The Egg Packing Pressure Index of Calanoid Copepod as a Novel Eco-Indicator in Diverse Geographical Ecosystems
Abstract
Highlights
- It is the first confirmation of a true egg sac membrane through a multigenerational assay.
- Packing pressure index depends on geographical locations rather than genetic similarities.
- Packing constraints increase with increasing temperature and salinity.
- An index is introduced to gauge the reproductive effort and maximal reproductive capacity of copepods.
- Packing constraints provide an as yet unrecognized indicator for ecosystem health.
Abstract
1. Introduction
2. Material and Methods
2.1. Eurytemora affinis: Isolation and Culture
2.2. Volume Estimation
2.3. Packing Constraint Estimation
2.4. Data Preparation and Statistical Analyses
3. Results
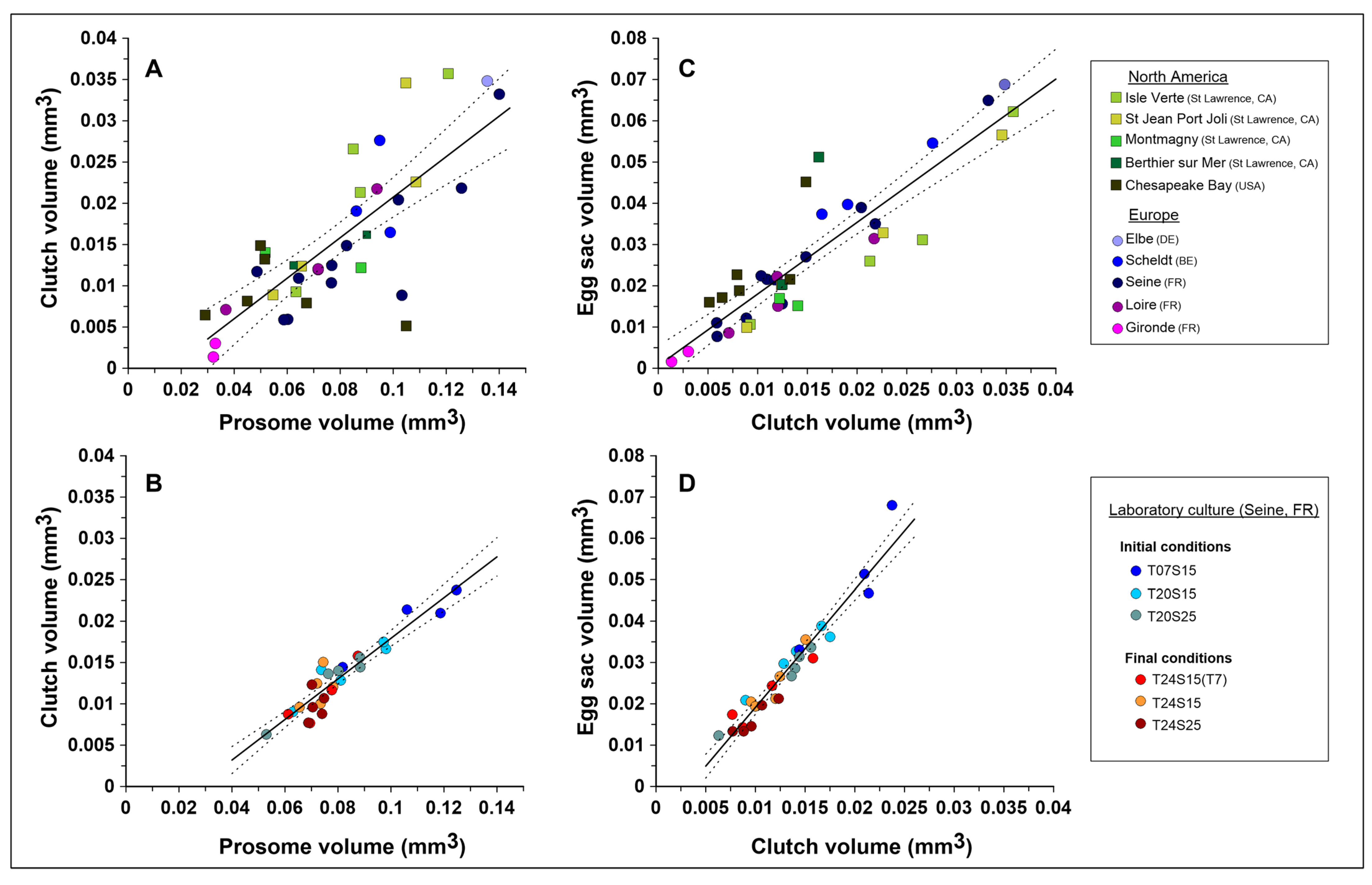
3.1. Relationship Between the Reproductive Effort and Morphological Reproductive Traits
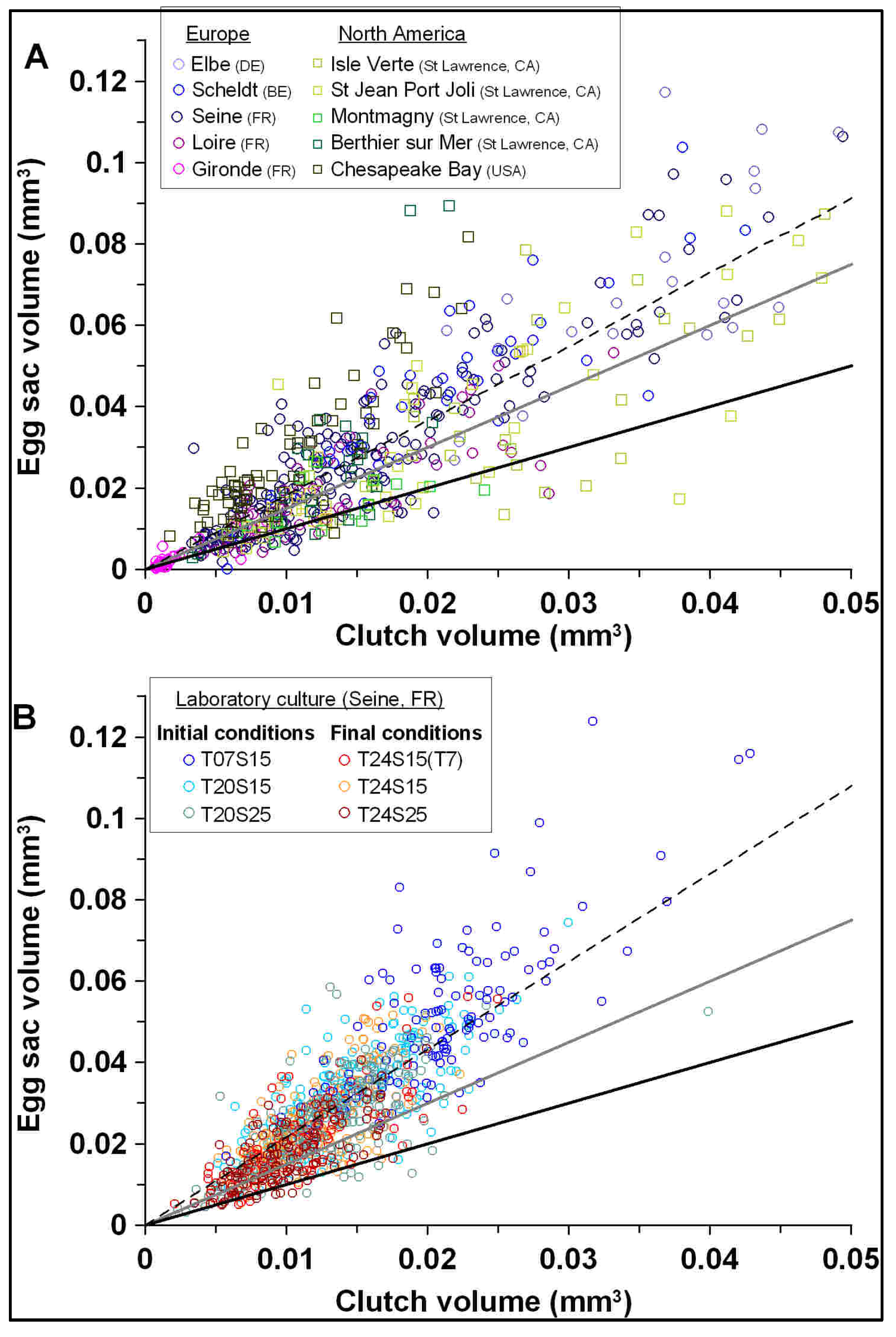
3.2. Egg Packing Constraint in Field and Laboratory Conditions
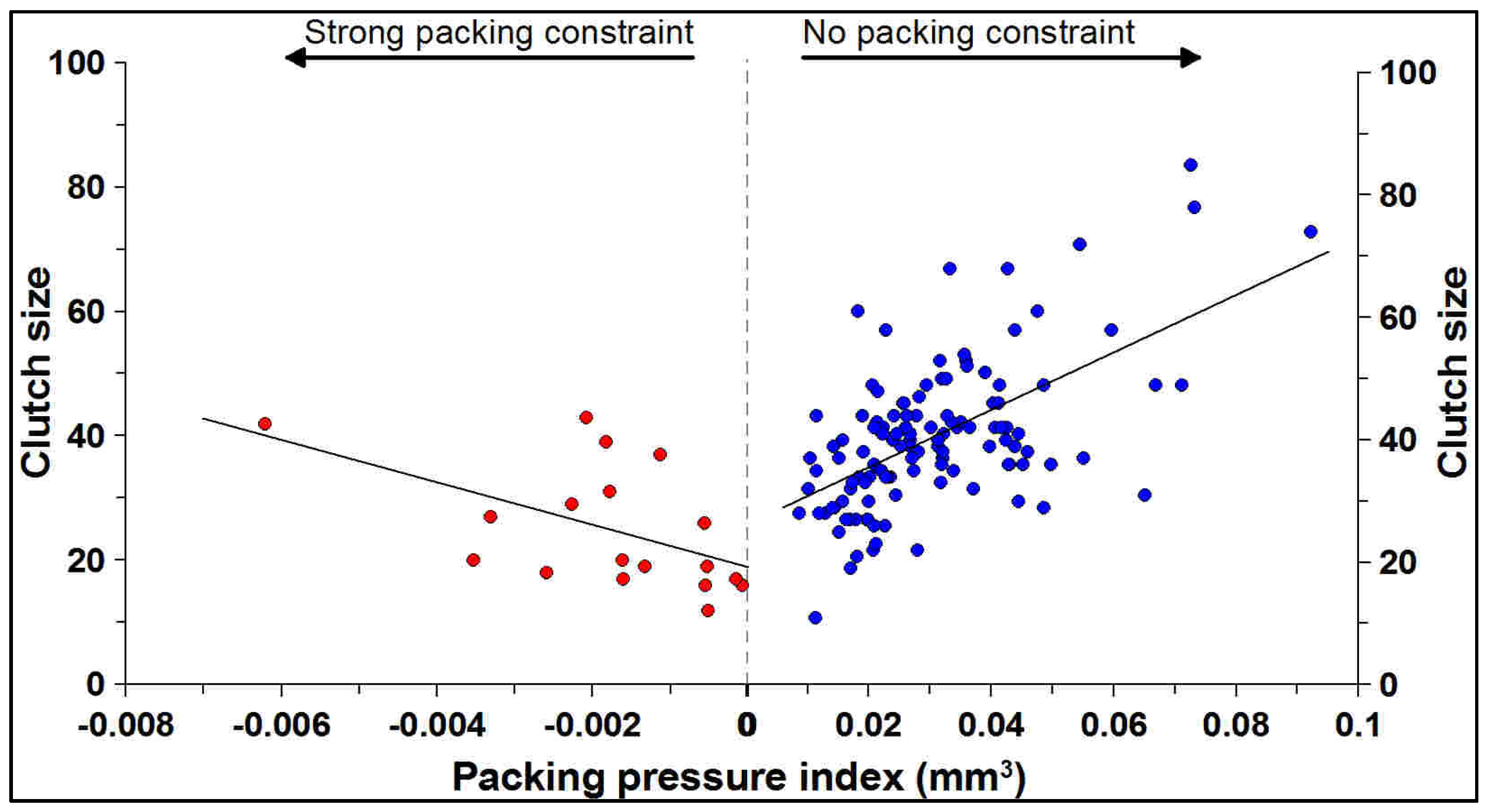
3.3. Relationship Between Packing Pressure Index and Clutch Size
4. Discussion
4.1. Presence of True Egg Sac with External Membrane in Eurytemora affinis
4.2. Packing Constraint in E. affinis Populations
4.3. Effect of the Packing Constraint on the Relationship Between ESv and Key Morphological and Reproductive Traits
4.4. Importance of an Egg Sac in Egg-Bearing Copepods: Generalities with Egg Packing Constraint
4.5. Ecological Significance of Packing Constraint
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosobokova, K.N.; Hirche, H.-J.; Hopcroft, R.R. Reproductive biology of deep-water calanoid copepods from the Arctic Ocean. Mar. Biol. 2007, 151, 919–934. [Google Scholar] [CrossRef]
- Bradford-Grieve, J.M. Colonization of the pelagic realm by calanoid copepods. Hydrobiologia 2002, 485, 223–244. [Google Scholar] [CrossRef]
- Logerwell, E.A.; Ohman, M.D. Egg-brooding, body size and predation risk in planktonic marine copepods. Oecologia 1999, 121, 426–431. [Google Scholar] [CrossRef]
