Diversity Patterns of Alien Plant Species in Mountainous Areas: A Case Study from the Central Balkans
Abstract
1. Introduction
2. Materials and Methods
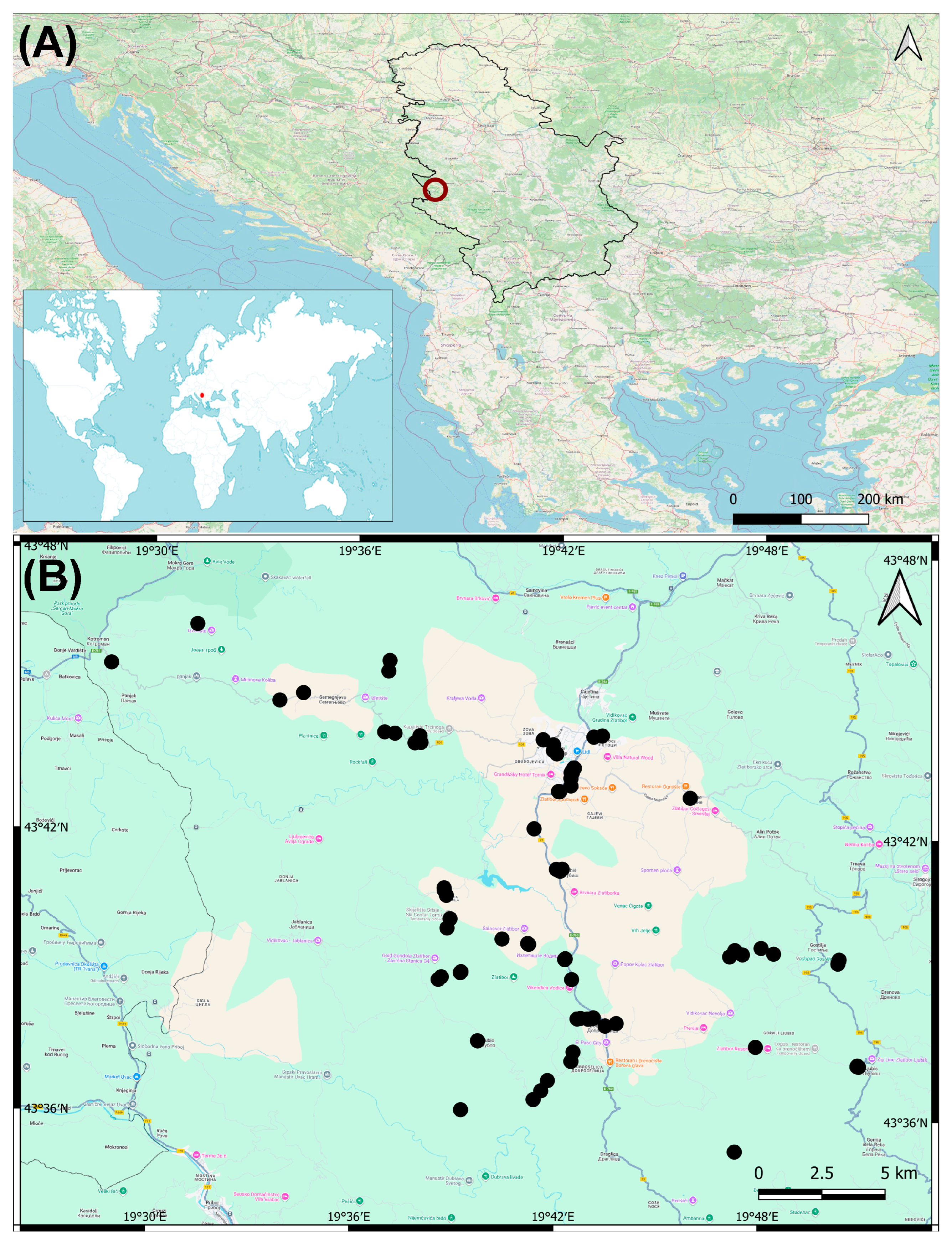
