Abstract
In Mesoamerica, maize is one of the most important food crops, with México being the center of its origin, domestication, and diversity. The state of Chiapas in southern Mexico is one of the areas with the highest maize landrace diversity. However, information on its genetic diversity, conservation status, and the potential use of maize landraces throughout the entire Chiapas region is lacking. One region where local farmers use and preserve a wide diversity of maize landraces is the Zoque region. Until now, however, the genetic diversity of these maize landraces has not been studied. The aim of this study was to analyze the diversity and genetic structure of maize cultivated in the Zoque region, from Chiapas, Mexico, by using 17 landraces and 48 ISSR loci. The analysis revealed two genetic groups based on geographical origin. The genetic diversity level was moderate (Hbay = 0.29 and I = 0.36) and distributed mainly within landraces (70%). The maize landrace blanco belongs to the Tuxpeño race and Bacalito blanco belongs to the Olotillo race from the Miguel Hidalgo municipality have greater diversity values (Hbay = 0.36, I = 0.45 and Hbay = 0.35, I = 0.45, respectively). The results indicated that the maize landraces cultivated in the Zoque region, Chiapas, Mexico, constitute a valuable genetic resource that can be used for genetic improvement and in conservation programs.
1. Introduction
Maize (Zea mays L.) is one of the most widely used grains in the world, particularly in Mesoamerica, where it is one of the most widely distributed and diverse crops. The center of origin, domestication and diversity of maize is in Mexico, where a wide genetic diversity is expressed through the different landraces resulting from natural and sociocultural processes [1,2,3,4]. Looking at the southern region of Mexico, Orozco-Ramírez et al. [5] considered Chiapas as one of the areas with the highest maize diversity, with 18 different races. The most commonly cultivated among them are Oloton, Olotillo, Tuxpeño, Zapalote chico, Zapalote grande, and Comiteco. Over time, the management, cultivation and uses of these races by local farmers have allowed the development of a wide diversity of maize landraces; these can be distinguished by different characteristics such as grain color, ear size, and cultivation cycle [6].
Despite the importance of maize landrace diversity in Chiapas, very few studies have focused on genetic diversity throughout the entire region [7]. Instead, the Frailesca region, which covers six municipalities (Villaflores, Villa Corzo, El Parral, Angel Albino Corzo, La Concordia, and Montecristo de Guerrero), has received all the attention. Such studies have covered various aspects related to maize landraces, such as ethnobotany [8], morphological diversity [6,9,10], historical or cultural processes [3], and genetic diversity [4,11]. The results of these works offer a general overview of the diversity present in the maize landraces cultivated in the Frailesca region of Chiapas, highlighting the importance of further evaluating other regions.
One region where local farmers use and preserve a wide diversity of maize landraces is the Zoque region, which covers 12 municipalities (Ocotepec, Tapilula, Tapalapa, Rayón, Tecpatán, Chapultenango, Francisco León, Ixhuatán, Jitotol, Ostuacán, Pantepec, and Copainalá) [12]. In this region, a high percentage of the population speak the indigenous language, and most families rely on small-scale agriculture—or “Milpa”—for their subsistence. In this production system, the farmers plant a group of basic crops such as maize (Zea mays L.), squash (Cucurbita spp.), and beans (Phaseolus vulgaris L.) [13,14]. In the case of maize grown in the milpa, several common management features contribute to the diversity of the crop, including the persistence of local maize types, cultivation of different maize landraces, acquisition of new seeds from neighbors or more distant sources, and selection of seeds from harvested ears [15]. However, until now, the genetic diversity of maize landraces cultivated in the Zoque region has not been studied. In this regard, a series of genetic markers have been developed to analyze and evaluate the genetic diversity of plants [16]. Among the different molecular markers used to study genetic diversity in maize, inter-simple sequence repeats (ISSRs) have been widely used [12,17,18]. ISSR markers are dominant, allowing the detection of polymorphism without previous knowledge of DNA sequences. They are also rapid, simple, and cheap for assessing diversity and genetic structures; as such, the application of an adequate number of ISSR markers has gained acceptance for genetic diversity evaluation in maize landraces [19,20,21,22]. In this study, ISSR markers were used for the first time to analyze the diversity and genetic structure of maize landraces cultivated in the Zoque region of Chiapas, Mexico. To generate basic information that can be used in participatory breeding and to develop in situ and ex situ conservation programs in this important cultural region.