- Belmonte, G. The Suspected Contradictory Role of Parental Care in the Adaption of Planktonic Calanoida to Temporary Freshwater. Water 2021, 13, 100. [Google Scholar] [CrossRef]
- Webb, D.G.; Weaver, A.J. Predation and the Evolution of Free Spawning in Marine Calanoid Copepods. Oikos 1988, 51, 189–192. [Google Scholar] [CrossRef]
- Barth-Jensen, C.; Koski, M.; Varpe, Ø.; Glad, P.; Wangensteen, O.S.; Præbel, K.; Svensen, C. Temperature-dependent egg production and egg hatching rates of small egg-carrying and broadcast-spawning copepods Oithona similis, Microsetella norvegica and Microcalanus pusillus. J. Plankton Res. 2020, 42, 564–580. [Google Scholar] [CrossRef]
- Bunker, A.J.; Hirst, A.G. Fecundity of marine planktonic copepods: Global rates and patterns in relation to chlorophyll a, temperature and body weight. Mar. Ecol. Prog. Ser. 2004, 279, 161–181. [Google Scholar] [CrossRef]
- Hopp, U.; Maier, G.; Bleher, R. Reproduction and adult longevity of five species of planktonic cyclopoid copepods reared on different diets: A comparative study. Freshw. Biol. 1997, 38, 289–300. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zeng, C.; Wang, Y.; Zeng, C. Effects of Food Concentration and Photoperiod on Egg Production, Female Life Expectancy and Population Dynamics of the Paracalanid Copepod, Bestiolina amoyensis. Front. Mar. Sci. 2021, 8, 788744. [Google Scholar] [CrossRef]
- Lindley, J.A. Eggs and Their Incubation as Factors in the Ecology of Planktonic Crustacea. J. Crustac. Biol. 1997, 17, 569–576. [Google Scholar] [CrossRef]
- Neffati, N.; Daly Yahia-Kefi, O.; Bonnet, D.; Carlotti, F.; Daly Yahia, M.N. Reproductive traits of two calanoid copepods: Centropages ponticus and Temora stylifera, in autumn in Bizerte Channel. J. Plankton Res. 2013, 35, 80–96. [Google Scholar] [CrossRef]
- Alajmi, F.; Zeng, C. Evaluation of microalgal diets for the intensive cultivation of the tropical calanoid copepod, arvocalanus crassirostris. Aquac. Res. 2015, 46, 1025–1038. [Google Scholar] [CrossRef]
