Epiphytic Habit and Spatial Distribution Patterns of Phalaenopsis deliciosa and Phalaenopsis hainanensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Data Processing and Analysis
2.2.1. Plant Community Structure
2.2.2. The Eepiphytic Selectivity Index
2.2.3. Horizontal Spatial Distribution Pattern
2.2.4. Vertical Spatial Distribution Pattern
3. Results
3.1. Community Structure Composition
3.1.1. Habitat Community Composition of P. deliciosa
3.1.2. The Composition of the Plant Community in P. hainanensis Plots
3.2. Community Floristic Characteristics
3.3. Epiphytic Habits
3.4. Spatial Distribution Pattern
4. Discussion
4.1. Plant Community Structure and Floristic Characteristics
4.2. Epiphytic Habit
4.3. Spatial Distribution
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Family | Genus | Species |
|---|---|---|
| Liliaceae | Dracaena | Dracaena angustifolia |
| Dracaena cambodiana | ||
| Flacourtiaceae | Scolopia | Scolopia saeva |
| Hydnocarpus | Hydnocarpus hainanensis | |
| Homalium | Homalium hainanense | |
| Euphorbiaceae | Croton | Croton laevigatus |
| Koilodepas | Koilodepas hainanense | |
| Suregada | Suregada glomerulata | |
| Cleistanthus | Cleistanthus concinnus | |
| Drypetes | Drypetes hainanensis | |
| Drypetes indica | ||
| Drypetes perreticulata | ||
| Breynia | Breynia fruticosa | |
| Baccaurea | Baccaurea ramiflora | |
| Bischofia | Bischofia javanica | |
| Alchornea | Alchornea rugosa | |
| Sapium | Sapium insigne | |
| Antidesma | Antidesma hainanense | |
| Bridelia balansae | ||
| Actephila | Actephila merrilliana | |
| Mallotus | Mallotus hookerianus | |
| Mallotus peltatus | ||
| Mallotus yunnanensis | ||
| Aporusa | Aporusa dioica | |
| Erismanthus | Erismanthus sinensis | |
| Fabaceae | Ormosia | Ormosia semicastrata |
| Dalbergia | Dalbergia peishaensis | |
| Dalbergia tsoi | ||
| Dalbergia odorifera | ||
| Bowringia | Bowringia callicarpa | |
| Millettia | Millettia tsui | |
| Derris | Derris trifoliata | |
| Annonaceae | Polyalthia | Polyalthia laui |
| Fissistigma | Fissistigma oldhamii | |
| Fissistigma polyanthum | ||
| Desmos | Desmos chinensis | |
| Alphonsea | Alphonsea mollis | |
| Mitrephora | Mitrephora thorelii | |
| Artabotrys | Artabotrys hainanensis | |
| Dasymaschalon | Dasymaschalon trichophorum | |
| Uvaria | Uvaria macclurei | |
| Menispermaceae | Diploclisia | Diploclisia glaucescens |
| Burseraceae | Canarium | Canarium album |
| Ancistrocladaceae | Ancistrocladus | Ancistrocladus tectorius |
| Mimosaceae | Albizia | Albizia corniculata |
| Albizia odoratissima | ||
| Acacia | Acacia pennata | |
| Gramineae | Schizostachyum | Schizostachyum pseudolima |
| Apocynaceae | Wrightia | Wrightia pubescens |
| Ervatamia | Ervatamia hainanensis | |
| Alyxia | Alyxia sinensis | |
| Hunteria | Hunteria zeylanica | |
| Violaceae | Rinorea | Rinorea bengalensis |
| Malvaceae | Pterospermum | Pterospermum heterophyllum |
| Microcos | Microcos paniculata | |
| Sterculia | Sterculia lanceolata | |
| Firmiana | Firmiana hainanensis | |
| Fagaceae | Lithocarpus | Lithocarpus corneus |
| Castanopsis | Castanopsis formosana | |
| Meliaceae | Turraea | Turraea pubescens |
| Aglaia | Aglaia odorata var. microphyllina | |
| Toona | Toona sinensis | |
| Amoora | Amoora tsangii | |
| Dipterocarpaceae | Hopea | Hopea hainanensis |
| Vatica | Vatica mangachapoi | |
| Pandanaceae | Pandanus | Pandanus tectorius |
| Asclepiadaceae | Hoya | Hoya carnosa |
