Abstract
This study reports two new species of Ampharete and one new record of a species of Amphicteis (Ampharetidae) from the Korean subtidal zone, based on an integrative approach. Ampharete koreana sp. nov. is characterized by paleal chaetae several times thicker than notochaetae, with short filamentous tips. It more closely resembles A. finmarchica but differs in the shape of its paleae, interbranchial gap, thoracic uncini, and pygidial cirri and shows a 2% genetic divergence in histone H3 and in 18% of mtCOI genes. Ampharete namhaensis sp. nov. shares with A. petersenae the absence of paleae and the presence of 16 abdominal uncinigers, a rare combination of traits among known Ampharete species. However, A. petersenae differs in having a marked interbranchial gap, prominent eyes on the prostomium, double the number of abdominal uncini and buccal tentacles, and in its distribution (southern Korea for A. namhaensis sp. nov. vs. North Atlantic and Arctic-adjacent waters for A. petersenae). The Korean Amphicteis specimens examined in this study closely resemble A. glabra in key morphological features, including fine paleae tips, 14 thoracic and 15 abdominal uncinigers, and approximately eight paleae. However, given the need for a comprehensive revision of Amphicteis and the potential for cryptic diversity within the genus, these specimens are provisionally designated as Amphicteis sp. cf. A. glabra.
1. Introduction
Ampharetidae are primarily tentaculate surface-deposit feeding, tube-dwelling polychaetes, inhabiting muddy, sandy, and organically enriched sediments [1,2]. They predominantly consume diatoms, green algae, and multicellular organic detritus, mixed with sediment particles, thereby playing a critical role in benthic organic matter recycling [1,3]. In Gorgan Bay, ampharetids serve as food for commercially important fish, such as sturgeons [4]. Ampharetids are globally distributed from intertidal to deep-sea zones deeper than 8000 m, including Polar Regions [2,5], with their remarkable adaptability allowing them to thrive in brackish and even fresh waters. Certain species, highly sensitive to organic pollution and hypoxia, are effective bioindicators of benthic ecosystems’ health [2,6]. Others, such as those inhabiting hydrothermal vents, produce unique compounds, underscoring their value as industrial and pharmaceutical resources [7,8]. In particular, the secondary metabolites secreted during tube-building possess novel chemical molecules with potential applications in drug development [7].
Ampharetidae, first established by Malmgren (1866) [9], show a typical hood-shaped prostomium, commonly bear four pairs of branchiae and numerous peribuccal feeding tentacles, and have built tubes with fine particles bounded with mucus [1,10]. Early generic classification primarily relied on branchial number and arrangement, paleae characteristics, and the number of thoracic uncinigers [11]. More recently, the classification incorporates prostomium types (11 recognized forms), the presence or absence of ridges on the 4th–5th thoracic notopodia (4–5 TN), and detailed parapodial characteristics [5,12,13,14]. This highly diverse family currently includes approximately 300 species grouped in 52 genera, 28 of them monospecific [5,8,14]. This emphasizes the need for considerable expertise to accurately distinguish genera and species [2], although recent molecular and morphological advancements have improved the taxonomic resolution [15,16,17,18].
The genus Ampharete, one of the most speciose within the family, currently includes 48 species [2]. However, increasing scrutiny of its polyphyletic nature and evolution of diagnostic criteria has prompted species revisions [14,18]. These include reexamining the cosmopolitanism of Ampharete finmarchica (M. Sars, 1865) [2,14,19,20], with detailed morphological assessment of paleae, branchiae, parapodia, and pygidial cirri revealing frequent misidentifications of its sister taxa [20,21].
In contrast, taxonomic studies of Korean Ampharetidae remain limited, with only five species having been documented over the past three decades, primarily based on brief descriptions and single photographs [22,23,24]. More recently, Lee et al. [25] added Paramphicteis weberi (Caullery, 1944). The Korean species Ampharete arctica sensu stricto Paik 1979 [23] has been widely reported across various depths, being a bioindicator of relatively healthy conditions. However, it has been recently synonymized with A. finmarchica, whose sister species have been globally misidentified [20,21]. Despite lacking robust taxonomic validation, the species is continuously referred to in ecological studies in Korea, with the single exception of Kim et al. [26], who cautiously reported it as Ampharete cf. finmarchica.
This study aims to re-evaluate the records of A. finmarchica (previously as A. arctica) and to assess the diversity of Ampharetidae in Korean waters, providing detailed descriptions, illustrations, and molecular data for the identified species, and therefore contributing to understanding their taxonomy and distribution in Korean waters.
2. Materials and Methods
2.1. Morphological Analysis
Samples were collected from 13 sublittoral stations in semi-enclosed bays of southern Korean coasts and the East China Sea near the Korean peninsula (Figure 1) using a 0.05 m2 Van Veen grab. Sediment samples were elutriated over a 0.5 mm sieve into a 30 L seawater container and transferred to a 1 L jar with 7% MgCl2 solution for anesthesia. Relaxed specimens were fixed in a buffered 10% formalin solution within one hour and subsequently preserved in 80% ethanol. Additionally, 112 preserved specimens of A. arctica and Ampharete sp. were borrowed from the Marine Biodiversity Institute of Korea (MABIK).
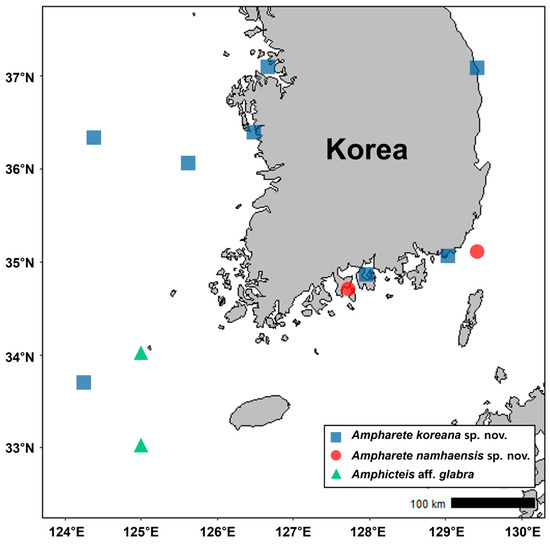
Figure 1.
Map of Korea showing the collection sites and the ampharetids newly reported at each site.
