Benthic Producers, Methane Carbon, and Diazotrophic Nitrogen as Sources of Nutrients in the Food Web of a Subarctic Lake
Abstract
1. Introduction
2. Materials and Methods
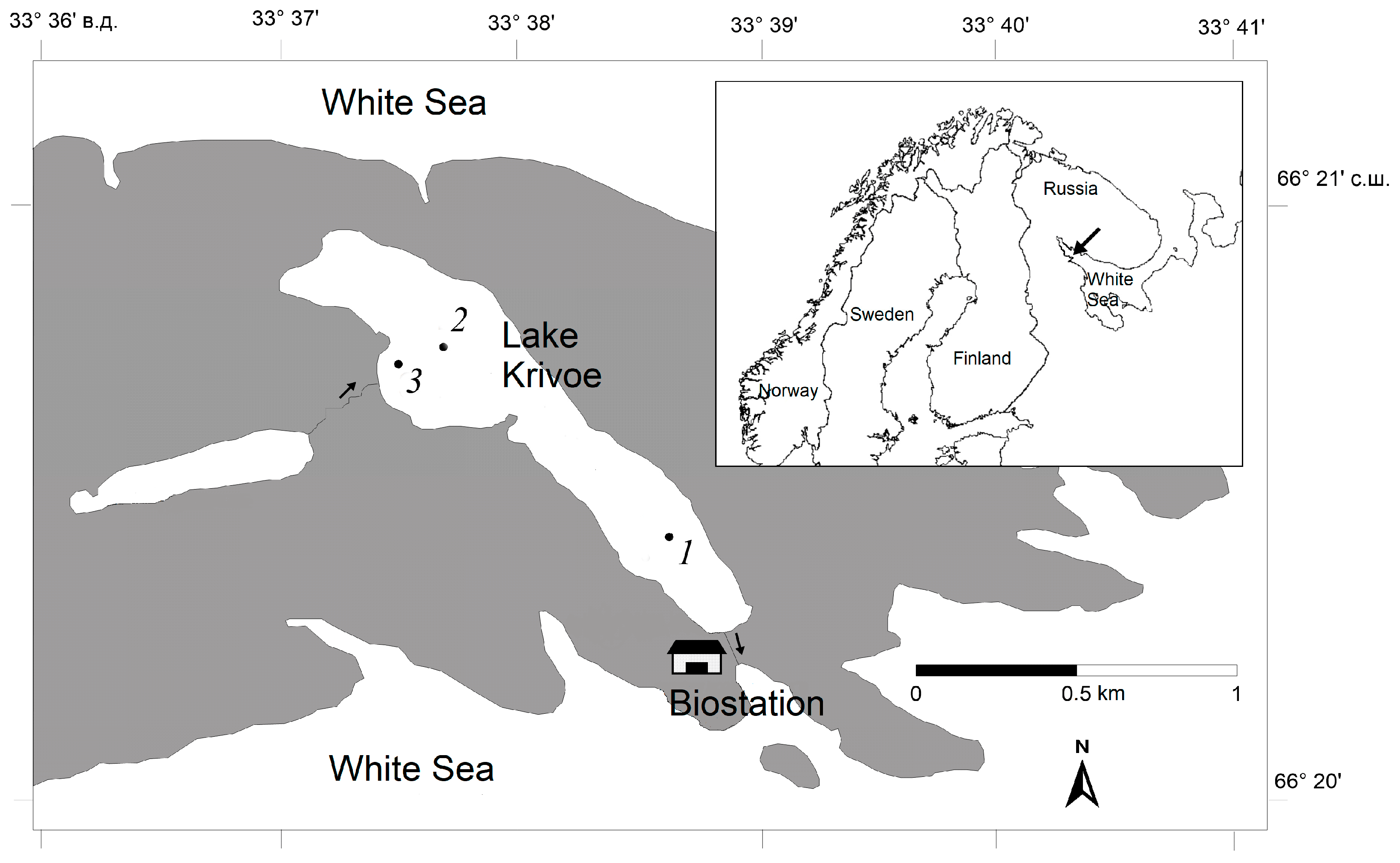
2.1. Study Lakes
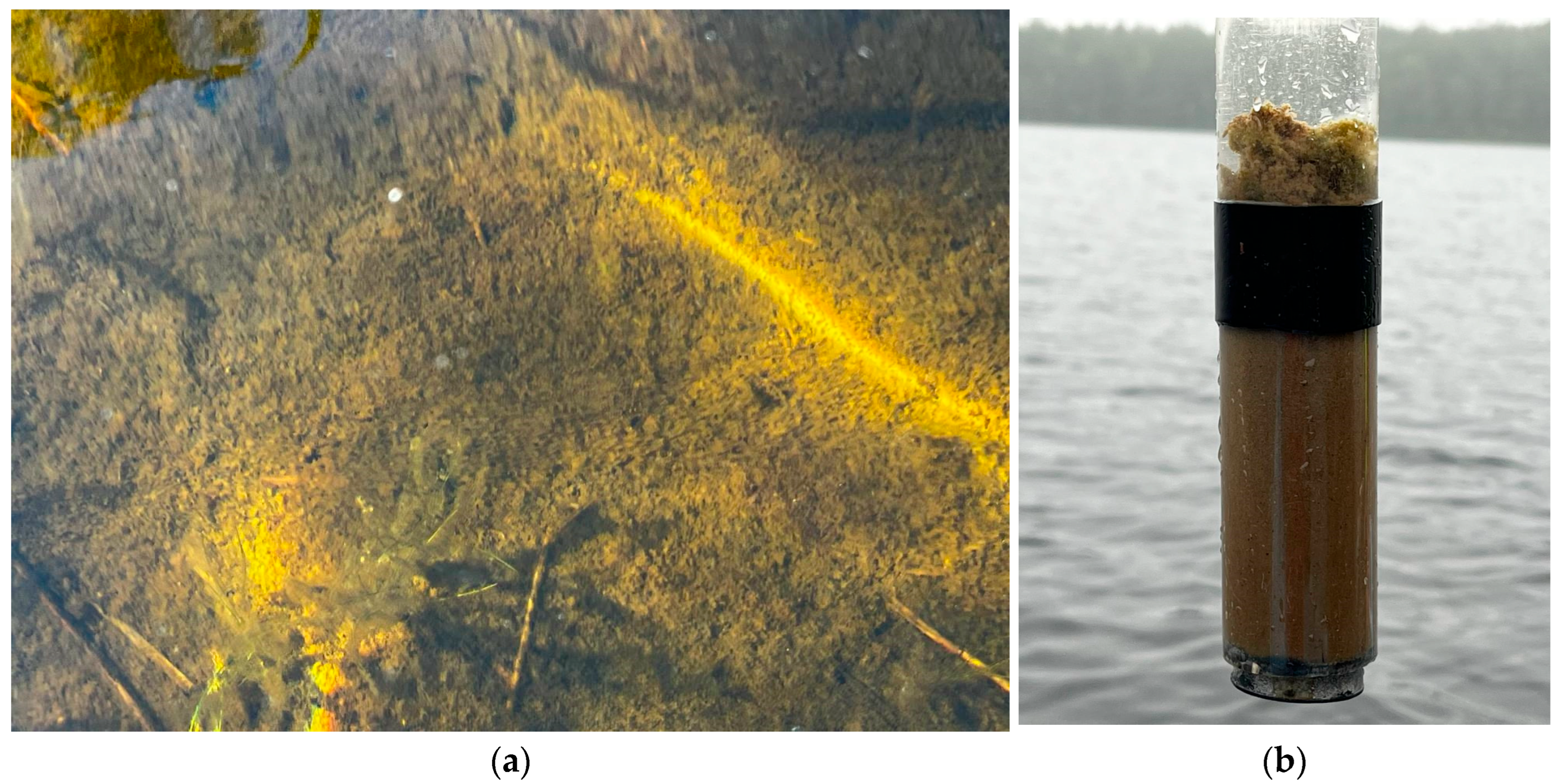
2.2. Field Sampling and Laboratory Procedures
2.3. Stable Isotope Analysis
3. Results
3.1. Structure of the Lake’s Food Web
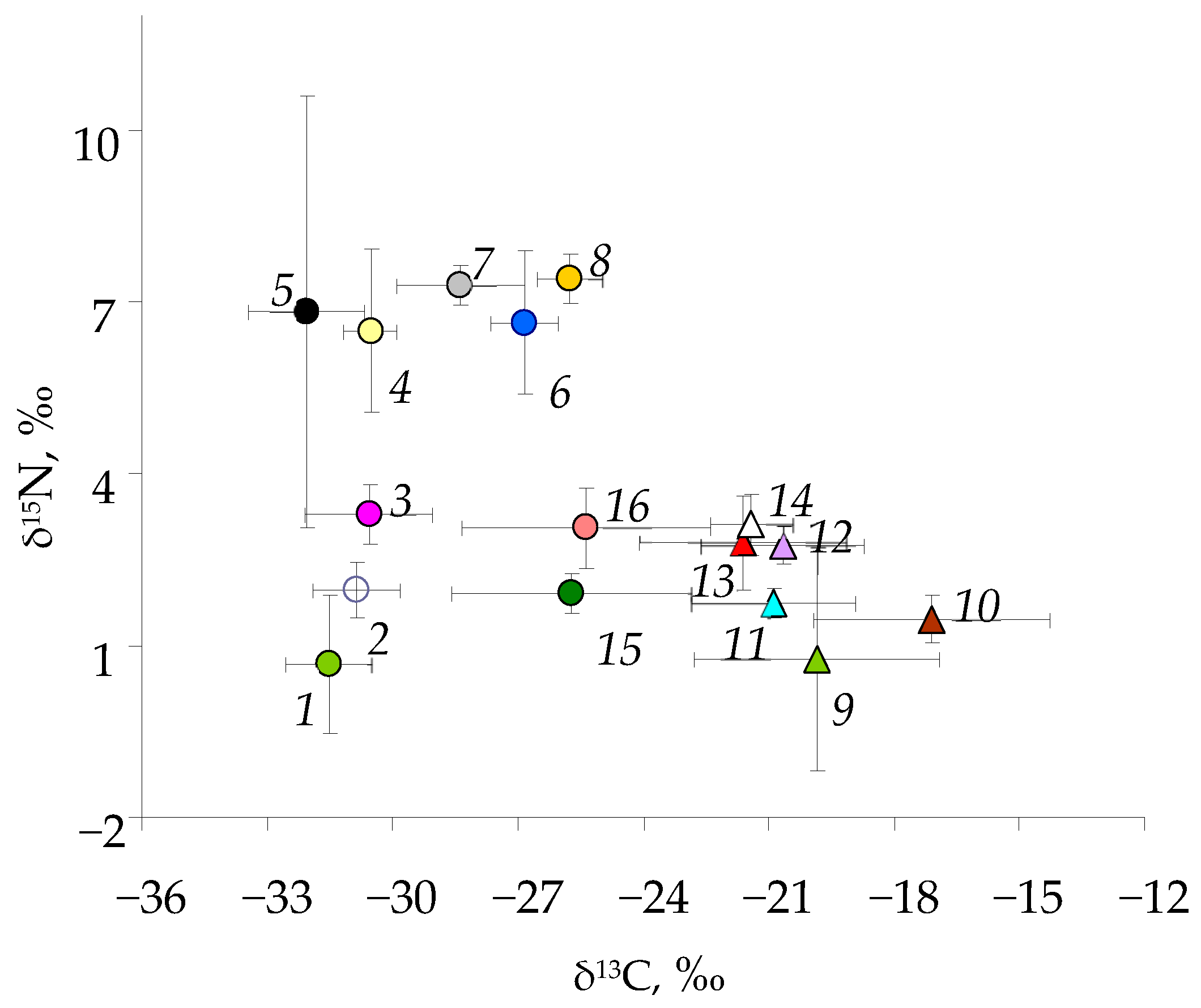
3.2. Carbon and Nitrogen Stable Isotopes’ Composition
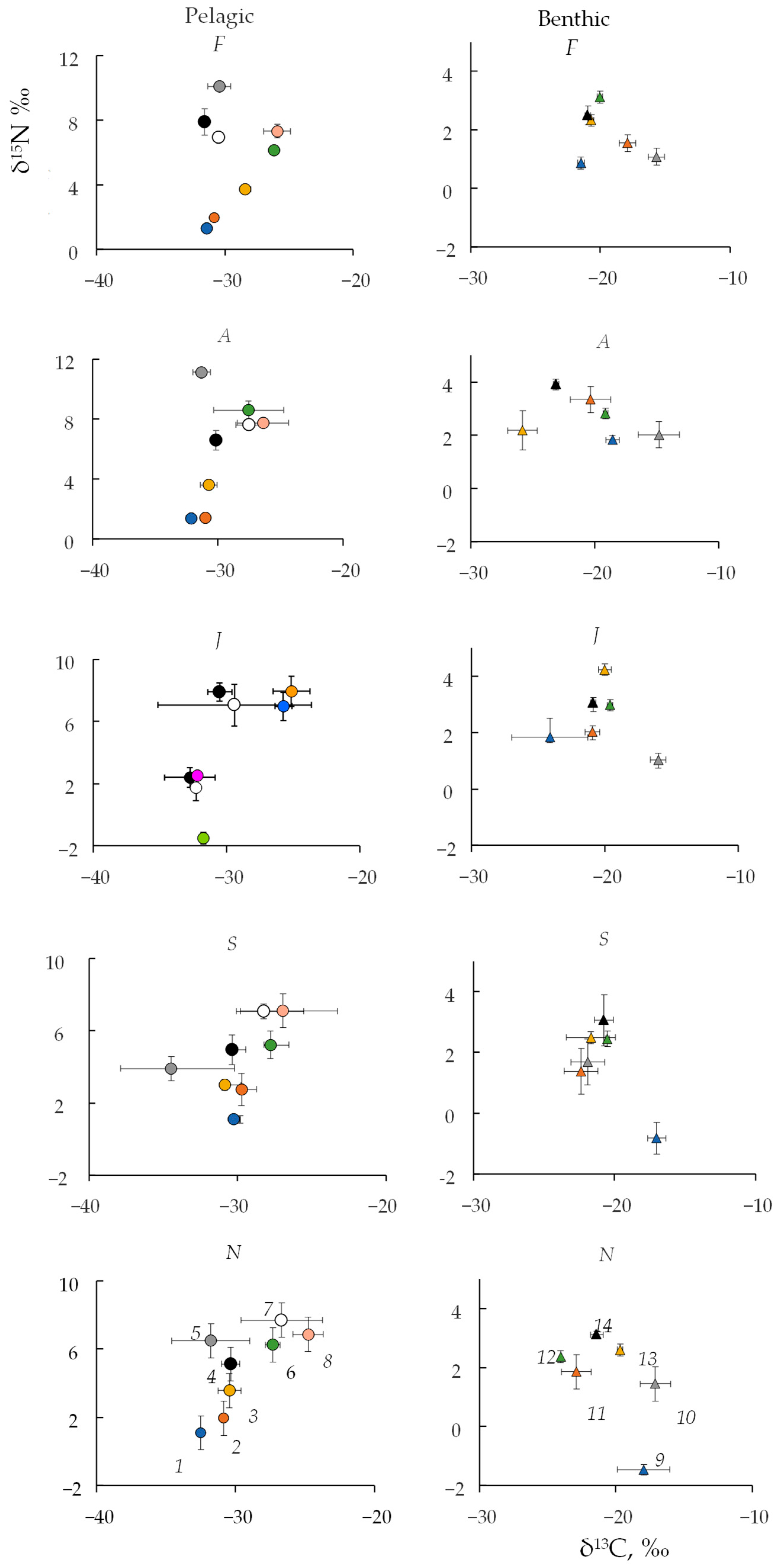
3.3. Seasonal Changes in Carbon and Nitrogen Stable Isotopes
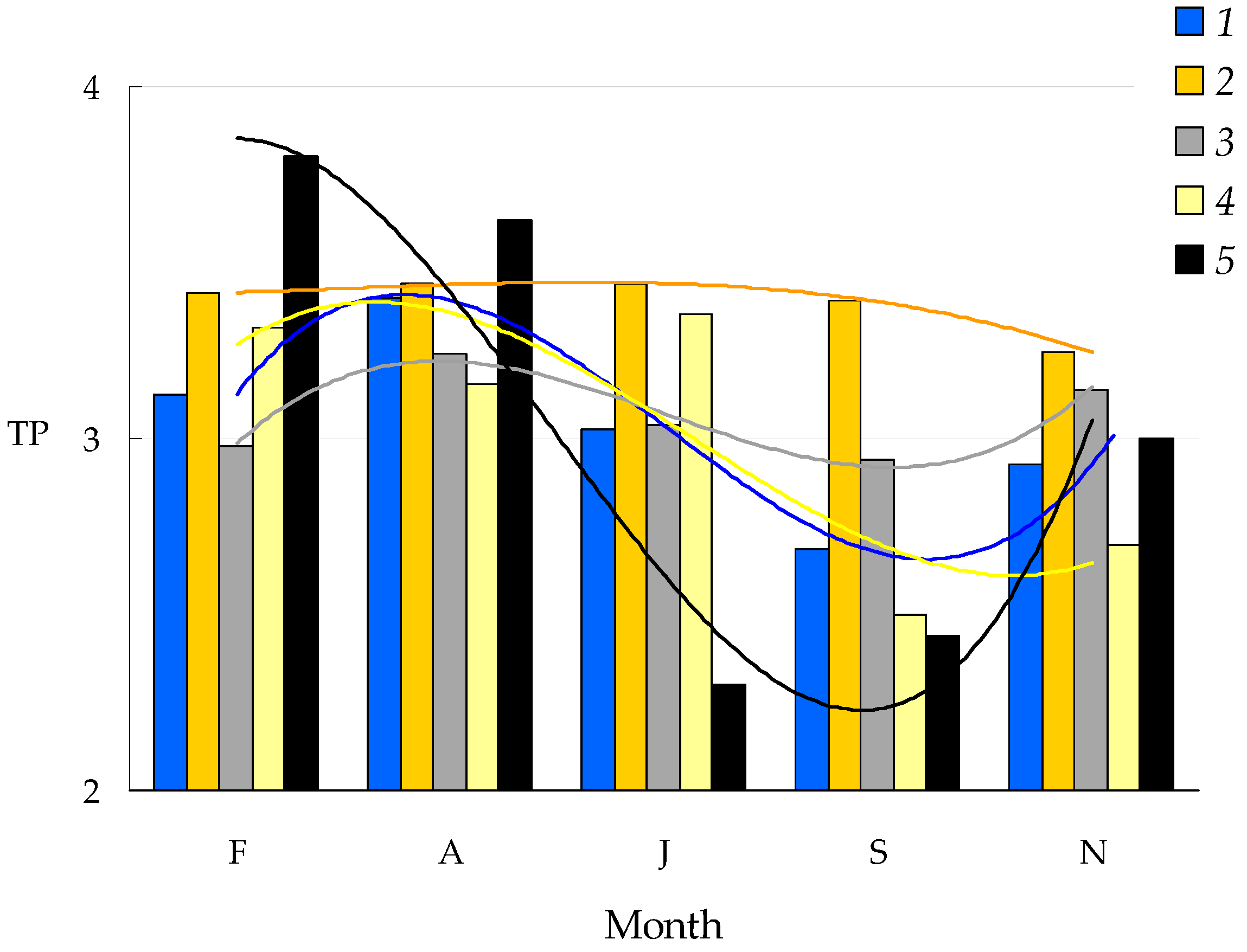
3.4. Trophic Position of Predatory Consumers
4. Discussion
4.1. Structural Specificity of the Lake’s Food Web
4.2. Possible Reasons for Seasonal Changes in δ13C Values
4.3. Seasonal Variations in δ15N Values and Trophic Positions of Consumers
4.4. Predators’ Omnivory and Seasonal Changes in Trophic Position
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huser, B.J.; Futter, M.N.; Bogan, D.; Brittain, J.E.; Culp, J.M.; Goedkoop, W.; Lento, J. Spatial and temporal variation in Arctic freshwater chemistry—Reflecting climate-induced landscape alterations and a changing template for biodiversity. Freshw. Biol. 2022, 67, 14–29. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Van de Bogert, M.; Bade, D.L.; Bastviken, D.; Gille, C.M.; Hodgson, J.R.; Kitchell, J.F.; Kritzberg, E.S. Ecosystem subsidies: Terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology 2005, 86, 2737–2750. [Google Scholar] [CrossRef]
- Karlsson, J.; Bergström, A.-K.; Byström, P.; Gudasz, C.; Rodríguez, P.; Hein, C. Terrestrial organic matter input suppresses biomass production in lake ecosystems. Ecology 2015, 96, 2870–2876. [Google Scholar] [CrossRef]
- Taipale, S.J.; Galloway, A.W.; Aalto, S.L.; Kahilainen, K.K.; Strandberg, U.; Kankaala, P. Terrestrial carbohydrates support freshwater zooplankton during phytoplankton deficiency. Sci. Rep. 2016, 6, 30897. [Google Scholar] [CrossRef]
- Grey, J. The incredible lightness of being methane-fuelled: Stable isotopes reveal alternative energy pathways in aquatic ecosystems and beyond. Front. Ecol. Evol. 2016, 4, 8. [Google Scholar] [CrossRef]
