Assessing the Potential Risk of Invasion of the Neophyte Pluchea ovalis (Pers.) DC. (Asteraceae) in the Canarian Archipelago Using an Ensemble of Species Distribution Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Occurrence Data
2.3. Predictors
2.4. Habitat Suitability Modeling (HSM)
2.5. Future Projections
2.6. Extrapolation Assessment
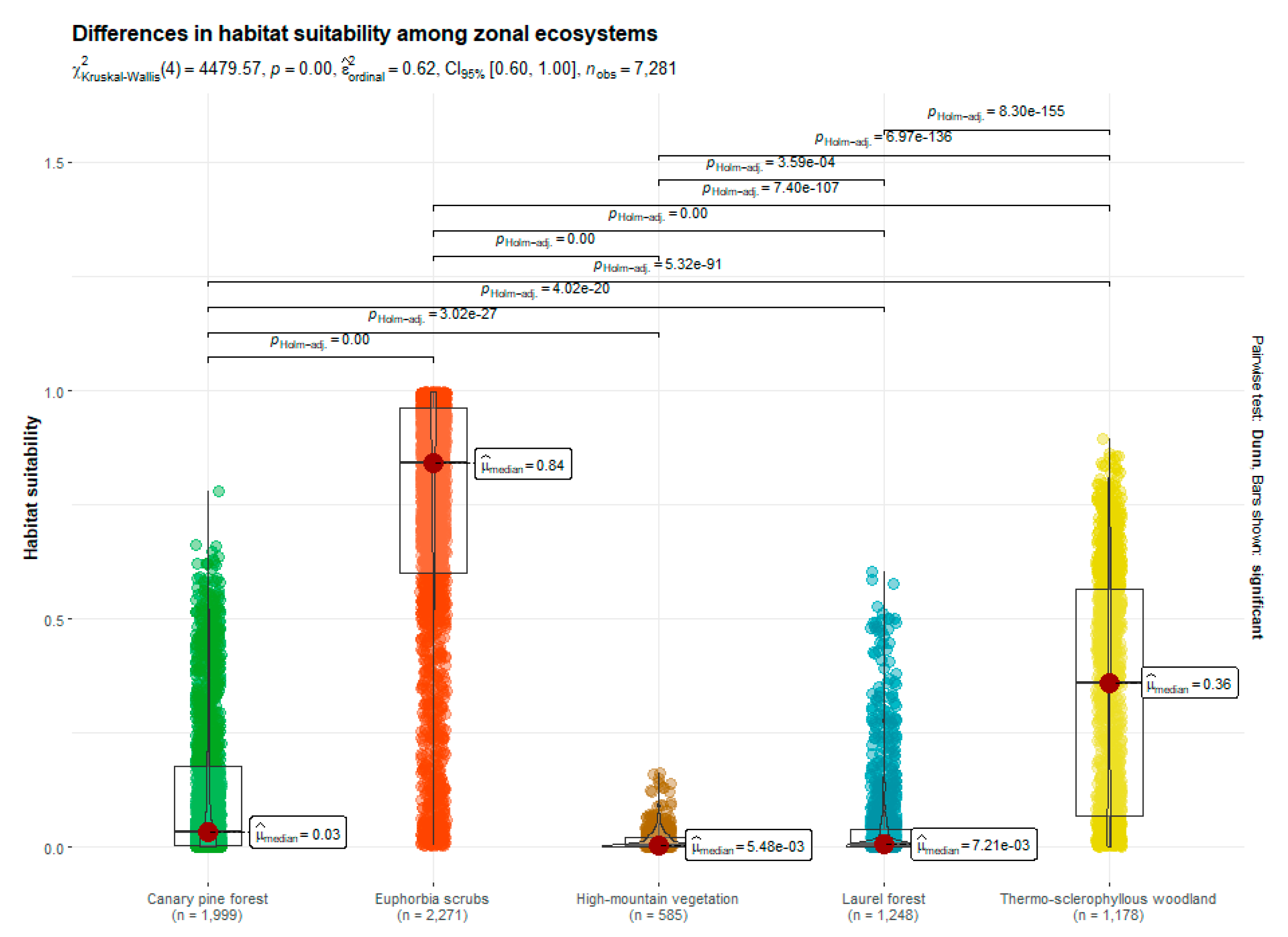
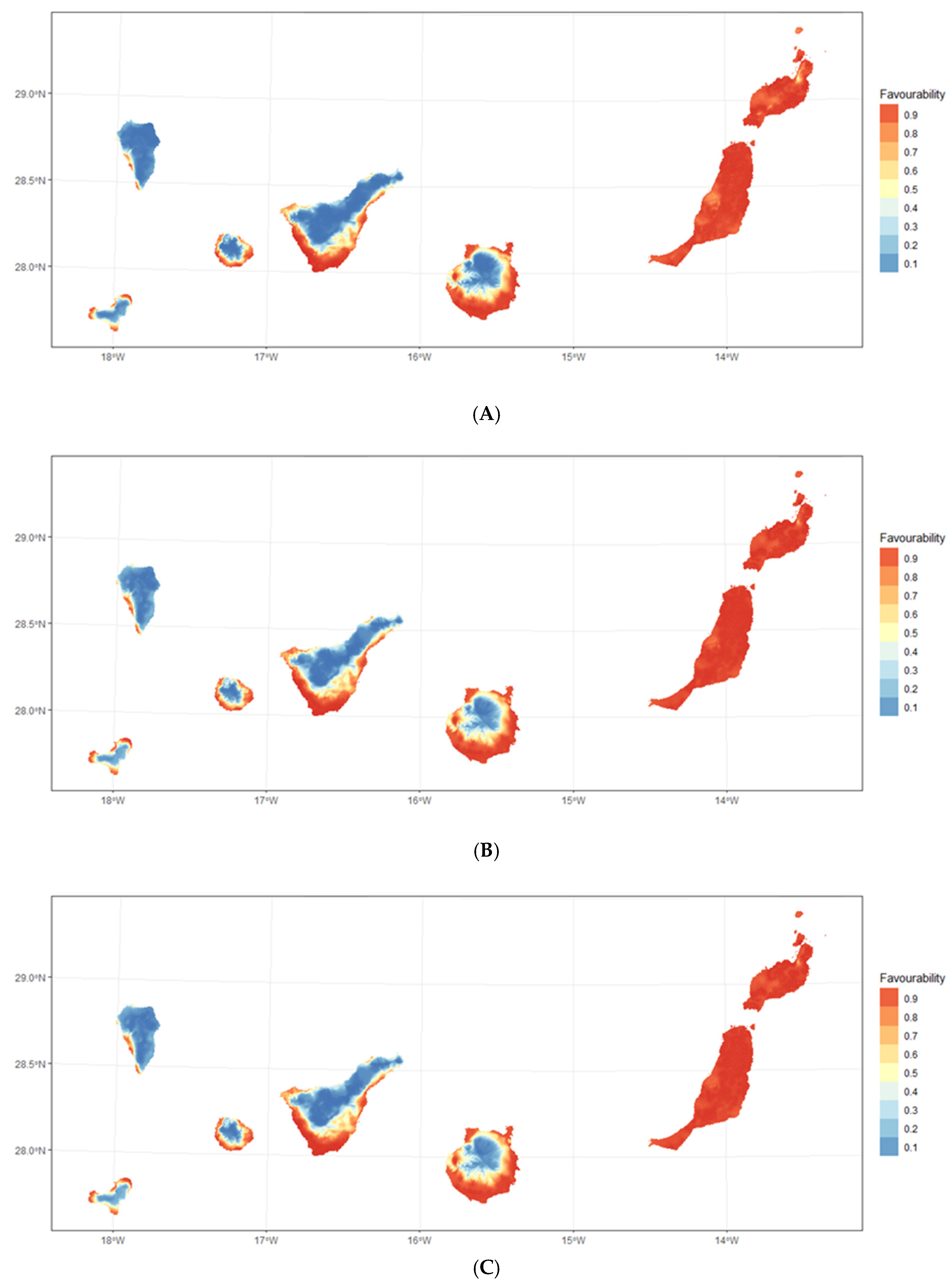
3. Results
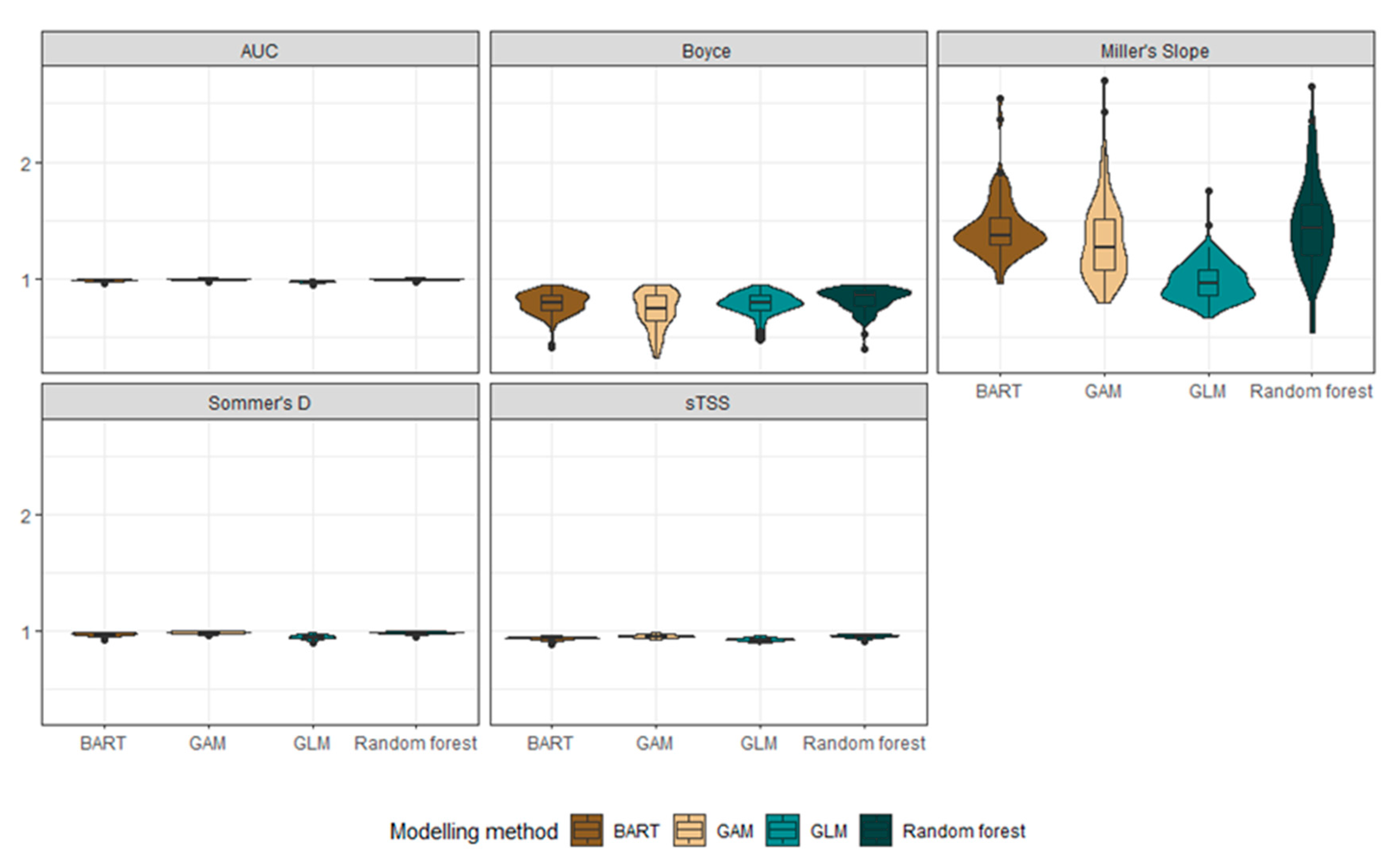
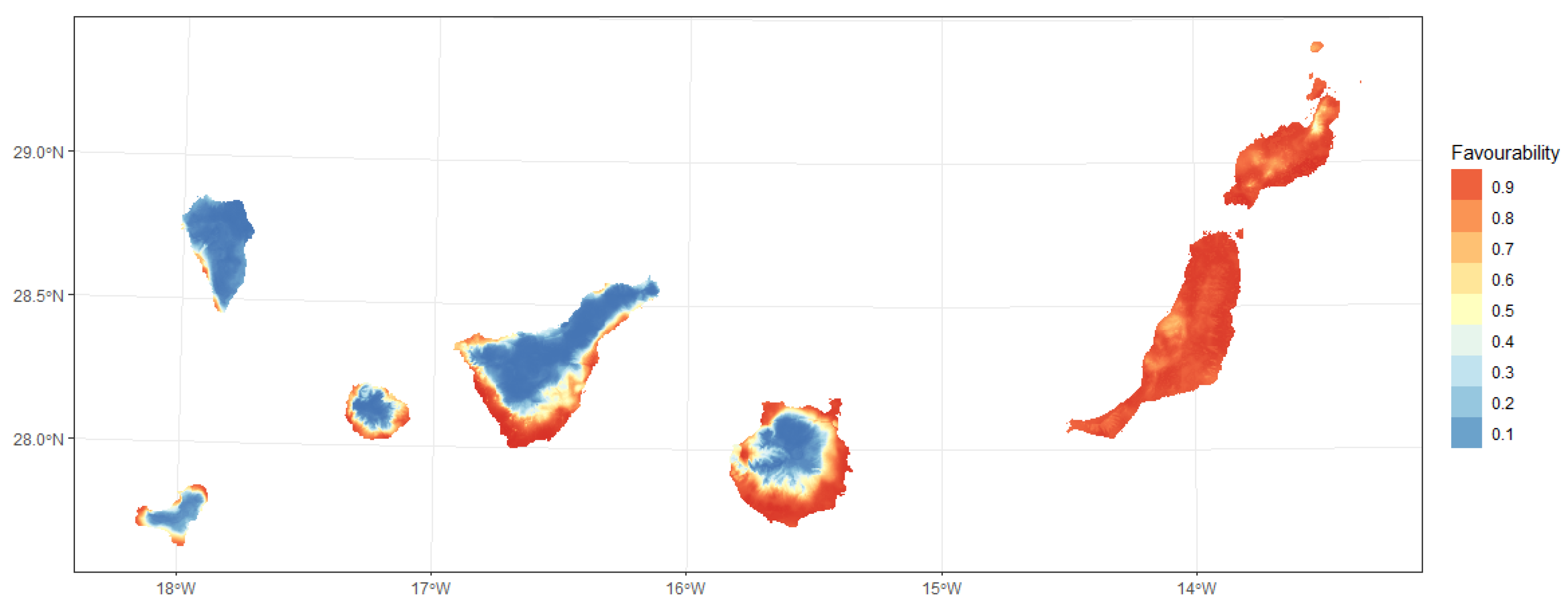
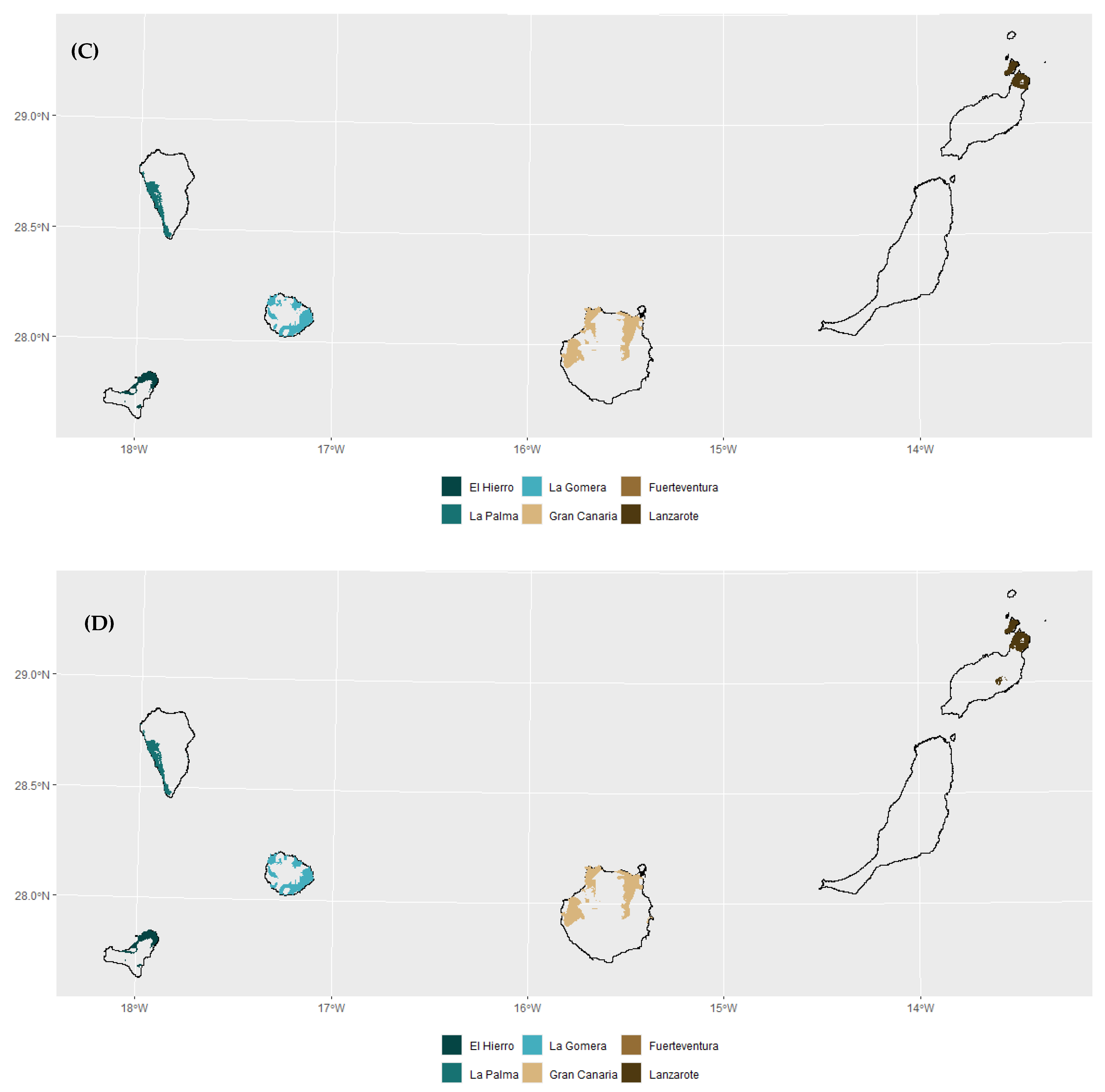
3.1. Habitat Suitabiliy Modeling
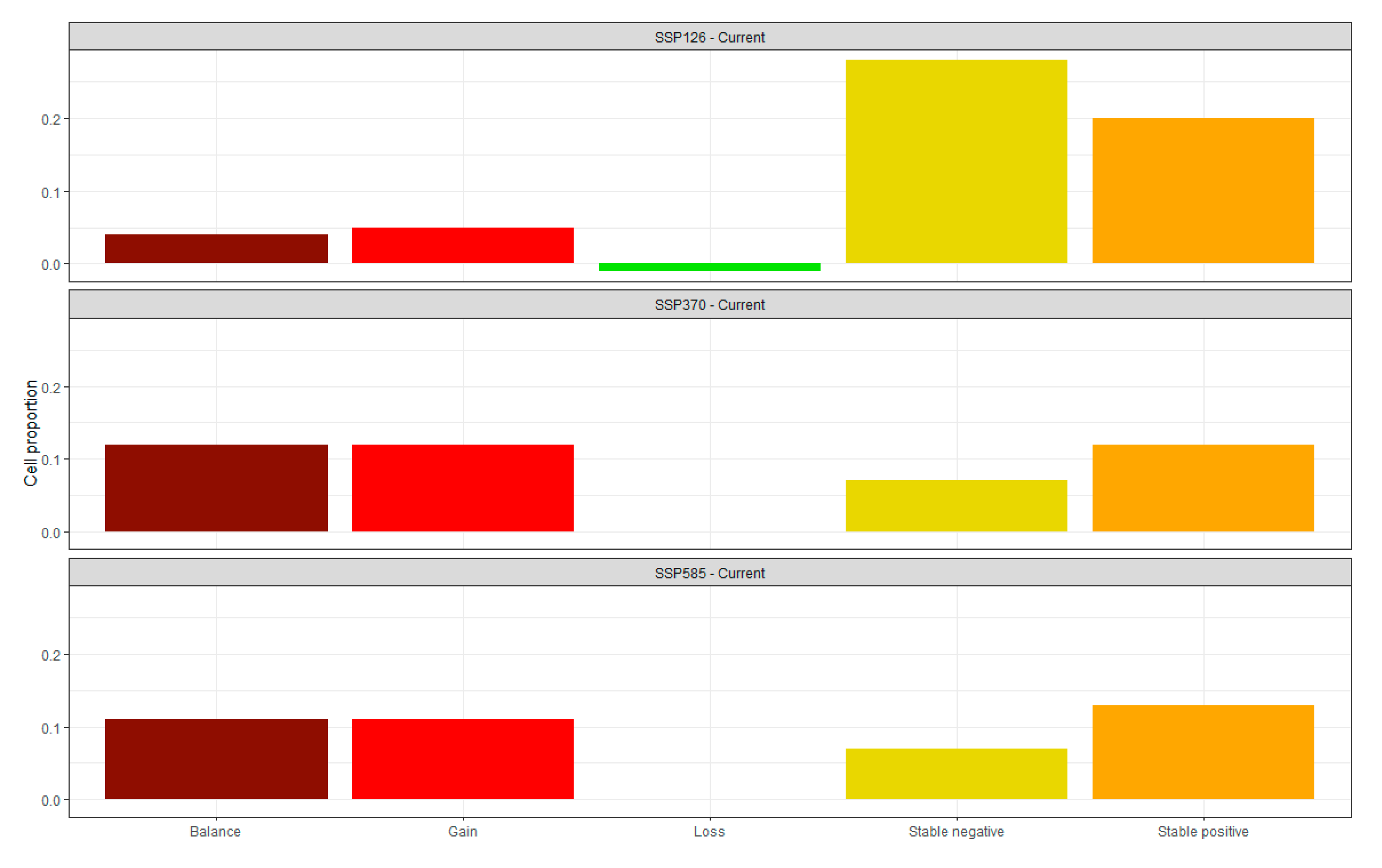
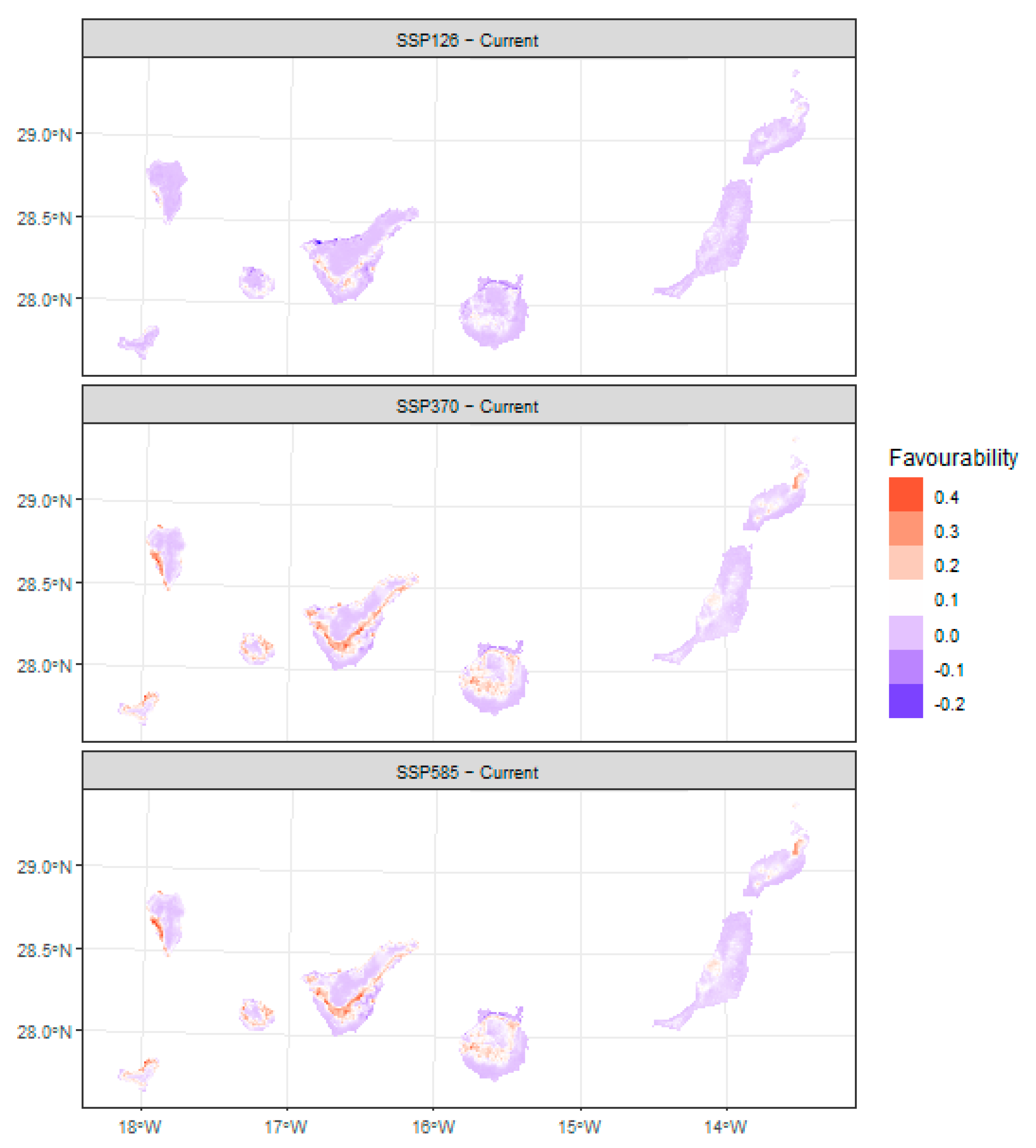
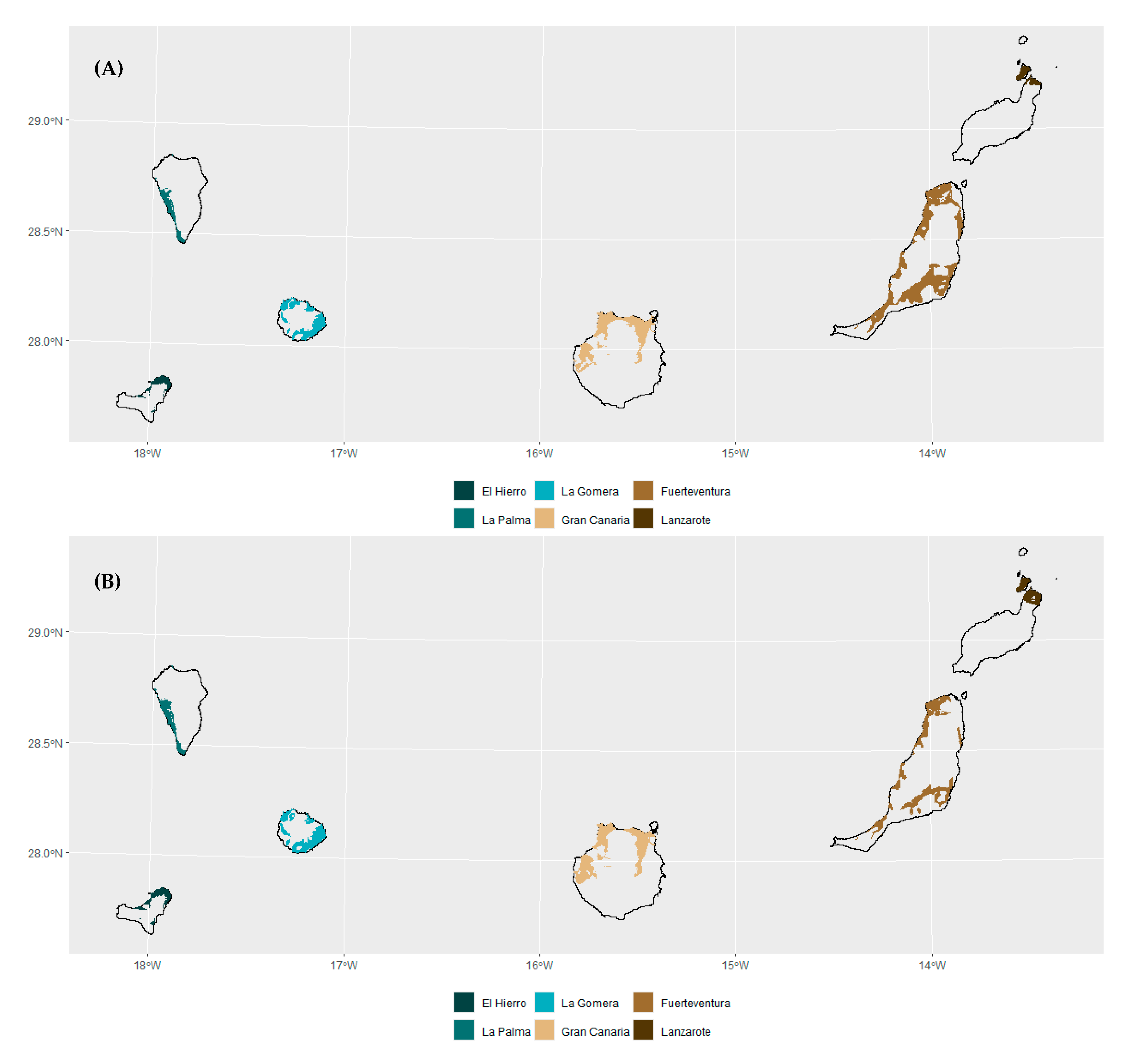
3.2. Future Projections and Changes in Site Suitability
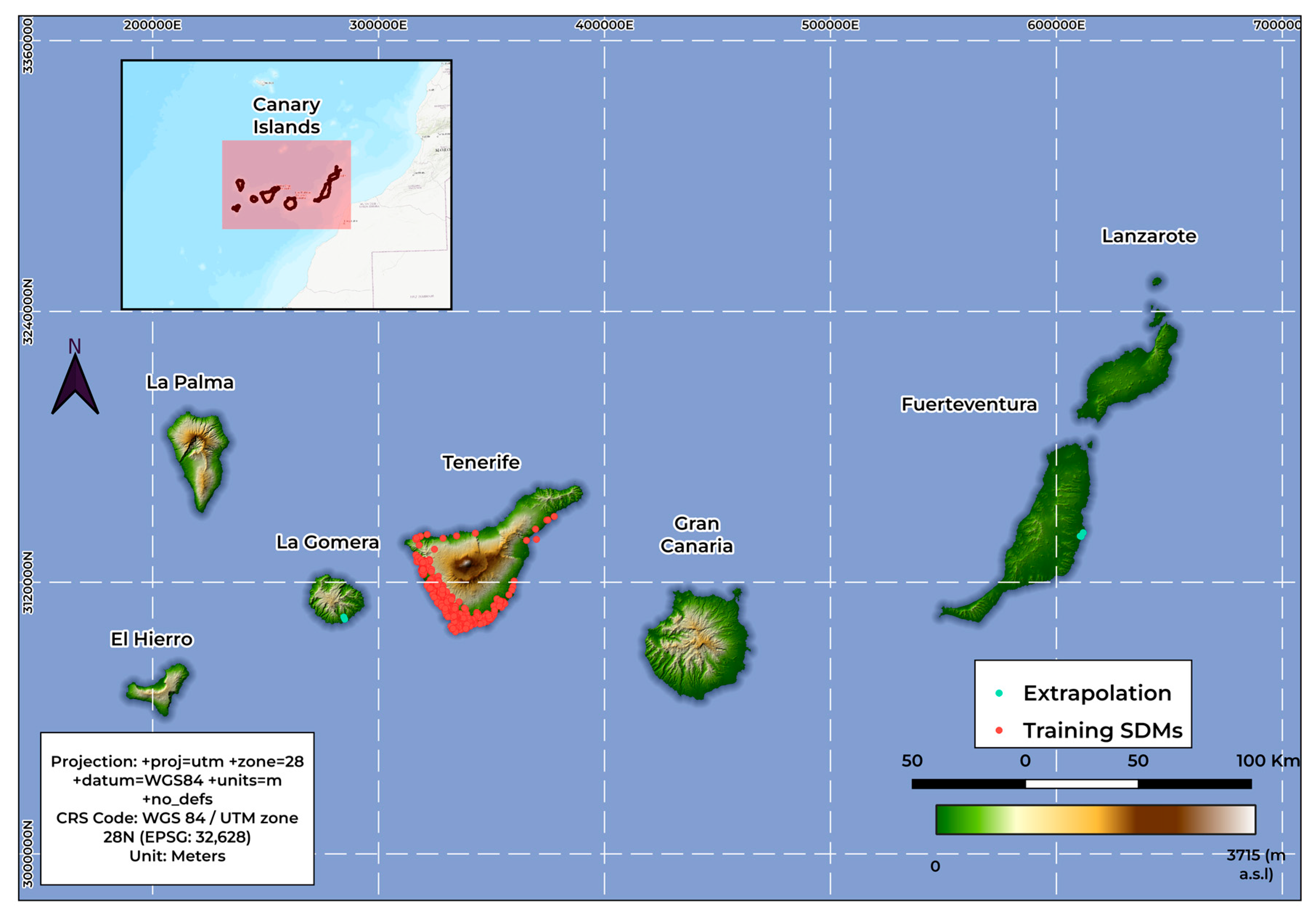
3.3. Extrapolation Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
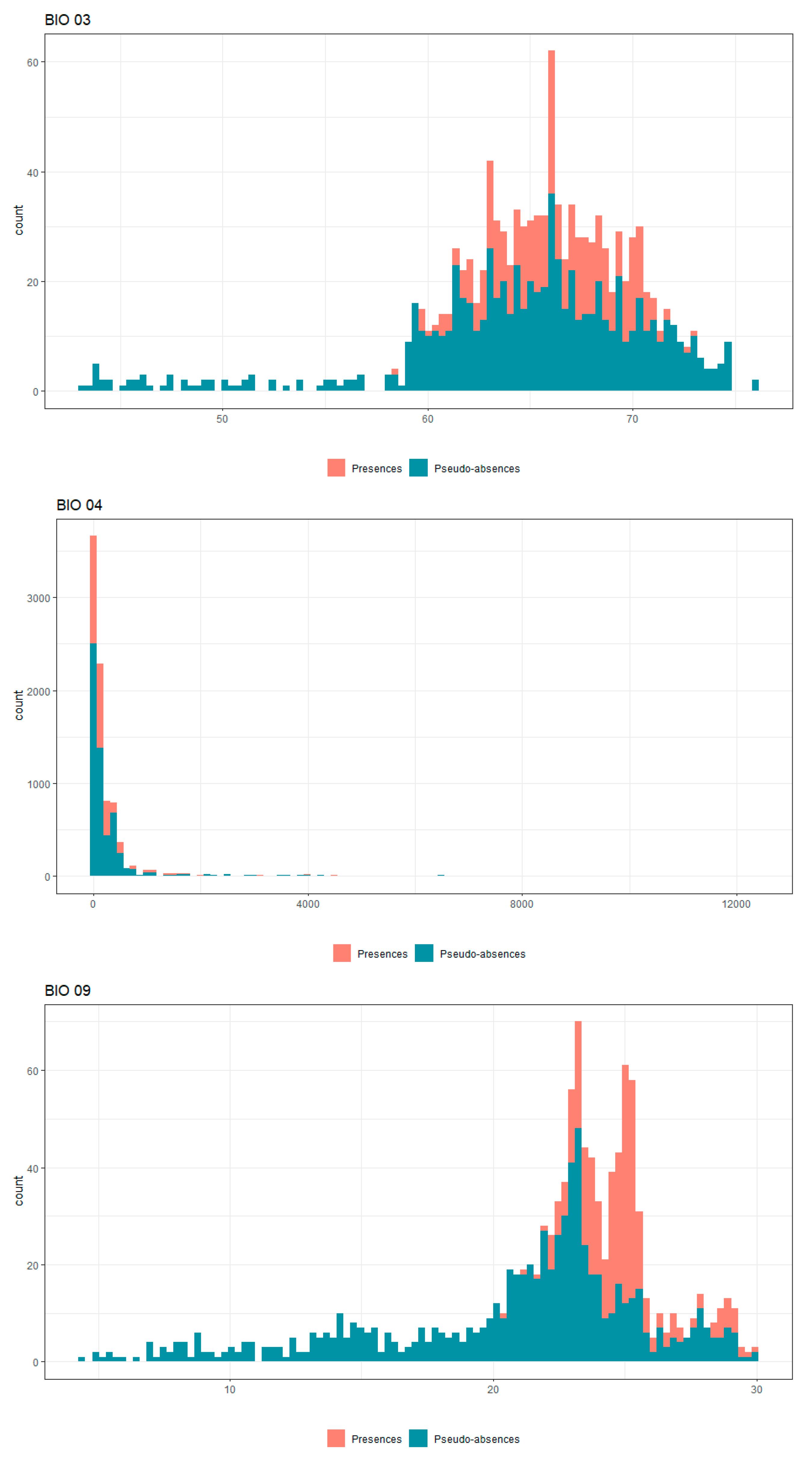

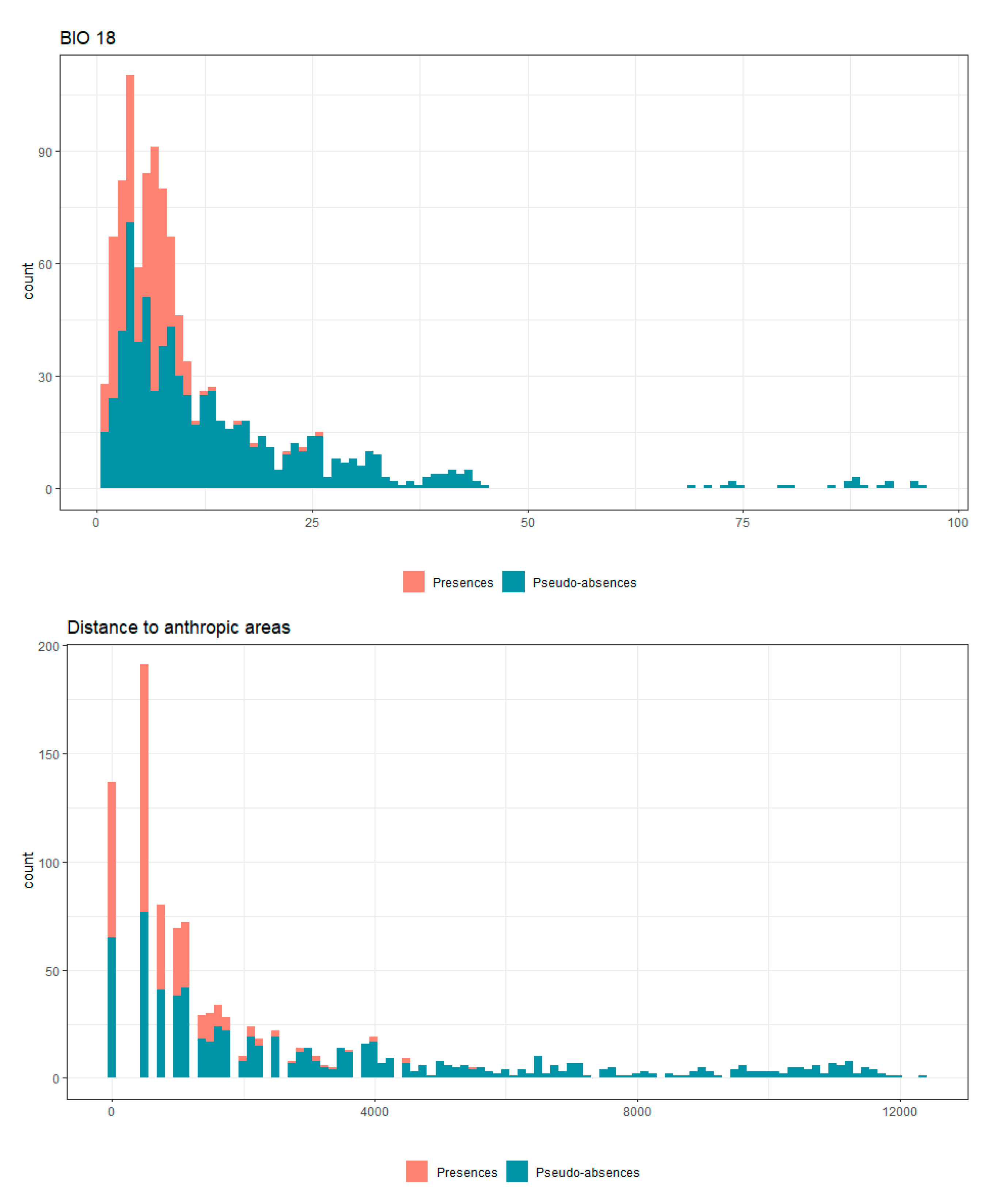
Appendix B
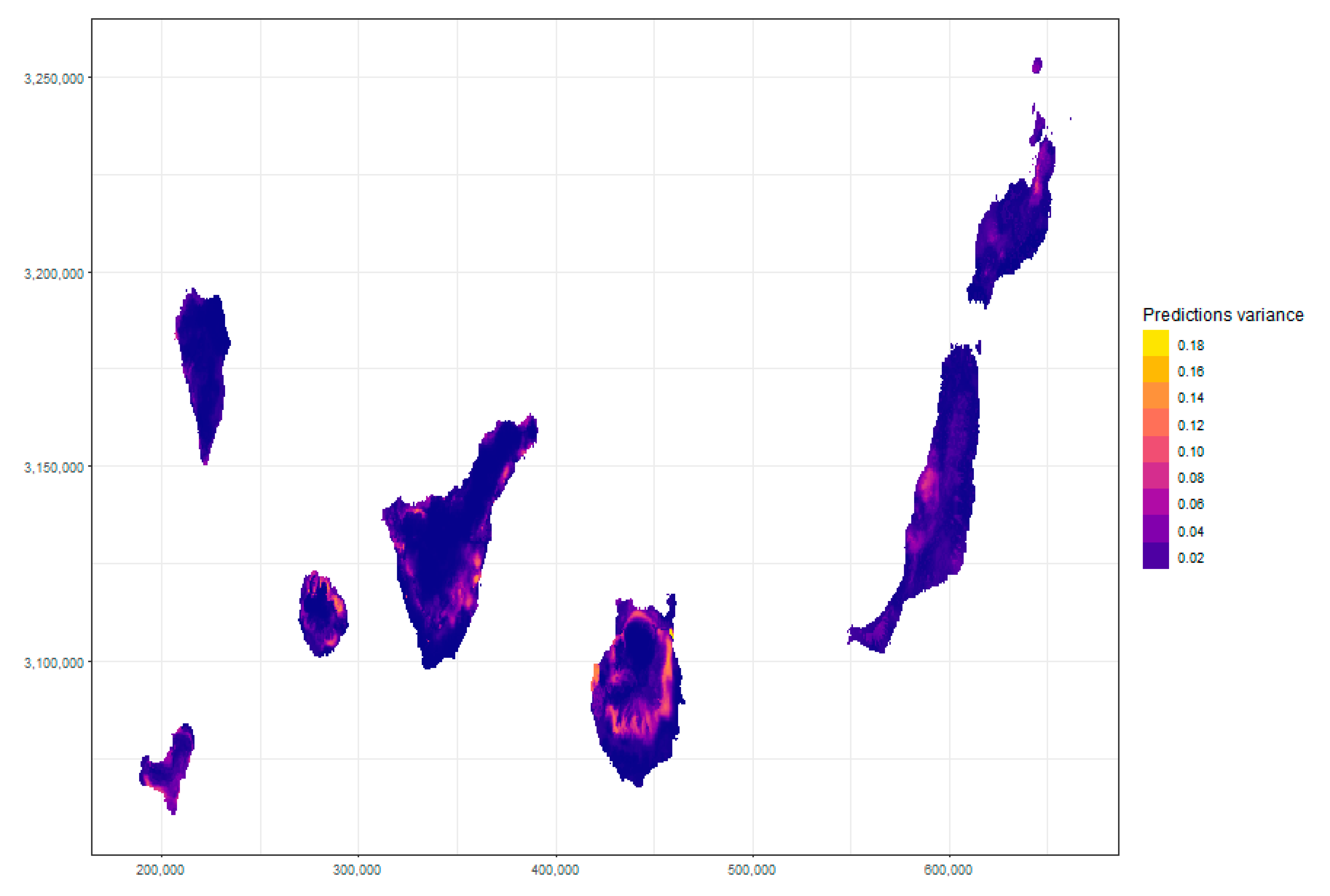
Appendix C
| Variable | Importance (%) |
|---|---|
| BIO3 | 11.5 |
| BIO4 | 12.9 |
| BIO9 | 12.4 |
| BIO12 | 14.4 |
| BIO14 | 11.5 |
| BIO15 | 13.1 |
| BIO18 | 12 |
| Distance to anthropic areas | 11.9 |
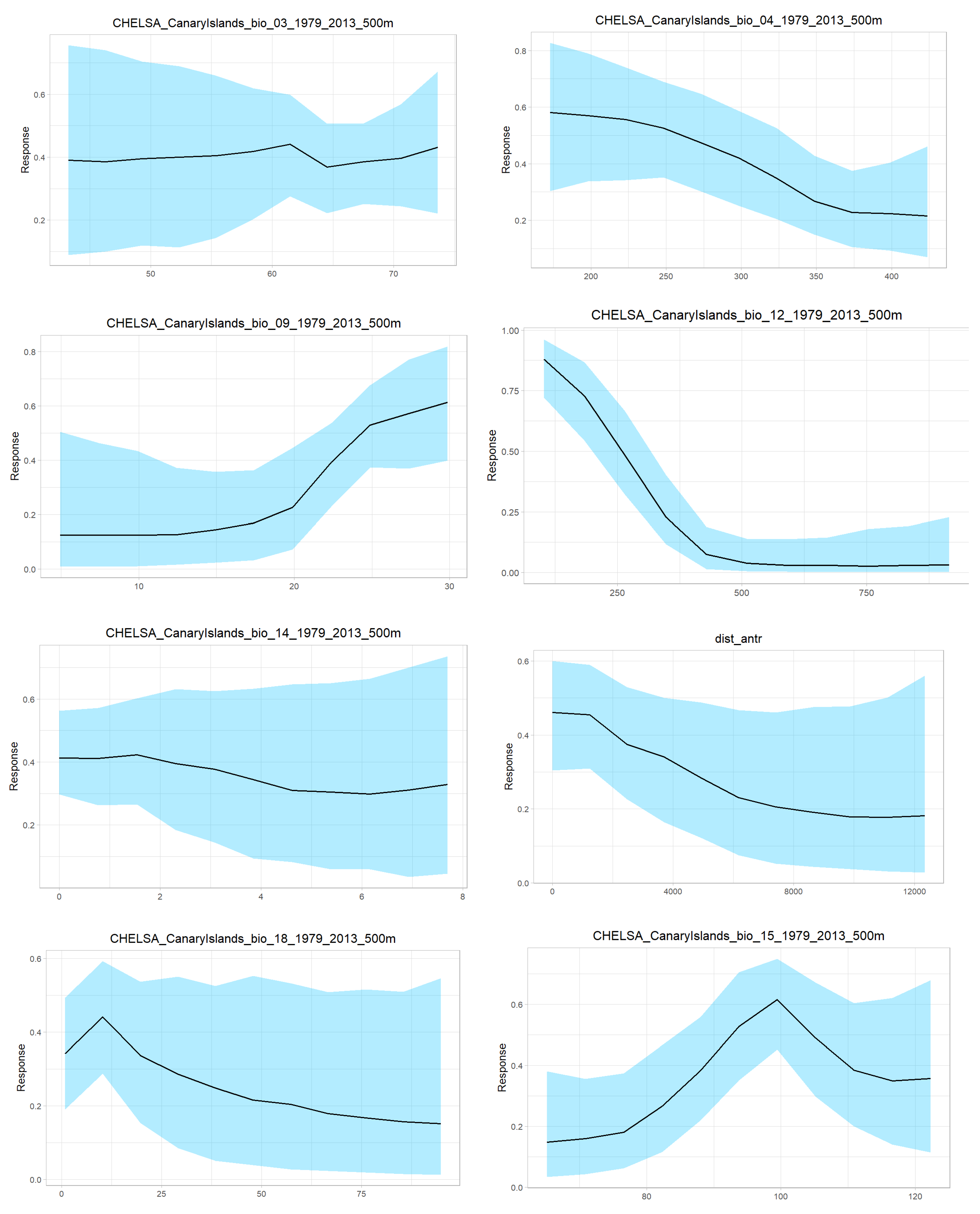
Appendix D
References
- van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Pfadenhauer, W.G.; DiRenzo, G.V.; Bradley, B.A. Human activity drives establishment, but not invasion, of non-native plants on islands. Ecography 2024, 2024, e07379. [Google Scholar] [CrossRef]
- Carford, J.A.; Vesk, P.A.; Richardson, D.M.; Pysek, P. Quantifying levels of biological invasion: Towards the objective classification of invaded and invasible ecosystems. Glob. Change Biol. 2012, 18, 44–62. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver or recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondizio, E.S., Settele, J., Díaz, S., Ngo, H.T., Eds.; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar] [CrossRef]
- Gallardo, B.; Bacher, S.; Barbosa, A.M.; Gallier, L.; González-Moreno, P.; Martínez-Bolea, V.; Sorte, C.; Vimercanti, G.; Vilá, M. Risk posed by invasive species to the provision of ecosystem services in Europe. Nat. Commun. 2024, 15, 2631. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography. Ecology, Evolution and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Fernández-Palacios, J.M.; Otto, R.; Borregaard, M.K.; Kreft, H.; Price, J.P.; Steinbauer, M.J.; Weigelt, P.; Whittaker, R.J. Evolutionary winners are ecological losers among oceanic islands plants. J. Biogeogr. 2021, 48, 2186–2198. [Google Scholar] [CrossRef]