In the laboratory, ampharetids were sorted using a stereomicroscope (Stemi 305; ZEISS, Oberkochen, Germany) and identified to species level under a stereomicroscope (SMZ745T; Nikon, Tokyo, Japan). Specimens were stained with Shirlastain A solution (SDLATLAS, Rock Hill, SC, USA) or methylene green zinc chloride double salt solution (M7766-25G; Sigma, St. Louis, MO, USA) to increase contrast and reveal staining patterns and photographed and measured using an Eclipse Ci-L camera (Nikon, Tokyo, Japan) coupled with the OSUN 2.0 software (Osun Hightech, Goyang-si, Republic of Korea). Terminology for specimens used in this study followed Jirkov [5,12].
The examined materials were deposited in MABIK collection in Seocheon (Republic of Korea) and in the Marine Annelida collection at Chonnam National University (JUMA), Yeosu, Republic of Korea (Table 1).

Table 1.
List of species names examined in this study, as well as their NCBI accession numbers, collection sites, and associated references.
2.2. Molecular Analysis
Genomic DNA was extracted from ethanol-preserved specimens by partially dissecting branchial tissues, which were incubated in 1.5 mL centrifuge tubes containing 45 μL of 10% Chelex suspension (Bio-Rad Laboratories Inc., Hercules, CA, USA) and 5 μL of Proteinase K (10 mg/mL; iNtRON Biotechnology, Seongnam-si, Republic of Korea) at 56 °C for 12 h. The extracted genomic DNA was used as a template to amplify the target gene region. Polymerase chain reaction (PCR) was performed on a TaKaRa PCR Thermal Cycler Dice® Gradient (TP600; Takara Co., Kusatsu, Japan) using the H3F and H3R primer pair for histone H3 [30]. The PCR mixture consisted of 16 μL of deionized water, 1 μL of each primer (10 μM), 2 μL of DNA template, and 20 μL of PCR premix (BiONEER Co., Daejeon, Republic of Korea). The temperature profile protocol for amplification followed [31,32]. Purification and sequencing of the PCR products were conducted by Macrogen Inc. (Seoul, Republic of Korea). Forward and reverse sequences were edited using Chromas version 2.3 (Technelysium Pty. Ltd., South Brisbane, Australia). Partial sequences of the histone H3 gene were aligned with available sequences from related species retrieved from the NCBI database (www.ncbi.nlm.nih.gov/Genbank, accessed on 22 December 2024) using MEGA software (version 11.0) [33]. Aligned sequences were analyzed to estimate genetic distances using Kimura’s two-parameter (K2P) model [34]. K2P distances were used to evaluate interspecific differences between all examined taxa.
3. Results
3.1. Systematics
Family Ampharetidae Malmgren, 1866 [9].
Subfamily Ampharetinae Malmgren, 1866 [9].
3.2. Ampharete koreana sp. nov.
Ampharete arctica Paik 1979; figure B.
Figure 2A–P.
urn:lsid:zoobank.org:act:78A19256-5C08-4C1D-B6ED-19390BFB8E91.
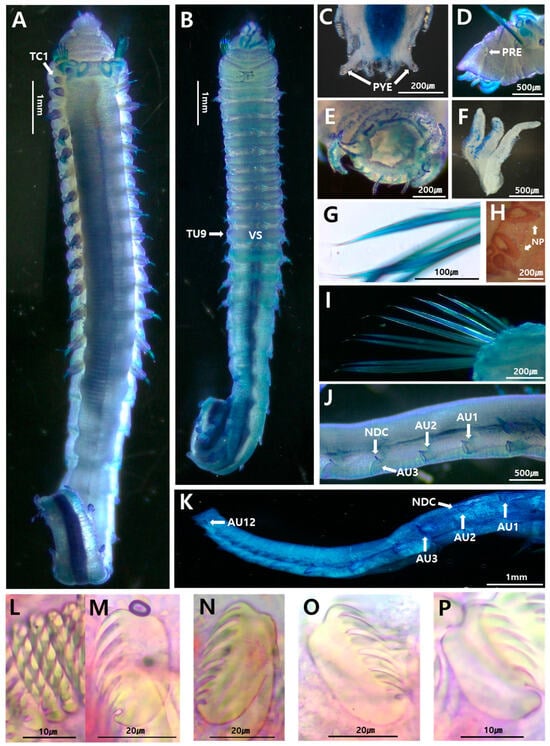
Figure 2.
Ampharete koreana sp. nov.: (A) entire body, dorsal view; (B) thorax, ventral view; (C) posterior end with pygidium, ventral view; (D) anterior end with prostomial eye, lateral view; (E) pygidium, frontal view; (F) buccal tentacles; (G) bilimbated capillary notochaetae on thoracic chaetiger 5; (H) nephridial papillae; (I) paleae; (J) thorax–abdomen junction, right lateral view of last thoracic segment and first three abdominal segments; (K) abdomen, right lateral view; (L) uncini of TU1, frontal view; (M–P) uncini of TU1, TU5, AU1, AU12, respectively, lateral view. AU, abdominal unciniger; NDC, neuropodial dorsal cirri; NP, nephridial papillae; PRE, prostomial eye; PYE, pygidial eye; TU, thoracic unciniger; VS, ventral shields. (A–J) were stained with methylene green, while (K–O) were stained with Shirlastain A for imaging.
3.2.1. Material Examined
Holotype: MABIKNA00058075, Republic of Korea, Uljin-gun (37°06′2.21″ N, 126°39′15.81″ E); subtidal zone, 10 m depth; collected by Byoung-Mi Choi, November 2011. Paratype: MABIKNA00058059, Republic of Korea, Uljin-gun (37°05′50″ N, 129°24′19.36″ E); subtidal zone, 27 m depth; collected by Byoung-Mi Choi, October 2011.
Additional material: two specimens from Republic of Korea used for morphological and DNA analysis; one from Gangjin-gun (34°52′39.71″ N, 127°56′48.47″ E; 6 m depth; collected by Jong Rae Kim, May 2024) and one from the southwestern boundary of Korean Exclusive Economic Zone (EEZ) (33°42′12.96″ N, 124°14′54.92″ E; 77 m depth; collected by Dae-Hun Kim and Jong Rae Kim, June 2024).