- de Wit, H.A.; Valinia, S.; Weyhenmeyer, G.A.; Futter, M.N.; Kortelainen, P.; Austnes, K.; Hessen, D.O.; Räike, A.; Laudon, H.; Vuorenmaa, J. Current browning of surface waters will be further promoted by wetter climate. Environ. Sci. Technol. Lett. 2016, 3, 430–435. [Google Scholar] [CrossRef]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; De Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J.; et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Guzeva, A.V.; Slukovskii, Z.I. Geochemical characterization of humic acids isolated from tundra lakes sediments of Murmansk Region, Russia. Water Sect. Russia Prob. Technol. Manag. 2023, 1, 78–92. (In Russian) [Google Scholar] [CrossRef]
- Jones, R.I.; Grey, J. Biogenic methane in freshwater food webs. Freshw. Biol. 2011, 56, 213–229. [Google Scholar] [CrossRef]
- Jones, S.E.; Lennon, J.T. Evidence for limited microbial transfer of methane in a planktonic food web. Aquat. Microb. Ecol. 2009, 58, 45–53. [Google Scholar] [CrossRef]
- Schilder, J.; van Hardenbroek, M.; Bodelier, P.; Kirilova, E.P.; Leuenberger, M.; Lotter, A.F.; Heiri, O. Trophic state changes can affect the importance of methane-derived carbon in aquatic food webs. Proc. R. Soc. B 2017, 284, 20170278. [Google Scholar] [CrossRef]
- Deines, P.; Bodelier, P.L.; Eller, G. Methane-derived carbon flows through methane oxidizing bacteria to higher trophic levels in aquatic systems. Environ. Microbiol. 2007, 9, 1126–1134. [Google Scholar] [CrossRef]
- DelVecchia, A.G.; Stanford, J.A.; Xu, X. Ancient and methane-derived carbon subsidizes contemporary food webs. Nat. Commun. 2016, 7, 13163. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Winberg, G.G.; Alimov, A.F.; Boulion, V.V.; Ivanova, M.B.; Korobtzova, E.V.; Kuzmitzkaya, N.K.; Nikulina, V.N.; Finogenova, N.P.; Fursenko, M.V. Biological productivity of two subarctic lakes. Freshw. Biol. 1973, 3, 177–197. [Google Scholar] [CrossRef]
- Bouillon, V.V. Relationship between growth rates of planktonic algae and bacteria. Sci. South Russia 2023, 19, 61–72. (In Russian) [Google Scholar] [CrossRef]
- Litvinchuk, L.F.; Sharov, A.N.; Chernova, E.N.; Smirnov, V.V.; Berezina, N.A. Mutual links between microcystins-producing cyanobacteria and plankton community in clear and brown northern lakes. Food Webs 2023, 35, e00279. [Google Scholar] [CrossRef]