| Verbenaceae | Vitex | Vitex quinata |
| Sphenodesme | Sphenodesme pentandra | |
| Callicarpa | Callicarpa longissima | |
| Loganiaceae | Strychnos | Strychnos cathayensis |
| Strychnos angustiflora | ||
| Strychnos umbellata | ||
| Magnoliaceae | Paramichelia | Paramichelia baillonii |
| Oleaceae | Fraxinus | Fraxinus griffithii |
| Osmanthus | Osmanthus matsumuranus | |
| Jasminum | Jasminum grandiflorum | |
| Jasminum nervosum var. elegans | ||
| Connaraceae | Ellipanthus | Ellipanthus glabrifolius |
| Rourea | Rourea Microphylia | |
| Pandaceae | Microdesmis | Microdesmis caseariifolia |
| Anacardiaceae | Spondias | Spondias pinnata |
| Spondias lakonensis | ||
| Lannea | Lannea coromandelica | |
| Toxicodendron | Toxicodendron succedaneum | |
| Lythraceae | Lagerstroemia | Lagerstroemia balansae |
| Rubiaceae | Lasianthus | Lasianthus chinensis |
| Lasianthus hirsutus | ||
| Paederia | Paederia scandens | |
| Psychotria | Psychotria straminea | |
| Psychotria rubra | ||
| Tarennoidea | Tarennoidea wallichii | |
| Ixora | Ixora hainanensis | |
| Antirhea | Antirhea chinensis | |
| Fagerlindia | Fagerlindia depauperata | |
| Aidia | Aidia oxyodonta | |
| Catunaregam | Catunaregam spinosa | |
| Wendlandia | Wendlandia uvariifolia subsp. chinensis | |
| Chasalia | Chasallia curviflora | |
| Tarenna | Tarenna mollissima | |
| Canthium | Canthium horridum | |
| Rosaceae | Photinia | Photinia benthamiana |
| Moraceae | Antiaris | Antiaris toxicaria |
| Streblus | Streblus ilicifolius | |
| Streblus taxoides | ||
| Theaceae | Eurya | Eurya nitida |
| Capparaceae | Capparis | Capparis dasyphylla |
| Capparis | Capparis zeylanica | |
| Capparis hainanensis | ||
| Sapotaceae | Chrysophyllum | Chrysophyllum roxburghii |
| Sarcosperma | Sarcosperma laurinum | |
| Combretaceae | Combretum | Combretum punctatum |
| Terminalia | Terminalia hainanensis | |
| Ebenaceae | Diospyros | Diospyros strigosa |
| Diospyros cathayensis | ||
| Rhamnaceae | Sageretia | Sageretia thea |
| Myrtaceae | Syzygium | Syzygium chunianum |
| Cleistocalyx | Cleistocalyx operculatus | |
| Clusiaceae | Garcinia | Garcinia oblongifolia |
| Samydaceae | Casearia | Casearia vililimba |
| Olacaceae | Erythropalum | Erythropalum scandens |
| Celastraceae | Euonymus | Euonymus chinensis |
| Euonymus laxiflorus | ||
| Sapindaceae | Arytera | Arytera littoralis |
| Litchi | Litchi chinensis | |
| Dimocarpus | Dimocarpus longan | |
| Sapindus | Sapindus mukurossi | |
| Dilleniaceae | Tetracera | Tetracera asiatica |
| Scrophulariaceae | Paulownia | Paulownia kawakamii |
| Convolvulaceae | Erycibe | Erycibe schmidtii |
| Argyreia | Argyreia acuta | |
| Melastomataceae | Memecylon | Memecylon scutellatum |
| Ulmaceae | Gironniera | Aphananthe cuspidata |
| Caesalpiniaceae | Sindora | Peltophorum dasyrrhachis |
| Rutaceae | Murraya | Murraya exotica |
| Glycosmis | Glycosmis pentaphylla | |
| Lauraceae | Cryptocarya | Cryptocarya metcalfiana |
| Dehaasia | Dehaasia hainanensis | |
| Litsea | Litsea baviensis | |
| Litsea variabilis | ||
| Litsea monopetala | ||
| Beilschmiedia | Beilschmiedia appendiculata | |
| Machilus | Machilus suaveolens | |
| Machilus chinensis | ||
| Cinnamomum | Cinnamomum tsoi | |
| Cinnamomum burmannii | ||
| Boraginaceae | Carmona | Carmona microphylla |
| Myrsinaceae | Maesa | Maesa japonica |
| Rapanea | Rapanea neriifolia | |
| Ardisia | Ardisia densilepidotula | |