2.1. Study Area
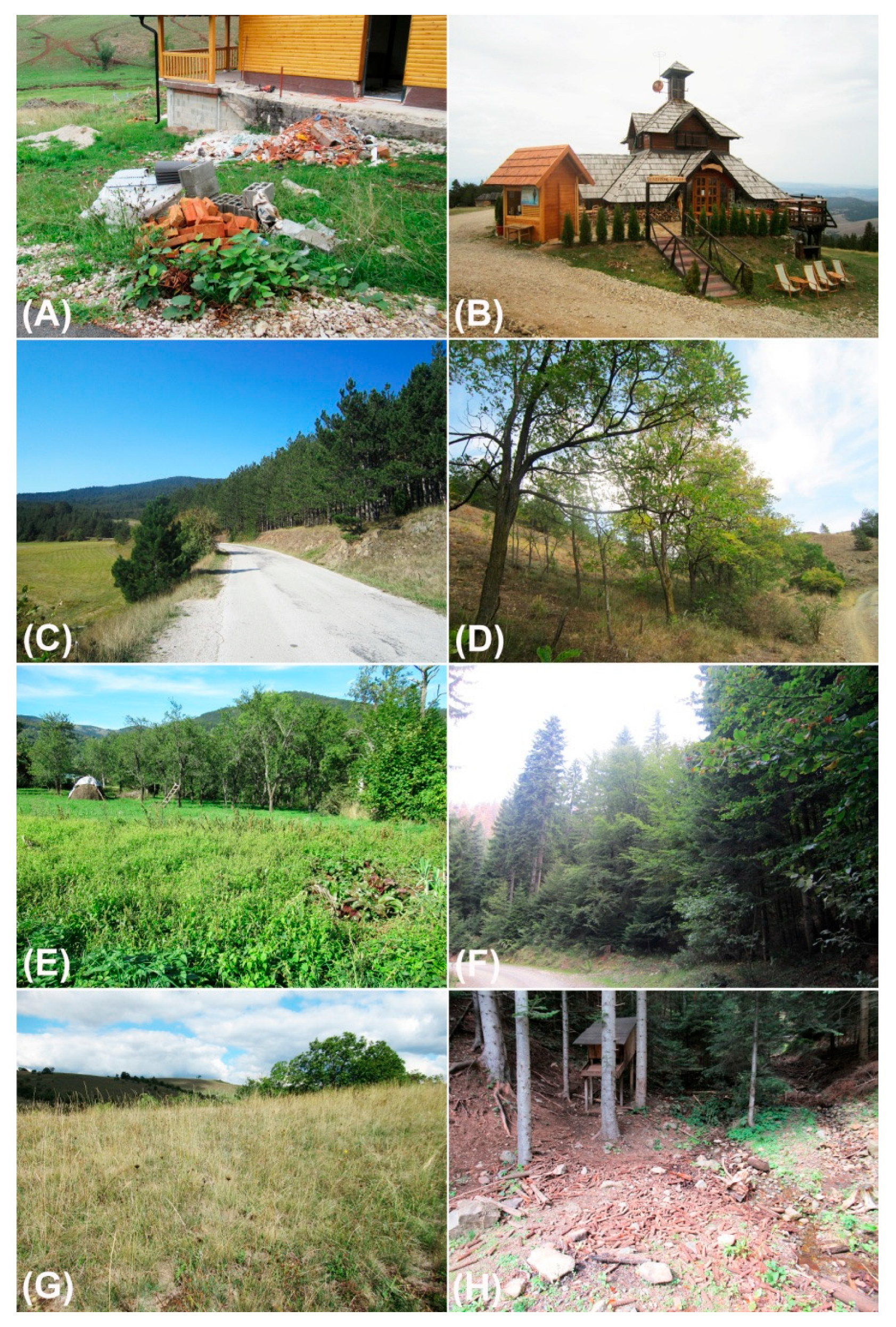
2.2. Data Collection
2.3. Data Analysis
