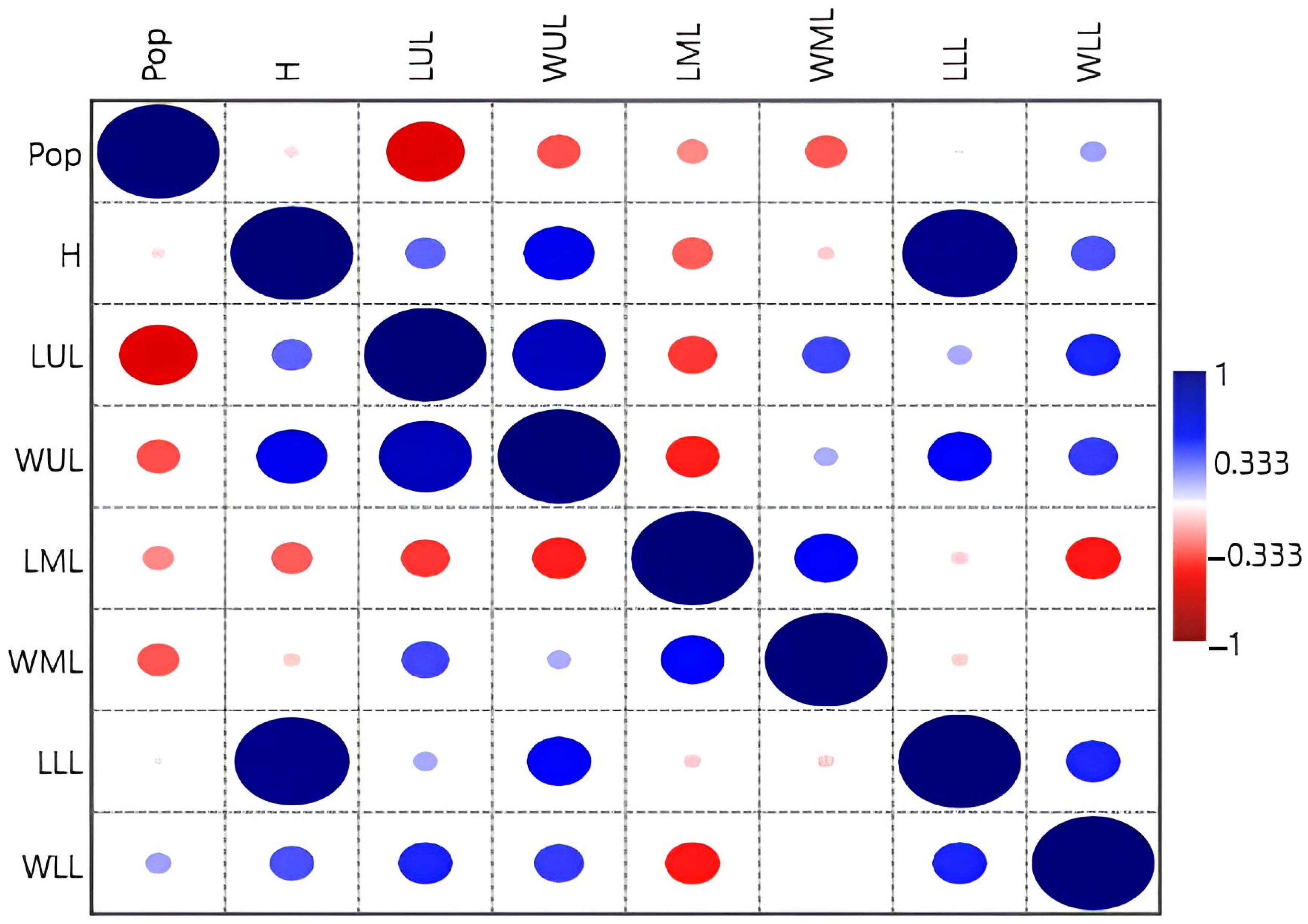
3.4. Morphological Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Species Name | Abundance on the Brown–Blanke Scale | Life Span | Life Form | Ecological Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pop1 | Pop2 | Pop3 | Pop4 | Pop5 | Pop6 | Pop7 | ||||
| Achillea salicifolia Besser | + | Pe | He | GM | ||||||
| Agropyron cristatum (L.) Gaertn. | 2 | Pe | He | M | ||||||
| Agrostis gigantea Roth | 2 | Pe | He | M | ||||||
| Alisma plantago-aquatica L. | 1 | Pe | Cr | GG | ||||||
| Allium angulosum L. | 1 | + | Pe | Cr | MG | |||||
| Alopecurus aequalis Sobol. | + | A-B | Te | MG | ||||||
| Alopecurus arundinaceus Poir. | 2 | 1 | Pe | He | M | |||||
| Artemisia abrotanum L. | 1 | 2 | 1 | 3 | Pe | Ha | M | |||
| Artemisia nitrosa Web. ex Stechm. | 2 | + | + | 2 | Pe | Ha | MX | |||
| Artemisia pontica L. | + | Pe | He | M | ||||||
| Asperugo procumbens L. | 1 | A-B | Te | M | ||||||
| Atriplex verrucifera Bieb. | + | Pe | Ha | MX | ||||||
| Beckmannia syzigachne (Steud.) Fern. | 1 | Pe | He | M | ||||||
| Bolboschoenus maritimus (L.) Palla | 2 | Pe | He | GG | ||||||
| Bromus inermis Leyss. | 3 | 2 | Pe | Cr | GM | |||||
| Calamagrostis epigejos (L.) Roth | 1 | 3 | 2 | 3 | Pe | He | M | |||
| Carex diluta Bieb | 1 | Pe | He | MG | ||||||
| Carex riparia Curtis | 1 | Pe | He | GG | ||||||
| Carex stenophylla Wahlenb. | 3 | Pe | He | M | ||||||
| Carex supina Willd. ex Wahlenb. | + | + | Pe | He | MX | |||||
| Carex vesicaria L. | + | 1 | Pe | He | MG | |||||
| Cenolophium fischeri (Spreng) W.P.J. Koch | 1 | Pe | He | M | ||||||
| Centaurea glastifolia subsp. intermedia (Boiss.) L.Martins | 1 | + | Pe | He | XM | |||||
| Chenopodium album L | + | + | 1 | A-B | Te | MX | ||||
| Cirsium arvense var. arvense | 1 | + | + | + | Pe | He | MX | |||
| Cirsium arvense var. vestitum Wimm. & G | + | Pe | Cr | MX | ||||||
| Eleocharis palustris (L.) Roem. et Schult. | 2 | Pe | Cr | G | ||||||
| Elymus repens (L.) Gould | 3 | 2 | 1 | 1 | 2 | 4 | + | Pe | He | MG |
| Euphorbia virgata Waldst. et Kit. | + | + | Pe | He | MX | |||||
| Festuca valesiaca Schleich. ex Gaudin | + | Pe | He | MX | ||||||
| Filipendula ulmaria (L.) Maxim. | 1 | Pe | He | M | ||||||
| Galatella dahurica DC. | 2 | Pe | He | XM | ||||||
| Geranium collinum Stephan ex Willd. | 1 | 1 | Pe | He | XM | |||||
| Glycyrrhiza uralensis Fisch. ex DC. | 1 | + | + | 2 | 1 | Pe | He | XM | ||
| Grubovia sedoides (Pall.) G.L.Chu | + | A-B | Te | X | ||||||
| Gypsophila perfoliata L. | + | Pe | He | M | ||||||
| Iris halophila Pall. | + | Pe | Cr | XM | ||||||
| Juncus compressus Jacq. | 1 | 1 | Pe | Cr | GM | |||||
| Juncus gerardii Loisel. | 1 | 2 | + | 3 | 1 | Pe | He | GM | ||
| Koeleria pyramidata (Lam.) P.Beauv. | 1 | Pe | He | MX | ||||||
| Lactuca tatarica (L.) C.A. Mey. | 1 | + | + | + | Pe | He | XM | |||
| Lepidium latifolium L. | 1 | + | 1 | Pe | He | M | ||||
| Limonium gmelinii (Willd.) O. Kuntze | + | + | + | + | + | Pe | He | XM | ||
| Lythrum virgatum L. | + | Pe | He | M | ||||||
| Oenanthe aquatica (L.) Poir. | 1 | Pe | Cr | GG | ||||||
| Pentanema caspicum (F.K.Blum ex Ledeb.) G.V.Boiko, Korniy. & Mosyakin | + | Pe | He | M | ||||||
| Petrosimonia triandra (Pall.) Simonk. | + | A-B | Te | X | ||||||
| Phalaroides arundinacea (L.) Rauschert | 1 | Pe | He | G | ||||||
| Phragmites australis (Cav.) Trin. ex Steud. | 1 | 1 | 3 | Pe | Cr | G | ||||
| Plantago media L. | + | Pe | He | M | ||||||
| Plantago salsa Pall. | 1 | Pe | He | XM | ||||||
| Plantago urvillei Opiz. | 1 | + | Pe | He | XM | |||||
| Poa palustris L. | 1 | Pe | He | GM | ||||||
| Poa pratensis L. | + | Pe | He | M | ||||||
