Characterization of Pollinators Associated with Cocoa Cultivation and Their Relationship with Natural Effective Pollination
Abstract
1. Introduction
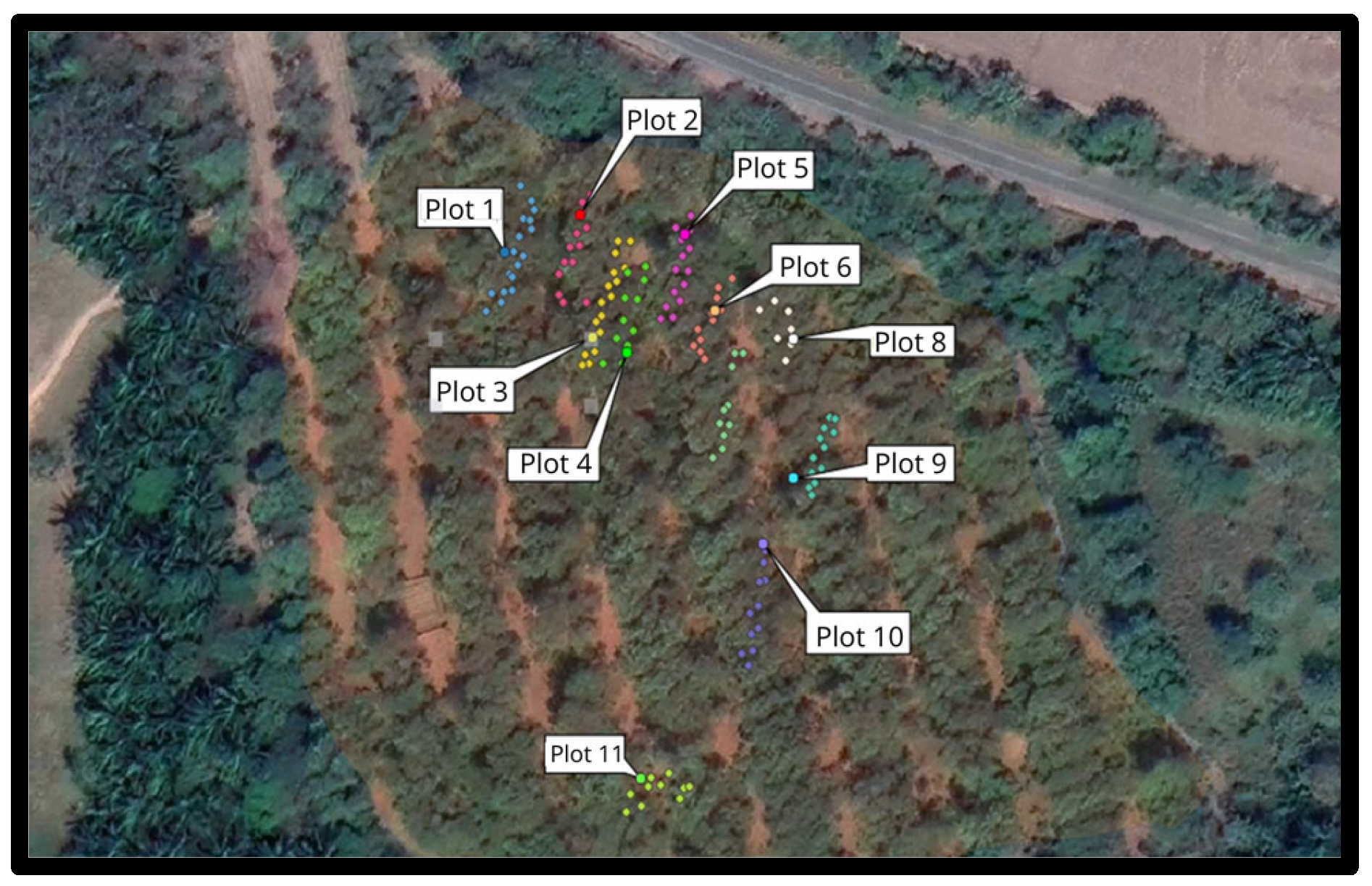
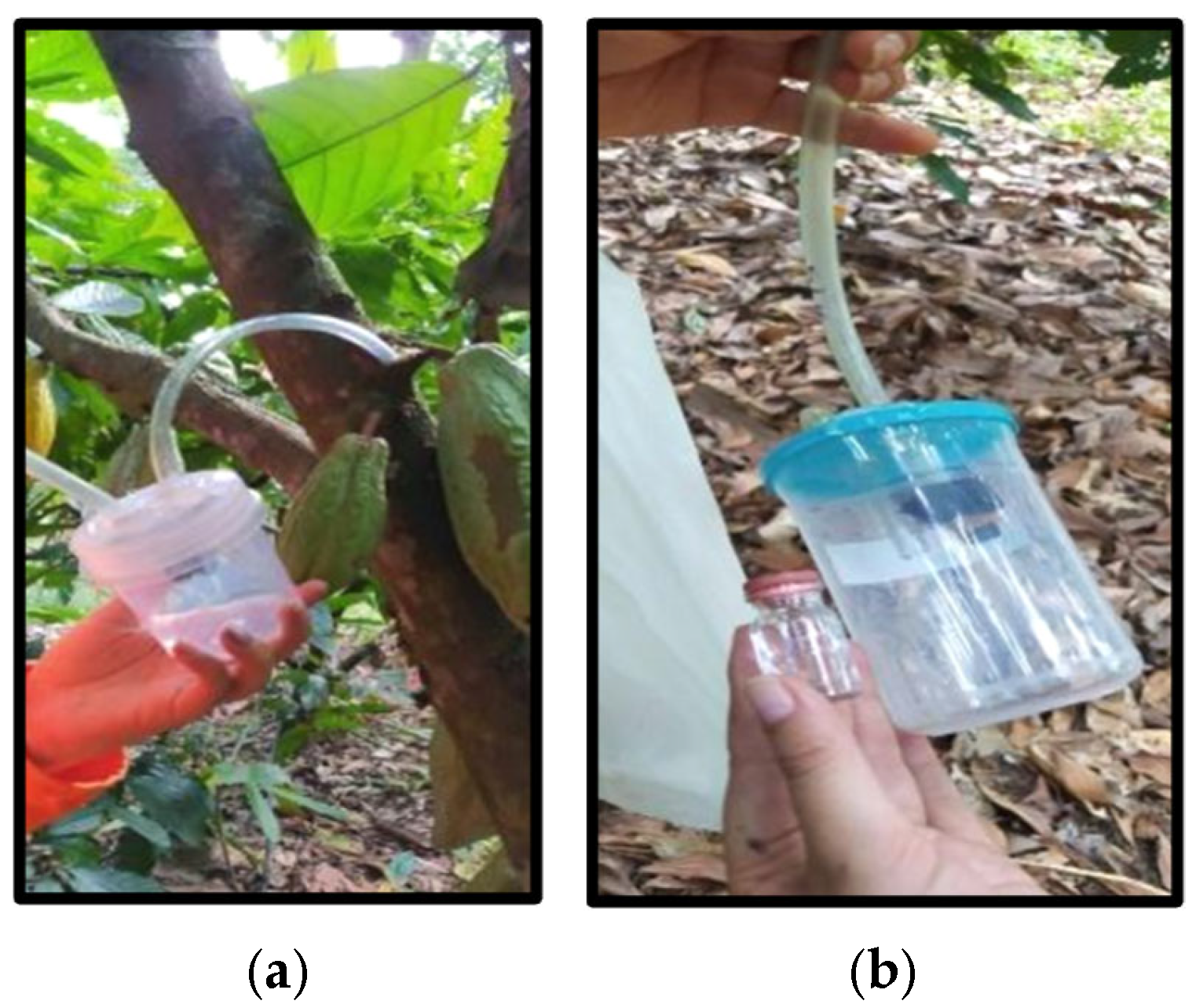
2. Materials and Methods
- Y = represents the response variable (pollinated flowers, fertilized flowers, aborted flowers, and flowers transitioning to fruit);
- X = represents pollinator abundance;
- = is the intercept;
- = is the regression coefficient;
- = is the error term.
