Relationship Between Tree Species Diversity and Soil Ecological Biochemistry Characteristics in Urban Wetland: A Case Study of International Important Wetland in Hangzhou, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Overview and Sampling Methods
2.2. Method for Calculating Tree Species Diversity Index
2.3. Methods for Determining Indicators
2.3.1. Soil N Components, C and P Contents
2.3.2. Soil Physicochemical Properties
2.4. Data Analysis Methods
3. Results
3.1. Species Composition Analysis
3.2. Correlation Between Tree Species Diversity, Soil Physicochemical Properties, and Chemical Ratios
3.3. Contribution of Tree Species Diversity and Soil Physicochemical Properties to Stoichiometric Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Midgley, G.F. Biodiversity and ecosystem function. Science 2012, 335, 174–175. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kang, J.H.; Choi, H.K.; Jang, J.E.; Lee, H.J. The assessment and application of biodiversity for freshwater ecosystems in Seoraksan and Odaesan National Parks using phylogenetic diversity. Environ. Biol. Res. 2024, 42, 593–608. [Google Scholar] [CrossRef]
- Shi, X.Z.; Wang, J.Q.; Huang, Z.Q.; He, J.Z. Research progresses in the effects of tree species diversity on soil microbial communities and biogeochemical cycling of elements. Acta Ecol. Sin. 2022, 42, 6092–6102. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Beßler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.; Scherber, C.; Steinbeiss, S.; et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef]
- Ma, Y.H.; Xu, D.Y.; Huang, Z.M.; Xu, F.; Xiang, M.; Wei, J.; Sheng, H.; Wu, Z.; Aguila, L.C.R.; Zhang, L.; et al. Mixed forest conversion from moso bamboo forests in wetland parks increases understory species diversity and improves soils. Glob. Ecol. Conserv. 2025, 57, e03386. [Google Scholar] [CrossRef]
- Hisano, M.; Searle, E.B.; Chen, H.Y.H. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol. Rev. Camb. Philos. 2018, 93, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, f8957. [Google Scholar] [CrossRef]
- Astel, A. Chemometrics based on fuzzy logic principles in environmental studies. Talanta 2007, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; He, S.; Wu, L.F.; Yan, Y.F.; Weng, S.F.; Liu, J.; Wang, W.Q.; Zeng, C.S. Characteristics of stoichiometric homeostasis of three plant species in wetlands in Minjiang Estuaryin Estuary. Wetl. Sci. 2014, 12, 293–298. [Google Scholar]
- Maynard, J.J.; Johnson, M.G. Applying fingerprint Fourier transformed infrared spectroscopy and chemometrics to assess soil ecosystem disturbance and recovery. J. Soil Water Conserv. 2018, 73, 443–451. [Google Scholar] [CrossRef]