| Ardisia crenata | ||
| Bignoniaceae | Radermachera | Radermachera hainanensis |
| Markhamia | Markhamia stipulata var. kerrii | |
| Palmae | Arenga | Arenga westerhoutii |
| Daemonorops | Daemonorops margaritae | |
| Calamus | Calamus simplicifolius | |
| Caryota | Caryota ochlandra | |
| Licuala | Licuala spinosa | |
| Licuala fordiana | ||
| 53 Family | 134 Genus | 159 Species |
| Family | Genus | Species |
|---|---|---|
| Aspleniaceae | Neottopteris | Neottopteris nidus |
| Liliaceae | Dracaena | Dracaena cambodiana |
| Flacourtiaceae | Scolopia | Scolopia saeva |
| Euphorbiaceae | Croton | Croton cascarilloides |
| Euphorbia | Euphorbia hainanensis | |
| Drypetes | Drypetes perreticulata | |
| Actephila | Actephila merrilliana | |
| Mallotus | Mallotus tenuifolius | |
| Mallotus yunnanensis | ||
| Annonaceae | Alphonsea | Alphonsea mollis |
| Alphonsea monogyna | ||
| Artabotrys | Artabotrys hexapetalus | |
| Balsaminaceae | Impatiens | Impatiens hainanensis |
| Davalliaceae | Davallia | Davallia mariesii |
| Elaeagnaceae | Elaeagnus | Elaeagnus gonyanthes |
| Drynariaceae | Pseudodrynaria | Pseudodrynaria coronans |
| Zingiberaceae | Pommereschea | Pommereschea lackneri |
| Hamamelidaceae | Distylium | Distylium racemosum |
| Malvaceae | Erythropsis | Erythropsis pulcherrima |
| Sterculia | Sterculia lanceolata | |
| Acanthaceae | Barleria | Barleria cristata |
| Fagaceae | Quercus | Quercus bawanglingensis |
| Gesneriaceae | Chirita | Chirita heterotricha |
| Orchidaceae | Coelogyne | Coelogyne fimbriata |
| Luisia | Luisia morsei | |
| Eria | Eria quinquelamellosa | |
| Eria gagnepainii | ||
| Dendrobium | Dendrobium aurantiacum var. denneanum | |
| Dendrobium aduncum | ||
| Pholidota | Pholidota yunnanensis | |
| Cryptochilus | Cryptochilus roseus | |
| Vanda | Vanda subconcolor | |
| Liparis | Liparis viridiflora | |
| Liparis yunnanensis | ||
| Meliaceae | Aglaia | Aglaia odorata var. microphyllina |
| Loganiaceae | Fagraea | Fagraea ceilanica |
| Magnoliaceae | Paramichelia | Paramichelia baillonii |
| Oleaceae | Fraxinus | Fraxinus griffithii |
| Osmanthus | Osmanthus matsumuranus | |
| Jasminum | Jasminum nervosum var. elegans | |
| Anacardiaceae | Pistacia | Pistacia chinensis |
| Rubiaceae | Antirhea | Antirhea chinensis |
| Begoniaceae | Begonia | Begonia peltatifolia |
| Moraceae | Streblus | Streblus ilicifolius |
| Streblus taxoides | ||
| Ficus | Ficus subpisocarpa | |
| Ficus parvifolia | ||
| Ficus tinctoria subsp. gibbosa | ||
| Sapotaceae | Planchonella | Planchonella obovata |
| Myrtaceae | Decaspermum | Decaspermum gracilentum |
| Cela | Mi | |
| Euonymus | Euonymus laxiflorus | |
| Ar | Sc | |
| Ulmaceae | Celtis | Celtis sinensis |
| Ulmus | Ulmus tonkinensis | |
| Caesalpiniaceae | Gleditsia | Gleditsia sinensise |
| Rutaceae | Clausena | Clausena excavate |
| Micromelum | Micromelum falcatum | |
| Lauraceae | Dehaasia | Dehaasia hainanensis |
| Myrsinaceae Bignoniaceae | Rapanea | Rapanea linearis |
| Radermachera | Radermachera frondosa | |
| 34 Famliy | 53 Genus | 61 Species |
Appendix B
Appendix B.1. Method for Estimating the Vertical Projected Area of Epiphytic Individuals
Appendix B.2. Supplementary Notes on Herb Layer Assessment
References
- Liu, G.-F.; Ding, Y.; Zang, R.-G.; Xu, Y.-Y.; Lin, C.; Li, X.-C. Diversity and Distribution of Vascular Epiphytes in the Tropical Natural Coniferous Forest of Hainan Island, China. Chin. J. Plant Ecol. 2010, 34, 1283–1293. [Google Scholar]