- Schrader, J.; Weigelt, P.; Cai, L.; Westoby, M.; Fernández-Palacios, J.M.; Cabezas, F.J.; Plunkett, G.M.; Ranker, T.M.; Triantis, K.M.; Trigas, P.; et al. Islands are key for protecting the world’s plant endemism. Nature 2024, 634, 868–874. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Kreft, H.; Irl, S.D.H.; Norder, S.; Ah-Peng, C.; Borges, P.A.V.; Burns, K.C.; de Nascimento, L.; Meyer, J.Y.; Montes, E.; et al. Scientists’ warning—The outstanding biodiversity of islands is in peril. Glob. Ecol. Conserv. 2021, 31, e01847. [Google Scholar] [CrossRef]
- Simberloff, D. Why do introduced species appear to devastate islands more than mainland areas? Pac. Sci. 1995, 49, 87–97. [Google Scholar]
- Otto, R.; Fernández-Lugo, S.; Blandino, C.; Manganelli, G.; Chiarucci, A.; Fernández-Palacios, J.M. Biotic homogenization of oceanic islands depends on taxon, spatial scale and the quantification approach. Ecography 2020, 43, 747–758. [Google Scholar] [CrossRef]
- Strandberg, N.A.; Steinbauer, M.J.; Walentowitz, A.; Gosling, W.D.; Fall, P.L.; Prebble, M.; Stevenson, J.; Wilmshurst, J.M.; Sear, D.A.; Langdon, P.G.; et al. Floristic homogenization of South Pacific islands commenced with human arrival. Nat. Ecol. Evol. 2024, 8, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Cubas, J.; Martín-Esquivel, J.L.; Nogales, M.; Irl, S.D.H.; Hernandez-Hernandez, R.; Lopez-Darias, M.; Marrero-Gomez, M.V.; Del Arco, M.J.; Gonzalez-Mancebo, J.M. Contrasting effects of invasive rabbits on endemic plants driving vegetation change in a subtropical alpine insular environment. Biol. Invasions 2018, 20, 793–807. [Google Scholar] [CrossRef]
- Essl, F.; Dawson, W.; Kreft, H.; Pergl, J.; Pysek, P.; van Kleunen, M.; Weigelt, P.; Mang, T.; Dullinger, S.; Lenzner, B.; et al. Drivers of the relative richness of naturalized and invasive plant species on Earth. AoB Plants 2019, 11, plz051. [Google Scholar] [CrossRef]
- Loh, R.K.; Daehler, C.C. Influence of woody invader control methods and seed availability on native and invasive species establishment in a Hawaiian forest. Biol. Invasions 2008, 10, 805–819. [Google Scholar] [CrossRef]