- Zanden, J.; Vadeboncoeur, Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 2002, 83, 2152–2161. [Google Scholar] [CrossRef]
- Berezina, N.A.; Terentjev, P.M.; Zubova, E.M.; Tsurikov, S.M.; Maximov, A.A.; Sharov, A.N. Seasonal diet changes and trophic links of cold-water fish (Coregonus albula) within a northern lake ecosystem. Animals 2024, 14, 394. [Google Scholar] [CrossRef]
- Mayer, B.; Wassenaar, L.I. Isotopic characterization of nitrate sources and transformations in Lake Winnipeg and its contributing rivers, Manitoba, Canada. J. Great Lakes Res. 2012, 38, 135–146. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Duberg, J.; Motwani, N.H.; Hogfors, H.; Klawonn, I.; Ploug, H.; Svedén, J.B.; Garbaras, A.; Sundelin, B.; Hajdu, S.; et al. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. AMBIO 2015, 44, 413–426. [Google Scholar] [CrossRef]
- Berezina, N.A.; Tiunov, A.V.; Tsurikov, S.M.; Kurbatova, S.A.; Korneva, L.G.; Makarova, O.S.; Bykova, S.N. Cyanobacteria as a food source for invertebrates: Results of a model experiment. Russ. J. Ecol. 2021, 52, 247–252. [Google Scholar] [CrossRef]
- Davis, A.M.; Blanchette, M.L.; Pusey, B.; Jardine, T.D.; Pearson, R.G. Gut content and stable isotope analyses provide complementary understanding of ontogenetic dietary shifts and trophic relationships among fishes in a tropical river. Freshw. Biol. 2012, 57, 2156–2172. [Google Scholar] [CrossRef]
- Rautio, M.; Mariash, H.; Forsström, L. Seasonal shifts between autochthonous and allochthonous carbon contributions to zooplankton diets in a subarctic lake. Limnol. Oceanogr. 2011, 56, 1513–1524. [Google Scholar] [CrossRef]
- Laske, S.; Rosenberger, A.; Wipfli, M.; Zimmerman, C. Generalist feeding strategies in Arctic freshwater fish: A mechanism for dealing with extreme environments. Ecol. Freshw. Fish 2018, 27, 767–784. [Google Scholar] [CrossRef]
- Perga, M.E. Potential of δ13C and δ15N of cladoceran subfossil exoskeletons for paleo-ecological studies. J. Paleolimnol. 2010, 44, 387–395. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Bazin, S.; Domaizon, I.; Barouillet, C.; Frossard, V.; Sentis, A. Seasonal variations in planktonic food web structure affect stability by shifting the distribution of energy fluxes. Oikos 2025, e11528. [Google Scholar] [CrossRef]