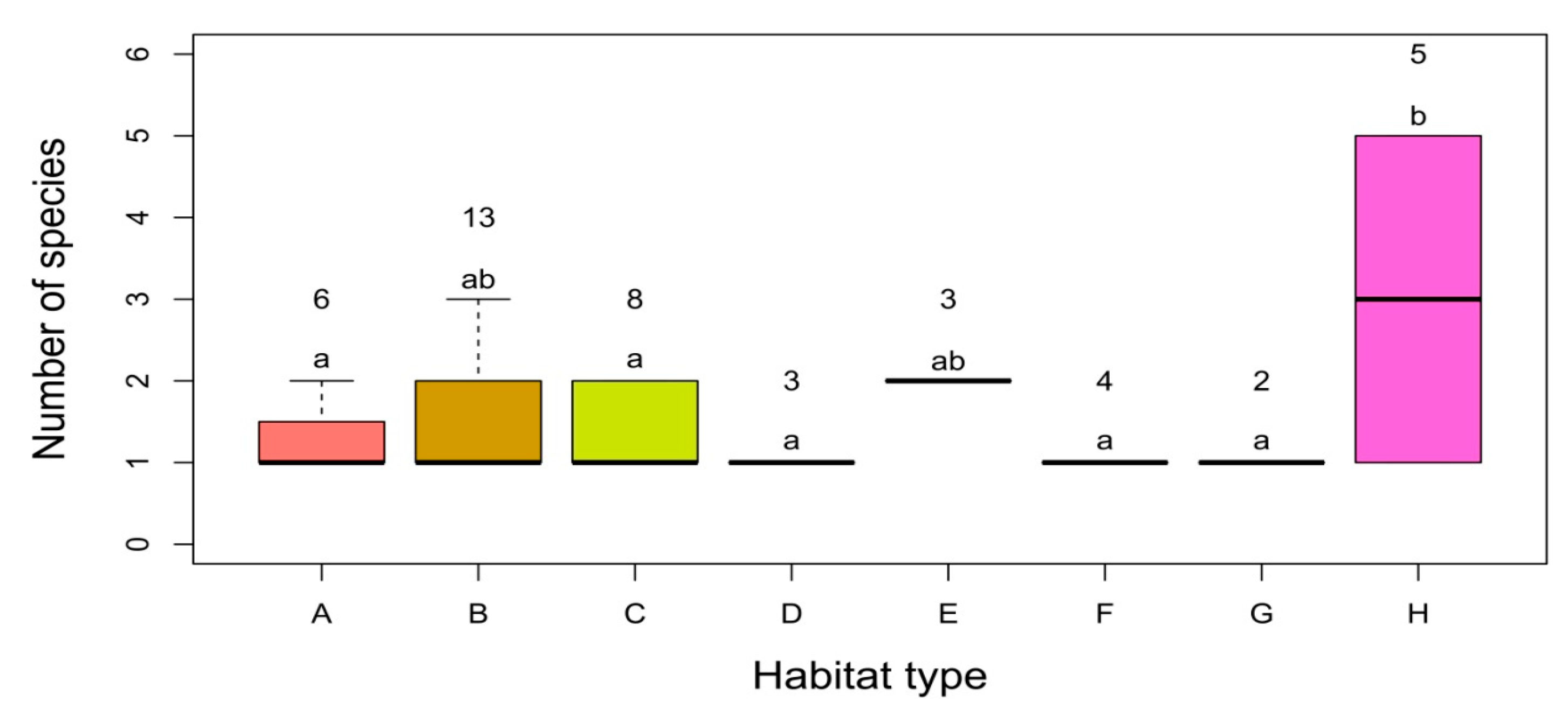
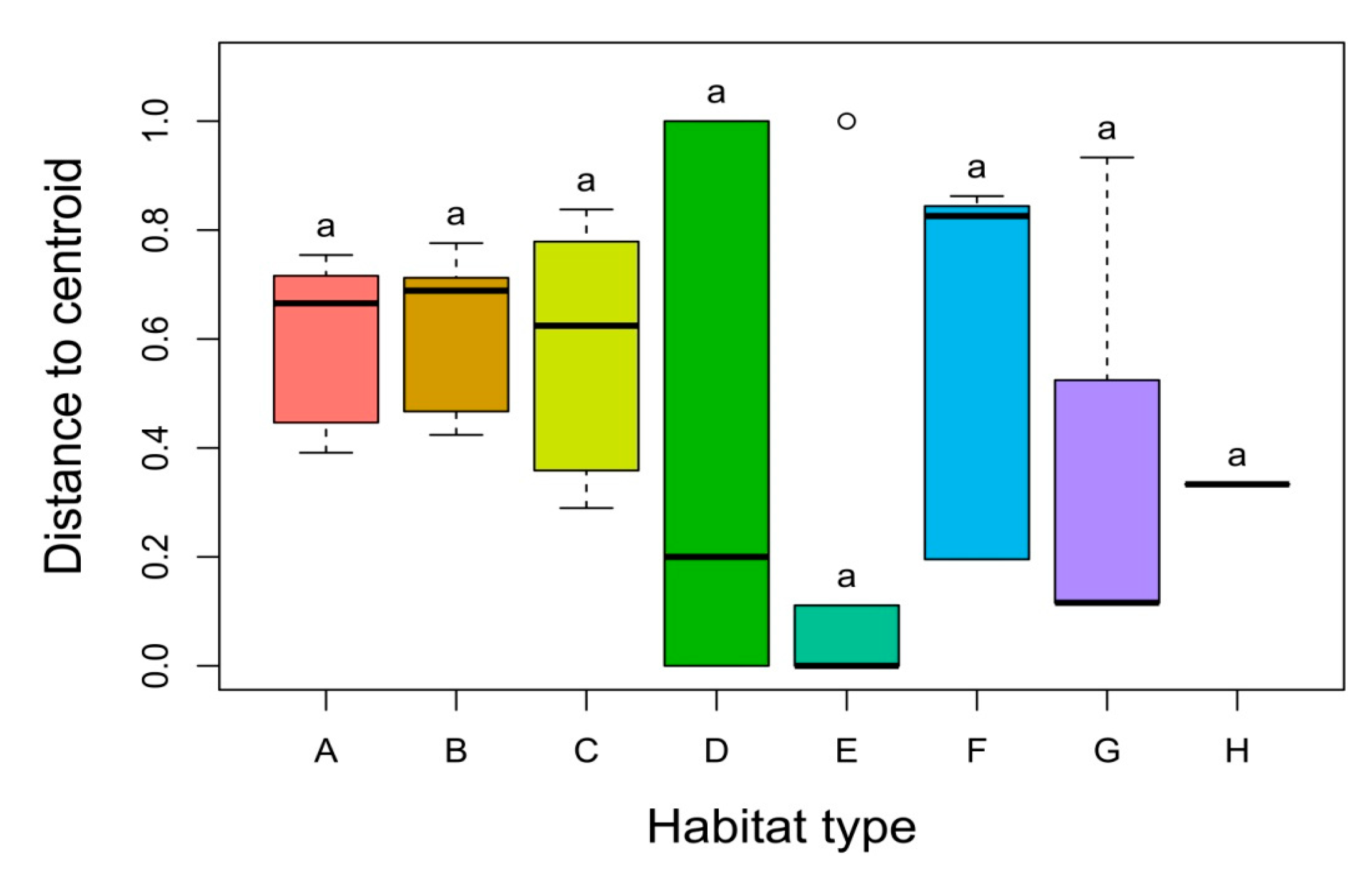