- Guerrero-Campos, M.; Mendes, S.B.; Marrero, P.; Romero, J.; Nieves, C.; Hervías-Parejo, S.; González-Mancebo, J.M.; Nogales, M. Introduced rabbits as seed-dispersing frugivores: A study case on a environmentally diverse oceanic island (Tenerife, Canaries). Biol. Invasions 2023, 25, 2117–2129. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Knapp, D.E.; Kenedy-Bowdoin, T. Effects of Morella faya tree invasion on aboveground carbon storage in Hawaii. Biol. Invasions 2010, 12, 477–494. [Google Scholar] [CrossRef]
- Berio-Fortini, L.; Leopold, C.R.; Perkins, K.S.; Chadwick, O.A.; Yelenik, S.; Jacobi, J.D.; Bishaw, K.; Gregg, M. Landscape level effects of invasive plants and animals on water infiltration through Hawaiian tropical forests. Biol. Invasions 2021, 23, 2155–2172. [Google Scholar] [CrossRef]
- Berio-Fortini, L.; Chen, Q.; Uyehara, Y.; Bogner, K.; Sprague, J.; Sprague, R. Fine-resolution land cover mapping over large and mountainous areas for Lāna‘i, Hawaii using posterior probabilities, and expert knowledge. Int. J. Remote Sens. 2024, 45, 1949–1971. [Google Scholar] [CrossRef]
- Devkota, R.S.; Field, R.; Hoffmann, S.; Walentowitz, A.; Medina, F.M.; Vetaas, O.R.; Chiarucci, A.; Weiser, F.; Jentsch, A.; Beierkuhnlein, C. Assessing the Potential Replacement of Laurel Forest by a Novel Ecosystem in the Steep Terrain of an Oceanic Island. Remote Sens. 2020, 12, 4013. [Google Scholar] [CrossRef]
- González-Montelongo, C.; Pérez-Vargas, I. Is an invasive alien tree able to sustain a similar lichen diversity as the native forest? The case of the sweet chestnut (Castanea sativa Mill.) and the laurel forest in Macaronesia. For. Ecol. Manag. 2021, 448, 19009. [Google Scholar] [CrossRef]
- Koerner, L.M.; Chadwick, M.A.; Tebbs, E.J. Mapping invasive strawberry guava (Psidium cattleianum) in tropical forests of Mauritius with Sentinel-2 and machine learning. Int. J. Remote Sens. 2022, 43, 841–872. [Google Scholar] [CrossRef]
- Rocchini, D.; Andreo, V.; Förster, M.; Garzón-López, C.X.; Gutiérrez, A.P.; Gillespie, T.W.; Hauffe, H.C.; He, K.S.; Kleinschmit, B.; Mairota, P.; et al. Potential of remote sensing to predict species invasions: A modelling perspective. Prog. Phys. Geogr. 2015, 39, 283–309. [Google Scholar] [CrossRef]