2. Materials and Methods
2.1. Maize Collection
A total of 17 maize landraces were collected directly from fields (in traditional farming systems called “Milpa”) in two communities where Milpa production remains an important economic activity for families who sow and conserve a wide genetic diversity of maize landraces: these two communities are Benito Juárez and Miguel Hidalgo in the municipality of Copainala in the Zoque region, located in the northeastern part of the state of Chiapas in southeastern Mexico (Figure 1). The maize landraces were identified according to their collection number, local name and collection site. We collected seven maize landraces from fields in Benito Juárez and ten from Miguel Hidalgo (Table 1).
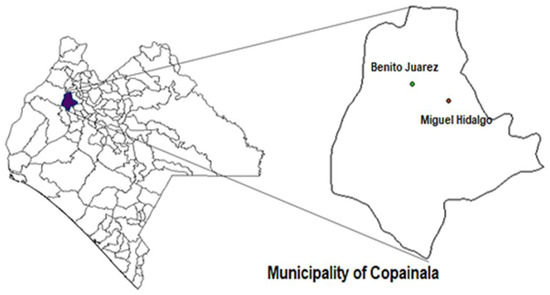
Figure 1.
Geographic location of the communities of the Zoque region in the northeastern area of Chiapas, Mexico, where the maize was collected.

Table 1.
Data from the 17 landraces collected from communities in Benito Juárez and Miguel Hidalgo from the municipality of Copainalá, Chiapas, Mexico.
2.2. Extraction and Amplification of DNA
Genomic DNA extraction was carried out at the Molecular Genetics Laboratory of Tecnológico Nacional of México, Conkal campus. Twenty seeds from each maize landrace were germinated using the between-paper method described by Rao et al. [23]. Genomic DNA was extracted from young leaves that were free of pests, diseases, and mechanical damage from a total of 12 individual plants for each maize landrace using DNeasy® Plant Mini Kit (QIAGEN, Hilden, Germany) following the supplier’s instructions. The quality of DNA was verified by electrophoresis in 1% agarose gel stained with 1 μL of Uview 6x loading dye (BioRad, Hercules, CA, USA) in 1x TBE buffer solution. For PCR amplification of the 15 ISSR primers tested, seven were selected that yielded good amplification and high polymorphism levels for maize [11,19,20,21] (Table 2). PCR amplifications were carried out in a total volume of 20 µL containing 10 µL of iTaq Universal SYBR Green Supermix (Bio-Rad), 2 µL of ISSR primer, 1 µL of template DNA (50 ng/reaction), and 7 µL of ultra-pure water. DNA amplification was performed in a C1000 Touch thermal cycler (BioRad, Hercules, CA, USA) programed for 4 min at 94 °C for initial denaturing, followed by 35 cycles of 2 min at 94 °C; 1.5 min of annealing at 52 or 54 °C depending on the primer used; 1 min at 72 °C; and a final extension of 7 min at 72 °C. The amplification products were separated by electrophoresis in 1% agarose gel stained with 1 μL of Uview 6x loading dye (BioRad) with 1x TBE buffer solution at a constant 110 V for 45 min. A 1 kb molecular marker standard was included in each gel, and the bands were visualized using the Gel Doc EZ Imager program (BioRad, Hercules, CA, USA).

Table 2.
ISSR primers utilized in the molecular characterization of 17 maize landraces from the Zoque region of Chiapas.
2.3. Data Analysis
Each ISSR band was considered as an independent locus, and polymorphic bands were scored as absent (0) or present (1) for all samples. Only clear and reproducible bands were used for the analysis.
2.3.1. Genetic Structure
The genetic structure was analyzed based on two methods. In the first, an individual assignment test was performed using Bayesian clustering approaches, as implemented in STRUCTURE v. 2.3.4 [24]. We used the admixture model with uncorrelated allele frequencies with a burn-in period of 100,000 and a run length of 1,000,000 Markov Chain Monte Carlo (MCMC) steps. Ten independent simulations were run for each value of K ranging from K = 1 to K = 5. The optimal value of K was determined according to Evanno et al. [25] using StructureSelector [26]. Finally, ancestry graphs for the optimum K value were generated using STRUCTURE. The second method used to analyze the genetic structure was an analysis of molecular variance (AMOVA) considering three levels—total landraces (including all maize landraces), between-maize landraces, and within-maize landraces—using the GenAlEx 6.5 software.
2.3.2. Genetic Relationships
To analyze the genetic relationships among maize landraces from the Zoque region, Chiapas, genetic data were analyzed with an UPGMA dendrogram using Euclidian distance. The tree topology was evaluated with 1000 bootstrap replicates with PAST [27].