- Mohamed Bakr, H.; Laabir, M.; Daly Yahia, M.N. A novel index based on planktonic copepod reproductive traits as a tool for marine ecotoxicology studies. Sci. Total Environ. 2020, 727, 138621. [Google Scholar] [CrossRef]
- Das, S.; Souissi, A.; Ouddane, B.; Hwang, J.-S.; Souissi, S. Trace metals exposure in three different coastal compartments show specific morphological and reproductive traits across generations in a sentinel copepod. Sci. Total Environ. 2023, 859, 160378. [Google Scholar] [CrossRef]
- Bollens, S.M.; Frost, B.W. Ovigerity, selective predation, and variable diel vertical migration in Euchaeta elongata (Copepoda: Calanoida). Oecologia 1991, 87, 155–161. [Google Scholar] [CrossRef]
- Svensson, J.-E. The influence of visibility and escape ability on sex-specific susceptibility to fish predation in Eudiaptomus gracilis (Copepoda, Crustacea). Hydrobiologia 1992, 234, 143–150. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Huys, R. A Homage to Homology: Patterns of Copepod Evolution. Acta Zool. 1992, 73, 327–334. [Google Scholar] [CrossRef]
- Mauchline, J. Egg and brood sizes of oceanic pelagic crustaceans. Mar. Ecol. Prog. Ser. 1988, 43, 251–258. [Google Scholar] [CrossRef]
- Hopkins, C.C.E. The relationship between maternal body size and clutch size, development time and egg mortality in Euchaeta norvegica (Copepoda: Calanoida) from Loch Etive, Scotland. J. Mar. Biol. Assoc. United Kingd. 1977, 57, 723–733. [Google Scholar] [CrossRef]
- Ohman, M.D.; Townsend, A.W. Egg strings in Euchirella pseudopulchra (Aetideidae) and comments on constraints on egg brooding in planktonic marine copepods. J. Mar. Syst. 1998, 15, 61–69. [Google Scholar] [CrossRef]
- Congdon, J.D.; Gibbons, J.W. Morphological constraint on egg size: A challenge to optimal egg size theory? Proc. Natl. Acad. Sci. USA 1987, 84, 4145–4147. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Smaller amphipod mothers show stronger trade-offs between offspring size and number. Ecol. Lett. 2000, 3, 142–149. [Google Scholar] [CrossRef]
- Beck, C.W.; Beck, R.E. The effect of packing constraints on optimal investment in offspring. Evol. Ecol. Res. 2005, 7, 1077–1088. [Google Scholar]