Examined MABIK voucher specimens: 82 previously identified as A. arctica deposited in MABIK.
3.2.2. Description
Holotype intact, measuring 26 mm long, 2.5 mm width (thorax). Prostomium trilobed; narrow, protruding median lobe delimited by deep lateral grooves; nuchal organs and prostomial glandular ridges absent. Two small, black circular eyespots on posterior side of median prostomial lobe near lateral grooves (Figure 2D). Peristomium visible laterally and ventrally; with well-developed buccal lip. Non-grooved buccal tentacles, bearing two ventrolateral rows of small papillae (Figure 2F). Four pairs of branchiae in fused segments II + III (SG2 + 3), in two groups with short median gap about one branchia wide; branchiophores not fused at base. Branchiae smooth, uniformly wide, tapering slightly at distal end, twice as long as prostomium, one-fourth as long as thorax, reaching thoracic chaetiger 5 (TC5); parallel ciliated rings absent; anterior three pairs of branchiae in transverse row; fourth pair most posterior, between second outermost and innermost branchiae. (Figure 2A). In total, 9–12 paleae on SG2 + 3, long; twice as thick and twice as long as regular notochaetae, with short, pointed tips (Figure 2H).
Thorax longer, wider than abdomen (Figure 2A). Fourteen thoracic chaetigers (=SG4–SG17) with notopodial capillary chaetae; last twelve segments with neuropodial tori bearing single rows of uncini. Nephridial papillae present (Figure 2A). Thoracic notopodia simple lobes, starting from SG4 (=TC1), up to three times longer than wide; first notopodia reduced. Notochaetae simple, spinulose bilimbated capillary chaetae, tapering to slender tips (Figure 2G). Thoracic neuropodia starting from SG6; anterior neuropodia oval-shaped, three times longer than wide. Cirri and papillae absent in thoracic parapodia. Thoracic uncini with 12–14 teeth arranged in two vertical rows above rostrum. Well-developed ventral shields to TU9, weakly developed in TU10, absent in TU11 (Figure 2B). Elevated or modified notopodia absent.
Abdomen shorter, thinner than thorax, with twelve uncinigers. Anterior two uncinigers (AU1–2) with neuropodia of thoracic type (=intermediate uncinigers) (Figure 2I). Remaining ten abdominal uncinigers (AU3–12) with enlarged neuropodial pinnules, bearing neuropodial dorsal cirrus (Figure 2J). Glandular pads above pinnules not observed in intermediate or abdominal uncinigers. Abdominal uncini of AU1–2 similar to thoracic ones; following abdominal uncini with typical abdominal shape, with 10–14 teeth arranged in two vertical rows above rostrum (Figure 2N,O). Pygidium crenulated, with low pygidial cirri and one pair of long lateral cirri (Figure 2E), each with pair of dark pigmented eyes at tip spots (Figure 2C).
3.2.3. Methyl Green Staining Patterns
Prostomium surface densely stained with light purple speckles (Figure 2A,D). Regions and segments 2–5 lightly stained compared to prostomium, appearing bright blue. From segment 6 onward, thoracic segments stained dark blue on surface, with the notopodial and neuropodial tori more intensely stained. Middle regions of stained thoracic segments appearing darker purple compared to surrounding areas; ventral shields are stained purple (Figure 2B,I). Abdomen and pygidium staining lighter blue compared to thorax. Edges of pygidial cirri staining a darker blue than pygidium; pygidial eyes and surrounding areas remain unstained (Figure 2E). Paleae and notochaetae staining a darker blue (Figure 2A,G,H).
3.2.4. Etymology
The specific epithet “koreana”, in its Latinized form, is a gentilic derived from Korea, which highlights its broad distribution in subtidal waters surrounding the southern Korean peninsula.
3.2.5. Remarks
Jirkov referred to Ampharete species characterized by having paleae with short filamentous tips and being several times thicker than thoracic notochaetae as the superspecies finmarchica, presenting eight species within this group [14]. The newly discovered Ampharete koreana sp. nov. from Korea aligns with this species group in terms of the characteristics of its paleae. Among the eight species included in the finmarchica group, A. koreana sp. nov. most closely resembles A. finmarchica by having prostomial eyes, bilimbated notochaetae, fewer than 20 paleae, and neuropodial dorsal cirri starting from the third abdominal unciniger. However, A. koreana sp. nov. differs by exhibiting an interbranchial gap and pygidial eyes, and possessing 12 abdominal uncinigers, 12–14 teeth on thoracic uncini, and 70–90 buccal tentacles (13, 10–12, and 40–50, respectively, in A. finmarchica). All 82 MABIK specimens identified as ‘A. arctica’ consistently agree with the morphology of A. koreana sp. nov.
Among the recorded Ampharete species lacking the distinctive characteristics of paleae mentioned earlier, A. koreana sp. nov. most closely resembles A. acutifrons and A. baltica with a pair of prostomial eyespots, fewer than 12 paleae, and cirriform pygidial papillae. However, the holotype of A. acutifrons lacks both prostomial eyespots and abdominal neuropodial dorsal cirri [35], and has 8 pygidial cirri, which distinguishes it from A. koreana sp. nov. (which has a minimum of 10–16 cirri). Ampharete baltica can be easily distinguished by its paleae being significantly longer than the prostomium and lack paleae with short filamentous tips.
Only A. lindstroemi and A. oculicirrata share the presence of prostomial eyespots and pygidial eyes with A. koreana. However, these species clearly differ in the shape of paleae and pygidial papillae, and the absence of abdominal neuropodial dorsal cirri. Furthermore, A. oculicirrata is easily distinguishable from A. koreana sp. nov. by having only 11 abdominal uncinigers.
In terms of geographic distribution, A. acutifrons, A. baltica, A. lindstroemi, and A. oculicirrata are primarily found in the North Atlantic, which contrasts with the distribution of A. koreana sp. nov. in the waters surrounding Korea.
3.2.6. Distribution and Ecology
Ampharete koreana sp. nov was collected subtidally from 4 to 77 m depth in the southern and eastern part of South Korea in May, June, October and November. In the station form mid-southern Korea region, surface sediments consisted of 45.9% of silt and 53.2% of clay and the salinity ranged from 30 to 32 PSU. The 82 MABIK specimens (now assigned to A. koreana sp. nov.) allowed confirming it broad distribution in the subtidal zone around Korea, from semi-enclosed bays to the southwestern Korean EEZ boundary. This species has not been reported outside Korean waters.