2.3.3. Genetic Diversity
Genetic diversity was evaluated on two levels—maize landraces and observed groups—through the following allelic richness indices: percentage of polymorphic loci (% P), observed number of alleles (na), effective number of alleles (ne), and the better estimators of genetic diversity for analyzing dominant marker data, the Shannon–Weaver diversity index (I) and expected heterozygosity with a Bayesian approach (HBay). The allelic richness indices and Shannon–Weaver diversity index were obtained using POPGENE v. 1.31 [28]. The expected heterozygosity (HBay) was calculated using AFLPSURV v. 1.0 [29].
3. Results
3.1. Genetic Structure
The Evanno method results [25] showed a maximum point of inflection at K = 2 for all accessions analyzed, indicating the existence of two genetically distinct groups (Figure 2).
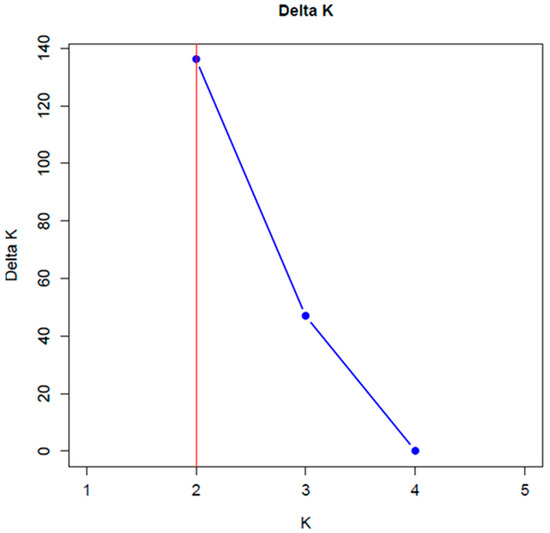
Figure 2.
Graph of Delta K to estimate the number of genetic groups among 17 maize landraces from Chiapas, Zoque region.
The bar graph (Figure 3) shows the ancestry coefficients of the 204 samples analyzed in the 17 maize landraces studied. Two genetically differentiated groups can be observed. Group I (in green) was the most diverse and included eleven maize landraces, eight of which are white seed (six of which are part of the Olotillo race named locally as Bacalito 1, 5, 12, 23, 25, and 28), two belong to the Tuxpeño race (locally called Blanco 18 and 22), one purple-colored maize landrace (which is part of the Olotillo race, Bacalito morado, landrace 2), one yellow seed landrace (belonging to the Olotillo race, Bacalito amarillo, landrace 3), and the quechulteco landrace (belonging to the Tehua race landrace 6). Of these eleven accessions, six were collected in the Benito Juárez locality (landraces 1, 2, 3, 5, 6, and 12) and five in Miguel Hidalgo (landraces 18, 22, 23, 25, and 28). Group II (in red) grouped six maize landraces, of which three corresponded to white seed maize landraces belonging to the Olotillo race (Bacalito blanco, landraces 7, 26, and 29), one cream seed landrace from the Olotillo race (named Bacalito crema, landrace 20), and two maize landraces of colored seeds belonging to the Tuxpeño race (named Pinto 24 and 27). This group was less diverse and included five accessions collected in the Miguel Hidalgo area and one in Benito Juárez.
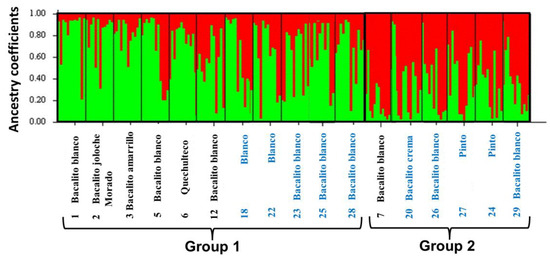
Figure 3.
The number of groups generated in the genetic structure calculated for K = 2. Each maize landrace is represented by a vertical bar. Maize landraces marked with the numbers 1, 2, 3, 5, 6, 7, and 12 in black color belong to Benito Juarez locality and those marked with the numbers 18, 20, 22, 23, 24, 25, 26, 27, 28, and 29 in blue color belong to Miguel Hidalgo locality.
A molecular variance analysis (AMOVA) at the maize landrace level indicated that the greatest genetic variability is distributed within the maize landraces with 70% of the total variability, while only 30% of the observed variability was distributed between accessions. At the level of genetic groups, greater variability was observed within the formed groups (96%) compared to that observed between groups (4%) (Table 3).