- Souissi, S.; Souissi, A. Promotion of the Development of Sentinel Species in the Water Column: Example Using Body Size and Fecundity of the Egg-Bearing Calanoid Copepod Eurytemora affinis. Water 2021, 13, 1442. [Google Scholar] [CrossRef]
- Das, S.; Ouddane, B.; Hwang, J.-S.; Souissi, S. Intergenerational effects of resuspended sediment and trace metal mixtures on life cycle traits of a pelagic copepod. Environ. Pollut. 2020, 267, 115460. [Google Scholar] [CrossRef]
- Lee, C.E. Rapid and Repeated Invasions of Fresh Water by the Copepod Eurytemora Affinis. Evolution 1999, 53, 1423–1434. [Google Scholar] [CrossRef]
- Lee, C.E.; Frost, B.W. Morphological stasis in the Eurytemora affinis species complex (Copepoda: Temoridae). Hydrobiologia 2002, 480, 111–128. [Google Scholar] [CrossRef]
- Lee, C.E. Global Phylogeography of a Cryptic Copepod Species Complex and Reproductive Isolation Between Genetically Proximate “Populations”. Evolution 2000, 54, 2014–2027. [Google Scholar] [CrossRef]
- Winkler, G.; Dodson, J.J.; Lee, C.E. Heterogeneity within the native range: Population genetic analyses of sympatric invasive and noninvasive clades of the freshwater invading copepod Eurytemora affinis. Mol. Ecol. 2008, 17, 415–430. [Google Scholar] [CrossRef]
- Beyrend-Dur, D.; Souissi, S.; Devreker, D.; Winkler, G.; Hwang, J.-S. Life cycle traits of two transatlantic populations of Eurytemora affinis (Copepoda: Calanoida): Salinity effects. J. Plankton Res. 2009, 31, 713–728. [Google Scholar] [CrossRef]
- Souissi, A.; Hwang, J.-S.; Souissi, S. Reproductive trade-offs of the estuarine copepod Eurytemora affinis under different thermal and haline regimes. Sci. Rep. 2021, 11, 20139. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ouddane, B.; Souissi, S. Multigenerational study of life history traits, bioaccumulation, and molecular responses of Pseudodiaptomus annandalei to cadmium. Ecotoxicol. Environ. Saf. 2022, 230, 113171. [Google Scholar] [CrossRef]