- Gaedke, U.; Li, X.; Guill, C.; Hemerik, L.; de Ruiter, P.C. Seasonal shifts in trophic interaction strength drive stability of natural food webs. Ecol. Lett. 2025, 28, e70075. [Google Scholar] [CrossRef]
- Bertilsson, S.; Burgin, A.; Carey, C.C.; Fey, S.B.; Grossart, H.-P.; Grubisic, L.M.; Jones, I.D.; Kirillin, G.; Lennon, J.T.; Shade, A. The under-ice microbiome of seasonally frozen lakes. Limnol. Oceanogr. 2013, 58, 1998–2012. [Google Scholar] [CrossRef]
- Vigneron, A.; Lovejoy, C.; Cruaud, P.; Kalenitchenko, D.; Culley, A.; Vincent, W.F. Contrasting winter versus summer microbial communities and metabolic functions in a permafrost thaw lake. Front. Microbiol. 2019, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Pålsson, C.; Graneli, W. Diurnal and seasonal variations in grazing by bacterivorous mixotrophs in an oligotrophic clearwater lake. Arch. Hydrobiol. 2003, 157, 289–307. [Google Scholar] [CrossRef]
- Sanders, R.; Porter, K.G. Phagotrophic Phytoflagellates. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1988; Volume 10. [Google Scholar] [CrossRef]
- Pereira, I.; Rangel, A.; Chagas, B.; de Moura, B.; Urbano, S.; Sassi, R.; Castro, C. Microalgae Growth Under Mixotrophic Condition Using Agro-Industrial Waste: A Review; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kristiansen, J.; Skaloud, P. Chrysophyta; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Roberts, E.; Laybourn-Parry, J. Mixotrophic cryptophytes and their predators in the Dry Valley lakes of Antarctica. Freshwat. Biol. 2001, 41, 737–746. [Google Scholar] [CrossRef]
- Tuchman, N.C.; Schollett, M.A.; Rier, S.T.; Geddes, P. Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 2006, 561, 167–177. [Google Scholar] [CrossRef]
- Pelroy, R.A.; Bassham, J.A. Photosynthetic and dark carbon metabolism in unicellular blue-green algae. Arch. Für Mikrobiologie. 1972, 86, 25–38. [Google Scholar] [CrossRef]