- Yan, C.; Li, Y.Y.; Gao, J.J.; Wang, X. Characteristics of soil and plant ecological stoichiometry of carbon, nitrogen, and phosphorus in different wetland types of the Yellow River. Sustainability 2025, 17, 3276. [Google Scholar] [CrossRef]
- Zhang, Z.X. Contents and Stoichiometric Characteristics of Carbon and Nutrient Elements in Leaf Litter, Roots and Soils in Subtropical Forest. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2022. [Google Scholar]
- Wang, S.Q.; Yu, G.R. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sin. 2008, 27, 3937–3947. [Google Scholar]
- Chen, C.; Zhang, S.J.; Li, L.D.; Liu, Z.D.; Chen, J.L.; Gu, X.; Wang, L.F.; Fang, X. Carbon, nitrogen and phosphorus stoichiometry in leaf, litter and soil at different vegetation restoration stages in the mid-subtropical region of China. Chin. J. Plant Ecol. 2019, 43, 658–671. [Google Scholar] [CrossRef]
- Lange, M.; Roth, V.N.; Eisenhauer, N.; Roscher, C.; Dittmar, T.; Fischer-Bedtke, C.; González Macé, O.; Hildebrandt, A.; Milcu, A.; Mommer, L.; et al. Plant diversity enhances production and downward transport of biodegradable dissolved organic matter. J. Ecol. 2021, 109, 1284–1297. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.; Elser, J.; He, N.; Wu, H.; Zhang, G.; Wu, J.; Bai, Y.; Han, X. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.T.; Zhang, H.; Huang, B.B.; Liu, C.H.; Jiang, Z.K.; Ma, X.Q. Soil C∶N∶P stoichiometry and its relationship with the soil physicochemical properties of different aged Chinese fir (Cunninghamia lanceolata) plantations. Acta Ecol. Sin. 2019, 39, 2520–2531. [Google Scholar]
- Zhang, S.; Landuyt, D.; Dhiedt, E.; De Frenne, P.; Verheyen, K. Tree species diversity affects litter decomposition via modification of the microenvironment. Ecosystems 2024, 27, 508–522. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Hedo, J.; Cerdá, A.; Candel-Pérez, D.; Viñegla, B. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. ssp. salzmannii) Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peuelas., J.; Shi, X.; Brearley, F.Q.; Lucas-Borja, M.E.; Leng, P.; Huang, Z. Tree species richness improves soil net nitrogen mineralization rates in a young biodiversity-ecosystem function experiment. Catena 2024, 243, 8. [Google Scholar] [CrossRef]
- Wang, Z.B. Fine Root Traits and Their Response to Soilproperties and Tree Species Diversity Incoastal Reclaimed Soil. Ph.D. Thesis, East China Normal University, Shanghai, China, 2020. [Google Scholar]
- Huang, Y.; Fang, X.; Chu, W.K.; Chen, M. Bacterial diversity and community structure in sediments of Xixi Wetland, Hangzhou. Oceanol. Et Limnol. Sin. 2015, 46, 1202–1209. [Google Scholar]