- Tande, K.S.; Hopkins, C.C.E. Ecological investigations of the zooplankton community of Balsfjorden, northern Norway: The genital system in Calanus finmarchicus and the role of gonad development in overwintering strategy. Mar. Biol. 1981, 63, 159–164. [Google Scholar] [CrossRef]
- Das, S.; Ouddane, B.; Souissi, S. Responses of the copepod Eurytemora affinis to trace metal exposure: A candidate for sentinel to marine sediment resuspension effects. Mar. Pollut. Bull. 2022, 181, 113854. [Google Scholar] [CrossRef]
- Souissi, A.; Souissi, S.; Devreker, D.; Hwang, J.-S. Occurence of intersexuality in a laboratory culture of the copepod Eurytemora affinis from the Seine estuary (France). Mar. Biol. 2010, 157, 851–861. [Google Scholar] [CrossRef]
- Devreker, D.; Souissi, S.; Molinero, J.C.; Beyrend-Dur, D.; Gomez, F.; Forget-Leray, J. Tidal and annual variability of the population structure of Eurytemora affinis in the middle part of the Seine Estuary during 2005. Estuar. Coast. Shelf Sci. 2010, 89, 245–255. [Google Scholar] [CrossRef]
- Souissi, A.; Souissi, S.; Hansen, B.W. Physiological improvement in the copepod Eurytemora affinis through thermal and multi-generational selection. Aquac. Res. 2016, 47, 2227–2242. [Google Scholar] [CrossRef]
- Das, S.; Tseng, L.-C.; Chou, C.; Wang, L.; Souissi, S.; Hwang, J.-S. Effects of cadmium exposure on antioxidant enzymes and histological changes in the mud shrimp Austinogebia edulis (Crustacea: Decapoda). Environ. Sci. Pollut. Res. 2019, 26, 7752–7762. [Google Scholar] [CrossRef]
- Altaff, K.; Chandran, M.R. Oviducal gland of planktonic copepod, Heliodiaptomus viduus Gurney—A new report. Curr. Sci. 1994, 66, 81–83. [Google Scholar]
- Lee, C.E.; Remfert, J.L.; Chang, Y.-M. Response to selection and evolvability of invasive populations. Genetica 2007, 129, 179–192. [Google Scholar] [CrossRef]
- Alekseev, V.R.; Souissi, A. A new species within the Eurytemora affinis complex (Copepoda: Calanoida) from the Atlantic Coast of USA, with observations on eight morphologically different European populations. Zootaxa 2011, 2767, 41–56. [Google Scholar] [CrossRef]
