Deeper Insights into Species Diversity and Ecological Characterisation of the Macrosporum Group of the Genus Tuber
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Study
2.2. Molecular Methods
2.3. Phylogenetic Analysis
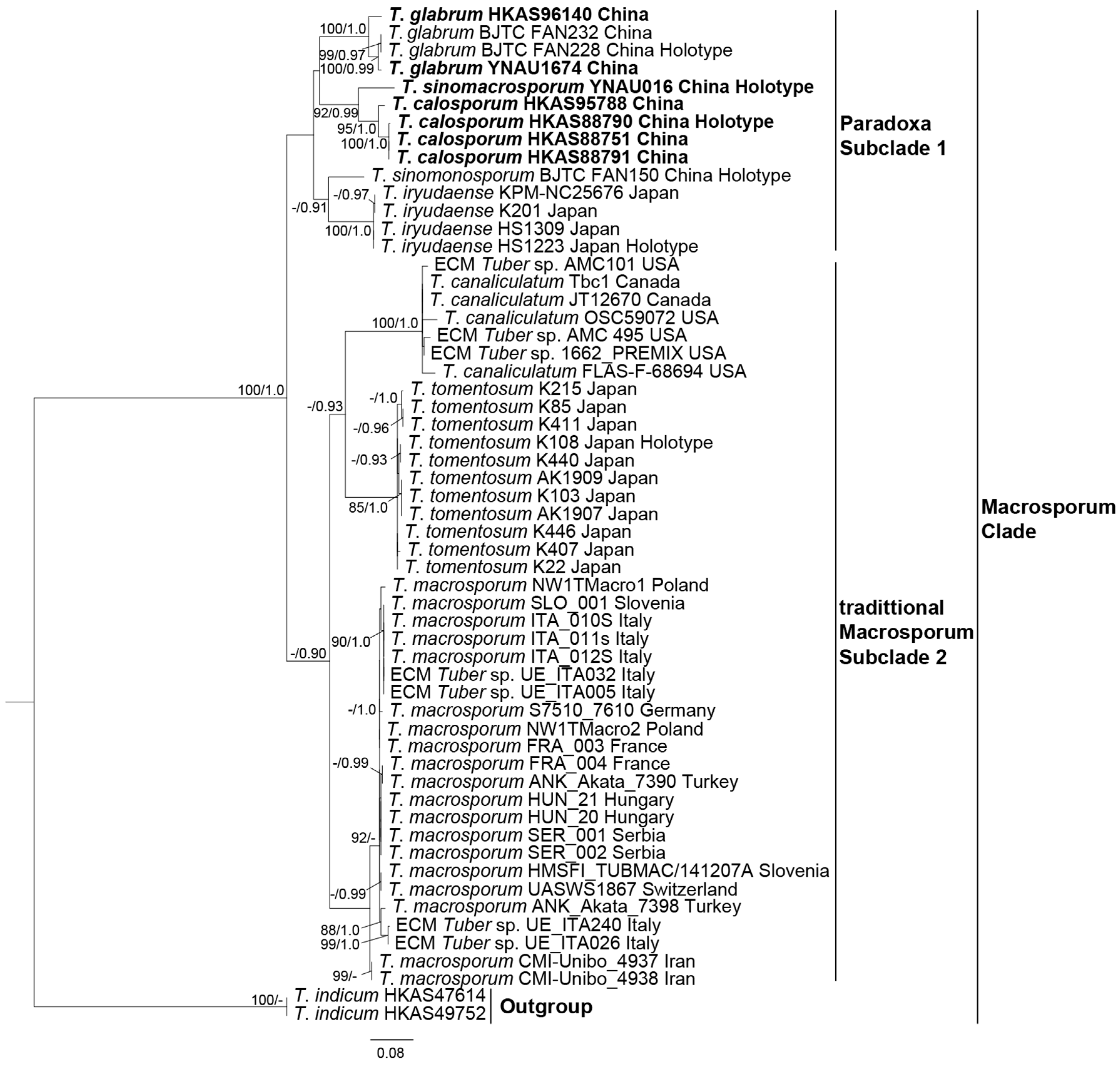
3. Results
3.1. Morphological Characteristics Observation and Analysis
3.1.1. Tuber sinomacrosporum S. P. Wan, and F. Q. Yu, sp. nov. Phytotaxa, (4), 2023
3.1.2. Tuber calosporum S.P. Wan, Mycoscience, 57: 393–399, 2016
3.1.3. Tuber glabrum L. Fan and S. Feng, Mycological Progress, 13(2): 241–246, 2014
3.2. Phylogenetic Analysis Results
4. Discussion
5. Conclusions
6. Species Identification Key for the Macrosporum Clade
| 1. The ascomata are distinctly cracked, with irregular plate-like warts....................T. macrosporum [3] 1. The ascomata are relatively smooth, without cracks.............................................................................2 2. The ascomata are without hairs................................................................................................................3 2. The ascomata have hairs............................................................................................................................4 3. The ascomata have a smooth surface, with spherical spores............................T. sinomonosporum [6] 3. The ascomata have small flaky warts in the grooves on the surface, with elliptical spores.............................................................................................................T. calosporum ([15], this article) 4. The ascomata have localized hairs in the grooves on the surface........................................................5 4. The ascomata are densely covered with hairs on the surface...............................................................6 5. The asci contain 1–3 spores, which are relatively small...................................T. canaliculatum [12,34] 5. The asci contain 1–2 spores, which are relatively large...................................T. sinomacrosporum [10] 6. The spores are spherical to nearly spherical................................................................T. tomentosum [9] 6. The spores are elliptical..............................................................................................................................7 7. The asci contain 1–2 spores, with surface ornamentation height < 2 μm........................T. glabrum ([14], this article) 7. The asci contain 1 spore, with surface ornamentation height < 5 μm........................T. iryudaense [9] |
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeandroz, S.; Murat, C.; Wang, Y.J.; Bonfante, P.; Tacon, F. Molecular phylogeny and historical biogeography of the genus Tuber, the ‘true truffles’. J. Biogeogr. 2008, 35, 815–829. [Google Scholar] [CrossRef]
- Guerin-Laguette, A. Successes and challenges in the sustainable cultivation of edible mycorrhizal fungi—Furthering the dream. Mycoscience 2021, 62, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Zambonelli, A.; Iotti, M.; Murat, C. True Truffle (Tuber spp.) in the World; Soil Biology Series 47; Springer: Cham, Switzerland, 2016; pp. 3–18. [Google Scholar]