Table 3.
Molecular variance analysis (AMOVA) between and within accessions and groups.
3.2. Genetic Relationships
The results of the UPGMA analysis (Figure 4) grouped the 17 maize landraces into two main groups, with a general grouping based on geographical origin. The first group is made up of ten landraces, of which seven belong to the Olotillo race (landraces 7 Bacalito blanco, 20 Bacalito crema, 12 Bacalito blanco, 2 Bacalito morado, 1 Bacalito blanco, 5 Bacalito blanco, and 3 Bacalito amarillo), one belongs to the Tehua race (landrace 6 quechulteco), and two belong to the Tuxpeño race (landraces 18 blanco and 22 blanco). These landraces were collected in the Benito Juárez area, with only landraces 18, 20, and 22 being collected in Miguel Hidalgo. The second group was composed of seven maize landraces, five of which have white seeds and belong to the Olotillo race (landraces 25 Bacalito blanco, 23 Bacalito blanco, 28 Bacalito blanco, 29 bacalito blanco, and 26 bacalito) and the remaining two have colorful seeds and belong to the Tuxpeño race (Maize landraces 24 and 27 named Pinto). All the accessions that belong to this group were collected in the Miguel Hidalgo area. With some exceptions, the topology observed in UPGMA (Figure 4) is consistent with the groups observed in the structure analysis (Figure 3). The exceptions are the inclusion of maize landraces 7 and 20 in group 1 of the UPGMA, whereas, in the structure analysis, these two accessions were grouped together in group 2. By contrast, maize landraces 23, 25, and 28 were included in group 2 of the UPGMA, but, in the structure analysis, these three accessions were in group 1.
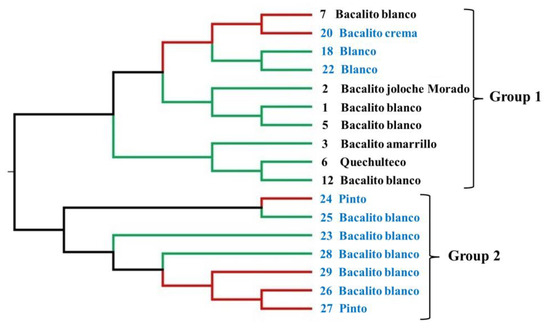
Figure 4.
The UPGMA dendrogram of the genetic relationships of the maize landraces of two regions in Chiapas, Mexico. Branch colors correspond to the colors assigned for each maize landrace in the structure analysis in Figure 3. Maize landraces marked with the numbers 1, 2, 3, 5, 6, 7, and 12 in black color belong to Benito Juarez locality and those marked with the numbers 18, 20, 22, 23, 24, 25, 26, 27, 28, and 29 in blue color belong to Miguel Hidalgo locality.
3.3. Genetic Diversity
The seven ISSR primers generated a total of 48 loci, 32 of which were polymorphic. Table 4 shows the indices of allelic richness and genetic diversity of the 17 maize landraces collected in the two communities of the Zoque region, Chiapas, with a general average of 31 polymorphic loci corresponding to 66% of the total. The maize landrace 28 Bacalito blanco presented the highest number of polymorphic loci, while the landraces 6 Quechulteco and 12 Bacalito blanco presented the lowest numbers of polymorphic loci, with 50% in both.

Table 4.
Genetic diversity index of 17 maize landraces collected from Benito Juárez and Miguel Hidalgo communities, Zoque region, Chiapas, México.
The average number of alleles observed in the whole sample was 1.86 ± 0.46, with the maize landrace 28 Bacalito blanco presenting the highest at 1.83 ± 0.37; meanwhile, landraces 6 Quechulteco and 12 Bacalito blanco presented the lowest number of observed alleles. The average number of effective alleles was 1.43 ± 0.38, with maize landrace 22 Bacalito blanco presenting the highest number at 1.56 ± 0.38, while that the landraces 1 Bacalito blanco and 24 Pinto obtained the lowest numbers of effective alleles with 1.30 in both landraces. With respect to the index of genetic diversity, as evaluated by the expected heterozygosity under a Bayesian approach (Hbay), an overall average of 0.29 ± 0.01 was found. The maize landrace 22 Bacalito blanco presented the greatest genetic diversity with 0.36 ± 0.02, while maize landrace 12 Bacalito blanco presented the least genetic diversity with 0.24 ± 0.02. When genetic diversity was evaluated using the Shannon index (I), the average was 0.36 and landraces 22 and 28, both Bacalito blanco, were those with the highest genetic diversity at 0.45 (Table 4).