- Sukhikh, N.; Souissi, A.; Souissi, S.; Winkler, G.; Castric, V.; Holl, A.-C.; Alekseev, V. Genetic and morphological heterogeneity among populations of Eurytemora affinis (Crustacea: Copepoda: Temoridae) in European waters. C. R. Biol. 2016, 339, 197–206. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Sautour, B.; Chardy, P. The paradox between the long-term decrease of egg mass size of the calanoid copepod Eurytemora affinis and its long-term constant abundance in a highly turbid estuary (Gironde estuary, France). J. Plankton Res. 2007, 29, 377–389. [Google Scholar] [CrossRef][Green Version]
- Mialet, B.; Azémar, F.; Maris, T.; Sossou, C.; Ruiz, P.; Lionard, M.; Van Damme, S.; Lecerf, A.; Muylaert, K.; Toumi, N.; et al. Spatial spring distribution of the copepod Eurytemora affinis (Copepoda, Calanoida) in a restoring estuary, the Scheldt (Belgium). Estuar. Coast. Shelf Sci. 2010, 88, 116–124. [Google Scholar] [CrossRef]
- Mahjoub, M.-S.; Souissi, S.; Michalec, F.-G.; Schmitt, F.G.; Hwang, J.-S. Swimming kinematics of Eurytemora affinis (Copepoda, Calanoida) reproductive stages and differential vulnerability to predation of larval Dicentrarchus labrax (Teleostei, Perciformes). J. Plankton Res. 2011, 33, 1095–1103. [Google Scholar] [CrossRef]
- Belmonte, G. Calanoida (Crustacea: Copepoda) of the Italian fauna: A review. Eur. Zool. J. 2018, 85, 273–289. [Google Scholar] [CrossRef]
- Caley, M.J.; Schwarzkopf, L.; Shine, R. Does total reproductive effort evolve independently of offspring size? Evolution 2001, 55, 1245–1248. [Google Scholar] [CrossRef]
- Poulin, R. Clutch size and egg size in free-living and parasitic copepods: A comparative analysis. Evolution 1995, 49, 325–336. [Google Scholar] [CrossRef]
- Glippa, O.; Alekseev, V.R.; Souissi, S. Effects of photoperiod on egg production in Eurytemora affinis Poppe, 1880 (Copepoda: Calanoida) from the Seine Estuary (France). Ital. J. Zool. 2013, 80, 518–525. [Google Scholar] [CrossRef]
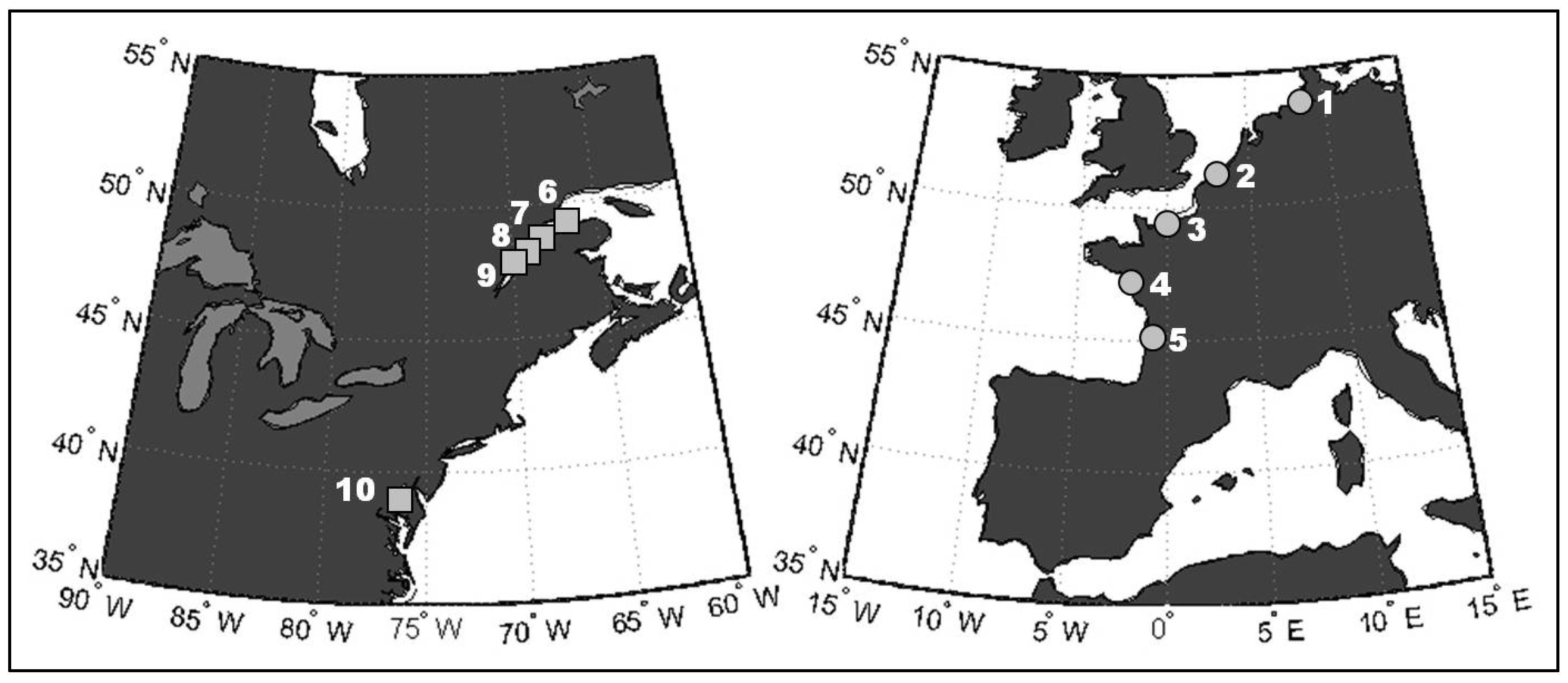