- Flöder, S.; Hansen, T.; Ptacnik, R. Energy–dependent bacterivory in ochromonas minima–A strategy promoting the use of substitutable resources and survival at insufficient light supply. Protist 2006, 157, 291–302. [Google Scholar] [CrossRef]
- Glagolev, M.V. Methane emission: Ideology and methodology of the “standard model” for Western Siberia. Dyn. Environ. Glob. Clim. Change 2008, S1, 176–190. [Google Scholar] [CrossRef]
- Grosbois, G.; Power, M.; Evans, M.; Koehler, G.; Rautio, M. Content, composition, and transfer of polyunsaturated fatty acids in an Arctic lake food web. Ecosphere 2022, 13, e03881. [Google Scholar] [CrossRef]
- Conrad, R. Control of microbial methane production in wetland rice fields. Nutr. Cycl. Agro-Ecosyst. 2002, 64, 59–69. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Summons, R.E.; Jahnke, L.L.; Roksandic, Z. Carbon isotopic fractionation in lipids from methanotrophic bacteria—Relevance for interpretation of the geochemical record of biomarkers. Geochim. Cosmochim. Acta 1994, 58, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Kankaala, P.; Taipale, S.; Grey, J.; Sonninen, E.; Arvola, L.; Jones, R.I. Experimental delta 13C evidence for a contribution of methane to pelagic food webs in lakes. Limnol. Oceanogr. 2006, 51, 2821–2827. [Google Scholar] [CrossRef]
- Deines, P.; Grey, J.; Richnow, H.H.; Eller, G. Linking larval chironomids to methane: Seasonal variation of the microbial methane cycle and chironomid delta C-13. Aquat. Microb. Ecol. 2007, 46, 273–282. [Google Scholar] [CrossRef]
- Agasild, H.; Zingel, P.; Tuvikene, L.; Tuvikene, A.; Timm, H.; Feldmann, T.; Salujõe, J.; Toming, K.; Jones, R.I.; Nõges, T. Biogenic methane contributes to the food web of a large, shallow lake. Freshw. Biol. 2014, 59, 272–285. [Google Scholar] [CrossRef]
- Jones, R.I.; Carter, C.E.; Kelly, A.; Ward, S.; Kelly, D.J.; Grey, J. Widespread contribution of methane-cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 2008, 89, 857–864. [Google Scholar] [CrossRef]
- Peter, H.; Sommaruga, R. An evaluation of methods to study the gut bacterial community composition of fresh-water zooplankton. J. Plankton Res. 2008, 30, 997–1006. [Google Scholar] [CrossRef]
- Barea-Arco, J.; Perez-Martinez, C.; Morales-Baquero, R. Evidence of a mutualistic relationship between an algal epibiont and its host, Daphnia pulicaria. Limnol. Oceanogr. 2001, 46, 871–881. [Google Scholar] [CrossRef]