4. Discussion
4.1. Genetic Structure
Based on the individual assignment test and UPGMA cluster analysis, the results show that the genetic structure of the maize landraces studied could be divided into two genetic groups, consistent with their geographical origin. Similar results have been reported in other studies, where a geographical clustering pattern was observed [11,30,31] rather than variations in maize landraces as evaluated via genotype characteristics, environmental adaptation, color, or grain type, as reported by Adu et al. [32]. In this study, the association between genetic groups and their geographical origin may be due to the fact that farmers from the communities of the Zoque region, Chiapas, Mexico, preserve their landraces because of the favorable characteristics related to the handling and consumption of the maize. These include, for example, easier kernel removal, kernels of greater weight and size, and shorter cooking times.
The AMOVA results show that the distribution of genetic diversity was higher within maize landraces and within observed genetic groups. This finding is consistent with the results of Dube et al. [33] and Da Silva et al. [34], who observed that the partition of genetic diversity between and within populations was significantly greater within populations. In general, this result has also been found in allogamous plants where there is a high genetic variation within populations and low among them [34,35]. In addition, in allogamous species, molecular markers typically reveal high heterozygosis within populations, which results in low differentiation between them [34].
4.2. Genetic Relationships
The UPGMA analysis of genetic relationships is in agreement with the formation of two genetic groups; however, it presented some differences with the individual assignment analysis (Figure 3 and Figure 4). Similar results were reported by Santos et al. [12] for maize landraces from southeastern Mexico. In the present work, five exceptions were found between the UPGMA and structure analysis (7, 20, 23, 25, and 28). In the case of the maize, landrace 7 Bacalito blanco was collected in the Benito Juárez area; however, in the structure analysis, it was grouped with those collected in the Miguel Hidalgo area. For landrace 20, the opposite occurred: it was collected in the Miguel Hidalgo area, but it was grouped with the accessions collected in Benito Juárez in the structure analysis. Similarly, landraces 23, 25, and 28 were collected in the Miguel Hidalgo area; however, in the structure analysis, these accessions were grouped with those collected in Benito Juárez. Cömertpay et al. [36] mention how one of the reasons for non-relatedness between landraces from the same region could be due to the unconscious selection of favorable alleles by farmers who select landraces with better adaptation to local agroclimatic conditions. Another reason could be the exchange of seeds with farmers from distant regions or the migration of landraces among regions, followed by mixing and introgression with pre-existing germplasms. On the other hand, Takur et al. [37] mention how the non-relatedness of landraces from the same region may reflect gene flow among different maize landraces in different regions or environments.
In this study, the five maize landraces which were not grouped with those of the same collection area are those belonging to the Olotillo race; they share the following characteristics: they are short-cycle maize landraces, present easier kernel removal and higher grain yields, have shorter cooking times, yield higher dough quantities, and produce better-tasting tortillas. These characteristics make farmers and housewives prefer them for sowing and consumption. A possible explanation for the lack of grouping between these five maize landraces (7, 20, 23, 35, and 28) and those belonging to the same area could be the exchange of seeds by farmers between the two studied localities with the aim of obtaining landraces with the best characteristics for sowing and consumption. Other explanations could be the relatively low number of ISSRs used or the effects of natural selection, genetic drift, unintentional outcrossing, and mutation [37].
4.3. Genetic Diversity
The seven ISSR primers used were successful in this study, presenting polymorphism in the maize landraces from the Zoque area of northeastern Chiapas. The percentage of general polymorphic loci was 66%, with a between-landraces range of 50 to 83%. Similar results have been reported in other genetic diversity and structural studies of maize landraces using dominant ISSR and RAPD markers [12,16,38]. Of the seven primers used in this study, four (GACA4, AG8C, AG8T, and CA8T) have demonstrated their usefulness in evaluating genetic polymorphism in native maize populations reaching polymorphism levels in the range of 85 to 100% [11,17]. The average levels of genetic diversity with both estimators (Hbay = 0.29 and I = 0.36) was moderate, showing that both estimators (Hbay and I) could be good genetic diversity estimators for dominant markers. These genetic diversity values were below those reported by other authors [4,30,31,33,38], but similar to those reported by Adu et al. [32] on inbred lines of tropical maize; by Da Silva et al. [34] on popcorn accessions from the Brazilian germplasm bank; by Soliman et al. [21] in accessions of maize from Genebank of the Institute of Plant Genetics and Crop Plant Research (IPK), Germany; and by Santos et al. [11] in maize landraces from Mexico. The variation between our results and the findings reported by the authors mentioned above could be attributed to the differences in the markers used, the area explored in each study, the number of landraces evaluated, and the number of markers used.