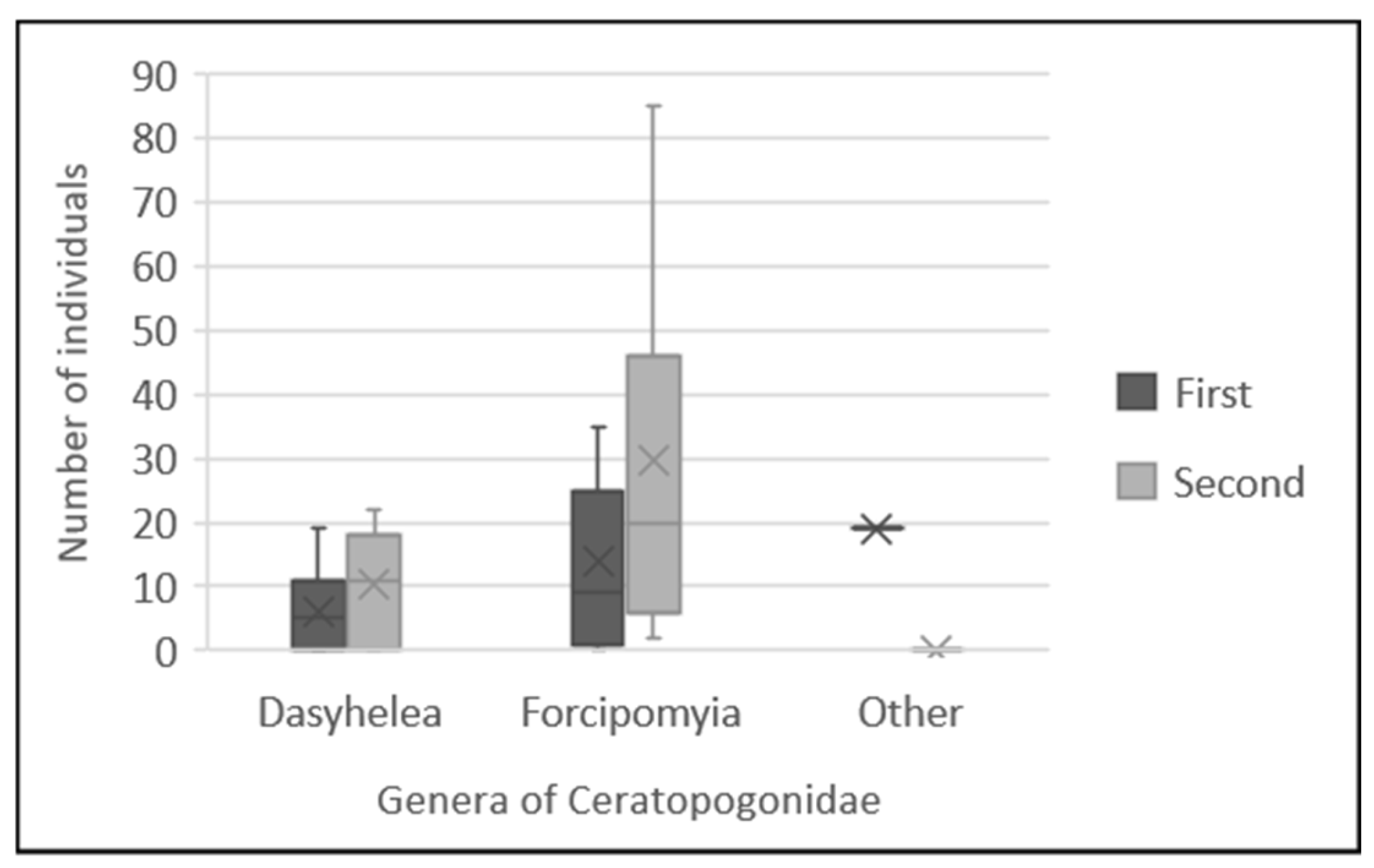
3. Results
3.1. Capture of Potential Pollinators and Floral Visitors
3.2. Presence of Pollinating Insects and Relationship with Natural Effective Pollination
4. Discussion
4.1. Assessing Abundance of Potential Pollinators and Floral Visitors
4.2. Impact of Pollinating Insect Presence on Natural Pollination Effectiveness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Cunningham, S.A.; Bos, M.; Steffan-Dewenter, I. Advances in pollination ecology from tropical plantation crops. Ecology 2008, 89, 935–946. [Google Scholar] [CrossRef] [PubMed]
- [FAO] Organización de las Naciones Unidas para la Agricultura y la Alimentación. FAOSTAT. Food Agric. Organ. Available online: http://www.fao.org/faostat/en/#data%0D (accessed on 4 August 2022).
- Jaimes-Suárez, Y.Y.; Carvajal-Rivera, A.S.; Galvis-Neira, D.A.; Carvalho Fabricio, E.L.; Rojas-Molina, J. Cacao agroforestry systems beyond the stigmas: Biotic and abiotic stress incidence impact. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Pabón, M. Caracterización socio-económica y productiva del cultivo de cacao en el departamento de Santander (Colombia). Rev. Mex. Agronegocios 2016, 38, 283–294. [Google Scholar]
- Groeneveld, J.H.; Tscharntke, T.; Moser, G.; Clough, Y. Experimental evidence for stronger cacao yield limitation by pollination than by plant resources. Perspect. Plant Ecol. 2010, 12, 183–191. [Google Scholar] [CrossRef]
- González, A. Identificación de Insectos Polinizadores del Cultivo de Cacao (Theobroma cacao L.), en la Finca Concepción, Municipio de Berlín, Departamento de Usulután. Licentiate Thesis, Biología, Universidad de El Salvador, San Salvador, El Salvador, 2018. [Google Scholar]