3.3. Ampharete namhaensis. sp. nov.
Figure 3A–O.
urn:lsid:zoobank.org:act:5E794EB2-75A6-4CA0-A718-9ED9C3A114A0.
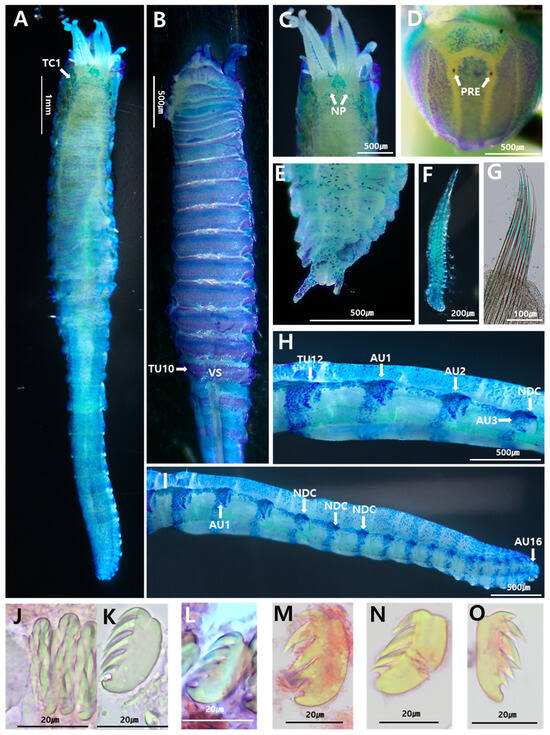
Figure 3.
Ampharete namhaensis sp. nov.: (A) entire body, dorsal view; (B) thorax, ventral view; (C) anterior end, dorsal view; (D) prostomium, frontal view; (E) posterior end with pygidium, ventral view; (F) buccal tentacle; (G) capillary notochaetae of thoracic chaetiger 6; (H) thorax–abdomen junction, left lateral view of the last one thoracic and three first abdominal segments; (I) abdomen, left lateral view; (J) uncini of TU1, frontal view; (K–O) uncini of TU1, TU12, AU1, AU2, AU12, respectively, lateral view. AU, abdominal unciniger; NDC, neuropodial dorsal cirri; NP, nephridial papillae; PRE, prostomial eye; TU, thoracic unciniger; VS, ventral shields. (A–I) were stained with methylene green, while (J–O) were stained with Shirlastain A for imaging.
3.3.1. Material Examined
Holotype: MABIKNA00126233, Republic of Korea, Busan (35°07′27.9″ N, 129°24′19.36″ E); subtidal zone, 115 m depth; collected by Byoung-Mi Choi, June 2009. Paratype: MABIKNA00126256, location and collector was same as holotype.
Additional material: two specimens from Republic of Korea used for morphological and DNA analysis were obtained from Yeosu (JUMA_20241219_002 and JUMA_20241206_019; 34°43′22″ N, 127°47′9″ E; 20 m depth; collected by Jong Rae Kim and Dae-Hun Kim, May 2024). Voucher specimens: 30 previously identified as unidentified Ampharete sp.1 deposited in MABIK.
3.3.2. Description
Holotype intact, measuring 11 mm long, 2.5 mm in wide (thorax). Prostomium trilobed; narrow, protruding median lobe delimited by deep lateral grooves; nuchal organs and prostomial glandular ridges absent. Two small, black circular eyespots on posterior side of median prostomial lobe near lateral grooves (Figure 3D). Peristomium visible laterally and ventrally; with well-developed buccal lip. Non-grooved buccal tentacles, bearing two ventrolateral rows of small papillae (Figure 3F). Four pairs of branchiae in fused segments II + III (SG2 + 3), in two groups with short median gap about one branchia wide; branchiophores non fused at base. Branchiae smooth, uniformly wide, tapering slightly at distal end, twice as long as prostomium, one-fourth as long as thorax, reaching TC5; parallel ciliated rings present; anterior three pairs of branchiae in transverse row; fourth pair most posterior, between second outermost and innermost branchiae. (Figure 3A). Paleal chaetae absent on fused segments II + III (SG2 + 3) (Figure 3C).
Thorax longer, wider than abdomen (Figure 3A). Fourteen thoracic chaetigers (SG4–SG17) with notopodial and capillary chaetae; last twelve segments with neuropodial tori bearing single rows of uncini. Small nephridial papillae present, between the posterior branchiae (Figure 3C). Thoracic notopodia simple lobes, starting from SG4 (=TC1), up to twice as longer than wide; first notopodia reduced. Notochaetae simple, spinulose capillary chaetae, tapering to slender tips (Figure 3G). Thoracic neuropodia starting from SG6; anterior neuropodia oval-shaped, twice longer than wide. Cirri and papillae absent in thoracic parapodia. Thoracic uncini with eight teeth arranged in two vertical rows above rostrum (Figure 3J,K). Well-developed ventral shields to TU10, weakly developed in TU11, absent in TU12 (Figure 3B). Elevated or modified notopodia absent.
Abdomen shorter, thinner than thorax, with sixteen uncinigers. Anterior two uncinigers (AU1–2) with neuropodia of thoracic type (=intermediate uncinigers) (Figure 3H). Remaining fourteen abdominal uncinigers (AU3–12) with enlarged neuropodial pinnules, bearing neuropodial dorsal cirrus (Figure 3I). Glandular pads above pinnules not ob-served in intermediate or abdominal uncinigers. Abdominal uncini of AU1–2 similar to thoracic ones; following abdominal uncini with typical abdominal shape, with 8–10 teeth arranged in two vertical rows above rostrum (Figure 3M–O). Pygidium crenulated, with low pygidial papillae and one pair of long lateral cirri (Figure 3E).
3.3.3. Methyl Green Staining Patterns
Anterior region and lateral parts of prostomium stained purple; dorsal part stained blue. Deep lateral grooves housing prostomial eye unstained (Figure 3D). Branchiae and dorsum of first five thoracic segments faintly stained light blue; dorsum of posterior thorax slightly darker than anterior segments (Figure 3A,C). From 6th thoracic segment onward, parapodial tori, segment boundaries, and ventral shields stained dark purple (Figure 3B). Posterior margins of abdominal chaetigers stained as dark blue bands on ventral side (Figure 3H,I). Pygidium stained lighter blue than thorax; lateral pygidial cirri stained darker blue than pygidium (Figure 3E).