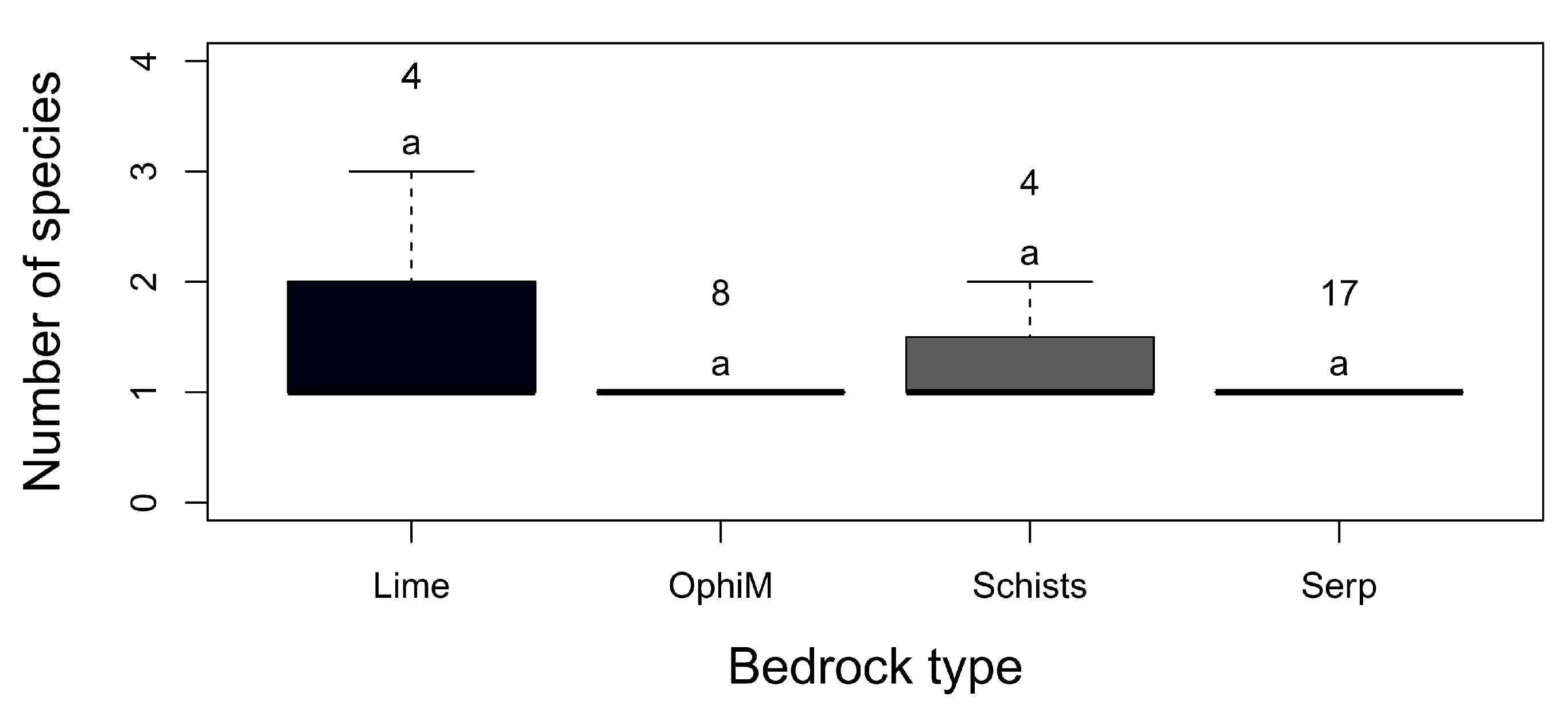
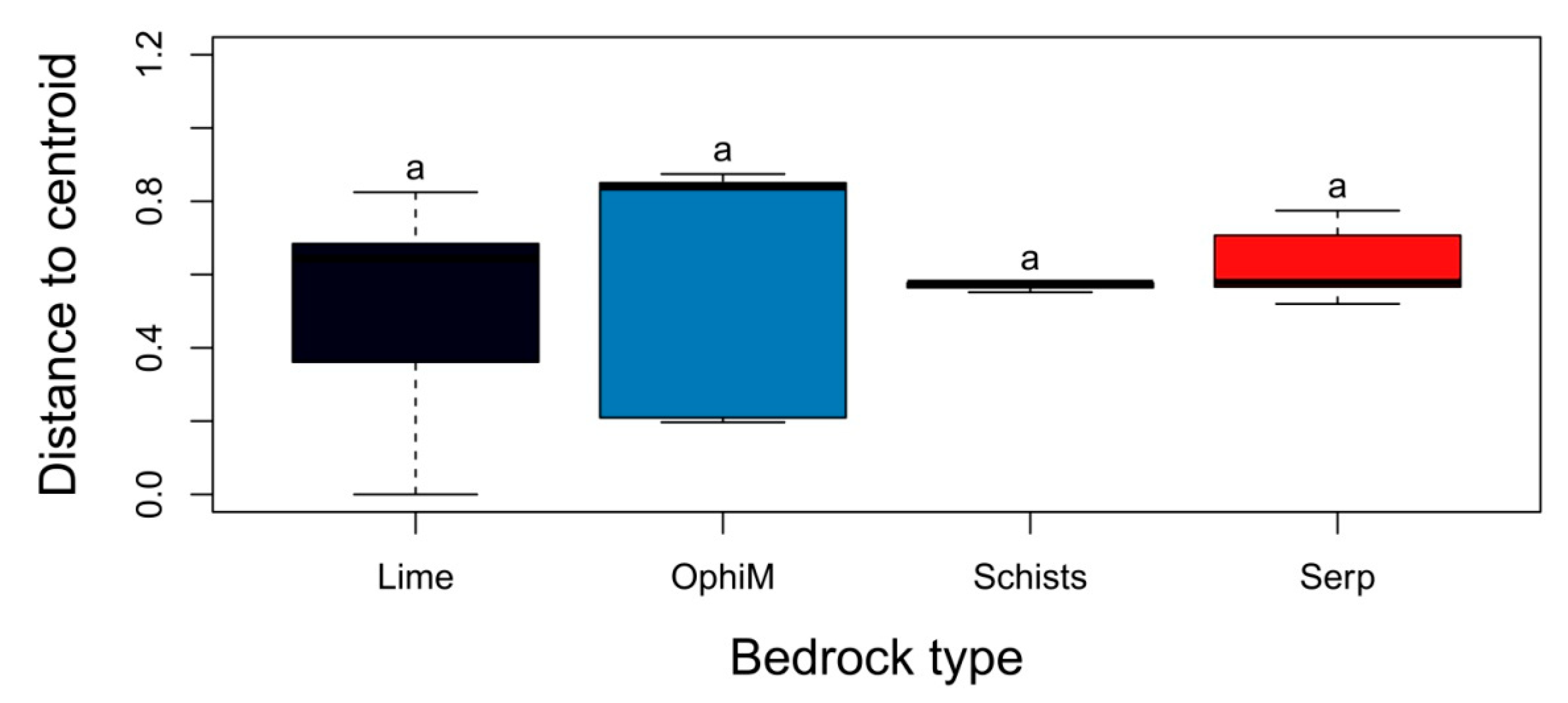
3. Results