| Puccinellia tenuissima Litv. ex V.I.Krecz. | 1 | Pe | He | M | ||||||
| Rhaponticum altaicum (Fisch. ex Spreng.) Soskov | 1 | 2 | + | + | 1 | 1 | + | Pe | Cr | MG |
| Rumex stenophyllus Ledeb. | 1 | Pe | He | GM | ||||||
| Saussurea amara (L.) DC. | 1 | 1 | 1 | 1 | 1 | 2 | Pe | He | M | |
| Saussurea salsa (Pall.) Spreng. | + | Pe | He | MX | ||||||
| Scorzonera pratorum (Krasch.) Stankov | + | + | Pe | He | M | |||||
| Sium latifolium L. | + | Pe | He | MG | ||||||
| Suaeda acuminata (C.A.Mey.) Moq. | + | A-B | Te | X | ||||||
| Takhtajaniantha austriaca (Willd.) Zaika, Sukhor. & N.Kilian | 1 | Pe | He | XM | ||||||
| Taraxacum officinale F.H.Wigg | + | Pe | He | M | ||||||
| Thalictrum minus L. | 1 | + | Pe | He | M | |||||
| Triglochin maritima L. | 1 | Pe | He | M | ||||||
| Typha angustifolia L. | + | Pe | Cr | G | ||||||
References
- Grudzinskaya, L.M.; Gemejieva, N.G.; Nelina, N.V.; Karzhaubekova, J.J. Annotated list of medicinal plants of Kazakhstan. Almaty Kazakhstan 2014, 20, 200. [Google Scholar]
- Zhang, X.-P.; Zhang, J.; Dong, M.; Zhang, M.-L.; Huo, C.-H.; Shi, Q.-W.; Gu, Y.-C. Chemical constituents of plants from the genus Rhaponticum. Chem. Biodivers. 2010, 7, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Mamyrova, S.A.; Kupriyanov, A.N.; Ivashchenko, A.A.; Kubentayev, S.A. Taxonomic Revision of the Genus Rhaponticum (Asteraceae) of Kazakhstan. OnLine J. Biol. Sci. 2024, 24, 802–815. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Izbastina, K.S.; Borodulina, O.V.; Kubentayeva, B.B. Revised checklist of endemic vascular plants of Kazakhstan. PhytoKeys 2024, 238, 241–279. [Google Scholar] [CrossRef]
- Myrzagaliyeva, A.; Irsaliyev, S.; Tustubayeva, S.; Samarkhanov, T.; Orazov, A.; Alemseitova, Z. Natural Resources of Rhaponticum carthamoides in the Tarbagatai State National Nature Park. Diversity 2024, 16, 676. [Google Scholar] [CrossRef]
- Kokoska, L.; Janovska, D. Chemistry and Pharmacology of Rhaponticum carthamoides: A Review. Phytochemistry 2009, 70, 842–855. [Google Scholar] [CrossRef]
- Kosović, E.; Lino, K.; Kuchař, M. HPLC-MS Methodology for Rh. carthamoides Extract Quality Evaluation: A Simultaneous Determination of Eight Bioactive Compounds. Diversity 2022, 14, 880. [Google Scholar] [CrossRef]
- Havlik, J.; Budesinsky, M.; Kloucek, P.; Kokoska, L.; Valterova, I.; Vasickova, S.; Zeleny, V. Norsesquiterpene hydrocarbon, chemical composition and antimicrobial activity of Rhaponticum carthamoides root essential oil. Phytochemistry 2009, 70, 414–418. [Google Scholar] [CrossRef]
- Roumanille, R.; Vernus, B.; Brioche, T.; Descossy, V.; Van Ba, C.T.; Campredon, S.; Philippe, A.G.; Delobel, P.; Bertrand-Gaday, C.; Chopard, A.; et al. Acute and Chronic Effects of Rhaponticum carthamoides and Rhodiola rosea Extracts Supplementation Coupled to Resistance Exercise on Muscle Protein Synthesis and Mechanical Power in Rats. J. Int. Soc. Sports Nutr. 2020, 17, 58. [Google Scholar] [CrossRef]
- Skała, E.; Sitarek, P.; Różalski, M.; Krajewska, U.; Szemraj, J.; Wysokińska, H.; Śliwiński, T. Antioxidant and DNA Repair Stimulating Effect of Extracts from Transformed and Normal Roots of Rhaponticum carthamoides against Induced Oxidative Stress and DNA Damage in CHO Cells. Oxid. Med. Cell Longev. 2016, 2016, 5753139. [Google Scholar] [CrossRef]
- Todorova, V.; Savova, M.S.; Ivanova, S.; Ivanov, K.; Georgiev, M.I. Anti-Adipogenic Activity of Rhaponticum carthamoides and Its Secondary Metabolites. Nutrients 2023, 15, 3061. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Ecdysterones from Rhaponticum carthamoides (Willd.) Iljin reduce hippocampal excitotoxic cell loss and upregulate mTOR signaling in rats. Fitoterapia 2017, 119, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xian, Y.; Jin, Z.; Yao, F.; Liu, Y.; Deng, Y.; Wang, B.; Chen, D.; Yang, J.; Ren, L.; et al. Rhaponticum carthamoides Improved Energy Metabolism and Oxidative Stress through the SIRT6/Nrf2 Pathway to Ameliorate Myocardial Injury. Phytomedicine 2022, 105, 154197. [Google Scholar] [CrossRef] [PubMed]