- Da Re, D.; Tordoni, E.; Negrín-Pérez, Z.; Fernández-Palacios, J.M.; Arévalo, J.R.; Otto, R.; Rocchini, D.; Bacaro, G. A spatially explicit model of alien plant richness in Tenerife (Canary Islands). Ecol. Complex. 2018, 38, 75–82. [Google Scholar] [CrossRef]
- Feounillas, P.; Ah-Peng, C.; Amy, E.; Bracco, I.; Dafreville, S.; Gosset, M.; Ingrassia, F.; Lavergne, C.; Lequette, B.; Notter, J.C.; et al. Quantifying invasion degree by alien plants in Reunion Island. Aust. Ecol. 2021, 46, 1125–1137. [Google Scholar] [CrossRef]
- Santamarina, S.; Alfaro-Saiz, E.; Llamas, F.; Acedo, C. Different approaches to assess the local invasion risk on a threatened species: Opportunities of using high-resolution species distribution models by selecting the optimal model complexity. Glob. Ecol. Conserv. 2019, 20, e00767. [Google Scholar] [CrossRef]
- Herrera, C.; Jurado-Rivera, J.A.; Leza, M. Ensemble of small models as a tool for alien invasive species management planning: Evaluation of Vespa velutina (Hymenoptera: Vespidae) under Mediterranean island conditions. J. Pest Sci. 2022, 96, 359–371. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and geographic distributions. In Monographs in Population Biology; Princeton University Press: Princeton, NJ, USA, 2011; MPB-49. [Google Scholar]
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from modeling studies on how climate change affects invasive alien species geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Chang. Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Benavides-Ríos, E.; Sadler, J.; Graham, L.; Matthews, T.J. Species distribution models and island biogeography. Glob. Ecol. Conserv. 2024, 51, e02943. [Google Scholar] [CrossRef]
- Bellard, C.; Leclerc, C.; Leroy, B.; Bakkenes, M.; Veloz, S.; Thuiller, W.; Courchamp, F. Vulnerability of biodiversity hotspots to global change. Glob. Ecol. Biogeogr. 2014, 23, 1376–1386. [Google Scholar] [CrossRef]
- Bello-Rodríguez, V.; Mateo, R.G.; Pellisier, L.; Cubas, J.; Cook, B.; González-Mancebo, J.M. Forecast increase in invasive rabbit spread into ecosystems of oceanic island (Tenerife) under climate change. Ecol. Appl. 2020, 31, e02206. [Google Scholar] [CrossRef] [PubMed]
- Baquero, R.A.; Barbosa, A.M.; Ayllón, D.; Guerra, C.; Sánchez, E.; Araujo, M.B.; Nicola, G.C. Potential distributions of invasive vertebrates in the Iberian Peninsula under projected changes in climate extreme events. Divers. Distrib. 2021, 27, 2262–2276. [Google Scholar] [CrossRef]