| Continent | Estuary | Latitude–Longitude | Dates (No. of Observations) | |||||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | Other Years | ||||
| Europe | Elbe | 53°32′06 N–09°47′31 E | 17 March (20) | |||||
| Scheldt | 51°21′06 N–04°14′58 E | 4 April (19) | 11 April (11) | 6 April (11) | ||||
| Seine | 49°28′33 N–00°27′54 W | 23 May (19) | 10 October (21)–6 November (16) | 8 March (19)–10 April (27) 8 June (34)–8 July (21) 20 September (11)–4 November (15) | 25 February (20) | 10 February 2010 (18) | ||
| Loire | 47°17′23 N–02°01′52 W | 18 April (20) | 15 November (31) | 16 April (9) | 25 June (16) | |||
| Gironde | 45°14′80 N–00°43′50 W | 20 April (20) | 15 April (15) | |||||
| North America | St La w rence | Isle Verte | 48°00′20 N–69°25′50 W | 18 May (15)–12 June(20) 11 July (26) | 22 May (7) | |||
| St Jean Port Joli | 47°12′59 N–70°16′22 W | 11 July (6) | 15 July (19) | 20 May (20) 2 June (33) | ||||
| Montmagny | 46°59′26 N–70°33′13 W | 14 June (20) | May (7) | |||||
| Berthier sur Mer | 46°56′07 N–70°44′07 W | 11 July (1) | 15 July (17) | 29 May (5) | ||||
| Chesapeake Bay | 39°23.81′ N–76°03.32′ W | 16 April (10)–15 December (19) | 6 February (22) –28 April (11) | May 2002 (6) April 2003 (4) | ||||
| Initial Experimental Conditions | Final Experimental Condition | ||||||
|---|---|---|---|---|---|---|---|
| Temperature | Salinity | No. of Generations | Total No. of Observations | Temperature | Salinity | No. of Generations | Total No. of Observations |
| 7 °C | 15 | 4 | 121 | 24 °C | 15 | 5 | 112 |
| 20 °C | 15 | 8 | 205 | 24 °C | 15 | 5 | 196 |
| 20 °C | 25 | 7 | 182 | 24 °C | 25 | 5 | 135 |
| Packing Constraint (Types) | Initial Condition | Final Condition | Initial Condition | Final Condition | Initial Condition | Final Condition |
|---|---|---|---|---|---|---|
| T7S15 | T24S15 | 20TS15 | T24S15 | T20S25 | T24S25 | |
| Strong packing constraint (%) | 1.6 | 3.4 | 0.5 | 2 | 4.3 | 6.9 |
| Intermediate packing constraint (%) | 1.6 | 20.7 | 10.2 | 14 | 12.9 | 32.4 |
| No packing constraint (%) | 96.7 | 75.9 | 89.3 | 84.0 | 82.8 | 60.7 |