- Córdoba-Correoso, C.T. Efecto de la Estructura de Sistemas Agroforestales de Cacao y de su Contexto Local, Sobre las Poblaciones de Dípteros Polinizadores del Cacao y Su Relación con la Producción en Bocas del Toro, Panamá. Master’s Thesis, Scientiae en Agricultura Ecológica, Centro Agronómico Tropical de Investigación y Enseñanza, Turrialba, Costa Rica, 2011. [Google Scholar]
- Falque, M.; Lesdalons, C.; Eskes, A.B. Comparison of two cacao (Theobroma cacao L.) clones for the effect of pollination intensity on fruit set and seed content. Sex. Plant Reprod. 1996, 9, 221–227. [Google Scholar] [CrossRef]
- Bos, M.M.; Veddeler, D.; Bogdanski, A.K.; Klein, A.-M.; Tscharntke, T.; Dewenter, S.I.; Tylianakis, J.M. Caveats to quantifying ecosystem services: Fruit abortion blurs benefits from crop pollination. Ecol. Appl. 2007, 17, 1841–1849. [Google Scholar] [CrossRef]
- De Almeida, A.A.F.; Valle, R.R. Ecophysiology of the cacao tree. Braz. J. Plant Physiol. 2007, 19, 425–448. [Google Scholar] [CrossRef]
- Montero-Cedeño, S.L.; Sánchez, P.; Solórzano-Faubla, R.; Pinargote-Borrero, A.; Cañarte-Bermúdez, E.G. Flowering and diversity of pollinator insects in a cacao monoculture system. Rev. Espamciencia 2019, 10, 1–10. [Google Scholar]
- Vanhove, W.; Yao, R.K.; N’Zi, J.C.; N’Guessan Toussaint, L.A.; Kaminski, A.; Smagghe, G.; Van Damme, P. Impact of insecticide and pollinator-enhancing substrate applications on cocoa (Theobroma cacao) cherelle and pod production in Côte d’Ivoire. Agric. Ecosyst. Environ. 2020, 293. [Google Scholar] [CrossRef]
- García-García, V.; Guzmán-Cedeño, Á. Incidencia de agentes polinizadores sobre la fecundación de la flor del cacao (Theobroma cacao L.). Rev. Espamciencia 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Decazy, B.; Massoux, F.; Tschiendji, C.; Misse, C. Ulilisation des Radio-Isotopes (32P) pour L’e’tude des Agents Pollinisateurs du Cacaoyer dans les Conditions Ecologiques du Sud-Cameroun. In Proceedings of the 6th International Cocoa Research Conference, Caracas, Venezuela; 1981; pp. 419–430. Available online: https://www.sidalc.net/search/Record/KOHA-OAI-BVE:67532 (accessed on 24 February 2025).