3.3.4. Etymology
The specific epithet “namhaensis” is a latinized toponymic derived from “Namhae”, the Korean term for the southern sea of Korea, where this new species is widely distributed.
3.3.5. Remarks
Ampharete namhaensis sp. nov. is the unique species of Ampharete lacking paleae and having 12 thoracic and 16 abdominal uncinigers. This closely resembles A. petersenae Jirkov, 1997. However, this species having the noticeable intebranchial gap between and the prominent prostomial eyes, shows 20 abdominal uncini per segment and 10 buccal tentacles (40 and 24, respectively, in A. namhaensis sp. nov.), and has a distribution restricted to the Northern Atlantic waters [36], while A. namhaensis sp. nov. occcurs exclusively along the southern coasts of Korea. All 30 MABIK specimens of ‘unidentified Ampharete sp.1’ consistently agree with the morphology of A. namhaensis sp. nov.
3.3.6. Distribution and Ecology
Ampharete namhaensis sp. nov. was collected subtidally at 4–77 m depth in southern and eastern part of South Korea in May, June, October, and November, mainly on sandy mud bottoms. Salinity ranged from 30 to 33PSU. The 30 MABIK specimens labeled as ‘unidentified Ampharete sp.1’ allowed for the confirmation of the presence of the species along the mid-southern Korean waters, being particularly numerous in the southeastern region near Busan. This species has not been reported outside of Korea.
3.4. Amphicteis sp. cf. A. glabra Moore, 1906 [37]
Amphicteis glabra Moore, 1906. Plate XLVI, Figures 5–8.
Figure 4A–O.
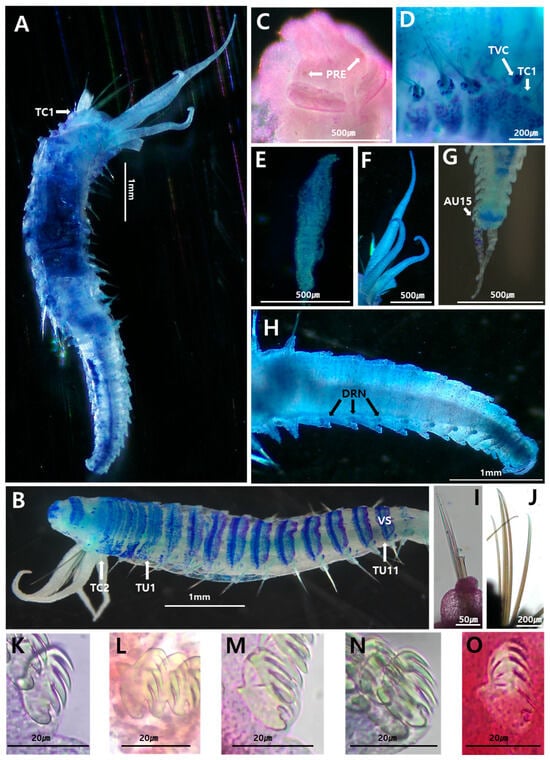
Figure 4.
Amphicteis aff. glabra (MABIK NA00158745, MABIK NA00158746): (A) entire body, dorsal view; (B) thorax, ventral view; (C) prostomium, frontal view; (D) thoracic chaetigers 1–5, right lateral view; (E) buccal tentacle; (F) branchiae, dorsal view; (G) posterior end with pygidium, ventral view; (H) abdomen, dorsal view; (I) capillary notochaetae of thoracic chaetiger 5; (J) paleae; (K–O) uncini of TU1, TU7, AU1, AU2, AU5, respectively, lateral view. AU, abdominal unciniger; DRN, digitiform rudimentary notopodia; PRE, prostomial eye; TC, thoracic chaetiger; TU, thoracic unciniger; TVC, tuberculate ventral cirri; VS, ventral shields. (A,B,D–H) were stained with methylene green, whereas (C,I–O) were stained with Shirlastain A for imaging.
3.4.1. Material Examined
The principal specimen used for description was MABIK NA00158746, Republic of Korea, Gageo-do (34° N, 125° E); subtidal zone, 33 m depth; collected by Jong Rae Kim, June 2024. An additional specimen, MABIK NA00158745, was dissected to observe detailed morphological characteristics; this specimen was collected from the Republic of Korea, Hong-do (35°0′59.96″ N, 124°59′35″ E), subtidal zone, 78 m depth, by Jong Rae Kim and Dae-Hun Kim in June 2009. The partial tissue of branchia from the specimen ‘MABIK NA00158746′ was used for DNA analysis.
3.4.2. Description
Complete specimen (MABIK NA00158746) measuring 7.7 mm long, 1.1 mm in width (thorax). Prostomium with paired longitudinal glandular ridges straight; gap between glandular ridges absent (Figure 4C). Paired nuchal ridges, separated by small median gap. Two small, black, circular eyespots on posterior side of median prostomial lobe near lateral grooves (Figure 4C). Peristomium visible laterally and ventrally; with well-developed buccal lip. Non-grooved buccal tentacles, smooth (Figure 4E). Four pairs of uniformly wide branchiae in fused segments II + III (SG2 + 3), in two groups with short median gap about one branchia wide; two pairs located anteriorly in SG2 + 3, with innermost branchiae in segment II and outermost in segment III; remaining two pairs posterior, with innermost branchiae in segment IV and outermost in segment V (Figure 4A,F). Branchiophores not fused at base. Branchiae smooth, uniformly wide, tapering slightly at distal end, six times as long as prostomium, two–three times as long as thorax, reaching TC12; parallel ciliated rings absent; anterior two pairs in transverse row; three pairs most posterior, between second outermost and innermost branchiae. (Figure 4A,F). Total of 6–8 paleae on SG2 + 3, twice as thick and four times as long as regular notochaetae, longer than following notochaetae, pointed tips (Figure 4J).