- Tuleuov, B.I. Technology of Phytosteroid Preparations; Glasir: Karaganda, Kazakhstan, 2017; 112p. [Google Scholar]
- Azimova, S.S. Natural Compounds. Phytoecdysteroids; Springer: New York, NY, USA, 2013; pp. 16–17. [Google Scholar] [CrossRef]
- Abubakirov, N.K. Ecdysteroids of flowering plants (Angiospermae). Chem. Nat. Compd. 1981, 6, 685–702. (In Russian) [Google Scholar] [CrossRef]
- Baltaev, U.A.; Abubakirov, N.K. Phytoecdysteroids of Rhaponticum integrifolium. Chem. Nat. Compd. 1977, 6, 813–819. (In Russian) [Google Scholar]
- Buděšínský, M.; Vokáč, K.; Harmatha, J.; Cvačka, J. Additional Minor Ecdysteroid Components of Leuzea carthamoides. Steroids 2008, 73, 502–514. [Google Scholar] [CrossRef]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The Phytochemical, Biological, and Medicinal Attributes of Phytoecdysteroids: An Updated Review. Acta Pharm. Sin. B 2021, 11, 1740–1766. [Google Scholar] [CrossRef]
- Geszprych, A.; Weglarz, Z. Composition of essential oil from underground and aboveground organs of Rhaponticum carthamoides [Willd.] Iljin. Herba Pol. 2002, 4, 188–192. [Google Scholar]
- Zughdani, M.; Soliman, H.Y.; Ekiz, G.; Linden, A.; Çalış, İ. Ecdysteroids from the underground parts of Rhaponticum acaule (L.) DC. Phytochemistry 2020, 180, 112530. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.-Q.; Bao, B.-Q.; Hang, H. Chemical constituents from flowers of Rhaponticum uniflorum. Chin. Tradit. Herb. Drugs 2016, 47, 2817–2821. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Qi, M.; Ge, L.; Tian, Z.; Li, J.; Zhang, M.; Wang, M.; Huang, L.; Tang, X. Anti-tumor effect of Rhaponticum uniflorum ethyl acetate extract by regulation of peroxiredoxin1 and epithelial-to-mesenchymal transition in oral cancer. Front. Pharmacol. 2017, 8, 870. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N. The Ethnopharmacological Uses, Metabolite Diversity, and Bioactivity of Rhaponticum uniflorum (Leuzea uniflora): A Comprehensive Review. Biomolecules 2022, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Lekouaghet, A.; Boutefnouchet, A.; Bensuici, C.; Gali, L.; Ghenaiet, K.; Tichati, L. In vitro evaluation of antioxidant and anti-inflammatory activities of the hydroalcoholic extract and its fractions from Leuzea conifera L. roots. S. Afr. J. Bot. 2020, 132, 103–107. [Google Scholar] [CrossRef]
- Hammoudi, A.; Zatla, A.T.; El Amine Dib, M. A Phytochemical and Antioxidant Study of the Hexanoic Extract of Rhaponticum acaule. Chem. Proc. 2023, 14, 81. [Google Scholar] [CrossRef]
- Mosbah, H.; Chahdoura, H.; Kammoun, J.; Hlila, M.B.; Louati, H.; Hammami, S.; Flamini, G.; Achour, L.; Selmi, B. Rhaponticum acaule (L.) DC essential oil: Chemical composition, in vitro antioxidant and enzyme inhibition properties. BMC Complement Altern. Med. 2018, 18, 79. [Google Scholar] [CrossRef]
- Berkenov, A.K. Modification of Ecdysteroids and Development of New Substances. Ph.D. Thesis, Kazakh National Medicinal University, Almaty, Kazakhstan, 2021. (In Kazakh). [Google Scholar]
- Berdin, A.G. Biological Active Compounds of RhapontiCum serratuloides (Georgi.) Bobr. and RhapontiCum Carthamoides (Willd.) Iljin. Ph.D. Thesis, Karaganda State University, Karaganda, Kazakhstan, 2000. (In Russian). [Google Scholar]