- Chang, N.; Jenkins, D.G. Plastid endosymbionts in the freshwater crustacean Daphnia obtusa. J. Crustac. Biol. 2000, 20, 231–238. [Google Scholar] [CrossRef]
- Montoya, J.P.; Carpenter, E.J.; Capone, D.G. Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol. Oceanogr. 2002, 47, 1617–1628. [Google Scholar] [CrossRef]
- Motwani, N.H.; Duberg, J.; Svedén, J.B.; Gorokhova, E. Grazing on cyanobacteria and transfer of diazotrophic nitrogen to zooplankton in the Baltic Sea: Cyanobacteria blooms support zooplankton growth. Limnol. Oceanogr. 2018, 63, 672–686. [Google Scholar] [CrossRef]
- Mehner, T.; Attermeyer, K.; Brauns, M.; Brothers, S.; Hilt, S.; Scharnweber, K.; van Dorst, R.M.; Vanni, M.J.; Gaedke, U. Trophic Transfer Efficiency in Lakes. Ecosystems 2022, 25, 1628–1652. [Google Scholar] [CrossRef]
- Berezina, N.A.; Zhgareva, N.N.; Strelnikova, A.P. Feeding features of the nine-spined stickleback Pungitius pungitius (Gasterosteidae) in water bodies of the North-West of Russia. J. Ichthyol. 2023, 63, 308–317. [Google Scholar] [CrossRef]
- Terentjev, P.M.; Berezina, N.A. Ecological and morphological characteristics and feeding of perch (Perca fluviatilis) in the autumn–winter period in dystrophic and oligotrophic lakes of northern Karelia (Russia). Inland Water Biol. 2022, 15, 915–928. [Google Scholar] [CrossRef]
- Bundy, M.H.; Vanderploeg, H.A.; Lavrentyev, P.J.; Kovalcik, P.A. The importance of microzooplankton versus phytoplankton to copepod populations during late winter and early spring in Lake Michigan. Can. J. Fish. Aquat. Sci. 2005, 62, 2371–2385. [Google Scholar] [CrossRef]
- Moore, M.; DeStasio, B.; Huizenga, K.; Silow, E. Trophic coupling of a microbial and classical food web in Lake Baikal, Siberia. Freshw. Biol. 2018, 64, 1–14. [Google Scholar] [CrossRef]
- Makarewicz, J.C.; Likens, G.E. Niche analysis of a zooplankton community. Science 1975, 190, 1000–1003. [Google Scholar] [CrossRef]
- Dussart, B.; Defaye, D. World Directory of Crustacea Copepoda of Inland Waters II—Cyclopiformes; Backhuys Publishers: Leiden, The Netherlands, 2006; 354p. [Google Scholar]