- Yi, X.M.; Hua, L.; He, H.; You, A.; Yin, H.; Chen, L.; Hu, J. Evaluation of water system connectivity in the Xixi Wetland based on graph theory. Wetl. Sci. 2023, 21, 951–960. [Google Scholar]
- Shi, J.; Liao, X.F.; Fang, X.B.; Zhou, L.Y.; Yu, H.X.; Yao, L.; Hong, Y.F. Causal analysis of the spatial-temporal variation of water quality in xixi wetland. Environ. Pollut. Control 2014, 36, 39–46. [Google Scholar]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Labs Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Zhou, W.J.; Nan, Q.R.; Yang, H.W.; Mo, Z.; Zhang, Y.; Zhao, L.Y. Species diversity and biomass of invasive communities of Alternathera philoxeroides in heterogeneous habitats. J. Hubei Univ. (Nat. Sci. Ed.) 2026. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Wang, J.R.; Zhou, J.J.; Zhu, G.F. A review of soil carbon, nitrogen, phosphorus and stoichiometry studies in China. Chin. J. Ecol. 2024, 43, 2493–2501. [Google Scholar]
- Li, W.T.; Hu, Z.Y.; Zhang, K.; Li, L.; Qiu, Y.; Guo, X. Effects of land-use types on soil carbon and nitrogen stocks in the Napahai wetlands. Chin. J. Ecol. 2024, 43, 1720–1727. [Google Scholar]
- Cao, R.; Yang, W.Q.; Yuan, J.; Li, H.; Tan, B. Changes of soil enzyme activities in soil organic layer and mineral soil layer in the Masson pine plantation with critical periods. Acta Ecol. Sin. 2022, 42, 8031–8040. [Google Scholar] [CrossRef]
- Tan, L.; He, Y.J.; Qin, L.; Chen, S.Z. Comparison of soil physical and chemical properties of pure castanopsis hystrix, pure pinus massoniana and mixed-species tree plantation in south subtropical area. J. West China For. Sci. 2014, 43, 35–41. [Google Scholar]
- Spohn, M.; Bagchi, S.; Biederman, L.A.; Borer, E.T.; Bråthen, K.A.; Bugalho, M.N.; Caldeira, M.C.; Catford, J.A.; Collins, S.L.; Eisenhauer, N.; et al. The positive effect of plant diversity on soil carbon depends on climate. Nat. Commun. 2023, 14, 6624. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; You, Y.; Wen, Y.; Wang, H.; Wang, J. Microbial community and associated enzymes activity influence soil carbon chemical composition in Eucalyptus urophylla plantation with mixing N-fixing species in subtropical China. Plant Soil 2017, 414, 199–212. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, J.; Shi, Z.; Lu, L.; Zeng, J.; Ming, A.; Tang, J.; Yu, H. Effects of tree species mixture on soil organic carbon stocks and greenhouse gas fluxes in subtropical plantations in China. For. Ecol. Manag. 2013, 300, 4–13. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Wang, Z.; Yu, C.Q.; Shen, Z.X.; Zhang, X.Z.; Hu, X.X. Soil C/P distribution characteristics of alpine steppe ecosystems in the Qinhai-Tibetan Plateau. Acta Prataculturae Sin. 2014, 23, 9–19. [Google Scholar]