- Armijos-Vásquez, V.E.; García-Cruzatty, L.C.; Castro-Olaya, J.; Martínez, M. Insectos polinizadores en sistemas de producción de Theobroma cacao L. en la zona central del litoral ecuatoriano. Cienc. Tecnol. 2020, 13, 23–30. [Google Scholar] [CrossRef]
- Kaufmann, T. Ecology and behavior of cocoa pollinating Ceratoponidae in Ghana, W. Africa. Enviromental Entomol. 1975, 4, 347–351. [Google Scholar] [CrossRef]
- Bravo, J.C.; Somarriba, E.; Arteaga, G. Factores que afectan la abundancia de insectos polinizadores del cacao en sistemas agroforestales. Rev. Cienc. Agrícolas 2011, 28, 119–131. [Google Scholar]
- Córdoba, C.; Cerda, R.H.; Deheuvels, O.; Hidalgo, E.; Declerck, F. Polinizadores, Polinización y Producción Potencial de Cacao en Sistemas Agroforestales de Bocas del Toro, Panamá. Agroforestería Américas 2013, 49, 26–32. Available online: https://repositorio.catie.ac.cr/handle/11554/6677 (accessed on 27 February 2025).
- Toledo-Hernández, M.; Tscharntke, T.; Tjoa, A.; Anshary, A.; Cyio, B.; Wanger, T.C. Landscape and farm-level management for conservation of potential pollinators in Indonesian cocoa agroforests. Biol. Conserv. 2021, 257. [Google Scholar] [CrossRef]
- Forbes, S.J.; Northfield, T. Increased pollinator habitat enhances cacao fruit set and predator conservation. Ecol. Appl. 2017, 27, 887–899. [Google Scholar] [CrossRef]
- Cañarte Bermúdez, E.G.; Montero Cedeño, S.L.; Navarrete Cedeño, J.B. Reconocimiento, Importancia y Cuidado de los Polinizadores en los Sistemas de Producción del Cacao (Guía No. 177). Instituto Nacional de Investigaciones Agropecuarias. 2021. Available online: https://repositorio.iniap.gob.ec/handle/41000/5749 (accessed on 27 February 2025).