In this work, we also found that maize landraces 22 and 28, called Bacalito blanco of the Olotillo race, had the greatest genetic diversity. This may occur because these types are the most cultivated by local farmers, and thus better adapted to the local conditions of the studied region. In general, the local varieties belonging to the Olotillo race were the most genetically diverse. This result is consistent with those of Barrera et al. [39], who observed that the populations of maize belonging to the Olotillo race presented high levels of diversity. In general, the levels of diversity observed in this study may largely be due to the fact that maize is an open-pollination species; this condition allows a high outcrossing rate between maize landraces, which may influence their maintenance and increase their genetic diversity [40]. In addition, maize landraces were sampled in two regions with variable environmental and geographical conditions, which could also influence the level of diversity observed.
5. Conclusions
The results of this study contribute insights into the structure and genetic diversity of maize landraces in the Zoque region, Chiapas, Mexico. The results showed that the genetic diversity of the local varieties in the Zoque region, Chiapas are structured into two genetic groups based on their geographic origin. The greatest genetic diversity is distributed within maize landraces and within observed genetic groups. Five maize landraces (7, 20, 23, 35, and 28) were not grouped according to their geographical origin. The level of total genetic diversity was moderate. The maize landraces 22 and 28, both Bacalitos part of the Olotillo race, showed the greatest genetic diversity. Our results indicate that the landraces collected in the Zoque region, Chiapas, Mexico, constitute a valuable genetic resource that can be used for genetic improvement and conservation programs both ex situ and in situ. In this sense, we recommend starting conservation programs that include a wide collection effort to save all maize landraces in a gene bank and promote the planting of all varieties among all producers and their families as part of an in situ conservation program.
Author Contributions
Conceptualization, L.L.-M., E.R.-S., G.R.P. and E.d.l.C.H.; methodology, E.d.l.C.H. and R.H.A.-N.; formal analysis, E.d.l.C.H., R.H.A.-N. and M.C.G.R.; investigation, E.d.l.C.H.; writing—original draft preparation, R.H.A.-N. and E.d.l.C.H.; writing—review and editing, R.H.A.-N., L.L.-M., E.R.-S., G.R.P. and M.C.G.R.; visualization, R.H.A.-N. and E.d.l.C.H.; supervision, L.L.-M., E.R.-S. and G.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank the farmers who donated genetic materials (maize landraces) and Emily Zamudio Moreno for their technical support. The first author thanks the Consejo Nacional de Humanidades, Ciencia y Tecnología-Mexico, for the postgraduate scholarship (scholarship number: 780593). All authors thank the Tecnologico Nacional de Mexico campus Conkal, for having allowed the development of the research in their postgraduate program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matsuoka, Y.; Vigouroux, Y.; Goodman, M.M.; Sanchez, G.J.; Buckler, E.; Doebley, J.A. Single domestication for maize shown by multilocus microsatellite genotyping. Proc. Natl. Acad. Sci. USA 2002, 99, 6080–6084. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, O.A.; Miranda, C.S.; Cuevas, S.J.A.; Santacruz, V.A..; Sánchez, D.S. Resistencias, Prehistoria, Historia y Diferencias de Teocintle a Maíz; Impresos América: Texcoco, Mexico, 2009; pp. 7–32. [Google Scholar]
- Caballero, M.A.; Cordova, L.; Lopez, A.J. Validación empírica de la teoría multicéntrica del origen y diversidad del maíz en México. Rev. Fitotec. Mex. 2019, 42, 357–366. [Google Scholar] [CrossRef]