- Liu, S.; Li, G.; Yang, C.; Du, J.; Xu, W.; Xie, M. Seasonal variation of soil carbon, nitrogen and phosphorus stoichiometry under different vegetation types in Loess Hilly Region. J. Soil Water Conserv. 2021, 35, 343–349. [Google Scholar]
- Dong, X.; Xin, Z.M.; Huang, Y.R.; Li, X.L.; Hao, Y.G.; Liu, F.; Liu, M.H.; Li, W. Soil stoichiometry in typical shrub communities in the Ulan Buh Desert. Acta Ecol. Sin. 2019, 39, 6247–6256. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Bongers, F. Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 2015, 96, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Heděnec, P.; Nilsson, L.O.; Zheng, H.; Gundersen, P.; Schmidt, I.K.; Rousk, J.; Vesterdal, L. Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Sci. Total Environ. 2020, 748, 141–149. [Google Scholar] [CrossRef]
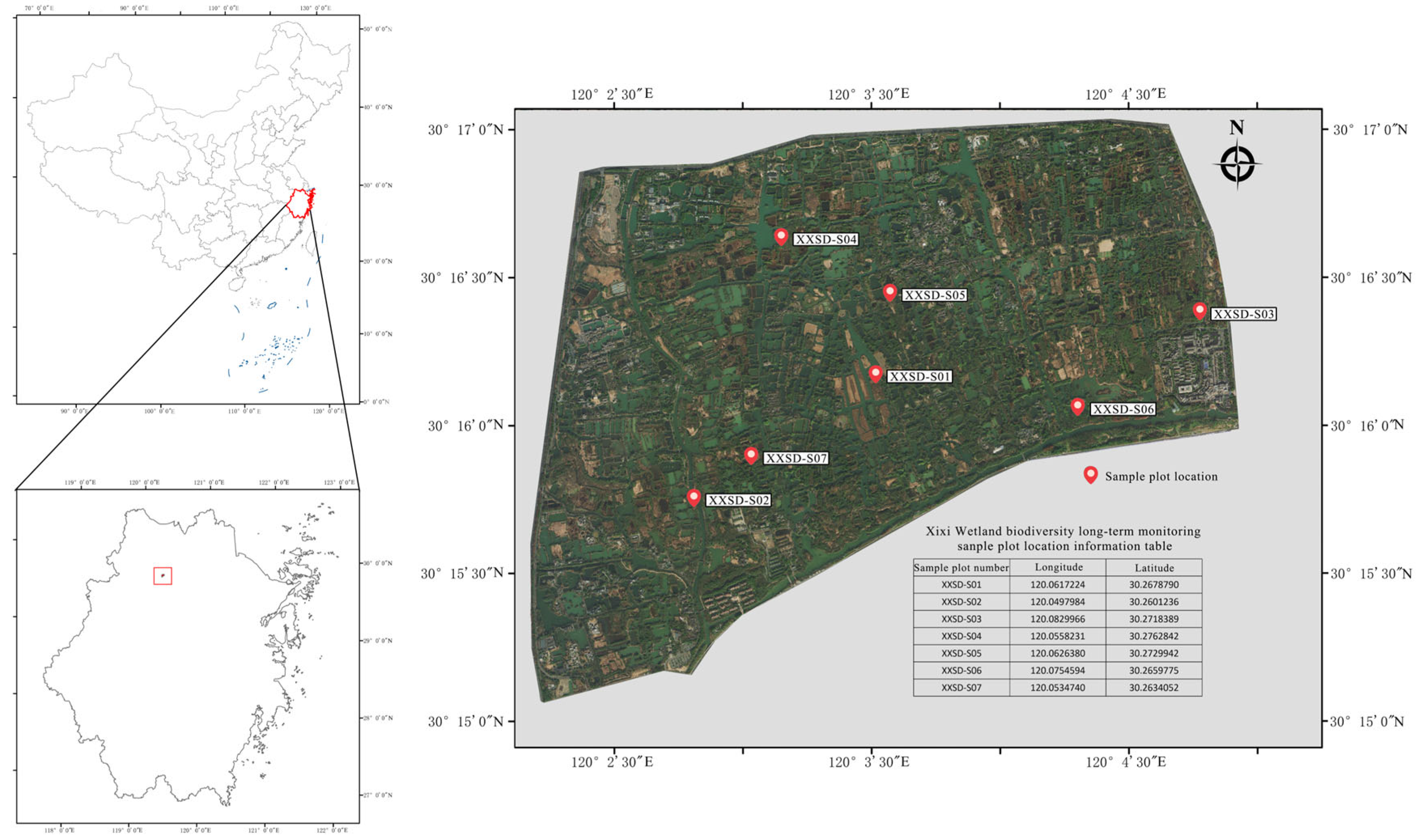

| Plot Name | Coordinates | Dominant Tree Species |
|---|---|---|
| XXSD-S01 | E 120.06172, N 30.26787 | Salix matsudana, Morus alba |
| XXSD-S02 | E 120.04979, N 30.26012 | Cinnamomum camphora, Prunus mume |
| XXSD-S03 | E 120.08299, N 30.27183 | Pterocarya stenoptera, Broussonetia papyrifera |
| XXSD-S04 | E 120.05582, N 30.27628 | Morus alba, Broussonetia papyrifera |
| XXSD-S05 | E 120.06263, N 30.27299 | Pterocarya stenoptera, Ulmus parvifolia |
| XXSD-S06 | E 120.07545, N 30.26597 | Cinnamomum camphora, Pterocarya stenoptera |
| XXSD-S07 | E 120.06172, N 30.26787 | Cinnamomum camphora, Celtis sinensis |
| Plot | R | H’ | D | Jsw |
|---|---|---|---|---|
| XXSD-S01 | 6 | 1.32 | 0.56 | 0.62 |
| XXSD-S02 | 8 | 1.36 | 0.42 | 0.56 |
| XXSD-S03 | 8 | 1.45 | 0.36 | 0.63 |
| XXSD-S04 | 7 | 1.23 | 0.58 | 0.58 |
| XXSD-S05 | 12 | 1.57 | 0.62 | 0.55 |
| XXSD-S06 | 9 | 1.22 | 0.49 | 0.62 |
| XXSD-S07 | 22 | 1.31 | 0.52 | 0.69 |
| Family | Genus | Species | Number | % |
|---|---|---|---|---|
| Cupressaceae | Metasequoia | Metasequoia glyptostroboides | 2 | 0.41 |
| Magnoliaceae | Yulania | Yulania denudata | 3 | 0.62 |
| Lauraceae | Phoebe | Phoebe sheareri | 1 | 0.21 |
| Camphora | Camphora officinarum | 62 | 12.86 | |
| Ulmaceae | Ulmus | Ulmusparvifolia | 7 | 1.45 |
| Cannabaceae | Celtis | Celtis sinensis | 19 | 3.94 |
| Moraceae | Morus | Morus alba | 129 | 26.76 |
| Broussonetia | Broussonetia papyrifera | 84 | 17.43 | |
| Juglandaceae | Pterocarya | Pterocarya stenoptera | 47 | 9.75 |
| Fagaceae | Castanopsis | Castanopsis jucunda | 1 | 0.21 |