- Gentry, A.H.; Dodson, C.H. Diversity and Biogeography of Neotropical Vascular Epiphytes. Ann. Mo. Bot. Gard. 1987, 74, 205–233. [Google Scholar] [CrossRef]
- Bramwell, D. How Many Plant Species Are There? Plant Talk 2002, 28, 32–33. [Google Scholar]
- Benzing, D.H. Vascular Epiphytes; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Bergstrom, B.J.; Carter, R. Host-Tree Selection by an Epiphytic Orchid, Epidendrum magnoliae Muhl. (Green Fly Orchid), in an Inland Hardwood Hammock in Georgia. Southeast. Nat. 2008, 7, 571–580. [Google Scholar] [CrossRef]
- Fay, M.F.; Chase, M.W. Orchid Biology: From Linnaeus via Darwin to the 21st Century. Ann. Bot. 2009, 104, 359–364. [Google Scholar] [CrossRef]
- Gravendeel, B.; Smithson, A.; Slik, F.J.W.; Schuiteman, A. Epiphytism and Pollinator Specialization: Drivers for Orchid Diversity? Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1523–1535. [Google Scholar] [CrossRef]
- Givnish, T.J.; Spalink, D.; Ames, M.; Lyon, S.P.; Hunter, S.J.; Zuluaga, A.; Iles, W.J.D.; Clements, M.A.; Arroyo, M.T.K.; Leebens-Mack, J.; et al. Orchid Phylogenomics and Multiple Drivers of Their Extraordinary Diversification. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151553. [Google Scholar] [CrossRef]
- Zotz, G. How Fast Does an Epiphyte Grow? Selbyana 1998, 16, 150–154. [Google Scholar]
- Winkler, M.; Hietz, P. Population Structure of Three Epiphytic Orchids (Lycaste aromatica, Jacquiniella leucomelana and J. teretifolia) in a Mexican Humid Montane Forest. Selbyana 2001, 22, 27–33. [Google Scholar]
- Callaway, R.M.; Reinhart, K.O.; Moore, G.W.; Moore, D.J.; Pennings, S.C. Epiphyte Host Preferences and Host Traits: Mechanisms for Species-Specific Interactions. Oecologia 2002, 132, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.C.E.; Willems, J.H. (Eds.) Population Ecology of Terrestrial Orchids; SPB Academic Publishing: The Hague, The Netherlands, 1991. [Google Scholar]
- Otero, J.T.; Bayman, P.; Ackerman, J.D. Variation in Mycorrhizal Performance in the Epiphytic Orchid Tolumnia variegata In Vitro: The Potential for Natural Selection on Fungal Effectiveness. Aust. J. Bot. 2005, 53, 353–361. [Google Scholar] [CrossRef]
- Jersáková, J.; Malinová, T. Spatial Aspects of Seed Dispersal and Seedling Recruitment in Orchids. New Phytol. 2007, 176, 237–241. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. The Interrelations of Certain Analytic and Synthetic Phytosociological Characters. Ecology 1951, 32, 434–455. [Google Scholar] [CrossRef]
- IUCN SSC Orchid Specialist Group. Global Action Plan for Orchid Conservation; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Hu, X.-Y.; Zhu, J.; Song, X.-Q.; He, R.-X. Orchid Diversity in China’s Hainan Island: Distribution and Conservation. Collect. Bot. 2015, 34, e007. [Google Scholar] [CrossRef]
- Chen, X.; Wood, J.J. Phalaenopsis . In Flora of China, Volume 25 (Orchidaceae); Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2009; pp. 466–469. [Google Scholar]
- Chen, X.; Liu, Z.; Zhu, G.; Lang, K.; Ji, Z.; Luo, Y. Phalaenopsis . In Flora of China; Wu, Z.-Y., Raven, P.H., Hong, D.-Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2009; Volume 25, pp. 478–483. [Google Scholar]
- Long, W.-X.; Zang, R.-G.; Ding, Y. Community Characteristics of Tropical Forests in Bawangling, Hainan. Chin. J. Plant Ecol. 2013, 37, 597–608. (In Chinese) [Google Scholar]