- Berdin, A.G.; Adekenov, S.M.; Raldugin, V.A.; Shakirov, M.M.; Druganov, A.G.; Kulyyasov, A.T.; Tolstikov, G.A. Rhaposerine and Rhaserolide, new Sesquiterpene Lactones from Rhaponticum serratuloides. Russ. Chem. Bull 1999, 48, 1987–1991. (In Russian) [Google Scholar] [CrossRef]
- Volodina, S.O.; Volodin, V.V.; Gorovoi, P.G.; Tkachenko, K.G.; Novozhilova, E.V.; Ishmuratova, M.M.; Chadin, I.F.; Kanev, V.A. Ecdysteroids from plants of Ural, Caucasus, Russian Far East and China (separated screening). Turczaninowia 2012, 4, 58–75. (In Russian) [Google Scholar]
- Krasnoborov, I.M. Flora of Siberia; Scientific Publishers, Inc.: Plymouth, UK, 2007; Volume 13. [Google Scholar] [CrossRef]
- Revyakina, N.V.; Kozyreva, Y.V. Rhaponticum serratuloides (Georgi) Bobr. In The Steppes of Eurasia, Proceedings of the VIII International Symposium Steppes of Northern Eurasia; Institute of Steppe of the Ural Branch of the Russian Academy of Sciences: Orenburg, Russia, 2018; pp. 812–813. (In Russian) [Google Scholar]
- Ostapko, V.M.; Boyko, A.V.; Mosyakin, S.L. Vascular Plants of South-East Ukraine; Knowledge: Donetsk, Ukraine, 2010; 247p. (In Russian) [Google Scholar]
- Cherepanov, S.K. Rhaponticum—Rhaponticum Hill. In Flora of the European Part of the USSR; N. Tsvelev, N., Ed.; Nauka: Saint Petersburg, Russia, 1994; Volume 7, p. 256. (In Russian) [Google Scholar]
- Korchikov, E.C. Rhaponticum serratuloides (Georgi) Bobr. In Red Book of the Samara Region; Rare Species of Plants and Fungi; Samara State Academy: Samara, Russia, 2017; Volume I, p. 56. [Google Scholar]
- Litvinskaya, S.A.; Pikalova, N.A. Stemmacantha serratuloides—Stemmacantha serratuloides (Georgi) M. Dittrich. In Red Data Book of Krasnodar Territorry; Plants and Fungi: Krasnodar, Russia, 2017; pp. 421–422. [Google Scholar]
- Panin, A.V.; Shilova, I.V. Stemmacantha serratuloides (Georgi) M. Dittrich. In Red Data Book of the Saratov Region: Fungi. Lichens. Plants. Animals, 3rd ed.; Shlyakhtin, G.V., Ed.; Papyrus: Saratov, Russia, 2021; p. 87. (In Russian) [Google Scholar]
- Sviridenko, B.F.; Sviridenko, T.V. Rhaponticum serratuloides (Georgi) Bobr. In Red Data Book of the Omsk Region; Omsk State Pedagogical University: Omsk, Russia, 2015; 453p. (In Russian) [Google Scholar]
- Baitulin, I.O. (Ed.) The Red Book of Kazakhstan (Plants); Art Print XXI: Astana, Kazakhstan, 2014; 452p. (In Russian) [Google Scholar]
- Orazova, A. Genus Rhaponticum Adans. In Flora of Kazakhstan; Pavlov, N.V., Ed.; AN KazSSR: Almaty, Kazakhstan, 1966; pp. 368–373. (In Russian) [Google Scholar]
- Rabotnov, T.A. Life cycle of perennial herbaceous plants in forest cenoses. In Proceedings of BIN of the USSR Academy of Sciences; 1950; Volume 6, pp. 7–204. (In Russian) [Google Scholar]
- Uranov, A.A. Age spectrum of phytocenopopulations as a function of time and energy processes. Biol. Sci. 1975, 2, 7–34. (In Russian) [Google Scholar]
- Smirnova, O.V.; Zaugolnova, L.B.; Toropova, N.A.; Falikov, L.D. Criteria for distinguishing age states and features of ontogenesis in plants of different biomorphs. In Cenopopulations of Plants (Basic Concepts and Structure); Nauka: Moscow, Russia, 1976; pp. 14–44. (In Russian) [Google Scholar]
- Zverev, A.A. Information Technologies in Studies of Vegetation Cover; TML-Press: Tomsk, Russia, 2007; 304p. (In Russian) [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934; 721p. [Google Scholar]
- Shennikov, A.P. Ecology of Plants; Sovetskaya nauka: Moscow, Russia, 1979; 375p. (In Russian) [Google Scholar]
- Serebryakov, I.G. Ecological morphology of plants. In Life Forms of Angiosperms and Conifers; Vyschaya Shkola: Moscow, Russia, 1962; 380p. [Google Scholar]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. Available online: http://www.plantsoftheworldonline.org/ (accessed on 9 April 2023).