| Theaceae | Camellia | Camellia sinensis | 1 | 0.21 |
| Elaeocarpaceae | Elaeocarpus | Elaeocarpus sylvestris | 3 | 0.62 |
| Salicaceae | Salix | Salix matsudana | 27 | 5.60 |
| Ebenaceae | Diospyros | Diospyros kaki var. silvestris | 10 | 2.07 |
| Rosaceae | Eriobotrya | Eriobotrya japonica | 5 | 1.04 |
| Prunus | Prunus mume | 13 | 2.70 | |
| Fabaceae | Robinia | Robinia pseudoacacia | 5 | 1.04 |
| Nyssaceae | Camptotheca | Camptotheca acuminata | 4 | 0.83 |
| Celastraceae | Euonymus | Euonymus maackii | 8 | 1.66 |
| Aquifoliaceae | Ilex | Ilex chinensis | 2 | 0.41 |
| Euphorbiaceae | Triadica | Triadica sebifera | 2 | 0.41 |
| Sapindaceae | Koelreuteria | Koelreuteria bipinnata var. integrifoliola | 1 | 0.21 |
| Anacardiaceae | Rhus | Rhus chinensis | 7 | 1.45 |
| Meliaceae | Melia | Melia azedarach | 8 | 1.66 |
| Rutaceae | Citrus | Citrus maxima | 1 | 0.21 |
| Araliaceae | Fatsia | Fatsia japonica | 1 | 0.21 |
| Lamiaceae | Clerodendrum | Clerodendrum trichotomum | 14 | 2.90 |
| Oleaceae | Ligustrum | Ligustrum lucidum | 12 | 2.49 |
| Oleaceae | Osmanthus | Osmanthus fragrans | 3 | 0.62 |
| Plot | pH | BD | EC | SM | Community | TN | TOC | TP | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g/cm3 | μs/cm | % | g/kg | g/kg | g/kg | ||||||
| S01 | 5.69 c | 1.36 a | 0.04 c | 42.40 a | SMC | 17.16 b | 1.87 c | 0.57 d | 9.16 c | 30.11 a | 3.28 ab |
| S02 | 5.57 c | 1.30 ab | 0.04 c | 32.10 b | CPC | 13.96 b | 1.46 c | 0.68 bcd | 9.54 c | 20.72 b | 2.18 c |
| S03 | 6.98 b | 1.30 ab | 0.21 a | 27.75 bc | PBC | 22.03 a | 2.09 c | 0.60 cd | 10.51 bc | 36.67 a | 3.49 a |
| S04 | 7.56 a | 1.17 b | 0.06 bc | 13.15 d | MBC | 2.25 c | 30.13 ab | 0.83 ab | 13.75 a | 37.17 a | 2.76 bc |
| S05 | 7.72 a | 1.24 ab | 0.28 a | 16.50 d | PUC | 2.06 c | 25.03 b | 0.77 abc | 12.45 ab | 32.58 a | 2.65 bc |
| S06 | 6.57 b | 1.20 ab | 0.17 ab | 16.68 d | CPC | 2.73 c | 34.19 a | 0.94 a | 12.54 ab | 36.24 a | 2.89 abc |
| S07 | 5.44 c | 1.34 ab | 0.04 c | 20.65 cd | CCC | 2.48 c | 28.36 ab | 0.83 ab | 11.36 bc | 34.35 a | 3.02 ab |
| Sample ID | Sampling Point ID | pH | TC | TN | TP | Available P | NH4+-N | NO3−-N |
|---|---|---|---|---|---|---|---|---|
| g/kg | g/kg | g/kg | mg/kg | mg/kg | mg/kg | |||
| A | S01-1.1 | 6.6 | 12.71 | 1.38 | 0.52 | 11.4 | 3.84 | 14.12 |
| B | S01-3.1 | 5.4 | 15.67 | 1.79 | 0.55 | 13.23 | 2.29 | 36.29 |
| C | S01-3.3 | 5.3 | 18.02 | 1.93 | 0.55 | 17.93 | 1.13 | 27.16 |
| D | S01-1.3 | 5.47 | 22.23 | 2.38 | 0.64 | 12.47 | 1.91 | 14.25 |
| A | S02-1.1 | 5.39 | 15.64 | 1.65 | 0.7 | 12.27 | 0.75 | 1.53 |
| B | S02-3.1 | 5.95 | 12.89 | 1.35 | 0.72 | 13.55 | 0.67 | 2.59 |