Thorax longer, wider than abdomen (Figure 4A). Seventeen thoracic chaetigers (SG4–SG17) with notopodia and capillary chaetae; last fourteen segments with neuropodial tori bearing single row of uncini. Nephridial papillae not observed (Figure 4A). Tuberculate ventral cirri present (Figure 4D). Thoracic notopodia lobes, starting from SG4 (=TC1), up to twice as long as they are wide; first notopodia reduced. Notochaetae simple, spinulose capillary chaetae, tapering to slender tips (Figure 4I). Thoracic neuropodia starting from SG7; anterior neuropodia oval-shaped, three times longer than wide. Cirri and papillae absent in thoracic parapodia. Thoracic uncini with four–five teeth arranged in one vertical row above rostrum; subrostral process present. Well-developed ventral shields in TU11, weakly developed in TU12, absent in TU13 (Figure 4B). Elevated or modified notopodia absent.
Abdomen shorter, thinner than thorax, with fifteen uncinigers. Eleven anterior abdominal uncinigers with digitiform rudimentary notopodia (Figure 4H). Remaining abdominal uncinigers (AU1–15) with enlarged neuropodial pinnules, without dorsal neuropodial cirrus (Figure 4H). Glandular pads above pinnules not observed in intermediate or abdominal uncinigers. Following abdominal uncini with typical abdominal shape, with four–five teeth arranged in vertical rows above rostrum (Figure 4M–O). Pygidium crenulated, with low pygidial papillae and one pair of long lateral cirri (Figure 4G).
3.4.3. Methyl Green Staining Patterns
Prostomium stained light blue; longitudinal glandular ridges and paired nuchal ridges stained dark blue. Branchiae stained lighter than prostomium, appearing sky blue; color faded rapidly within minutes (Figure 4B). Dorsum of thorax stained blue; thoracic noto- and neuropodia and tuberculate ventral cirri stained darker blue than surroundings (Figure 4A,D). Ventral side of thorax exhibited more prominent staining than dorsal side. Ventral shield stained blue, with central part forming a darker blue band (Figure 4B). Abdomen stained lighter blue than thorax; abdominal neuropodia and digitiform rudimentary notopodia stained darker blue (Figure 4H). Pygidium and pygidial long lateral cirri stained dark blue (Figure 4D). Notochaetae temporarily stained sky blue; color faded quickly.
3.4.4. Remarks
The specimens of Amphicteis found in Korean waters consistently match the original description of A. glabra, particularly in having prostomial eyes, paleae with fine tips and a length that extends beyond the prostomium, 14 thoracic and 15 abdominal uncinigers, and fewer than 10 paleae. However, A. glabra was originally described from Alaskan waters and subsequently reported from California. Notably, the species has not been recorded from Russian waters [38]. The morphological characteristics of the Korean specimens observed in this study are also consistent with other species of the genus Amphicteis. Given the limited taxonomic resolution within this genus, a broader systematic revision of Amphicteis is necessary to clarify species boundaries. Furthermore, we did not observe any distinct morphological differences that would justify treating these specimens as a separate species at this time. However, the possibility that this represents a new species cannot be overlooked, particularly given the taxonomic complexity within Amphicteis. To determine whether the Korean specimens are truly distinct from A. glabra or from other closely related Amphicteis species, a comprehensive revision of the genus, including access to type material, is required. This led us to consider the Korean specimens as “Amphicteis aff. glabra”. The use of “aff.” is appropriate in this case, as it acknowledges the strong morphological resemblance to A. glabra while recognizing the existing taxonomic uncertainty within the genus. We refrain from using “cf.” as there are no clear morphological differences observed that would justify such a designation.
3.4.5. Distribution and Ecology
Amphicteis aff. glabra were sampled subtidally at 82–92 m depth near the boundary of southwestern Korean territorial waters in June 2021 and June 2024, mainly on sandy mud sediments, and the salinity ranged from 31 to 33 PSU. Amphicteis glabra is known to occur near the Alaskan EEZ, and the western coast of North America [37].
3.5. Molecular Comparison
The DNA barcodes for the histone H3 region of Ampharte koreana sp. nov. (PQ799499), A. namhaesis sp. nov. (PQ799501), and Amphicteis aff. glabra (PQ799502) were obtained for the first time in this study and have been made publicly available in the NCBI database. The histone H3 sequences of three Korean species and the sequences of registered ampharetids available in the NCBI database show clear differences at the species and genus levels, as detailed in Table 2. The average KP2 intrageneric H3 divergence in Amphicteis was 9%, while it was 4% in the other seven genera, and the average intergeneric distance was 8%. Ampharete koreana sp. nov. and A. namhaensis sp. nov. differed from 2% to 15% when compared with 18 other recorded species within the family, being closest to four species of Ampharete (4.5% in average) and most divergent from Amphicteis dalmatica (14.6% and 13%, respectively). The closest species to A. koreana sp. nov. were the Norwegian A. finmarchica (1.9%) and Icelandic Ampharete cirrata (2.2%, recorded as A. acutifrons in NCBI [35]), which agrees with our morphological analysis. Ampharete namhaensis sp. nov. could not be directly compared with other paleae lacking species of Ampharete, such as A. petersenae, due to lacking available public genetic data. However, it was closest to Ampharete californica (4.1%). H3 genetic divergence among cryptic polychaete species ranges from 2% to 9% [31,32,39,40], suggesting that the observed sequence differences fall within the range commonly associated with species-level differentiation in polychaetes. While we acknowledge that molecular divergence alone cannot serve as an absolute criterion for species delineation, when considered alongside distinct morphological characteristics, it provides supporting evidence for species differentiation. The morphological and genetic data presented in this study strongly suggest that A. koreana sp. nov. and A. namhaensis sp. nov. represent new species in Korean waters.

Table 2.
Genetic divergences between examined ampharetids and Korean specimens based on K2P distance.
Amphicteis aff. glabra was genetically closest to Amphicteis obscurior (6.7%) and Amphicteis gunneri (9.8%), and most divergent from Neosabellides elongatus (13.7%). Nevertheless, A. aff. glabra clearly differed genetically from A. gunneri, the only previously known species of Amphicteis in Korea (Table 2).