- Braun-Blanquet, J. Pflanzensociologie, 3rd ed.; Aufl. Wien: New York, NY, USA, 1964; 865p. [Google Scholar]
- Smirnova, E.B.; Shatakhanov, B.D.; Kabanov, S.V.; Ponomareva, A.L.; Shevchenko, E.N.; Zaigralova, G.N. Phytocenotic confinement and the structure of coenopopulation of the rare medicinal plant Stemmacantha serratuloides (Georgi) M. Dittrich. In the Balashovo municipality of the Saratov region. Ann. Trop. Med. Public Health-Spec. Issue 2018, 9, S616–S618. [Google Scholar]
- Demchenko, L.A. Vegetation cover of Kustanay region. Mater. Flora Veg. Kazakhstan 1961, 10, 57–60. (In Russian) [Google Scholar]
- Ivashchenko, A.A.; Mamyrova, S.A.; Nelina, N.V. RhapontiCum serratuloides (Georgi) Bobr. In the flora of the State Nature Reserve “Altyn-Dala”. In Proceedings of the 3rd International Scientific Conference, Biological Diversity of Asian Steppe, A. Baitursynov KRU, Kostanay, Kazakhstan, 14 April 2022; Bragina, T., Isakaev, Y., Eds.; KSPI: Kostanay, Kazakhstan, 2022; pp. 61–67. (In Russian). [Google Scholar]
- Rachkovskaya, E.I.; Nelina, N.V. Vegetation of the Altyn-Dala reserve. In Study, Conservation and Rational Use of the Flora of Eurasia; Almaty, Kazakhstan, 2017; pp. 396–400. (In Russian) [Google Scholar]
- Mânzu, C.C.; Irimia, I.; Cîșlariu, A.G.; Chinan, V. Chorological data for some rare plant species from ROSCI0222 Sărăturile Jijia Inferioară-Prut and ROSPA0042 Eleșteele Jijiei Și Miletinului (Iași County). Acta Horti Bot. Bucurestiensis 2020, 46, 35–54. [Google Scholar]
- Aslyamova, E.R.; Ilyina, I.V.; Ishmuratova, M.M. Age spectra of Stemmacantha serratuloides (Georgi) M. Dittrich. In the conditions of the Bashkir Trans-Urals. Rep. Bashkir Univ. 2019, 4, 381–385. (In Russian) [Google Scholar] [CrossRef]
- Zhmud, E.V.; Achimova, A.A.; Kuban, I.N.; Yamitrov, M.B.; Dorogina, O.V. Rhaponticum carthamoides (Asteraceae) in the Altai Republic: Assesment of the state of the plant affected of human activities. J. Sib. Fed. Univ. Biol. 2022, 15, 92–106. [Google Scholar] [CrossRef]
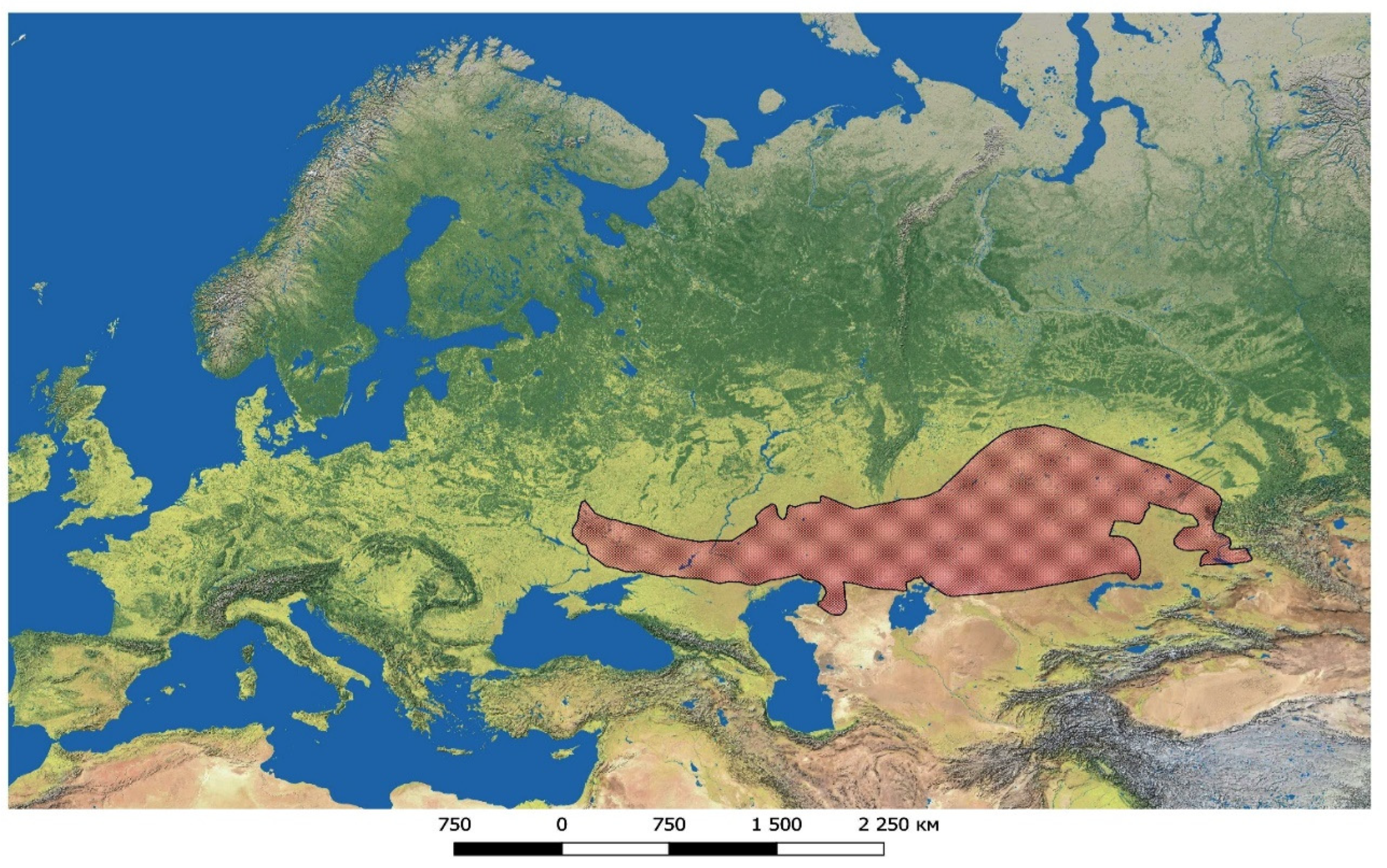

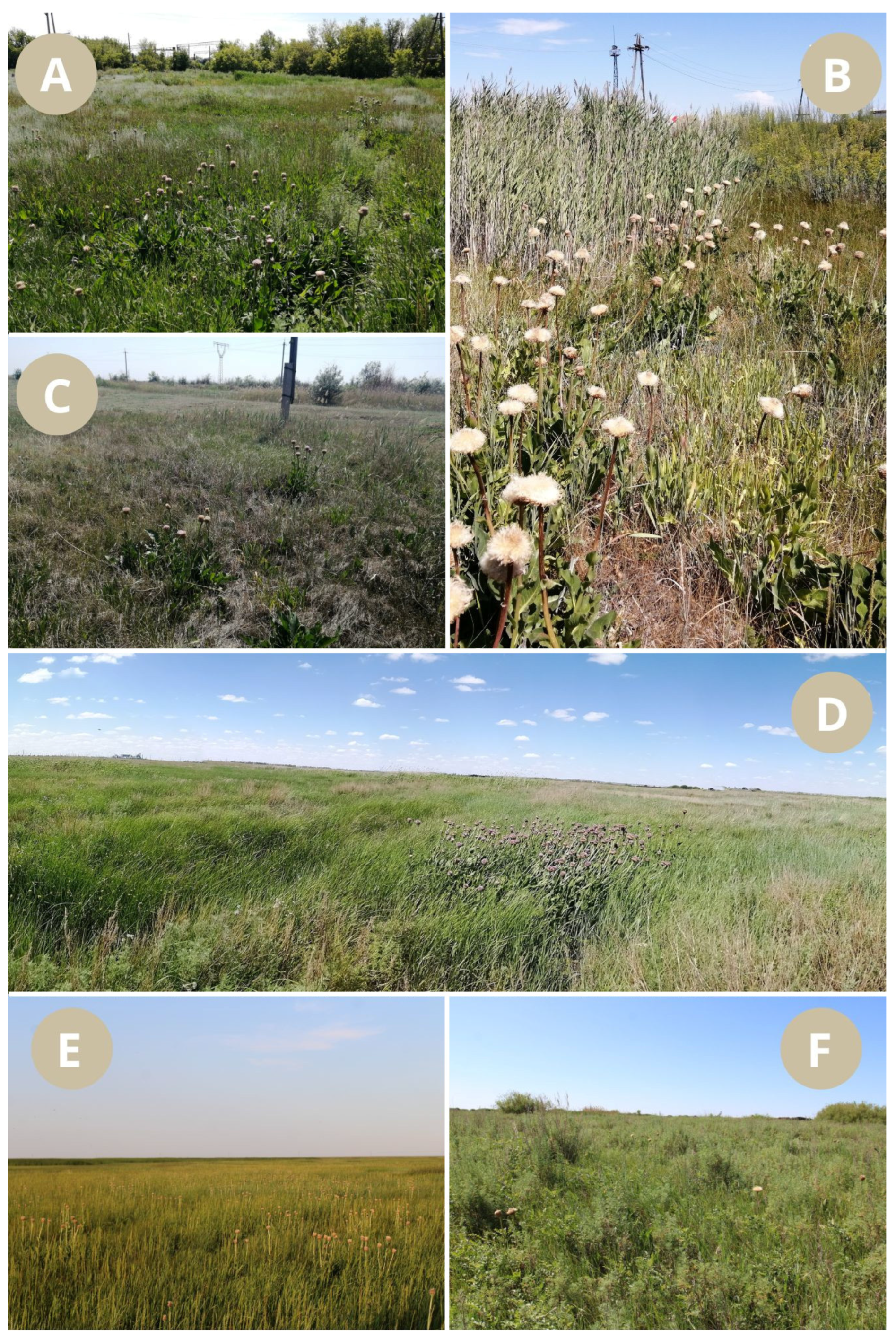

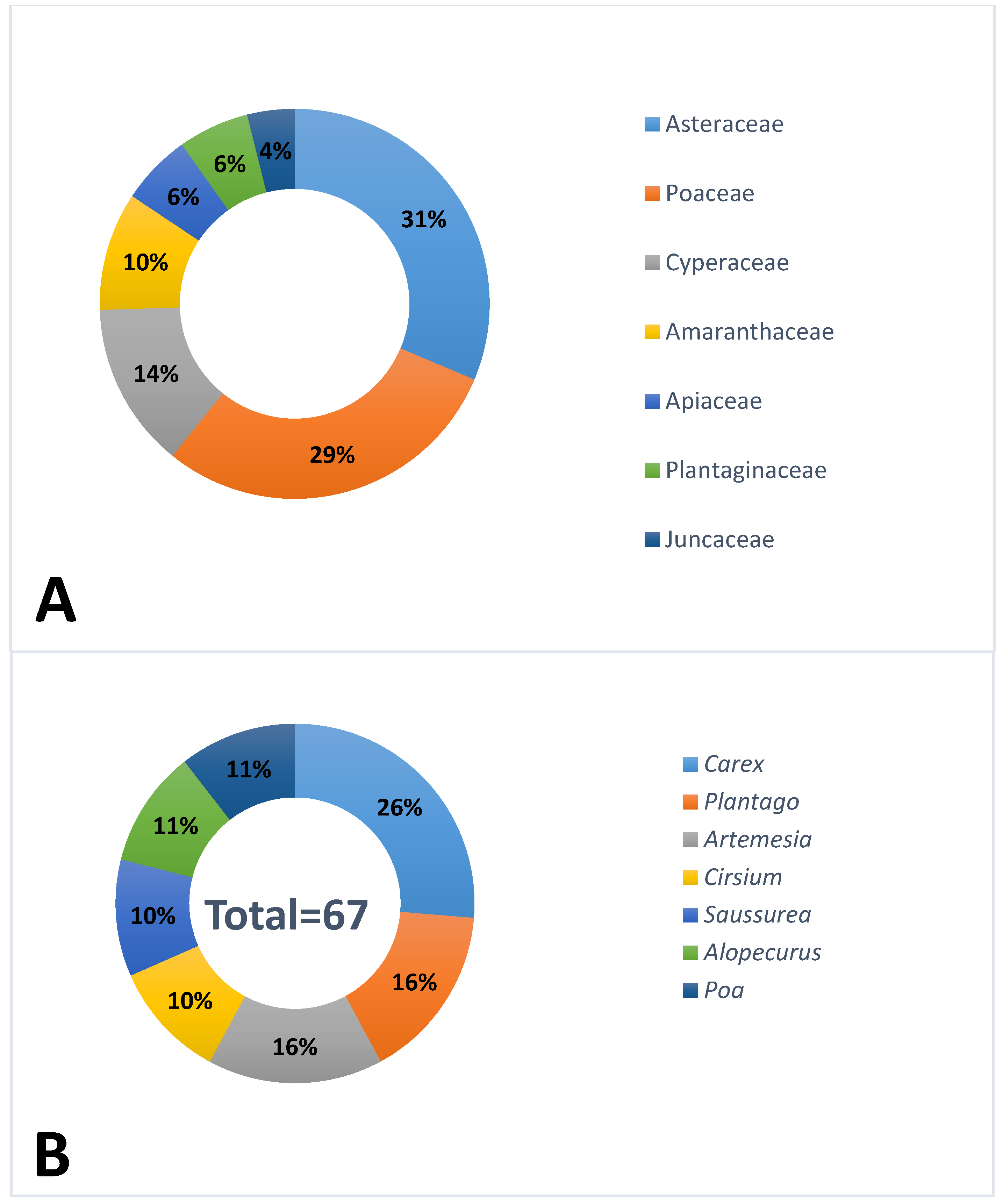
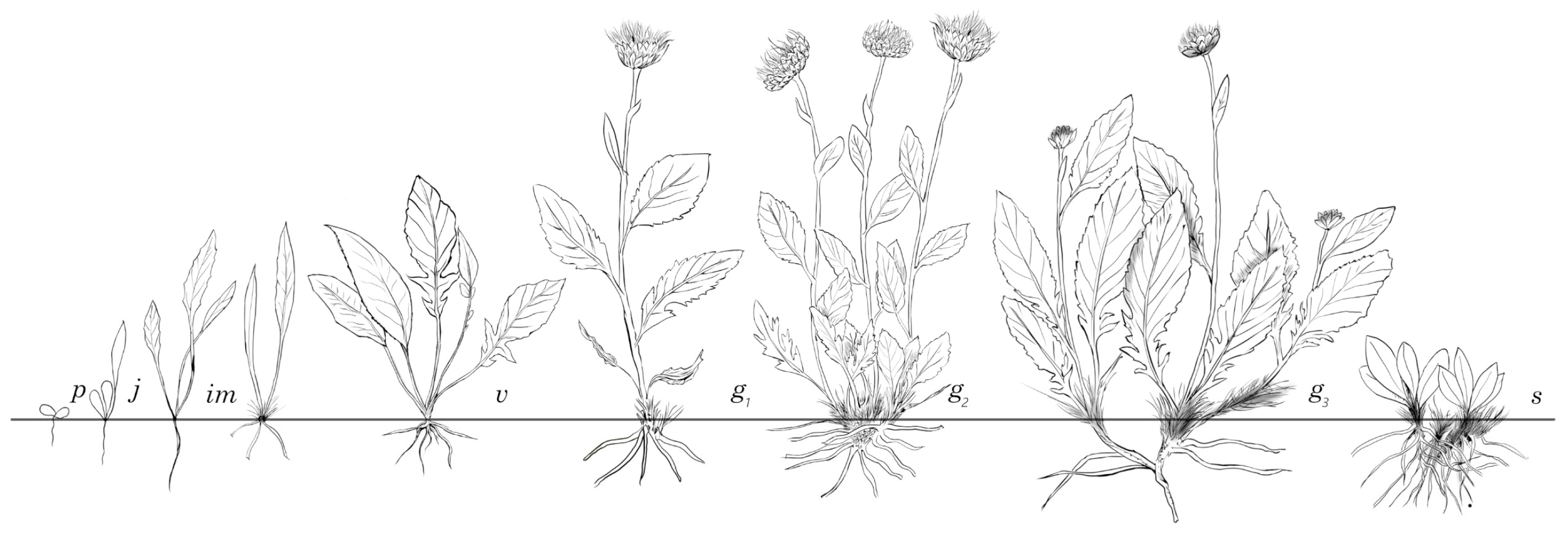