- Li, Y.; Guo, S.X.; Chen, J. Research progress on chemical constituents and pharmacological effects of Tuber (Pezizales, Ascomycota). Mycosystema 2021, 40, 873–894. [Google Scholar]
- Bonito, G.; Smith, M.E.; Nowak, M.; Healy, R.A.; Guevara, G.; Cazares, E.; Kinoshita, A.; Nouhra, E.R.; Dominguez, L.S.; Tedersoo, L.; et al. Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. PLoS ONE 2013, 8, E52765. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, T.; Xu, Y.Y.; Yan, X.Y. Species diversity, phylogeny, endemism and geography of the truffle genus Tuber in China based on morphological and molecular data. Persoonia 2022, 48, 175–202. [Google Scholar] [CrossRef]
- Montecchi, A.; Sarasini, M. Funghi ipogei d’Europa; Associazione Micologica Bresadola Fondazione centro studi micologici: Trento, Italy, 2000; p. 714. [Google Scholar]
- Castellano, M.A.; Trappe, J.M.; Luoma, D.L. Sequestrate fungi. In Biodiversity of Fungi; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Inventory and Monitoring Methods; Elsevier: Amsterdam, The Netherlands, 2004; pp. 197–213. [Google Scholar]
- Kinoshita, A.; Sasaki, H.; Orihara, T.; Nakajima, M.; Nara, K. Tuber iryudaense and T. tomentosum: Two new truffles encased in tomentose mycelium from Japan. Mycologia 2021, 113, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.P.; Liu, W.; Cui, M.J.; Wang, R.; Wang, Y.Y.; Liu, M.J.; Wang, R.X.; Yu, C.J.; Yu, F.Q. Morphological and molecular evidence support a new truffle, Tuber sinomacrosporum, in Macrosporum group. Phytotaxa 2023, 591, 283–291. [Google Scholar] [CrossRef]
- Vittadini, C. Monographia Tuberacearum; Ex Typographia Felicis Rusconi: Milano, Italy, 1831; p. 35. [Google Scholar]
- Gilkey, H.M. Two new truffles. Mycologia 1920, 12, 99–101. [Google Scholar] [CrossRef]
- Kinoshita, A.; Sasaki, H.; Nara, K. Phylogeny and diversity of Japanese truffles (Tuber spp.) inferred from sequences of four nuclear loci. Mycologia 2011, 103, 779–794. [Google Scholar] [CrossRef]
- Fan, L.; Feng, S.; Cao, J.Z. The phylogenetic position of Tuber glabrum sp. nov. and T. sinomonosporum nom. nov. two Paradoxa-like truffle species from China. Mycol. Prog. 2014, 13, 241–246. [Google Scholar] [CrossRef]
- Wan, S.P.; Wang, X.H.; Zheng, Y.; Yu, F.Q. Tuber shidianense and T. calosporum, two new truffle species from southwest China. Mycoscience 2016, 57, 393–399. [Google Scholar] [CrossRef]
- Fortier, D.; Seguin, J.C.; Voyer, N. Characterization of the Volatilome of Tuber canaliculatum Harvested in Quebec, Canada. ACS Omega 2022, 7, 29038–29045. [Google Scholar] [CrossRef]
- Vezzola, V. La coltivazione di Tuber macrosporum. In Atti del 3 congresso internazionale di Spoleto sul tartufo; Donnini, D., Ed.; Comunita Montana dei Monti Martani, Serano e Subasio: Valtopina, Italy, 2010; pp. 694–697. [Google Scholar]
- Milenković, M.; Marjanović, Z. Current Results on Tuber spp. Research in Yugoslavia; Federation Francaise des Trufficulteurs: Uzès, France, 2001; pp. 218–225. [Google Scholar]
- Csorbainé, A.G.; Bratek, Z.; Illyés, Z.; Dimény, J. Studies on Tuber macrosporum Vittad. Natural Truffle Habitats in the Carpatho-Pannon Region; Comunità Montana dei Monti Martani, Serano e Subasio: Valtopina, Italy, 2011; pp. 182–188. [Google Scholar]