- Torres, B.; Rocandio, M.; Santacruz, A.; Córdova, L.; Coutiño, B.; López, H. Genetic diversity characterization of maize populations using molecular markers. Ital. J. Agron. 2023, 18, 2206. [Google Scholar] [CrossRef]
- Orozco, Q.; Perales, H.; Hijmans, R.J. Geographical distribution and diversity of maize (Zea mays L. subsp. mays) races in Mexico. Genet. Resour. Crop Evol. 2017, 64, 855–865. [Google Scholar] [CrossRef]
- Coutiño, B.; Vidal, V.A.; Cruz, C.; Gómez, M. Características eloteras y de grano de variedades nativas de maíz de Chiapas. Rev. Mex. Cienc. Agrícolas 2015, 6, 1119–1127. [Google Scholar]
- Perales, H.; Golicher, D. Mapping the Diversity of Maize Races in Mexico. PLoS ONE 2014, 9, e114657. [Google Scholar] [CrossRef]
- Guevara-Hernández, F.; Hernández, M.A.; Bastarrechea, J.L.; Fonseca, M.A.; Delgado, F.; Ocaña, M.; Acosta, R. Riqueza de maíces locales (Zea mays L.) en la región Frailesca, Chiapas, México: Un estudio etnobotánico. Rev. Fac. Agron. 2020, 37, 223–243. [Google Scholar]
- Hernández-Ramos, M.A.; Guevara, F.; Basterrechea, J.L.; Coutiño, B.; O-Arias, M.; Pinto, R. Diversidad y conservación de maíces locales de la Frailesca, Chiapas, México. Rev. Fitotec. Mex. 2020, 43, 471–479. [Google Scholar] [CrossRef]
- Torres, B.; Rocandio, M.; Santacruz, A.; Córdova, L.; Coutiño, B.; López, H. Morphological and agronomic diversity of seven corn races from the state of Chiapas. Rev. Mex. Cienc. Agrícolas 2022, 13, 687–699. [Google Scholar]
- Santos, L.P.; Andueza-Noh, R.H.; Ruíz, E.; Latournerie, L.; Garruña, R.; Mijangos, J.O.; Martínez, J. Characterization of the genetic structure and diversity of maize (Zea mays L.) landrace populations from Mexico. Maydica 2017, 62. [Google Scholar]
- Sánchez-Cortés, M.S.; Lazos Chavero, E. Desde dónde y cómo se construye la identidad zoque: La visión presente en dos comunidades de Chiapas. Península 2009, 4, 55–79. [Google Scholar] [CrossRef]
- Geck, M.S.; García, A.J.R.; Casu, L.; Leonti, M. Acculturation and ethnomedicine: A regional comparison of medicinal plant knowledge among the Zoque of southern Mexico. J. Ethnopharmacol. 2016, 187, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Trejo, J.; Ocampo-González, P.; Coutiño-Hernández, P.R. Uso de los mamíferos silvestres en el municipio de Copainalá, región Zoque, Chiapas; México. Quehacer Científico Chiapas 2014, 9, 3–9. [Google Scholar]
- Perales, H.R.; Benz, B.F.; Brush, S.B. Maize diversity and ethnolinguistic diversity in Chiapas, Mexico. Proc. Natl. Acad. Sci. USA 2005, 102, 949–954. [Google Scholar] [CrossRef]
- Li, S.; Gan, X.; Han, H. Low within-population genetic diversity and high genetic differentiation among populations of the endangered plant Tetracentron sinense Oliver revealed by inter-simple sequence repeat analysis. Ann. For. Sci. 2018, 75, 74. [Google Scholar] [CrossRef]
- Hernández, M.A.; Rodríguez, L.A.; Guevara, F.; Rosales, M.Á.; Pinto, R.; Ortiz, R. Caracterización molecular de maíces locales de la Reserva de la Biosfera La Sepultura, México. Agron. Mesoam. 2017, 28, 69–83. [Google Scholar] [CrossRef]
- Marcón, F.; Martínez, E.J.; Rodríguez, G.R.; Zilli, A.L.; Brugnoli, E.A.; Acuña, C.A. Genetic distance and the relationship with heterosis and reproductive behavior in tetraploid bahiagrass hybrids. Mol. Breed. 2019, 39, 89. [Google Scholar] [CrossRef]
- Amoon, M.H.; Abdul-Hamed, Z.A. Determination genetic diversity of inbred lines and hybrids of maize using issr technic. Iraqi. J. Agri. Sci. 2020, 51, 269–277. [Google Scholar]
- Muhammad, R.W.; Qayyum, A.; Ahmad, M.Q.; Hamza, A.; Yousaf, M.; Ahmad, B.; Noor, E. Characterization of maize genotypes for genetic diversity on the basis of inter simple sequence repeats. Genet. Mol. Res. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Soliman, R.S.; El, H.H.; Börner, A.; Badr, A. Genetic diversity of a global collection of maize genetic resources in relation to their subspecies assignments, geographic origin, and drought tolerance. Breed. Sci. 2021, 71, 313–325. [Google Scholar] [CrossRef]