- Adjaloo, M.; Branoh Banful, B.K.; Oduro, W. Evaluation of breeding substrates for cocoa pollinator, Forcipomyia spp. and subsequent implications for yield in a tropical cocoa production system. Am. J. Plant Sci. 2013, 4, 203–210. [Google Scholar] [CrossRef]
- Arnold, S.E.J.; Bridgemohan, P.; Perry, G.B.; Spinelli, G.R.; Pierre, B.; Murray, F.; Haughton, C.; Dockery, O.; Grey, L.; Murphy, S.T.; et al. The significance of climate in the pollinator dynamics of a tropical agroforestry system. Agric. Ecosyst. Environ. 2018, 254, 1–9. [Google Scholar] [CrossRef]
- Briceño, G.A. Caracterización ecológica general de unidades de paisaje de la finca San José de Matadepantano (Yopal, Casanare). Épsilon 2014, 22, 1–10. [Google Scholar]
- Frimpong, E.A.; Gemmill-Herren, B.; Gordon, I.; Kwapong, P.K. Dynamics of insect pollinators as influenced by cocoa production systems in Ghana. J. Pollinat. Ecol. 2011, 5, 74–80. [Google Scholar] [CrossRef]
- Alexander, M.P.A. Versatile stain for pollen fungi, yeast and bacterium. Stain Technol. 1980, 5, 13–18. [Google Scholar] [CrossRef]
- Fiallos, G. La Correlación de Pearson y el proceso de regresión por el Método de Mínimos Cuadrados. Cienc. Lat. Rev. Científica Multidiscip. 2021, 5, 2491. [Google Scholar] [CrossRef]
- Vansynghel, J.; Ocampo-Ariza, C.; Maas, B.; Martin, E.A.; Thomas, E.; Hanf-Dressler, T.; Schumacher, N.; Ulloque-Samatelo, C.; Tscharntke, T.; Steffan-Dewenter, I. Cacao flower visitation: Low pollen deposition, low fruit set and dominance of herbivores. Ecol. Solut. Evid. 2022, 3, e12140. [Google Scholar] [CrossRef]
- Chumacero de Schawe, C.; Kessler, M.; Hensen, I.; Tscharntke, T. Abundance and diversity of flower visitors on wild and cultivated cacao (Theobroma cacao L.) in Bolivia. Agrofor. Syst. 2016, 92, 117–125. [Google Scholar] [CrossRef]
- Vandrommea, M.; Van de Sandea, E.; Pinceelb, T.; Vanhoved, W.; Trekelsa, H.; Vanschoenwinkel, B. Resolving the identity and breeding habitats of cryptic dipteran cacao flower visitors in a neotropical cacao agroforestry system. Basic Appl. Ecol. 2023, 68, 1439–1791. [Google Scholar] [CrossRef]
- Zakariyya, F.; Sulistyowati, E.; Suci Rahayu, D. Abundance of pollinator insect (Forcipomyia spp.) of cocoa under some shade trees. Pelita Perkeb. 2016, 32, 91–100. [Google Scholar] [CrossRef]
- Salazar-Díaz, R.; Torres-Coto, V. Study of the dynamics of cocoa (Theobroma cacao) pollinators in three production systems. Tecnol. Marcha 2017, 30, 90–100. [Google Scholar] [CrossRef][Green Version]
- Ramos-Serrano, R.M. Estudio de la Diversidad de Insectos Polinizadores en Sistemas Agroforestales de Cacao y su Relación Con la Productividad y Diversidad de Especies del Dosel. Bachelor’s Thesis, Universidad San Pedro Sula, Sula, Honduras, 2011. [Google Scholar]
- Young, A. Seasonal differences in abundance and distribution of cocoa-pollinating midges in relation to flowering and fruit set between shaded and sunny habitats of the La Lola cocoa farm in Costa Rica. J. Appl. Ecol. 2008, 20, 801–831. [Google Scholar] [CrossRef]
- Mendoza-Zambrano, G.F.; Romero-Cedeño, E.F. Actividad de los Polinizadores en la Fecundación de la Flor de Cacao (Theobroma cacao) Bajo Tres Sistemas de Producción en Portoviejo- Manabí. Trabajo de Grado. Escuela Superior Politécnica Agropecuaria de Manabí Manuel Félix López, Manabí, Ecuador. 2021. Available online: https://repositorio.iniap.gob.ec/handle/41000/6241 (accessed on 27 February 2025).