- Benucci, G.M.; Gógán, C.A.; Baciarelli, F.L.; Bencivenga, M.; Di, M.G.; Donnini, D. Mycorrhization of Quercus robur L., Quercus cerris L. and Corylus avellana L. seedlings with Tuber macrosporum Vittad. Mycorrhiza 2012, 22, 639–646. [Google Scholar] [CrossRef]
- Benucci, G.M.; Raggi, L.; Albertini, E.; Csorbai, A.G.; Donnini, D. Assessment of ectomycorrhizal biodiversity in Tuber macrosporum productive sites. Mycorrhiza 2014, 24, 281–292. [Google Scholar] [CrossRef]
- Berch, S.M. Advances in the Cultivation of Truffles in Canada. In Mushrooms, Humans and Nature in a Changing World; Pérez-Moreno, J., Guerin-Laguette, A., Flores Arzú, R., Yu, F.Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 287–296. [Google Scholar]
- Li, L.; Wan, S.; Wang, Y.; Thongklang, N.; Yang, M.; Liu, C.; Luo, Z.; Li, S. Three New Species of Tuber Discovered in Alpine Fir Forests in Yunnan, China. J. Fungi 2024, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Han, L.; Zhang, P.R.; Yan, X.Y. Molecular analysis of Chinese truffles resembling Tuber californicum in morphology reveals a rich pattern of species diversity with emphasis on four new species. Mycologia 2016, 108, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, G.; Li, Y.; Wang, R.; Yang, S.; Yuan, J.; Xie, X.; Shi, X.; Yu, J.; Pérez-Moreno, J.; et al. Three New Truffle Species (Tuber, Tuberaceae, Pezizales, and Ascomycota) from Yunnan, China, and Multigen Phylogenetic Arrangement within the Melanosporum Group. J. Fungi 2024, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.P.; Tang, L.; Zheng, Y.; Yu, F.Q. Two novel Tuber species (Tuberaceae, Pezizales) in the Latisporum Group from China. Mycoscience 2017, 58, 312–319. [Google Scholar] [CrossRef]
- Wan, S.P.; Xu, W.J.; Tan, N.; Wang, Y.; Zheng, Y.; Yu, F.Q. Three Excavatum species Tuber badium, T. depressum and T. verrucosivolvum from Sichuan Province, China. Phytotaxa 2017, 296, 228–238. [Google Scholar] [CrossRef]
- Li, Y.; Fan, L.; Wang, K.; Song, M.S.; Wang, X.C.; Zhuang, W.Y.; Yao, Y.J. Status and Protection of Threatened Truffle (Tuberaceae) Resources in China. Acta Edulis Fungi 2020, 27, 109–119. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Nylander, J.A.A. MrModeltest 2.3. Program Distributed by the Author. Evolutionary Biology Center; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Stamatakis, A. RAxML-VI-HPC: Maximum-likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.P. A new species of Tuber from China. Acta Mycol. Sin. 1991, 10, 263–265. [Google Scholar]
- Gilkey, H.M. Tuberales of North America. Oregon State Monographs. Stud. Bot. 1939, 1, 1–63. [Google Scholar]
- Riousset, G.; Riousset, L.; Chevalier, G.; Bardet, M.C. Truffle d’ Europe et de Chine; Institut National de la Recherche Agronomique: Paris, France, 2001; p. 181. [Google Scholar]
- Cacialli, G. Le specie europee del genere Tuber. Una revisione storica. Mycologist 2005, 19, 134–135. [Google Scholar] [CrossRef]
- Miko, M.; Jan, G.; Bratek, Z. Tuber macrosporum Vitt. and Tuber mesentericum Vitt. one hundred years neglected hypogeous fungi species in Slovak Republic. Acta Fytotechn Zootech 2006, 4, 85–90. [Google Scholar]