- Dutra, L.; Brito de Azevedo, E.; Vieira-Reis, F.; Bento, R.; Silva, L. Limitations of species distribution models based on available climate change data: A case of study in the Azorean Forest. Forests 2019, 10, 575. [Google Scholar] [CrossRef]
- Udo, N.; Darrot, C.; Atlan, A. From useful to invasive, the status of gorse in Reunion Island. J. Environ. Manag. 2019, 229, 166–173. [Google Scholar] [CrossRef]
- Poulin, J.; Weller, S.G.; Sakai, A.K. Genetic diversity does not affect the invasiveness of fountain grass (Pennisetum setaseum) in Arizona, California and Hawaii. Divers. Distrib. 2005, 11, 241–247. [Google Scholar] [CrossRef]
- Cordell, S.; Sandquist, D.R. The impact of an invasive African bunchgrass (Pennisetum setaceum) on water availability and productivity of canopy trees within a tropical dry forest in Hawaii. Funct. Ecol. 2008, 22, 1008–1017. [Google Scholar] [CrossRef]
- Massetii, A.; Menezes de Sequeira, M.; Pupo-Correia, A.; Figueiredo, A.; Guiomar, N.; Gil, A. Assessing the effectiveness of RapidEye multispectral imagery for vegetation mapping in Madeira Island (Portugal). Ital. J. Remote Sens. 2016, 49, 643–672. [Google Scholar] [CrossRef]
- Da Re, D.; Tordoni, E.; De Pascalis, F.; Negrín-Pérez, Z.; Fernández-Palacios, J.M.; Arévalo, J.R.; Rocchini, D.; Medina, F.M.; Otto, R.; Arlé, E.; et al. Invasive fountain grass (Pennisetum setaceum (Forssk.) Chiov.) increases its potential area of distribution in Tenerife Island under future climatic scenarios. Plant Ecol. 2020, 221, 867–882. [Google Scholar] [CrossRef]
- Da Re, D.; Tordoni, E.; Naranjo-Cigala, A.; Padrón-Mederos, M.A.; González, M.; González-Montelongo, C.; Arévalo, J.R. Assessing the impacts of land use and climate change on the distribution of Ulex europaeus L. (Fabaceae) in the Canary Islands. Biol. Invasions 2024, 26, 4363–4379. [Google Scholar] [CrossRef]
- Redexos. 2024. Available online: https://www3.gobiernodecanarias.org/cptss/sostenibilidad/biodiversidad/redexos/app/guide/species/pluchea-ovalis (accessed on 5 November 2024).
- Verloove, F. New xenophytes from Gran Canaria (Canary Islands, Spain), with emphasis on naturalized and (potentially) invasive species. Collect. Bot. 2013, 32, 59–82. [Google Scholar] [CrossRef]
- Available online: https://www.worldfloraonline.org/taxon/wfo-0000136883 (accessed on 24 February 2025).