- Montero-Cedeño, S.; Cañarte-Bermudez, E.; Navarrete-Cedeño, J.; Pinargote-Borrero, A.; Sánchez-Hernández, P. Ceratopogonidae: Their role in pollination and fertilization at various technological levels of Theobroma cacao L. production. Rev. Fac. Agron. Univ. Zulia 2022, 39, e223943. [Google Scholar] [CrossRef]
- Aneja, M.; Gianfagna, T.; Ng, E. The roles of abscisic acid and ethylene in the abscission and senescence of cocoa flowers. Plant Growth Regul. 1999, 27, 149–155. [Google Scholar] [CrossRef]
- Somarriba-Chávez, E.; Cerda-Bustillos, R.; Astorga-Domian, C.; Quesada-Chaverri, F.; Vásquez Morera, N. Reproducción Sexual del Cacao; Serie Técnica; Materiales de Extensión; CATIE: Turrialba, Costa Rica, 2010. [Google Scholar]
- Mena-Montoya, M.; García-Cruzatty, L.C.; Cuenca-Cuenca, E.; Vera Pinargote, L.D.; Villamar-Torres, R.; Mehdi Jazayeri, S. Pollen flow of Theobroma cacao and its relationship with climatic factors in the central zone of the Ecuadorian Littoral. Bioagro 2020, 32, 39–48. [Google Scholar]
- Lopes, U.V.; Nascimento, I.; Argolo-Magalhães, D.M.; dos Santos, R.P.; Figueiredo dos Santos, R. Number of pollen grains, ovules and pollen-ovule ratio in cacao clones. Agrotrópica 2022, 34, 95–106. [Google Scholar] [CrossRef]
- Bos, M.M.; Steffan-Dewenter, I.; Tscharntke, T. Shade tree management affects fruit abortion, insect pests and pathogens of cacao. Agric. Ecosyst. Environ. 2007, 120, 201–205. [Google Scholar] [CrossRef]
- Paulin, D.; Mossu, G.; Lachenaud, P.; Cilas, C. La sélection du cacaoyer en Cote d’Ivoire. Analyse du comportement de soixante- deux hybrides dans quatre localités. Café Cacao 1993, 37, 3–20. [Google Scholar]
- Cilas, C.; Machado, R.; Motamayor, J.C. Relations between sever- al traits linked to sexual plant reproduction in Theobroma cacao L.: Number of ovules per ovary, number of seeds per pod, and seed weight. Tree Genet. Genomes 2010, 6, 219–226. [Google Scholar] [CrossRef]
- Ambele, C.F.; Bisseleua, H.D.B.; Djuideu, C.T.L.; Akutsk, K.S. Managing insect services and disservices in cocoa agroforestry systems. Agrofor. Syst. 2023, 97, 965–984. [Google Scholar] [CrossRef]




| Sampling Period | Maximum Temperature (°C) | Minimum Temperature (°C) | Accumulated Precipitation (mm) | Average Relative Humidity (%) |
|---|---|---|---|---|
| First: 15–29 October 2021 | 31.37 | 22.90 | 37.90 | 83.92 |
| Second: 2–16 November 2021 | 30.00 | 22.54 | 43.80 | 85.42 |
| Order | N° of Individuals Captured | Percentage (%) |
|---|---|---|
| Diptera | 153 | 88.44 |
| Hemiptera | 1 | 0.58 |
| Hymenoptera | 7 | 4.05 |
| Psocoptera | 10 | 5.78 |
| Araneae | 2 | 1.16 |
| Total | 173 | 100 |
| Type of Sampling | Abundance of Insects | n | Mean | S.D. | CV | Minimum | Maximum | Median |
|---|---|---|---|---|---|---|---|---|