- Marjanović, Ž.; Grebenc, T.; Marković, M.; Glišić, A.; Milenković, M. Ecological specificity and molecular diversity of truffles (genus Tuber) originating from mid-west of the Balkan Peninsula. Sydowia 2010, 62, 67–87. [Google Scholar]
- Gógán, C.A. Studies on Cultivation Possibilities of Summer Truffle (Tuber aestivum Vittad.) and Smooth Black Truffle (Tuber macrosporum Vittad.) in Hungary. Ph.D. Thesis, SzentIstván University, Gödöllő, Budapest, 2011. [Google Scholar]
- Lefevre, C. Native and Cultivated Truffles of North America. In Edible Ectomycorrhizal Mushrooms; Zambonelli, A., Bonito, G.M., Eds.; Springer: Cham, Switzerland, 2012; pp. 220–221. [Google Scholar]


| Species | Voucher/Isolate | Origin | Tissue | ITS GenBank | Host |
|---|---|---|---|---|---|
| T. canaliculatum | JT12670 | Canada | ascocarp | GQ221455 | Quercus sp. Pinus sp. |
| T. canaliculatum | Tbc1 | Canada | ascocarp | OM948973 | - |
| T. canaliculatum | 1662_PREMIX | USA | ectomycorrhiza | OQ612551 | Quercus rubra |
| T. canaliculatum | AMC495 | USA | ectomycorrhiza | OQ207646 | Betula lenta |
| T. canaliculatum | AMC101 | USA | ectomycorrhiza | OP413007 | Quercus alba |
| T. canaliculatum | FLAS-F-68694 | USA | ascocarp | OM416870 | - |
| T. canaliculatum | OSC59072 | USA | ascocarp | HM485347 | - |
| T. macrosporum | ITA_011s | Italy | ascocarp | KP738396 | - |
| T. macrosporum | ITA_010S | Italy | ascocarp | KP738394 | - |
| T. macrosporum | HUN_21 | Hungary | ascocarp | KP738377 | - |
| T. macrosporum | HUN_20 | Hungary | ascocarp | KP738376 | - |
| T. macrosporum | FRA_004 | France | ascocarp | KP738363 | - |
| T. macrosporum | FRA_003 | France | ascocarp | KP738362 | - |
| T. macrosporum | SLO_001 | Slovakia | ascocarp | KP738360 | - |
| T. macrosporum | HMSFI_TUBMAC/141207A | Slovenia | ascocarp | FM205634 | - |
| T. macrosporum | SER_002 | Serbia | ascocarp | KP738358 | - |
| zT. macrosporum | SER_001 | Serbia | ascocarp | KP738357 | - |
| T. macrosporum | CMI-Unibo_4938 | Iran | ascocarp | MW884554 | - |
| T. macrosporum | CMI-Unibo_4937 | Iran | ascocarp | MW884553 | - |
| T. macrosporum | ANK_Akata_7398 | Turkey | ascocarp | MW362409 | - |
| T. macrosporum | ANK_Akata_7390 | Turkey | ascocarp | MW362408 | - |
| T. macrosporum | UASWS1867 | Switzerland | ascocarp | KY197983 | - |
| T. macrosporum | NW1TMacro2 | Poland | ascocarp | KJ524532 | - |
| T. macrosporum | NW1TMacro1 | Poland | ascocarp | KJ524529 | - |
| T. macrosporum | S7510_7610 | Germany | ascocarp | JF926121 | - |
| T. macrosporum | ITA_012S | Italy | ascocarp | KP738346 | - |
| T. macrosporum | UE_ITA032 | Italy | ectomycorrhiza | JX474822 | - |
| T. macrosporum | UE_ITA005 | Italy | ectomycorrhiza | JX474809 | - |
| T. macrosporum | UE_ITA026 | Italy | ectomycorrhiza | JX474821 | - |
| T. macrosporum | UE_ITA240 | Italy | ectomycorrhiza | JX474839 | - |
| T. tomentosum | K108 (holotype) | Japan | ascocarp | AB553349 | Quercus acutissima Quercus crispula Quercus serrata |
| T. tomentosum | AK1907 | Japan | ascocarp | LC570852 | Quercus serrata |
| T. tomentosum | AK1909 | Japan | ascocarp | LC570851 | Quercus sp. |