4. Discussion
Our study of three previously undescribed ampharetids from the subtidal zone of Korea provides morphological descriptions and supplementary molecular data that contribute to clarifying their taxonomic placement. Although mtCOI, 16S, and 18S rRNA barcodes are frequently used for species-level genetic identification of polychaetes that are morphologically similar to cryptic species, the histone H3 sequences obtained in this study effectively distinguished the ampharetids of Korea from closely related taxa available in the NCBI database at the genus and species levels. However, the limited availability of histone H3 sequences in public databases somewhat restricted its use in integrative species identification in this study. On the other hand, the methyl green staining patterns (MGSP) of Korean species identified in this study showed clear differences at the species level, and distinctive staining patterns on the ventral surface of each species were easily observable. If comprehensive morphological identification and the continuous acquisition of genetic barcodes and MGSP for a broader range of ampharetids are systematically conducted, it will significantly enhance our understanding of this highly diverse family.
Ampharete koreana sp. nov. morphologically resembles A. finmarchica. However, they clearly differed morphologically and molecularly, showing a 2% genetic divergence in histone H3 sequences and an 18% difference in mtCOI (authors, unpublished data), when compared with publicly available sequences of Norvergian A. finmarchica (accession number OR891684). Previous Korean records of A. arctica, a junior synonym of A. finmarchica, underscore our finding, as this was the only species of Ampharete recorded in Korean waters over the past four decades. Paik [23] described A. arctica in a short paper with a single illustration erroneously reporting three pairs of branchiae, which may likely be a typographical error, as the species of this genus typically have four pairs. Paik [23] did not mention the number of abdominal segments, but clearly illustrated 12 abdominal uncinigers, a distinct feature of A. koreana sp. nov. (13 in A. finmarchica). The 82 MABIK specimens labeled as A. arctica, collected from various regions across Korea, consistently exhibited the fine-tipped paleae and 12 abdominal segments typical of A. koreana sp. nov. This new species inhabits shallow coastal areas and small bays less than 100 m deep, whereas A. finmarchica is predominantly found in the lower sublittoral level at high-latitude regions [14]. Therefore, all previous records of A. arctica or A. finmarchica in Korean waters are likely misidentifications of A. koreana sp. nov. Concerning A. namhaensis sp. nov., direct molecular comparisons with morphologically similar species, such as A. petersenae, cannot be conducted due to the lack of publicly available data. Nevertheless, our molecular analyses confirmed it did not match any published ampharetid data, further supporting the acknowledgement of the new species.
Author Contributions
Conceptualization: J.-R.K. and M.-K.J.; methodology: M.-K.J. and D.-H.K.; formal analysis: D.-H.K. and J.-R.K.; investigation: D.-H.K. and J.-R.K.; visualization: D.-H.K., J.-R.K. and M.-K.J.; resources: M.-K.J.; supervision: M.-K.J.; writing—original draft preparation: J.-R.K. and M.-K.J.; writing—review and editing: M.-K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the management of Marine Fishery Bio-resources Center (2024), funded by the National Marine Biodiversity Institute of Korea (MABIK), and a part of the project titled “Research center for fishery resource management based on the information and communication technology” (2025, grant number 20180384), funded by the Ministry of Oceans and Fisheries, Korea. This study was financially supported by Chonnam National University (Grant number: 2023-0934).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the anonymous reviewers and the editor who made constructive and invaluable suggestions and comments. We also thank the captain and crew of the R/V Sae Dong Baek-Ho for their support at sea.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations
| AU | abdominal unciniger |
| DRN | digitiform rudimentary notopodia |
| JUMA | Chonnam National University |
| MABIK | Marine Biodiversity Institute of Korea |
| NDC | neuropodial dorsal cirri |
| NP | nephridial papillae |
| PRE | prostomial eyes |
| PYE | pygidium eyes |
| SG | segment |
| TN | thoracic notopodia |
| TU | thoracic unciniger |
| TVC | tuberculate ventral cirri |
| VS | ventral shields |
References
- Rouse, G.; Pleijel, F.; Tilic, E. Annelida; Oxford University Press: Oxford, UK, 2022; p. 432. [Google Scholar]
- Read, G.F.K. (Ed.) World Polychaeta Database. Ampharetidae Malmgren, 1866. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=981 (accessed on 22 December 2024).
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar] [CrossRef]
- Saghali, M.; Baqraf, R.; Hosseini, S.A.; Patimar, R. Benthic community structure in the Gorgan Bay (Southeast of the Caspian Sea, Iran): Correlation to water physiochemical factors and heavy metal concentration of sediment. Int. J. Aquat. Biol. 2013, 1, 245–253. [Google Scholar] [CrossRef]
- Jirkov, I.A. Discussion of taxonomic characters and classification of Ampharetidae (Polychaeta). Ital. J. Zool. 2011, 78, 78–94. [Google Scholar] [CrossRef]
- Borja, A.; Franco, J.; Pérez, V. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Reuscher, M.; Fiege, D.; Wehe, T. Four new species of Ampharetidae (Annelida: Polychaeta) from Pacific hot vents and cold seeps, with a key and synoptic table of characters for all genera. Zootaxa 2009, 2191, 1–40. [Google Scholar] [CrossRef]
- Malmgren, A.J. Nordiska Hafs-Annulater. Öfvers. Af K. Vetenskapsakademiens Förh. Stockh. 1866, 22, 355. [Google Scholar]
- Glasby, C.; Biriukova, O.; Martin, P.; Dyne, G.; Utevsky, S.; Wilson, R. Annelida–diagnoses, descriptions and keys to family-level taxa. ARPHA Prepr. 2024, 5, e137961. [Google Scholar] [CrossRef]