| Population No. | Location Name, Altitude, Coordinates | Habitat | General Information |
|---|---|---|---|
| Pop 1 | Karaganda region, the vicinity of the village of Karabas, 503 m a.s.l., 49°34′48.0″ N, 72°53’38.1″ E | Grass and Artemisia meadow near a road | Soils are anthropogenically disturbed, chestnut, meadow, almost not saline. Anthropogenic disturbance of the phytocenosis is high. The community is dominated by Elymus repens (L.) Gould and Artemisia nitrosa Web. ex Stechm., with 18 species in total. Artemisia abrotanum L., Plantago urvillei Opiz., Phragmites australis (Cav.) Trin. ex Steud., and Rh. altaicum make the largest contribution in addition to the dominant species. |
| Pop 2 | Karaganda region, near the city of Abay, 500 m a.s.l., 49°38’03.4″ N, 72°50’38.3″ E | Well-moistened hollow near a road | Soils are dark chestnut, meadow, slightly saline. The anthropogenic disturbance of the phytocoenosis is medium. The dominant species are Carex stenophylla Wahlenb., Juncus gerardii Loisel., Rh. altaicum. The community has 16 species in total. In addition to the dominant species, Agrostis gigantea Roth and Elymus repens make the largest contribution. |
| Pop 3 | Karaganda region, near the city of Abay, 49°40′31.3″ N, 72°51′50.3″ E | Mown meadow | The mown meadow, the plants are scattered, the anthropogenic disturbance of the phytocoenosis is medium. Soils are are dark chestnut, slightly saline. The community is dominated by Artemisia abrotanum and Bromus inermis Leyss.; there are 11 species in total. In addition to the dominant species, Agropyron cristatum (L.) Gaertn., Saussurea amara (L.) DC. and Elymus repens make the largest contribution. |
| Pop 4 | Karaganda region, between Abai and Saran, 485 m a.s.l., 49°40′57.6″ N, 72°51′37.2″ E | Flood meadow, on the outskirts of drying lakes | Soils are dark chestnut, meadow, medium saline. Anthropogenic disturbance of the phytocenosis is low. The dominant species are Calamagrostis epigejos (L.) Roth, Glycyrrhiza uralensis Fisch. ex DC., Bromus inermis. There are 16 species in total. Juncus compressus Jacq. also makes a significant contribution to the vegetation cover. |