| Continent | Europe | (North) America | ||||||
|---|---|---|---|---|---|---|---|---|
| Estuaries | S | L | G | SL1 | SL2 | SL3 | SL4 | |
| ESv vs. Cv | SPC | 0.88 | 0.90 | 0.95 | 0.67 | 0.76 | 0.96 | 0.97 |
| IPC | 0.98 | 0.96 | 0.996 | 0.98 | 0.97 | 0.88 | 0.97 | |
| NPC | 0.86 | 0.87 | 0.83 | 0.87 | 0.86 | - | 0.53 | |
| ESv vs. Ev | SPC | 10−5 | 0.03 | 0.62 | 0.07 | 0.73 | 0.35 | 0.21 |
| IPC | 0.04 | 0.01 | 0.003 | 0.27 | 0.01 | 0.13 | 0.54 | |
| NPC | 0.08 | 0.15 | 0.25 | 0.34 | 0.05 | - | 0.18 | |
| ESv vs. Pv | SPC | 0.06 | 0.53 | 0.004 | 0.21 | 0.60 | 0.71 | 0.22 |
| IPC | 0.10 | 0.70 | 0.21 | 0.65 | 0.10 | 0.14 | 0.49 | |
| NPC | 0.44 | 0.73 | 0.12 | 0.66 | 0.13 | 0.13 | 0.27 | |
| ESv vs. GSw | SPC | 0.06 | 0.15 | 0.007 | 0.31 | 0.33 | 0.002 | 0.03 |
| IPC | 0.20 | 0.06 | 0.52 | 0.15 | 0.26 | 0.04 | 0.94 | |
| NPC | 0.38 | 0.32 | 0.04 | 0.11 | 0.05 | 0.19 | 0.04 | |
| SPC (%) | 14.65 | 25.00 | 32.35 | 25.00 | 23.00 | 17.39 | 33.33 | |
| IPC (%) | 21.21 | 28.13 | 35.29 | 36.11 | 27.66 | 53.33 | 21.74 | |
| NPC (%) | 64.14 | 46.88 | 32.35 | 38.89 | 48.94 | 13.33 | 60.87 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souissi, A.; Das, S.; Hwang, J.-S.; Souissi, S. The Egg Packing Pressure Index of Calanoid Copepod as a Novel Eco-Indicator in Diverse Geographical Ecosystems. Diversity 2025, 17, 182. https://doi.org/10.3390/d17030182
Souissi A, Das S, Hwang J-S, Souissi S. The Egg Packing Pressure Index of Calanoid Copepod as a Novel Eco-Indicator in Diverse Geographical Ecosystems. Diversity. 2025; 17(3):182. https://doi.org/10.3390/d17030182
Chicago/Turabian StyleSouissi, Anissa, Shagnika Das, Jiang-Shiou Hwang, and Sami Souissi. 2025. "The Egg Packing Pressure Index of Calanoid Copepod as a Novel Eco-Indicator in Diverse Geographical Ecosystems" Diversity 17, no. 3: 182. https://doi.org/10.3390/d17030182
APA StyleSouissi, A., Das, S., Hwang, J.-S., & Souissi, S. (2025). The Egg Packing Pressure Index of Calanoid Copepod as a Novel Eco-Indicator in Diverse Geographical Ecosystems. Diversity, 17(3), 182. https://doi.org/10.3390/d17030182