- Greig-Smith, P. Quantitative Plant Ecology, 3rd ed.; University of California Press: Berkeley, CA, USA, 1983. [Google Scholar]
- Ye, W.-H.; Pan, Z.-C.; Wang, Z.-R.; Chen, C.-Y.; Pan, J.-S.; Xu, Z.-F. Method for Calculating Shrub Layer Coverage. Acta Phytoecol. Sin. 2008, 32, 112–119. (In Chinese) [Google Scholar]
- Wu, Z.-Y.; Sun, H.; Zhou, Z.-K.; Peng, H.; Li, D.-Z. The Geography of Seed Plant Flora of China; Science Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Song, X.-Q. Epiphytic Selectivity Index (ESI) for Host Preference of Epiphytes. J. Trop. Biol. 2005, 2, 45–50. (In Chinese) [Google Scholar]
- Wiegand, T.; Moloney, K.A. Rings, Circles, and Null-Models for Point Pattern Analysis in Ecology. Oikos 2004, 104, 209–229. [Google Scholar] [CrossRef]
- Bončina, Z.; Trifković, V.; Rosset, C.; Klopčič, M. Evaluation of Estimation Methods for Fitting the Three-Parameter Weibull Distribution to European Beech Forests. iForest 2022, 15, 484–490. [Google Scholar] [CrossRef]
- Bončina, Ž.; Rosset, C.; Klopčič, M. Modelling Diameter Distribution in Near-Natural European Beech Forests: Are Geo-Climatic Variables Alone Sufficient? Forests 2025, 16, 1556. [Google Scholar] [CrossRef]
- Nanos, N.; Sjöstedt de Luna, S. Fitting diameter distribution models to data from forest inventories with truncated diameter distributions. For. Syst. 2017, 26, e01S. [Google Scholar] [CrossRef]
- Cruz, A.C.R.; Corrêa, N.M.; Murakami, M.; Amorim, T.A.; Faria, A.P.G.; Matthews, S.J. Importance of the Vertical Gradient in the Variation of Epiphyte Community Structure in the Brazilian Atlantic Forest. Flora 2022, 295, 152137. [Google Scholar] [CrossRef]
- Zotz, G.; Bader, M.Y. Epiphytic Plants in a Changing World: Global Change Effects on Vascular and Non-Vascular Epiphytes. In Progress in Botany; Lüttge, U., Beyschlag, W., Büdel, B., Eds.; Springer: Berlin, Germany, 2009; Volume 70, pp. 147–170. [Google Scholar]
- Zotz, G. Plants on Plants—The Biology of Vascular Epiphytes; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Zotz, G. The Systematic Distribution of Vascular Epiphytes—A Critical Update. Bot. J. Linn. Soc. 2013, 171, 453–481. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids: From Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- McCormick, M.K.; Jacquemyn, H. Orchid recruitment: Key principles and research priorities. Plant Ecol. 2014, 215, 1297–1311. [Google Scholar]
- Trapnell, D.W.; Hamrick, J.L. Range-wide and fine-scale population genetic structure of Encyclia tampensis. Mol. Ecol. 2005, 14, 2657–2666. [Google Scholar]
- Zotz, G. The systematic distribution of vascular epiphytes. J. Veg. Sci. 2007, 18, 195–202. [Google Scholar]
- Arditti, J.; Ghani, A.K.A. Tansley Review No. 110. Numerical and Physical Properties of Orchid Seeds and Their Biological Implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef] [PubMed]
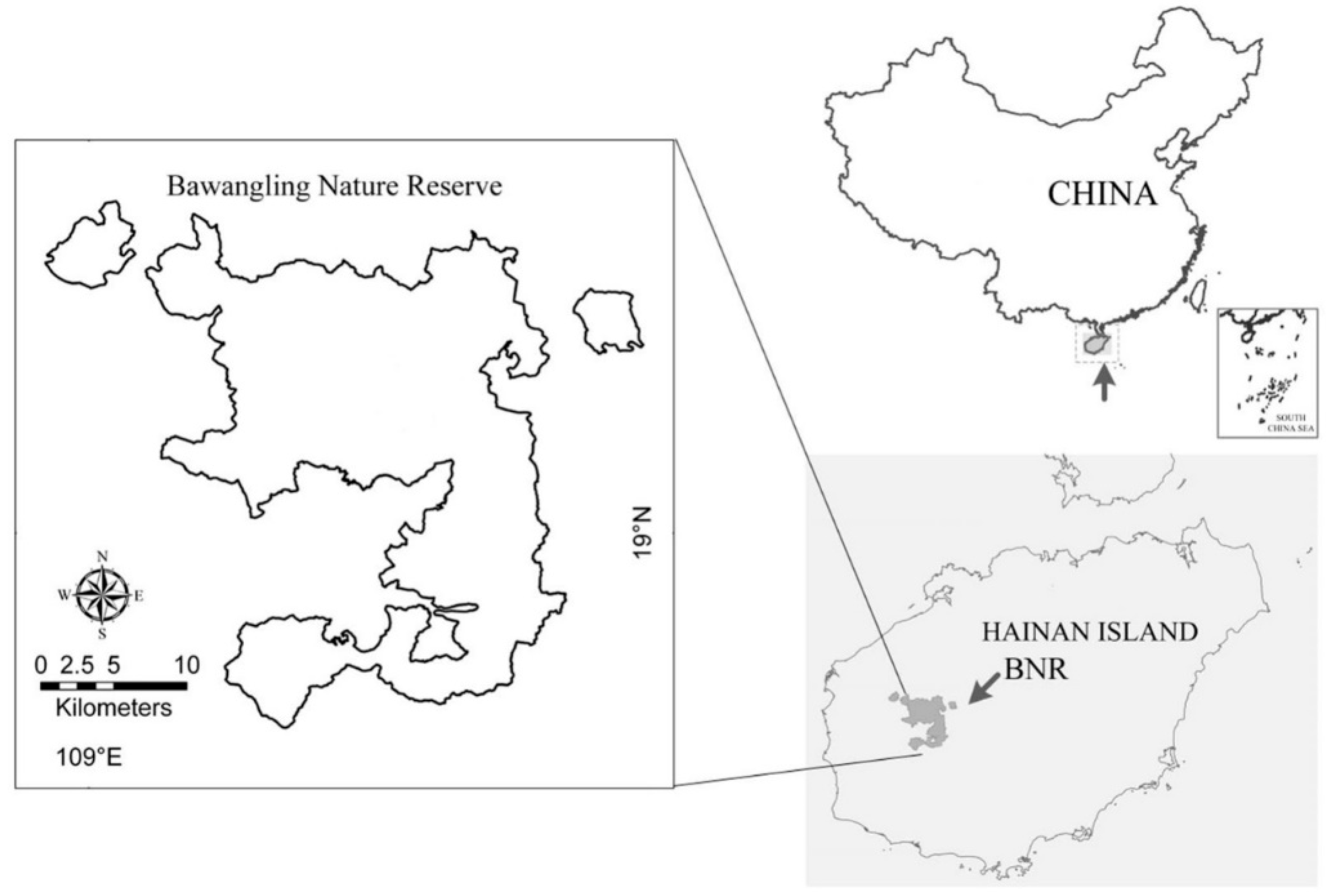

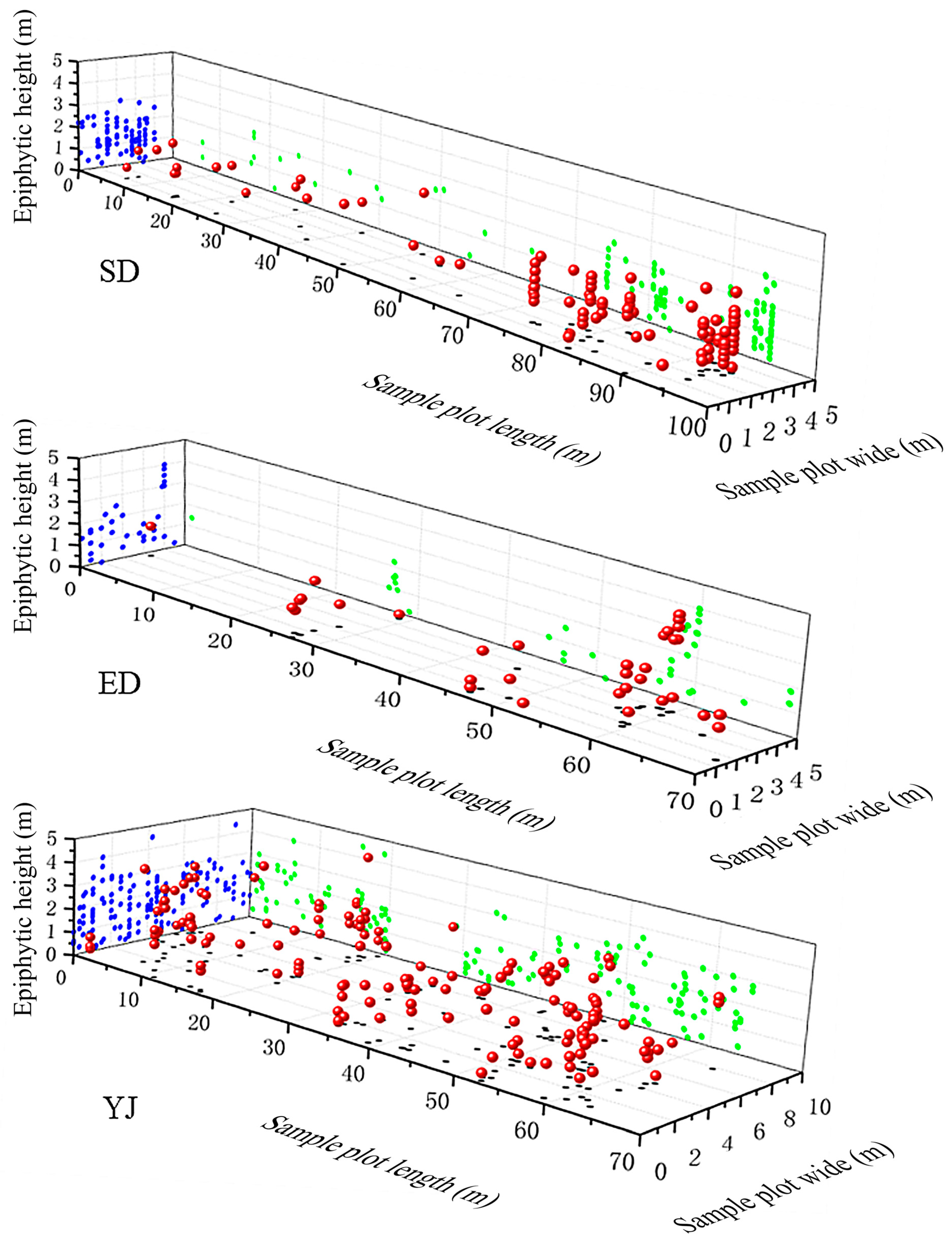
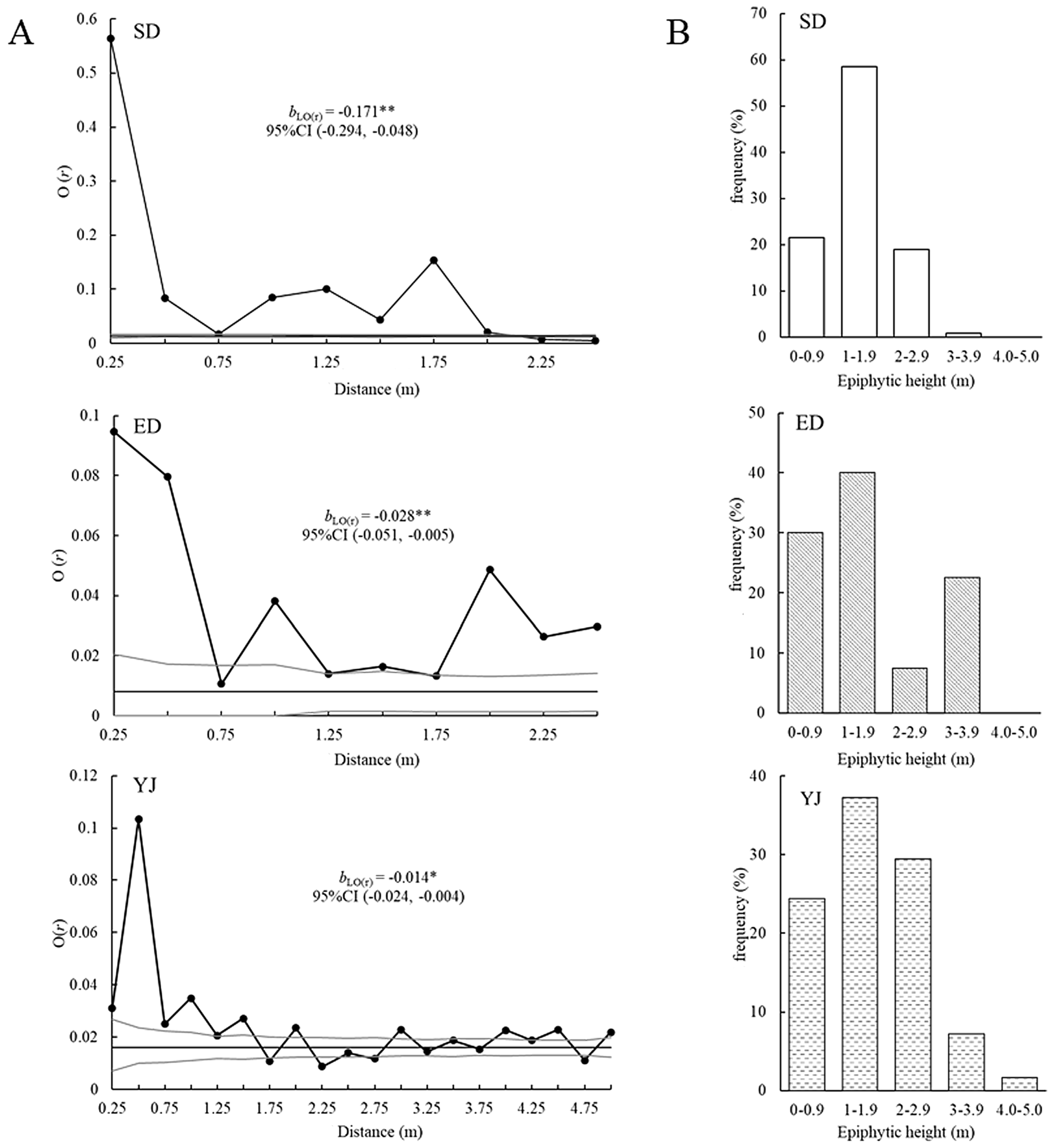


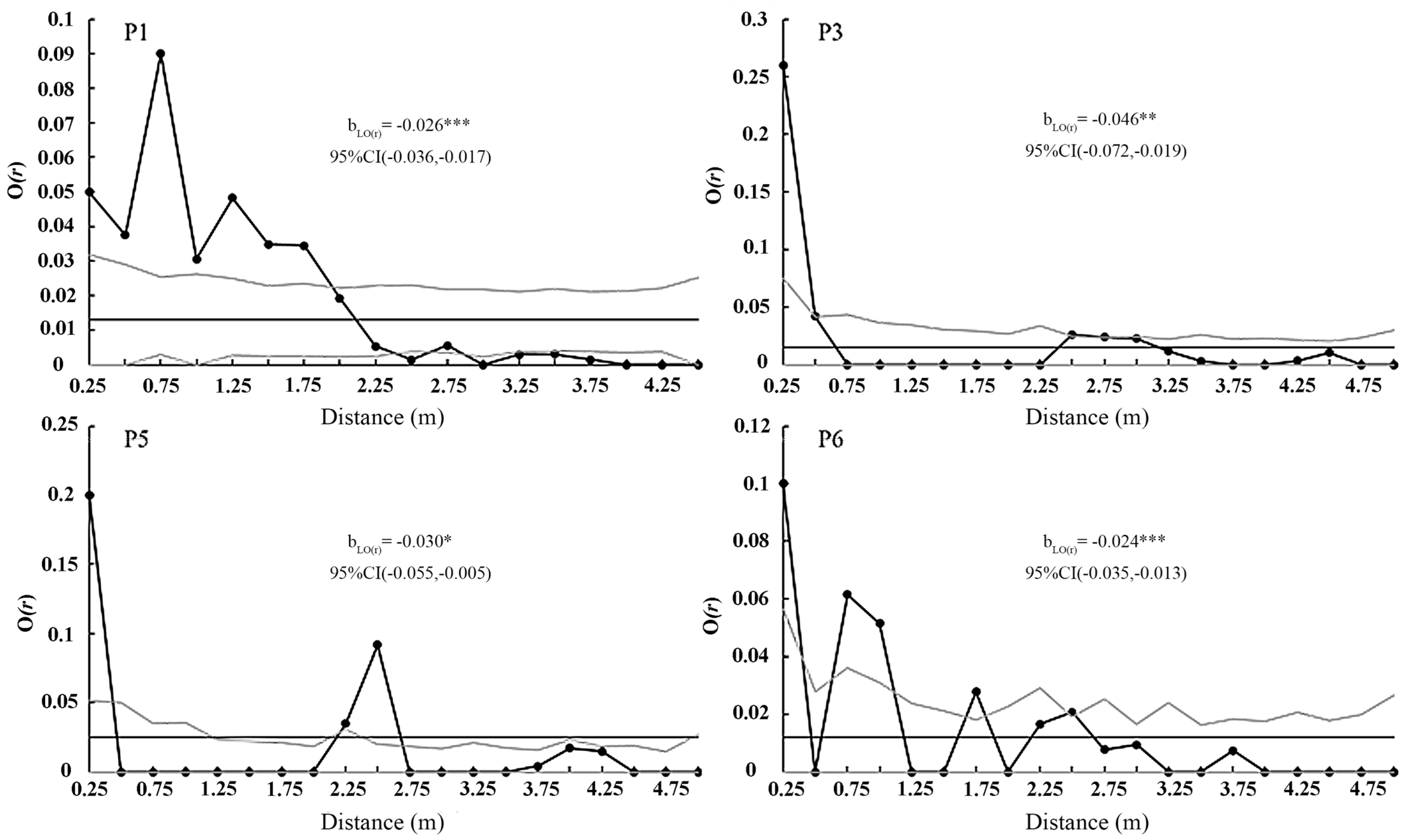
| Population | Longitude | Latitude | Altitude | Sample Area | No. of Quadrats |
|---|---|---|---|---|---|
| Phalaenopsisdeliciosa | |||||
| Third stage power station (SD) | E: 109° 6′3.77″ | N: 19° 6′39.97″ | 230 m | 5 m × 100 m | 1 |
| Second stage power station (ED) | E: 109° 6′56.33″ | N: 19° 5′59.18″ | 284 m | 5 m × 70 m | 1 |