- Fauchald, K. The Polychaete Worms: Definitions and Keys to the Orders, Families and Genera; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1977; p. 188. [Google Scholar]
- Jirkov, I. Revision of Ampharetidae (Polychaeta) with modified thoracic notopodia. Invertebr. Zool. 2008, 5, 111–132. [Google Scholar] [CrossRef]
- Imajima, M.; Reuscher, M.G.; Fiege, D. Ampharetidae (Annelida: Polychaeta) from Japan. Part II: Genera with elevated and modified notopodia. Zootaxa 2013, 3647, 137–166. [Google Scholar] [CrossRef] [PubMed]
- Jirkov, I. Revision of Ampharete (superspecies finmarchica)(Annelida: Ampharetidae). Invertebr. Zool. 2023, 20, 1–26. [Google Scholar] [CrossRef]
- Stiller, J.; Rousset, V.; Pleijel, F.; Chevaldonné, P.; Vrijenhoek, R.C.; Rouse, G.W. Phylogeny, biogeography and systematics of hydrothermal vent and methane seep Amphisamytha (Ampharetidae, Annelida), with descriptions of three new species. Syst. Biodivers. 2013, 11, 35–65. [Google Scholar] [CrossRef]
- Colgan, D.; Hutchings, P.; Brown, S. Phylogenetic relationships within the Terebellomorpha. J. Mar. Biol. Assoc. UK 2001, 81, 765–773. [Google Scholar] [CrossRef]
- Colgan, D.J.; Hutchings, P.A.; Braune, M. A multigene framework for polychaete phylogenetic studies. Org. Divers Evol. 2006, 6, 220–235. [Google Scholar] [CrossRef]
- Eilertsen, M.H.; Kongsrud, J.A.; Alvestad, T.; Stiller, J.; Rouse, G.W.; Rapp, H.T. Do ampharetids take sedimented steps between vents and seeps? Phylogeny and habitat-use of Ampharetidae (Annelida, Terebelliformia) in chemosynthesis-based ecosystems. Bmc. Evol. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Parapar, J.; Helgason, G.V.; Jirkov, I.; Moreira, J. Polychaetes of the genus Ampharete (Polychaeta: Ampharetidae) collected in Icelandic waters during the BIOICE project. Helgoland Mar. Res. 2012, 66, 331–344. [Google Scholar] [CrossRef]
- Blake, J.A.; Hilbig, B.; Scott, P.H. Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel: Vol. 7, The Annelida Part 4; Department of the Interior Minerals Management Service, Science Applications International Corporation: Reston, VA, USA, 1996. [Google Scholar]
- Jirkov, I. Polychaeta of the Arctic Ocean; Yanus-K: Moscow, Russia, 2001; p. 632. [Google Scholar]
- Paik, E.I. Illustrated Encyclopedia of Fauna and Flora of Korea; Volume 31: Polychaeta; Ministry of Education: Seoul, Republic of Korea, 1989; Volume 764, p. 102. [Google Scholar]
- Paik, E.I. New records of three polychaetous annelid species in Korea. Bull. Korean Fish. Soc. 1979, 12, 277–280. [Google Scholar]
- Paik, E.I. Taxonomic studies on polychaetous annelids in Korea. Res. Bull. Hyosung Women’s Univ. 1982, 24, 745–913. [Google Scholar]
- Lee, G.H.; Yoon, S.M.; Min, G.-S. A New Record of Paramphicteis weberi (Polychaeta: Terebellida: Ampharetidae) in Korean Fauna. Anim. Syst. Evol. Divers. 2021, 37, 165–170. [Google Scholar] [CrossRef]
- Kim, S.L.; Oh, K.-H.; Ra, K.; Yu, O.H. Effects of Freshwater Inflow during the Rainy Season on the Benthic Polychaete Community in the Geum River Estuary, South Korea. Diversity 2024, 16, 180. [Google Scholar] [CrossRef]
- Rousset, V.; Pleijel, F.; Rouse, G.W.; Erséus, C.; Siddall, M.E. A molecular phylogeny of annelids. Cladistics 2007, 23, 41–63. [Google Scholar] [CrossRef]
- Stiller, J.; Tilic, E.; Rousset, V.; Pleijel, F.; Rouse, G.W. Spaghetti to a tree: A robust phylogeny for Terebelliformia (Annelida) based on transcriptomes, molecular and morphological data. Biology 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Alalykina, I.L.; Polyakova, N.E. New deep-sea species of Anobothrus (Annelida: Ampharetidae) from the Kuril-Kamchatka Trench and adjacent abyssal regions. Prog. Oceanogr. 2020, 182, 102237. [Google Scholar] [CrossRef]
- Colgan, D.; McLauchlan, A.; Wilson, G.; Livingston, S.; Edgecombe, G.; Macaranas, J.; Cassis, G.; Gray, M. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Jeong, M.K.; Soh, H.Y.; Wi, J.H.; Suh, H.L. A new Notomastus (Annelida, Capitellidae) species from Korean waters, with genetic comparison based on three gene markers. Zookeys 2018, 754, 141–155. [Google Scholar] [CrossRef]
- Kim, D.H.; Soh, H.Y.; Jeong, M.-K. First report of a Paucibranchia (Polychaeta, Eunicidae) species without lateral palps in Korean subtidal waters, with genetic evidence for its taxonomic position. Diversity 2022, 14, 1131. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Krueger, L.; Dietrich, A.; Bastrop, R.; Bick, A. From synonym to valid species: Redescriptions of Ampharete acutifrons (Grube, 1860) and A. cirrata Webster & Benedict, 1887, and brief descriptions of A. baltica Eliason, 1955 and A. grubei Malmgren, 1865 (Annelida: Terebellida: Ampharetidae). Zootaxa 2022, 5174, 357–380. [Google Scholar] [CrossRef]
- Zirkov, I.A. Ampharete petersenae sp. n. (Ampharetidae, Polychaeta) from the Northern Atlantic. Zool Zh 1997, 76, 1418–1420. [Google Scholar]
- Moore, J.P. New species of Ampharetidae and Terebellidae from the North Pacific. Proc. Acad. Nat. Sci. Phila. 1905, 57, 846–860. [Google Scholar]
- Buzhinskaja, G. Polychaetes of the Far East Seas of Russia and adjacent waters of the Pacific Ocean: Annotated checklist and bibliography. In Program of Fundamental Investigations of Presidium RAS “Wildlife: Present State and Development” in Russian Academy of Sciences, AN Severtzov Institute of Ecology and Evolution, Zoological Institute; KMK Scientific Press: Moscow, Russia, 2013. [Google Scholar]
- Glasby, C.J.; Wei, N.W.V.; Gibb, K.S. Cryptic species of Nereididae (Annelida: Polychaeta) on Australian coral reefs. Invertebr. Syst. 2013, 27, 245–264. [Google Scholar] [CrossRef]
- Jeong, M.K.; Soh, H.Y.; Suh, H.L. Three new species of Heteromastus (Annelida, Capitellidae) from Korean waters, with genetic evidence based on two gene markers. Zookeys 2019, 869, 1–18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).