| Indirect—Wood trap | Diptera | 4 | 176.5 | 132.4 | 75.0 | 63.0 | 326.0 | 158.5 |
| Diptera: Ceratopogonidae | 4 | 31.0 | 24.9 | 80.2 | 5.0 | 59.0 | 30.0 | |
| Ceratopogonidae: Forcipomyia sp. | 4 | 27.8 | 24.2 | 87.2 | 3.0 | 59.0 | 24.5 | |
| Ceratopogonidae: Dasyhelea sp. | 4 | 3.3 | 5.3 | 161.6 | 0.0 | 11.0 | 1.0 | |
| Direct—Flower Suction | Diptera | 4 | 27.8 | 23.0 | 82.9 | 7.0 | 57.0 | 23.5 |
| Diptera: Ceratopogonidae | 4 | 8.0 | 3.7 | 45.6 | 4.0 | 12.0 | 8.0 | |
| Ceratopogonidae: Forcipomyia sp. | 4 | 5.0 | 4.2 | 83.3 | 0.0 | 10.0 | 5.0 |
| N° Plot | Number of Flowers Evaluated | Percentage of Flowers (%) | |||
|---|---|---|---|---|---|
| Pollinated | Fertilized | Aborted | Flower to Fruit | ||
| 1 | 122 | 27.32 | 4.10 | 68.03 | 0.82 |
| 2 | 61 | 45.90 | 9.84 | 40.98 | 3.28 |
| 3 | 79 | 35.44 | 5.06 | 59.49 | 0.00 |
| 4 | 99 | 34.34 | 13.13 | 50.51 | 2.02 |
| Dependent Variable | Independent Variable | Relationship | p Linear Regression | R2 of Linear Regression | Pearson Correlation Coefficient |
|---|---|---|---|---|---|
| Pollinated | Diptera: Ceratopogonidae | + | 0.45 | 0.31 | 0.55 |
| Ceratopogonidae: Forcipomyia sp. | + | 0.60 | 0.16 | 0.40 | |
| Fertilized | Diptera: Ceratopogonidae | + | 0.01 | 0.99 | 0.99 |
| Ceratopogonidae: Forcipomyia sp. | + | 0.02 | 0.96 | 0.98 | |
| Aborted | Diptera: Ceratopogonidae | - | 0.18 | 0.68 | −0.82 |
| Ceratopogonidae: Forcipomyia sp. | - | 0.30 | 0.49 | −0.70 | |
| Flower to Fruit | Diptera: Ceratopogonidae | + | 0.27 | 0.53 | 0.73 |
| Ceratopogonidae: Forcipomyia sp. | + | 0.42 | 0.33 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Moyano, D.K.; Rodríguez-Cruz, F.A.; Hormaza-Martínez, P.A.; Ramírez-Godoy, A. Characterization of Pollinators Associated with Cocoa Cultivation and Their Relationship with Natural Effective Pollination. Diversity 2025, 17, 189. https://doi.org/10.3390/d17030189
Ríos-Moyano DK, Rodríguez-Cruz FA, Hormaza-Martínez PA, Ramírez-Godoy A. Characterization of Pollinators Associated with Cocoa Cultivation and Their Relationship with Natural Effective Pollination. Diversity. 2025; 17(3):189. https://doi.org/10.3390/d17030189
Chicago/Turabian StyleRíos-Moyano, Diana Katherinne, Fredy Alexander Rodríguez-Cruz, Paola Andrea Hormaza-Martínez, and Augusto Ramírez-Godoy. 2025. "Characterization of Pollinators Associated with Cocoa Cultivation and Their Relationship with Natural Effective Pollination" Diversity 17, no. 3: 189. https://doi.org/10.3390/d17030189
APA StyleRíos-Moyano, D. K., Rodríguez-Cruz, F. A., Hormaza-Martínez, P. A., & Ramírez-Godoy, A. (2025). Characterization of Pollinators Associated with Cocoa Cultivation and Their Relationship with Natural Effective Pollination. Diversity, 17(3), 189. https://doi.org/10.3390/d17030189