| Yajia Power Station (YJ) | E: 109° 7′46.34″ | N: 19° 5′8.74″ | 476 m | 10 m × 70 m | 1 |
| Phalaenopsishainanensis | |||||
| Erxian Ridge (EXL) | E: 109° 6′34.26″ | N: 19° 0′50.89″ | 1 050 m | 10 m × 10 m | 6 |
| Coconut Village (YZC) | E: 109° 7′59.55″ | N: 19° 1′5.07″ | 600 m | 10 m × 10 m | 3 |
| Population | Host Species | Relative Abundance | Relative Frequency | Relative Coverage | Importance Value |
|---|---|---|---|---|---|
| YJ | Streblus taxoides | 19.13 | 8.72 | 9.28 | 12.37 |
| Erismanthus sinensis | 7.54 | 5.96 | 11.99 | 8.50 | |
| Streblus ilicifolius | 8.70 | 5.50 | 7.56 | 7.25 | |
| Hydnocarpus hainanensis | 7.25 | 7.80 | 5.38 | 6.81 | |
| ED | Mallotus peltatus | 14.29 | 8.41 | 10.15 | 10.95 |
| Erismanthus sinensis | 5.44 | 4.67 | 7.85 | 5.99 | |
| SD | Streblus ilicifolius | 35.62 | 13.71 | 21.11 | 23.48 |
| Streblus taxoides | 24.12 | 12.57 | 11.06 | 15.92 | |
| Terminalia hainanensis | 3.98 | 6.86 | 30.56 | 13.80 | |
| Dasymaschalon trichophorum | 6.86 | 9.14 | 4.49 | 6.83 | |
| Cleistanthus concinnus | 4.65 | 6.86 | 4.30 | 5.27 | |
| Total | Streblus ilicifolius | 20.13 | 7.20 | 10.80 | 12.71 |
| Streblus taxoides | 18.44 | 8.20 | 8.23 | 11.62 | |
| Terminalia hainanensis | 2.00 | 2.60 | 10.90 | 5.17 |
| Population | Host Species | Relative Abundance | Relative Frequency | Relative Coverage | Importance Value |
|---|---|---|---|---|---|
| YJ | Schizostachyum pseudolima | 30.28 | 13.51 | 58.64 | 34.14 |
| Streblus ilicifolius | 11.31 | 6.76 | 10.26 | 9.44 | |
| Streblus taxoides | 8.56 | 6.08 | 11.62 | 8.75 | |
| Dasymaschalon trichophorum | 7.95 | 9.46 | 4.20 | 7.20 | |
| ED | Schizostachyum pseudolima | 50.78 | 20.00 | 75.26 | 48.68 |
| Licuala fordiana | 8.59 | 7.27 | 11.68 | 9.18 | |
| SD | Schizostachyum pseudolima | 20.42 | 17.98 | 57.08 | 31.83 |
| Streblus ilicifolius | 25.46 | 23.60 | 13.43 | 20.83 | |
| Dasymaschalon trichophorum | 15.38 | 15.73 | 15.19 | 15.44 | |
| Streblus taxoides | 16.45 | 14.61 | 6.96 | 12.67 | |
| Actephila merrilliana | 16.98 | 14.61 | 4.48 | 12.02 | |
| Total | Schizostachyum pseudolima | 28.93 | 16.04 | 63.67 | 36.21 |
| Dasymaschalon trichophorum | 10.32 | 10.24 | 7.05 | 9.20 | |
| Streblus taxoides | 10.80 | 7.51 | 5.92 | 8.08 | |
| Streblus ilicifolius | 11.52 | 7.17 | 5.10 | 7.93 | |
| Actephila merrilliana | 8.76 | 6.14 | 2.07 | 5.66 |
| Population | Host Species | Relative Abundance | Relative Frequency | Relative Coverage | Importance Value |
|---|---|---|---|---|---|
| EXL | Quercus bawanglingensis | 15.84 | 5.41 | 19.37 | 13.54 |
| Streblus ilicifolius | 14.85 | 8.11 | 17.21 | 13.39 | |
| Mallotus yunnanensis | 19.80 | 5.41 | 11.28 | 12.16 | |
| Sterculia lanceolata | 8.91 | 8.11 | 6.48 | 7.83 | |
| Aglaia odorata var. microphyllina | 5.94 | 5.41 | 4.49 | 5.28 | |
| Osmanthus matsumuranus | 1.98 | 5.41 | 8.29 | 5.22 | |
| YZC | Streblus ilicifolius | 14.74 | 4.65 | 13.21 | 10.86 |
| Clausena excavata | 16.84 | 6.98 | 7.52 | 10.45 | |
| Dehaasia hainanensis | 7.37 | 9.30 | 12.13 | 9.60 | |
| Mallotus yunnanensis | 7.37 | 2.33 | 14.24 | 7.98 | |
| Total | Streblus ilicifolius | 14.87 | 6.33 | 14.43 | 11.88 |
| Mallotus yunnanensis | 13.85 | 3.80 | 13.46 | 10.37 | |
| Dehaasia hainanensis | 4.62 | 6.33 | 9.56 | 6.83 | |
| Clausena excavata | 8.72 | 5.06 | 6.31 | 6.70 | |
| Quercus bawanglingensis | 9.23 | 3.80 | 5.90 | 6.31 | |
| Sterculia lanceolata | 6.67 | 6.33 | 3.26 | 5.42 |