- Plants of the World Online. 2024. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:238870-1#distributions (accessed on 31 October 2024).
- Available online: https://identify.plantnet.org/es/k-world-flora/species/Pluchea%20ovalis%20(Pers.)%20DC./data (accessed on 24 February 2025).
- Verloove, F.; Reyes-Betancort, J.A. Additions to the flora of Tenerife (Canary Islands, Spain). Collect. Bot. 2011, 30, 63–78. [Google Scholar] [CrossRef]
- Clements, D.R.; Ditommaso, A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res. 2011, 51, 227–240. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Otto, R.; Capelo, J.; Caujapé-Castells, J.; de Nascimento, L.; Duarte, M.C.; Elias, R.B.; García-Verdugo, C.; Menezes de Sequeira, M.; Médail, F.; et al. In defence of the entity of Macaronesia as a biogeographical region. Biol. Rev. 2024, 99, 2060–2081. [Google Scholar] [CrossRef]
- Troll, V.R.; Carracedo, J.C. The Geology of the Canary Islands; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Del Arco, M.J.; Rodríguez-Delgado, O. The Vegetation of the Canary Islands; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Gobierno de Canarias. Banco de Datos de Biodiversidad de Canarias. 2024. Available online: https://www.biodiversidadcanarias.es/biota/ (accessed on 23 September 2024).
- Acevedo, P.; Real, R. Favourability: Concept, distinctive characteristics and potential usefulness. Naturwissenschaften 2012, 99, 515–522. [Google Scholar] [CrossRef]
- Da Re, D.; Tordoni, E.; Lenoir, J.; Lembrechts, J.J.; Vanwambeke, S.O.; Rocchini, D.; Bazzichetto, M. USE it: Uniformly sampling pseudo-absences within the environmental space for applications in habitat suitability models. Methods Ecol. Evol. 2023, 14, 2873–2887. [Google Scholar] [CrossRef]
- Patiño, J.; Collart, F.; Vanderpoorten, A.; Martin-Esquivel, J.L.; Naranjo-Cigala, A.; Mirolo, S.; Karger, D.N. Spatial resolution impacts projected plant responses to climate change on topographically complex islands. Divers. Distrib. 2023, 29, 1245–1262. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- CORINE Land Cover (Vector/Raster 100 m), Europe, 6-Yearly. 2018. Available online: https://sdi.eea.europa.eu/catalogue/copernicus/api/records/71c95a07-e296-44fc-b22b-415f42acfdf0?language=all (accessed on 26 October 2024).