| T. tomentosum | K22 | Japan | ascocarp | AB553345 | Quercus myrsinifolia |
| T. tomentosum | K440 | Japan | ascocarp | AB553365 | Quercus serrata Quercus crispula |
| T. tomentosum | K446 | Japan | ascocarp | AB553367 | Quercus serrata |
| T. tomentosum | K215 | Japan | ascocarp | AB553356 | Quercus acutissima |
| T. tomentosum | K85 | Japan | ascocarp | AB553347 | Abies firma Carpinus tschonoskii Quercus serrata |
| T. tomentosum | K103 | Japan | ascocarp | AB553348 | Pinus densiflora |
| T. tomentosum | K407 | Japan | ascocarp | AB553358 | Castanopsis sieboldii Quercus glauca |
| T. tomentosum | K411 | Japan | ascocarp | AB553360 | Pinus thunbergii |
| T. glabrum | BJTCFAN228 (holotype) | China: Yunnan | ascocarp | KF002731 | Pinus yunnanensis |
| T. glabrum | BJTCFAN232 | China: Yunnan | ascocarp | KF002727 | Pinus yunnanensis |
| T. glabrum | YNAU1674 | China: Yunnan | ascocarp | PQ181852 | Pinus armandii |
| T. glabrum | HKAS96140 | China: Yunnan | ascocarp | PQ181853 | Pinus armandii |
| T. calosporum | HKAS:88790 (holotype) | China: Yunnan | ascocarp | KT444598 | Pinus yunnanensis |
| T. calosporum | HKAS95788 | China: Yunnan | ascocarp | PQ168923 | - |
| T. calosporum | HKAS:88791 | China: Yunnan | ascocarp | KT444597 | Pinus yunnanensis |
| T. calosporum | HKAS:88751 | China: Yunnan | ascocarp | KT444600 | Pinus yunnanensis |
| T. sinomonosporum | BJTCFAN150 (holotype) | China: Yunnan | ascocarp | KF002729 | - |
| T. sinomacrosporum | YNAU016 (holotype) | China: Yunnan | ascocarp | OK625303 | Pinus sp. |
| T. iryudaense | HS1309 | Japan | ascocarp | LC570849 | Quercus serrata |
| T. iryudaense | HS1223 (holotype) | Japan | ascocarp | LC570848 | Quercus serrata Quercus salicina Castanopsis sieboldii |
| T. iryudaense | KPM-NC25676 | Japan | ascocarp | LC570847 | Quercus serrata |
| T. iryudaense | K201 | Japan | ascocarp | AB553344 | Quercus serrata |
| T. indicum | HKAS49752 | China | ascocarp | GU979079 | - |
| T. indicum | HKAS47614 | China | ascocarp | GU979077 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; E, L.; Wang, R.; Yu, C.; Yang, J.; Li, Y.; Jiang, X.; Song, J.; Yu, F.; Wang, R.; et al. Deeper Insights into Species Diversity and Ecological Characterisation of the Macrosporum Group of the Genus Tuber. Diversity 2025, 17, 92. https://doi.org/10.3390/d17020092
Liu R, E L, Wang R, Yu C, Yang J, Li Y, Jiang X, Song J, Yu F, Wang R, et al. Deeper Insights into Species Diversity and Ecological Characterisation of the Macrosporum Group of the Genus Tuber. Diversity. 2025; 17(2):92. https://doi.org/10.3390/d17020092
Chicago/Turabian StyleLiu, Ruilong, Lingfang E, Rui Wang, Chengjin Yu, Jingsheng Yang, Yuenan Li, Xuesong Jiang, Junyue Song, Fuqiang Yu, Ruixue Wang, and et al. 2025. "Deeper Insights into Species Diversity and Ecological Characterisation of the Macrosporum Group of the Genus Tuber" Diversity 17, no. 2: 92. https://doi.org/10.3390/d17020092
APA StyleLiu, R., E, L., Wang, R., Yu, C., Yang, J., Li, Y., Jiang, X., Song, J., Yu, F., Wang, R., & Wan, S. (2025). Deeper Insights into Species Diversity and Ecological Characterisation of the Macrosporum Group of the Genus Tuber. Diversity, 17(2), 92. https://doi.org/10.3390/d17020092