| Population | Host Species | Relative Abundance | Relative Frequency | Relative Coverage | Importance Value |
|---|---|---|---|---|---|
| EXL | Mallotus yunnanensis | 38.46 | 12.50 | 46.88 | 32.61 |
| Croton cascarilloides | 19.23 | 12.50 | 23.44 | 18.39 | |
| YZC | Schefflera arboricola | 16.67 | 50.00 | 67.57 | 44.74 |
| Paramichelia baillonii | 83.33 | 50.00 | 32.43 | 55.26 | |
| Total | Mallotus yunnanensis | 31.25 | 11.11 | 36.36 | 26.24 |
| Schefflera arboricola | 12.50 | 22.22 | 24.24 | 19.65 | |
| Croton cascarilloides | 15.63 | 11.11 | 18.18 | 14.97 | |
| Paramichelia baillonii | 15.63 | 11.11 | 7.27 | 11.34 |
| Geographical Areal-Types | Phalaenopsis deliciosa | Phalaenopsis hainanensis | ||
|---|---|---|---|---|
| No. of Genus | Proportion | No. of Genus | Proportion | |
| 1 Cosmopolitan | 1 | 0.75 | 3 | 5.66 |
| 2 Pantropic | 29 | 21.64 | 14 | 26.42 |
| 3 Trop. Asia & Trop. Amer. disjuncted | 6 | 4.48 | 1 | 1.89 |
| 4 Old World Tropics | 18 | 13.43 | 4 | 7.55 |
| 5 Trop. Asia toTrop. Australasia | 21 | 15.67 | 8 | 15.09 |
| 6 Trop. Asia to Trop. Africa | 13 | 9.7 | 1 | 1.89 |
| 7 Trop. Asia: India-Malasia | 41 | 30.6 | 13 | 24.53 |
| 8 North Temperate | 1 | 0.75 | 5 | 9.43 |
| 9 E. Asia & N. Amer. disjuncted | 2 | 1.49 | 1 | 1.89 |
| 12 Central Asia, West Asia to the Mediterranean | / | / | 1 | 1.89 |
| 14 E. Asia | 2 | 1.49 | 2 | 3.77 |
| Total | 134 | 100 | 53 | 100 |
| Shannon—Wiener Index | H′ = 1.818 | H′ = 1.985 | ||
| Species | Abundance | Frequence | Total Plants | Relative Abundance | Relative Frequency | Relative Dominance | Epiphytic Index |
|---|---|---|---|---|---|---|---|
| Streblus ilicifolius | 40 | 36 | 82 | 23.39 | 12.12 | 30.15 | 21.89 |
| Streblus taxoides | 34 | 41 | 55 | 19.88 | 13.80 | 20.22 | 17.97 |
| Mallotus peltatus | 11 | 15 | 17 | 6.43 | 5.05 | 6.25 | 5.91 |
| Erismanthus sinensis | 9 | 18 | 15 | 5.26 | 6.06 | 5.51 | 5.61 |
| Sphenodesme pentandra | 9 | 13 | 12 | 5.26 | 4.38 | 4.41 | 4.68 |
| Hydnocarpus hainanensis | 4 | 19 | 7 | 2.34 | 6.40 | 2.57 | 3.77 |
| Artabotrys hainanensis | 7 | 7 | 10 | 4.09 | 2.36 | 3.68 | 3.38 |
| Dasymaschalon trichophorum | 3 | 18 | 5 | 1.75 | 6.06 | 1.84 | 3.22 |
| Acacia pennata | 6 | 5 | 6 | 3.51 | 1.68 | 2.21 | 2.47 |
| Koilodepas hainanense | 4 | 8 | 6 | 2.34 | 2.69 | 2.21 | 2.41 |
| Antirhea chinensis | 3 | 6 | 7 | 1.75 | 2.02 | 2.57 | 2.12 |
| Euonymus laxiflorus | 3 | 9 | 3 | 1.75 | 3.03 | 1.10 | 1.96 |
| Aporusa dioica | 1 | 12 | 1 | 0.58 | 4.04 | 0.37 | 1.66 |
| Millettia tsui | 4 | 3 | 4 | 2.34 | 1.01 | 1.47 | 1.61 |
| Tetracera asiatica | 3 | 5 | 3 | 1.75 | 1.68 | 1.10 | 1.51 |
| Ellipanthus glabrifolius | 1 | 10 | 1 | 0.58 | 3.37 | 0.37 | 1.44 |
| Diospyros cathayensis | 1 | 8 | 2 | 0.58 | 2.69 | 0.74 | 1.34 |
| Tarenna mollissima | 1 | 8 | 1 | 0.58 | 2.69 | 0.37 | 1.22 |
| Pterospermum heterophyllum | 2 | 4 | 3 | 1.17 | 1.35 | 1.10 | 1.21 |
| Strychnos angustiflora | 1 | 5 | 3 | 0.58 | 1.68 | 1.10 | 1.12 |
| Derris trifoliata | 2 | 4 | 2 | 1.17 | 1.35 | 0.74 | 1.08 |
| Vitex quinata | 1 | 5 | 2 | 0.58 | 1.68 | 0.74 | 1.00 |
| Amoora tsangii | 1 | 6 | 1 | 0.58 | 2.02 | 0.37 | 0.99 |
| Aphananthe cuspidata | 1 | 4 | 2 | 0.58 | 1.35 | 0.74 | 0.89 |
| Diploclisia glaucescens | 2 | 2 | 2 | 1.17 | 0.67 | 0.74 | 0.86 |
| Alphonsea mollis | 1 | 3 | 2 | 0.58 | 1.01 | 0.74 | 0.78 |
| Dalbergia peishaensis | 2 | 1 | 2 | 1.17 | 0.34 | 0.74 | 0.75 |
| Litchi chinensis | 1 | 3 | 1 | 0.58 | 1.01 | 0.37 | 0.65 |