- Williams, R.J.; Martinez, N.D. Limits to trophic levels and omnivory in complex food webs: Theory and data. Am. Nat. 2004, 163, 458–468. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Value |
|---|---|
| Surface area, km2 | 0.5 |
| Mean (maximal) depth, m | 11.8 (32) |
| Transparency, m | 5.1–8.0 |
| Water color, Pt-Co | 25–30 |
| Water pH | 6.6–7.7 |
| Humic acids, µg/L | 30–31 |
| Organic carbon (water), mg/L | 3–6.4 |
| Total nitrogen (water), µg/L | 1860–4110 |
| Total phosphorus (water), µg/L | 14–74 |
| Total iron (water), µg/L | 4–280 |
| PP (phytoplankton), mg carbon/m2 day (0–5 m) | 85–150 |
| PP (periphyton), mg carbon/m2 day | 20–255 |
| Chl a (water), µg/L | 0.2–3 |
| Chl a + Phe (sediment), µg/cm2 (mean ± SD) | 25.6 ± 0.9 |
| Organic matter (sediment), % (mean ± SD) | 31.5 ± 1.5 |
| Trophic/Ecological Group | Group Name and Dominant Species |
|---|---|
| Producers | |
| Autotrophs Phytoplankton | Cyanobacteria Microcystis pulverea, Dolichospermum lemmermannii, Coelosphaerium kuetzingianum, and Merismopedia warmingiana; diatom Aulacoseira subarctica; green alga Botryococcus braunii |
| Autotrophs Phytoperiphyton | Cyanobacteria: Nostoc sp., Phormidiums sp., Aphanothece microscopica, Chroococcus minutus, Scytonema subtile, Rivularia coadunate, Tolypothrix distorta, and T. elenkinii; green algae: Coleochaete pulvinate, Chaetophora elegans, Mougeotia, Bulbochaete, Rhizoclonium, Ulothrix zonata, Klebsormidium subtile; diatoms: Achnanthes adnata, Diatoma elongatum, genera Amphora, Caloneis, Epithemia, Gomphonema, Fragillaria, Neidium, Pinnularia, Surirella |
| Mixotrophs Phytoplankton | Planktonic phytoflagellates, cryptophytes Komma and Cryptomonas spp., chrysophytes (genera Dinobryon and Uroglena) |
| Consumers | |
| Herbivores Zooplankton Zoobenthos | Cladoceran Bosmina longirostris; Copepod Eudiaptomus graciloides Gastropod Lymnaea stagnalis, bivalve mollusks Sphaerium corneum |
| Deposit-feeders, detritivores Zoobenthos | Crustaceans Gammarus lacustris and Monoporeia affinis Insect larvae of chironomids (Sergentia coracina), ceratopogonids Dasyella sp., megalopterans Sialis sp., ephemeropterans Ephemera vulgate, and Caenis horaria; trichopterans Phryganea bipunctata and Limnephilus spp. |