| C | S02-3.3 | 5.4 | 14.59 | 1.49 | 0.64 | 19.9 | 1.69 | 5.85 |
| D | S02-1.3 | 5.52 | 12.7 | 1.36 | 0.64 | 11.4 | 1.07 | 2.78 |
| A | S03-1.1 | 6.98 | 20.98 | 2.04 | 0.59 | 11.55 | 2.96 | 23.87 |
| B | S03-3.1 | 6.98 | 22.21 | 2.1 | 0.6 | 17.25 | 0.6 | 12.94 |
| C | S03-3.3 | 6.9 | 20.06 | 1.96 | 0.59 | 12.7 | 0.52 | 13.41 |
| D | S03-1.3 | 7.05 | 24.86 | 2.27 | 0.62 | 12.3 | 0.7 | 15.13 |
| A | S04-1.1 | 7.13 | 26.9 | 2.43 | 0.68 | 28.13 | 1.27 | 21.35 |
| B | S04-3.1 | 7.52 | 31.08 | 2.62 | 1.04 | 61.5 | 1.93 | 33.11 |
| C | S04-3.3 | 7.72 | 31.56 | 1.79 | 0.7 | 18.7 | 2.38 | 18.98 |
| D | S04-1.3 | 7.85 | 30.99 | 2.15 | 0.91 | 48.76 | 1.57 | 23.47 |
| A | S05-1.1 | 7.71 | 26.28 | 2.34 | 0.84 | 21.98 | 3.64 | 12.79 |
| B | S05-3.1 | 8.06 | 20.42 | 1.37 | 0.61 | 18.68 | 3 | 4.89 |
| C | S05-3.3 | 7.84 | 25.95 | 2.15 | 0.85 | 36.23 | 1.96 | 17.75 |
| D | S05-1.3 | 7.25 | 27.47 | 2.37 | 0.78 | 28.37 | 1.94 | 24.55 |
| A | S06-1.1 | 6.57 | 38.34 | 3.03 | 1.08 | 53.34 | 1.62 | 17.33 |
| B | S06-3.1 | 6.49 | 30.43 | 2.44 | 0.85 | 20.15 | 1.95 | 17.96 |
| C | S06-3.3 | 6.63 | 42.05 | 3.33 | 0.98 | 44.1 | 1.85 | 27.97 |
| D | S06-1.3 | 6.59 | 25.92 | 2.1 | 0.85 | 39.92 | 1.88 | 18.4 |
| A | S07-1.1 | 5.52 | 27.54 | 2.35 | 0.72 | 20.05 | 0.54 | 15.44 |
| B | S07-3.1 | 5.19 | 29 | 2.66 | 0.75 | 19.35 | 2.27 | 18.61 |
| C | S07-3.3 | 5.48 | 21.07 | 1.96 | 0.89 | 19.95 | 1.63 | 13.52 |
| D | S07-1.3 | 5.57 | 35.82 | 2.96 | 0.97 | 19.45 | 1.61 | 17.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, K.; Li, C.; Luo, Y.; Li, W. Relationship Between Tree Species Diversity and Soil Ecological Biochemistry Characteristics in Urban Wetland: A Case Study of International Important Wetland in Hangzhou, China. Diversity 2025, 17, 817. https://doi.org/10.3390/d17120817
Yao K, Li C, Luo Y, Li W. Relationship Between Tree Species Diversity and Soil Ecological Biochemistry Characteristics in Urban Wetland: A Case Study of International Important Wetland in Hangzhou, China. Diversity. 2025; 17(12):817. https://doi.org/10.3390/d17120817
Chicago/Turabian StyleYao, Kekan, Chuanliang Li, Yuheng Luo, and Weicheng Li. 2025. "Relationship Between Tree Species Diversity and Soil Ecological Biochemistry Characteristics in Urban Wetland: A Case Study of International Important Wetland in Hangzhou, China" Diversity 17, no. 12: 817. https://doi.org/10.3390/d17120817
APA StyleYao, K., Li, C., Luo, Y., & Li, W. (2025). Relationship Between Tree Species Diversity and Soil Ecological Biochemistry Characteristics in Urban Wetland: A Case Study of International Important Wetland in Hangzhou, China. Diversity, 17(12), 817. https://doi.org/10.3390/d17120817