| Rourea Microphylia | 1 | 2 | 1 | 0.58 | 0.67 | 0.37 | 0.54 |
| Sterculia lanceolata | 1 | 2 | 1 | 0.58 | 0.67 | 0.37 | 0.54 |
| Alyxia sinensis | 1 | 2 | 1 | 0.58 | 0.67 | 0.37 | 0.54 |
| Garcinia oblongifolia | 1 | 2 | 1 | 0.58 | 0.67 | 0.37 | 0.54 |
| Sarcosperma laurinum | 1 | 2 | 1 | 0.58 | 0.67 | 0.37 | 0.54 |
| Hunteria zeylanica | 1 | 2 | 1 | 0.58 | 0.67 | 0.37 | 0.54 |
| Argyreia acuta | 1 | 1 | 1 | 0.58 | 0.34 | 0.37 | 0.43 |
| Fissistigma oldhamii | 1 | 1 | 1 | 0.58 | 0.34 | 0.37 | 0.43 |
| Fraxinus griffithii | 1 | 1 | 1 | 0.58 | 0.34 | 0.37 | 0.43 |
| Murraya exotica | 1 | 1 | 1 | 0.58 | 0.34 | 0.37 | 0.43 |
| Ormosia semicastrata f. litchiifolia | 1 | 1 | 1 | 0.58 | 0.34 | 0.37 | 0.43 |
| Jasminum nervosum var. elegans | 1 | 1 | 1 | 0.58 | 0.34 | 0.37 | 0.43 |
| Species | Abundance | Frequence | Total Plants | Relative Abundance | Relative Frequency | Relative Dominance | Epiphytic Index |
|---|---|---|---|---|---|---|---|
| Streblus ilicifolius | 8 | 5 | 19 | 23.53 | 13.16 | 41.30 | 26.00 |
| Mallotus yunnanensis | 9 | 3 | 9 | 26.47 | 7.89 | 19.57 | 17.98 |
| Sterculia lanceolata | 2 | 5 | 2 | 5.88 | 13.16 | 4.35 | 7.80 |
| Ulmus tonkinensis | 1 | 5 | 1 | 2.94 | 13.16 | 2.17 | 6.09 |
| Clausena excavata | 1 | 4 | 1 | 2.94 | 10.53 | 2.17 | 5.21 |
| Osmanthus matsumuranus | 1 | 3 | 1 | 2.94 | 7.89 | 2.17 | 4.34 |
| Schefflera arboricola | 2 | 1 | 2 | 5.88 | 2.63 | 4.35 | 4.29 |
| Fraxinus griffithii | 1 | 2 | 1 | 2.94 | 5.26 | 2.17 | 3.46 |
| Antirhea chinensis | 1 | 2 | 1 | 2.94 | 5.26 | 2.17 | 3.46 |
| Erythropsis pulcherrima | 1 | 1 | 2 | 2.94 | 2.63 | 4.35 | 3.31 |
| Ficus subpisocarpa | 1 | 1 | 1 | 2.94 | 2.63 | 2.17 | 2.58 |
| Microtropis submembranacea | 1 | 1 | 1 | 2.94 | 2.63 | 2.17 | 2.58 |
| Radermachera frondosa | 1 | 1 | 1 | 2.94 | 2.63 | 2.17 | 2.58 |
| Jasminum nervosum var. elegans | 1 | 1 | 1 | 2.94 | 2.63 | 2.17 | 2.58 |
| Ficus parvifolia | 1 | 1 | 1 | 2.94 | 2.63 | 2.17 | 2.58 |
| Streblus taxoides | 1 | 1 | 1 | 2.94 | 2.63 | 2.17 | 2.58 |
| Artabotrys hexapetalus | 1 | 1 | 1 | 2.94 | 2.63 | 2.17 | 2.58 |
| Population | Sample Plot Number | Epiphytic Height (m) | Total | |||
|---|---|---|---|---|---|---|
| 0–0.9 | 1–1.9 | 2–2.9 | 3–4 | |||
| EXL | P1 | 5 | 7 | 8 | 1 | 21 |
| P2 | 5 | 1 | 0 | 0 | 6 | |
| P3 | 0 | 5 | 4 | 0 | 9 | |
| P4 | 0 | 0 | 1 | 9 | 10 | |
| P5 | 10 | 0 | 0 | 0 | 10 | |
| P6 | 0 | 0 | 10 | 0 | 10 | |
| YZC | P7 | 2 | 0 | 0 | 0 | 4 |
| P8 | 0 | 4 | 1 | 0 | 5 | |
| P9 | 3 | 0 | 1 | 0 | 4 | |
| Total | 25 | 17 | 25 | 10 | 77 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Li, W.; Chen, Z.; Zhang, Z. Epiphytic Habit and Spatial Distribution Patterns of Phalaenopsis deliciosa and Phalaenopsis hainanensis. Diversity 2025, 17, 818. https://doi.org/10.3390/d17120818
Zhong H, Li W, Chen Z, Zhang Z. Epiphytic Habit and Spatial Distribution Patterns of Phalaenopsis deliciosa and Phalaenopsis hainanensis. Diversity. 2025; 17(12):818. https://doi.org/10.3390/d17120818
Chicago/Turabian StyleZhong, Haotian, Wenchang Li, Zhiheng Chen, and Zhe Zhang. 2025. "Epiphytic Habit and Spatial Distribution Patterns of Phalaenopsis deliciosa and Phalaenopsis hainanensis" Diversity 17, no. 12: 818. https://doi.org/10.3390/d17120818
APA StyleZhong, H., Li, W., Chen, Z., & Zhang, Z. (2025). Epiphytic Habit and Spatial Distribution Patterns of Phalaenopsis deliciosa and Phalaenopsis hainanensis. Diversity, 17(12), 818. https://doi.org/10.3390/d17120818