- Hanz, D.; Cutts, V.; Barajas-Barbosa, M.P.; Algar, A.; Beierkuhnlein, C.; Collart, F.; Fernández-Palacios, J.M.; Field, R.; Karger, D.N.; Kienle, D.R.; et al. Effects of climate change on the distribution of plant species and plant functional strategies on the Canary Islands. Divers. Distrib. 2023, 29, 1157–1171. [Google Scholar] [CrossRef]
- Brun, P.; Thuiller, W.; Chauvier, Y.; Pellissier, L.; Wüest, R.O.; Wang, Z.; Zimmermann, N.E. Model Complexity Affects Species Distribution Projections under Climate Change. J. Biogeogr. 2020, 47, 130–142. [Google Scholar] [CrossRef]
- Zarzo-Arias, A.; Penteriani, V.; Gabor, L.; Simova, P.; Grattarola, F.; Moudry, V. Importance of data selection and filtering in species distribution models: A case study on the Cantabrian brown bear. Ecosphere 2022, 13, e4284. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Barbosa, A.M. fuzzySim: Applying fuzzy logic to binary similarity indices in ecology. Methods Ecol. Evol. 2015, 6, 853–858. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. 2002. Available online: https://CRAN.R-project.org/doc/Rnews/ (accessed on 1 November 2024).
- Carlson, C. embarcadero: Species Distribution Models with BART. R Package Version 1.2.0.1003, Commit 0c680394089ea93da1d2028caddb757a4ffd30c4. 2024. Available online: https://github.com/cjcarlson/embarcadero (accessed on 1 November 2024).
- Barbosa, A.M.; Real, R.; Munoz, A.R.; Brown, J.A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2021, 35, 213–226. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitatsuitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Newson, R. Confidence intervals for rank statistics: Somers’ D and extensions. Stata J. 2006, 6, 309–334. [Google Scholar] [CrossRef]
- Miller, M.E.; Hui, S.L.; Tierney, W.M. Validation techniques for logistic regression models. Stat. Med. 1991, 10, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models with Applications in R; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling. R Package Version 1.3-14. 2023. Available online: https://CRAN.R-project.org/package=dismo (accessed on 2 November 2024).
- Hernangómez, D. Using the tidyverse with terra objects: The tidyterra package. J. Open-Source Softw. 2023, 8, 5751. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open-Source Geospatial Foundation Project. 2024. Available online: http://qgis.osgeo.org (accessed on 26 October 2024).