- Lenka, D.; Tripathy, S.K.; Kumar, R.; Behera, M.; Ranjan, R. Assessment of genetic diversity in quality protein maize (QPM) inbreds using ISSR markers. J. Environ. Biol. 2015, 36, 985–992. [Google Scholar] [PubMed]
- Rao, N.K.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Novell, D.; Larinde, M. Manual of seed handling in genebanks. In Handbooks for Genebanks; Bioversity International: Rome, Italy, 2006; p. 8. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 1, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Resour. 2018, 18, 176–177. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeonton. Electron. 2001, 4, 4–9. [Google Scholar]
- Yeh, F.C.; Boyle, T.J. POPGENE v. 1.31. Microsoft Windows-Based Freeware for Population Analysis; University of Alberta and Centre for International Forestry Research; University of Alberta: Edmonton, AB, Canada, 1999. [Google Scholar]
- Vekemans, X. AFLP-SURV Version 1.0: Distributed by the Autor; Laboratoire de genetique et Ecologie Vegetale; Universite Libre de Bruxelles: Brussels, Belgium, 2002. [Google Scholar]
- Patel, R.; Memon, J.; Kumar, S.; Patel, D.A.; Sakure, A.A.; Patel, M.B.; Das, A.; Karjagi, C.G.; Patel, S.; Patel, U.; et al. Genetic Diversity and Population Structure of Maize (Zea mays L.) Inbred Lines in Association with Phenotypic and Grain Qualitative Traits Using SSR Genotyping. Plants 2024, 13, 823. [Google Scholar] [CrossRef]
- Aci, M.M.; Lupini, A.; Mauceri, A.; Morsli, A.; Khelifi, L.; Sunseri, F. Genetic variation and structure of maize populations from Saoura and Gourara oasis in Algerian Sahara. BMC Genet. 2018, 1, 51. [Google Scholar] [CrossRef]
- Adu, G.B.; Badu-Apraku, B.; Akromah, R.; Garcia-Oliveira, A.L.; Awuku, F.J.; Gedil, M. Genetic diversity and population structure of early-maturing tropical maize inbred lines using SNP markers. PLoS ONE 2019, 14, e0214810. [Google Scholar]
- Dube, S.P.; Sibiya, J.; Kutu, F. Genetic diversity and population structure of maize inbred lines using phenotypic traits and single nucleotide polymorphism (SNP) markers. Sci. Rep. 2023, 13, 17851. [Google Scholar] [CrossRef]
- Da Silva, T.A.; Cantagalli, L.B.; Saavedra, J.; Lopes, A.D.; Mangolin, C.A.; Da silva, M.F.P.; Scapim, C.A. Population structure and genetic diversity of Brazilian popcorn germplasm inferred by microsatellite markers. Electron. J. Biotech. 2015, 18, 181. [Google Scholar] [CrossRef]
- Huang, R.; Zong, Z.; Yu, W.; Ying, W. Genetic variation and genetic structure within metapopulations of two closely related selfing and outcrossing Zingiber species (Zingiberaceae). Aoplants 2021, 13, 1–15. [Google Scholar]
- Cömertpay, G.; Baloch, F.S.; Kilian, B. Diversity Assessment of Turkish Maize Landraces Based on Fluorescent Labelled SSR Markers. Plant. Mol. Biol. Rep. 2012, 30, 261–274. [Google Scholar] [CrossRef]
- Thakur, N.; Prakash, J.; Thakur, K.; Sharma, J.K.; Kumari, R.; Rana, M.; Lata, S. Genetic diversity and structure of maize accessions of north western Himalayas based on morphological and molecular markers. Proc. Natl. Acad. Sci. India Sec. B Biol. Sci. 2017, 87, 1385–1398. [Google Scholar] [CrossRef]
- Chňapek, M.; Balážová, Ž.; Špaleková, A. Genetic diversity of maize resources revealed by different molecular markers. Genet. Resour. Crop Evol. 2024, 71, 4685–4703. [Google Scholar] [CrossRef]
- Barrera, L.A.; Legaria, J.P.; Ortega, R. Diversidad genética en poblaciones de razas mexicanas de maíz. Rev. Fitotec. Mex. 2020, 43, 121–125. [Google Scholar]
- Bøhn, T.; Aheto, D.W.; Mwangala, F.S.; Fischer, K.; Bones, I.L.; Simoloka, C.; Breckling, B. Pollen-mediated gene flow and seed exchange in small-scale Zambian maize farming, implications for biosafety assessment. Sci. Rep. 2016, 6, 34483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).