| Pop 5 | Karaganda region, Temirtau district, 363 m a.s.l., 50°04′49″ N, 73°13′30″ E | Swampy meadow | Soils are chestnut, medium-saline. The anthropogenic disturbance of the phytocoenosis is medium. The dominant species are Phragmites australis, Juncus gerardi. There are 23 species in total: Calamagrostis epigeios, Alopecurus arundinaceus Poir., Artemisia nitrosa, Elymus repens also make a significant contribution. |
| Pop 6 | Akmola region, Zhaltyr village, 291 m a.s.l., 51°44′05.7″ N, 69°54′42.1″ E | Flooded meadow | Soils are slightly saline, dark chestnut. Anthropogenic disturbance of the phytocenosis is low. The dominant species is Elymus repens, there are 27 species in total: Bolboschoenus maritimus (L.) Palla, Eleocharis palustris (L.) Roem. et Schult., Rh. altaicum also make a significant contribution. |
| Pop 7 | Akmola region, Astana district, the vicinity of the village of Karazhar, the valley of the Nura River, the Astana–Malinovka highway, 341 m a.s.l., 51°05′27.9″ N, 71°11′05.3″ E | Swampy meadow | Soils are dark chestnut, medium-saline. Anthropogenic disturbance of the phytocenosis is high. The dominant species are Artemisia abrotanum, Calamagrostis epigeios. The total number of species is 18: Galatella dahurica DC, Saussurea amara also make a significant contribution. |
| Indicator | Pop 1 | Pop 2 | Pop 3 | Pop 4 | Pop 5 | Pop 6 | Pop 7 |
|---|---|---|---|---|---|---|---|
| Total projective vegetation cover, % | 73 | 90 | 80 | 65 | 70 | 98 | 95 |
| Projective cover of Rh. altaicum, % | 2 | 15 | 0.5 | 0.5 | 1.5 | 3.8 | 1.2 |
| Area, m2 | 450 | 300 | 1000 | 4000 | 800 | 60,000 | 2400 |
| Average density of individuals, pcs/m2 | 2.1 | 2.7 | 2.9 | 1.1 | 1.1 | 7.4 | 7.2 |
| No | Morphometric Features, cm | ||||||
|---|---|---|---|---|---|---|---|
| H | LUL | WUL | LML | WML | LLL | WLL | |
| Pop 1 | |||||||
| M ± m | 71.12 ± 3.83 | 7.74 ± 0.25 | 3.28 ± 0.25 | 11.57 ± 0.39 | 6.54 ± 0.61 | 15.12 ± 0.61 | 6.75 ± 0.49 |
| CV % | 16.18 | 9.82 | 22.42 | 10.33 | 27.77 | 12.24 | 21.67 |
| Pop 2 | |||||||
| M ± m | 74.95 ± 6.02 | 6.05 ± 0.46 | 2.89 ± 0.45 | 14.04 ± 0.94 | 5.99 ± 0.79 | 17.07 ± 1.11 | 6.44 ± 0.59 |
| CV % | 26.64 | 25.16 | 51.90 | 22.31 | 44.23 | 20.62 | 29.15 |
| Pop 3 | |||||||
| M ± m | 52.44 ± 3.2 | 5.51 ± 0.39 | 1.88 ± 0.14 | 12.22 ± 0.59 | 5.47 ± 0.21 | 13.41 ± 0.52 | 5.77 ± 0.48 |