- Lonsdale, W.M. Global patterns of plant invasions and the concept of invasibility. Ecology 1999, 80, 1522–1536. [Google Scholar] [CrossRef]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pysek, P.; Hobbs, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Chytry, M.; Jarosik, V.; Pysek, P.; Hajek, O.; Knollova, I.; Tichy, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef]
- Otto, R.; Arteaga, M.A.; Delgado, J.D.; Arévalo, J.R.; Blandino, C.; Fernández-Palacios, J.M. Road Edge effect and elevation pattern of native and alien species on an oceanic island (Tenerife, Canary Islands). Folia Geobot. 2014, 49, 65–82. [Google Scholar] [CrossRef]
- González-Montelongo, C.; Naranjo-Cigala, A.; Arévalo, J.R. Introduced species as a disturbance symptom. Rev. Cienc. Agrar. 2024, 47, 430–434. [Google Scholar]
- Del Arco, M.J.; González-González, R.; Garzón-Machado, V.; Pizarro-Hernández, B. Actual and potential natural vegetation on the Canary Islands and its conservation status. Biodivers. Conserv. 2010, 19, 3089–3140. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Schrader, J.; de Nascimento, L.; Irl, S.D.H.; Sánchez-Pinto, L.; Otto, R. Are plant communities on the Canary Islands resistant to plant invasion? Divers. Distrib. 2022, 29, 51–60. [Google Scholar] [CrossRef]
- Fenouillas, P.; Caubit, M.; Cazal, M.; Soleyen, C.A.; Strasberg, D.; Rouget, M. Standardising field-based assessment of invasion degree: A case study in two habitats of Reunion Island. Ecol. Solut. Evid. 2024, 5, e12314. [Google Scholar] [CrossRef]
- Piquet, J.C.; Warren, D.L.; Saavedra-Bolaños, J.F.; Sánchez-Rivero, J.M.; Gallo-Barneto, R.; Cabrera-Pérez, M.A.; Fisher, R.N.; Fisher, S.R.; Rochester, C.J.; Hinds, B.; et al. Could climate change benefit invasive snakes? Modelling the potential distribution of the California Kingsnake in the Canary Islands. J. Environ. Manag. 2021, 294, 112917. [Google Scholar] [CrossRef]
- Soley-Guardia, M.; Alvarado-Serrano, D.F.; Anderson, R.P. Top ten hazards to avoid when modeling species distributions: A didactic guide of assumptions, problems, and recommendations. Ecography 2024, 2024, e06852. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef]
- Diogo, I.; Sillero, N.; Capinha, C. Predicting the risk of invasion by broadleaf watermilfoil (Myriophyllum heterophyllum) in mainland Portugal. Hellyon 2024, 10, e34201. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Delgado, J.D.; Otto, R.; Naranjo-Cigala, A.; Salas-Pascual, M.; Fernández-Palacios, J.M. Distribution of alien vs native plant species in roadside communities along an altitudinal gradient in Tenerife and Gran Canaria (Canary Islands). Perspect. Plant Ecol. Evol. Syst. 2005, 7, 185–202. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Otto, R.; Escudero, C.; Fernández-Lugo, S.; Arteaga, M.; Delgado, J.D.; Fernández-Palacios, J.M. Do anthropogenic corridors homogenize plant communities at a local scale? A case studied in Tenerife (Canary Islands). Plant Ecol. 2010, 209, 23–35. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Afonso, L.; Naranjo-Cigala, A.; Salas-Pascual, M. Invasion of the Gran Canaria ravines ecosystems (Canary Islands) by the exotic species Acacia farnesiana. J. Plant Ecol. 2010, 206, 185–193. [Google Scholar] [CrossRef]
- Manzoor, S.A.; Griffiths, G.; Lukac, M. Land use and climate change interaction triggers contrasting trajectories of biological invasion. Ecol. Indic. 2021, 120, 106936. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Schweiger, A.H.; Steinbauer, M.J.; Ah-Peng, C.; Arévalo, J.R.; Beierkuhnlein, C.; Chiarucci, A.; Daehler, C.C.; Fernández-Palacios, J.M.; Flores, O.; et al. Human impact, climate and dispersal strategies determine plant invasion on islands. J. Biogeogr. 2021, 48, 1889–1903. [Google Scholar] [CrossRef]
- Richardson, D.M.; Thuiller, W. Home away from home—Objective mapping of high-risk source areas for plant introductions. Divers. Distrib. 2007, 13, 299–312. [Google Scholar] [CrossRef]
- Cardador, L.; Tella, J.L.; Louvrier, J.; Anadón, J.D.; Abellán, P.; Carrete, M. Climate matching and anthropogenic factors contribute to the colonization and extinction of local populations during avian invasions. Divers. Distrib. 2022, 28, 1908–1921. [Google Scholar] [CrossRef]
- King-Jones, S. Revision of Pluchea Cass. (Compositae, Plucheeae) in the Old World; Englera. 1; Freie Universität Berlin Botanic Garden and Botanical Museum; Dahlem: Berlin, Germany, 2001. [Google Scholar]
- Morente-López, J.; Arjona, Y.; Salas-Pascual, M.; Reyes-Betancort, J.A.; del Arco, M.J.; Emerson, B.C.; García-Gallo, A.; Jay-García, L.S.; Naranjo-Cigala, A.; Patiño, J. Biogeographic origins and drivers of alien plant invasions in the Canary Islands. J. Biogeogr. 2023, 50, 576–590. [Google Scholar] [CrossRef]
- Broennimann, O.; Treier, U.A.; Muller-Schaller, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climate niche shift during biological invasions. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef]
- Broennimann, O.; Guisan, A. Predicting current and future biological invasions: Both native and invaded ranges matter. Biol. Lett. 2008, 4, 585–589. [Google Scholar] [CrossRef]
- Sillero, N.; Ribeiro-Silva, J.; Arenas-Castro, S. Shifts in climatic realised niches of Iberian species. Oikos 2022, 2022, e08505. [Google Scholar] [CrossRef]
- Briscoe-Runquist, R.D.; Lake, T.; Tiffin, P.; Moeller, D.A. Species distribution models throughout the invasion history of Palmer amaranth predict regions at risk of future invasion and reveal challenges with modeling rapidly shifting geographic ranges. Sci. Rep. 2019, 9, 2426. [Google Scholar] [CrossRef]
- Fornoso-Freire, V.; Barbosa, A.M.; Baselga, A.; Gómez-Rodríguez, C. Predicting the spatio-temporal pattern of range expansion under lack of equilibrium with climate. Biol. Conserv. 2023, 288, 110361. [Google Scholar] [CrossRef]
- Berio-Fortini, L.; Kaiser, L.R.; Daehler, C.C.; Jacobi, J.D.; Dimson, M.; Gillespie, T.W. Exploring and integrating differences in niche characteristics across regional and global scales to better understand plant invasions in Hawaiʻi. Biol. Invasions 2024, 26, 1827–1843. [Google Scholar] [CrossRef]
- Mateo, R.G.; Morales-Barbero, J.; Zarzo-Arias, A.; Lima, H.; Gómez-Rubio, V.; Goicolea, T. sabinaNSDM: An R package for spatially nested hierarchical species distribution modelling. Methods Ecol. Evol. 2024, 5, 1796–1803. [Google Scholar] [CrossRef]
- Engelstad, P.; Jarnevich, C.S.; Hogan, T.; Sofaer, H.R.; Pearse, I.S.; Sieracki, J.L.; Frakes, N.; Sullivan, J.; Young, N.E.; Prevey, J.S.; et al. INHABIT: A web-based decision support tool for invasive plant species habitat visualization and assessment across the contiguous United States. PLoS ONE 2022, 17, e0263056. [Google Scholar] [CrossRef]
- Dimson, M.; Fortini, L.B.; Tingley, M.W.; Gillespie, T.W. Citizen science can complement professional invasive plant surveys and improve estimates of suitable habitat. Divers. Distrib. 2023, 29, 1141–1156. [Google Scholar] [CrossRef]
- Santana, J.; Sillero, N.; Ribeiro, J.; Capinha, C.; Lopes, R.J.; Reino, L. Predicting the expansion of invasive species: How much data do we need? NeoBiota 2024, 95, 109–132. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Young, N.E.; Talbert, M.; Talbert, C. Forecasting an invasive species’ distribution with global distribution data, local data, and physiological information. Ecosphere 2018, 9, e02279. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Alvarado, J.J.; Pestano-González, M.; González-Montelongo, C.; Naranjo-Cigala, A.; Arévalo, J.R. Assessing the Potential Risk of Invasion of the Neophyte Pluchea ovalis (Pers.) DC. (Asteraceae) in the Canarian Archipelago Using an Ensemble of Species Distribution Modelling. Diversity 2025, 17, 195. https://doi.org/10.3390/d17030195
García-Alvarado JJ, Pestano-González M, González-Montelongo C, Naranjo-Cigala A, Arévalo JR. Assessing the Potential Risk of Invasion of the Neophyte Pluchea ovalis (Pers.) DC. (Asteraceae) in the Canarian Archipelago Using an Ensemble of Species Distribution Modelling. Diversity. 2025; 17(3):195. https://doi.org/10.3390/d17030195
Chicago/Turabian StyleGarcía-Alvarado, Juan José, Miguel Pestano-González, Cristina González-Montelongo, Agustín Naranjo-Cigala, and José Ramón Arévalo. 2025. "Assessing the Potential Risk of Invasion of the Neophyte Pluchea ovalis (Pers.) DC. (Asteraceae) in the Canarian Archipelago Using an Ensemble of Species Distribution Modelling" Diversity 17, no. 3: 195. https://doi.org/10.3390/d17030195
APA StyleGarcía-Alvarado, J. J., Pestano-González, M., González-Montelongo, C., Naranjo-Cigala, A., & Arévalo, J. R. (2025). Assessing the Potential Risk of Invasion of the Neophyte Pluchea ovalis (Pers.) DC. (Asteraceae) in the Canarian Archipelago Using an Ensemble of Species Distribution Modelling. Diversity, 17(3), 195. https://doi.org/10.3390/d17030195