| Omnivores I Microphagues < 40 µm Zooplankton | Protista Amphileptus trachelioides, Didinium spp., Cyclotrichium viride, Bursella spumosa, Bursaridium pseudobursaria, Stokesia vernalis, Vorticella anabaena, and Strobilidium spp. Rotifer Asplanchna priodonta |
| Omnivores II Macrophagues > 50 µm Zooplankton Nekton | Copepod Cyclops scutifer Amphipod Gammaracanthus loricatus |
| Predator II Nekton | Fish Perca fluviatilis |
| Consumer | February | April | June | September | November | Average |
|---|---|---|---|---|---|---|
| Cladocera Bosmina longirostris | 0.7 | - | 3.4 | 1.9 | 0.9 | 1.7 |
| Amphipod Monoporeia afinis | 2.4 | 2.2 | 4 | 1.9 | 2.5 | 2.6 |
| Amphipod Gammarus lacustris | 0.6 | - | - | 2.2 | 3.4 | 2.1 |
| Chironomid Sergentia coracina | 1.8 | - | 1.2 | 3.4 | 3.9 | 2.6 |
| Ephemeropteran Ephemera vulgata | 2.3 | 0.8 | 1.3 | 2.8 | 4.8 | 2.4 |
| Stickleback Pingutius pingutius | 4.5 | 5.6 | 4.1 | 2.7 | 3.7 | 4.1 |
| Vendace Coregonus albula | 4.9 | 6.2 | 5.2 | 4.7 | 5.7 | 5.3 |
| Perch Perca fluviatilis | 5.8 | 5.9 | 5.9 | 5.7 | 5.1 | 5.7 |
| Amphipod Gammaracanthus loricatus | 5.9 | 5.2 | 6.1 | 2.3 | 3.1 | 4.5 |
| Copepod Cyclops scutifer | 5.0 | 5.1 | 1.5 | 2.2 | 5.0 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezina, N.A.; Gubelit, Y.I.; Maximov, A.A. Benthic Producers, Methane Carbon, and Diazotrophic Nitrogen as Sources of Nutrients in the Food Web of a Subarctic Lake. Diversity 2025, 17, 799. https://doi.org/10.3390/d17110799
Berezina NA, Gubelit YI, Maximov AA. Benthic Producers, Methane Carbon, and Diazotrophic Nitrogen as Sources of Nutrients in the Food Web of a Subarctic Lake. Diversity. 2025; 17(11):799. https://doi.org/10.3390/d17110799
Chicago/Turabian StyleBerezina, Nadezhda A., Yulia I. Gubelit, and Alexey A. Maximov. 2025. "Benthic Producers, Methane Carbon, and Diazotrophic Nitrogen as Sources of Nutrients in the Food Web of a Subarctic Lake" Diversity 17, no. 11: 799. https://doi.org/10.3390/d17110799
APA StyleBerezina, N. A., Gubelit, Y. I., & Maximov, A. A. (2025). Benthic Producers, Methane Carbon, and Diazotrophic Nitrogen as Sources of Nutrients in the Food Web of a Subarctic Lake. Diversity, 17(11), 799. https://doi.org/10.3390/d17110799