| CV % | 18.32 | 21.34 | 22.40 | 14.48 | 11.57 | 11.57 | 25.34 |
| Pop 4 | |||||||
| M ± m | 84.40 ± 3.66 | 6.58 ± 0.36 | 2.82 ± 0.40 | 10.64 ± 0.72 | 5.46 ± 0.47 | 17.54 ± 0.56 | 7.72 ± 0.52 |
| CV % | 13.69 | 16.48 | 42.62 | 21.51 | 27.10 | 10.11 | 21.42 |
| Pop 5 | |||||||
| M ± m | 54.38 ± 3.69 | 5.43 ± 0.40 | 1.59 ± 0.22 | 14.68 ± 0.90 | 6.33 ± 0.43 | 14.50 ± 0.58 | 6.90 ± 0.30 |
| CV % | 20.38 | 22.05 | 40.60 | 18.38 | 20.40 | 11.91 | 13.02 |
| Pop 6 | |||||||
| M ± m | 80.83 ± 3.37 | 5.23 ± 0.25 | 2.23 ± 0.19 | 12.24 ± 0.48 | 5.88 ± 0.27 | 16.55 ± 0.45 | 6.12 ± 0.45 |
| CV % | 12.52 | 14.17 | 25.62 | 11.83 | 13.54 | 8.12 | 21.96 |
| Pop 7 | |||||||
| M ± m | 61.5 ± 1.73 | 6.01 ± 0.49 | 3.02 ± 0.4 | 10.28 ± 0.48 | 5.80 ± 0.27 | 14.99 ± 0.7 | 7.17 ± 0.58 |
| CV % | 10.53 | 24.24 | 41.22 | 13.90 | 13.93 | 14.03 | 24.10 |
| m.av. | 65.52 | 6.08 | 2.53 | 12.24 | 5.92 | 15.60 | 6.70 |
| CVav, % | 16.89 | 19.04 | 35.25 | 16.12 | 22.65 | 12.66 | 22.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamyrova, S.; Kupriyanov, A.; Ishmuratova, M.; Ivashchenko, A.; Myrzagaliyeva, A.; Orazov, A.; Kubentayev, S. The Current State of Populations of Rhaponticum altaicum (Asteraceae) in the Northern and Central Kazakhstan. Diversity 2025, 17, 206. https://doi.org/10.3390/d17030206
Mamyrova S, Kupriyanov A, Ishmuratova M, Ivashchenko A, Myrzagaliyeva A, Orazov A, Kubentayev S. The Current State of Populations of Rhaponticum altaicum (Asteraceae) in the Northern and Central Kazakhstan. Diversity. 2025; 17(3):206. https://doi.org/10.3390/d17030206
Chicago/Turabian StyleMamyrova, Saule, Andrey Kupriyanov, Margarita Ishmuratova, Anna Ivashchenko, Anar Myrzagaliyeva, Aidyn Orazov, and Serik Kubentayev. 2025. "The Current State of Populations of Rhaponticum altaicum (Asteraceae) in the Northern and Central Kazakhstan" Diversity 17, no. 3: 206. https://doi.org/10.3390/d17030206
APA StyleMamyrova, S., Kupriyanov, A., Ishmuratova, M., Ivashchenko, A., Myrzagaliyeva, A., Orazov, A., & Kubentayev, S. (2025). The Current State of Populations of Rhaponticum altaicum (Asteraceae) in the Northern and Central Kazakhstan. Diversity, 17(3), 206. https://doi.org/10.3390/d17030206