3.1. Floristic Richness
3.2. Alpha, Beta and Gamma Diversity
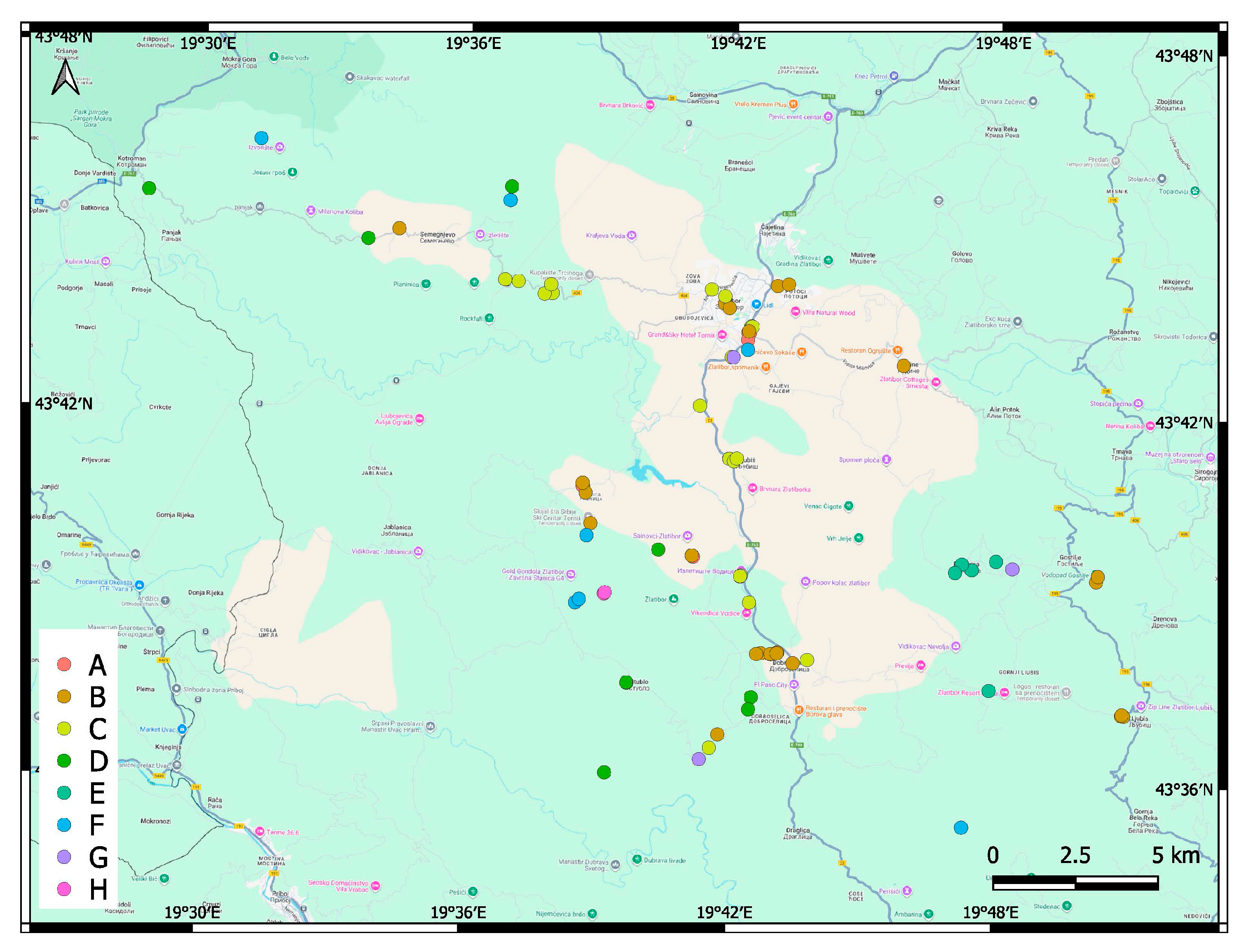
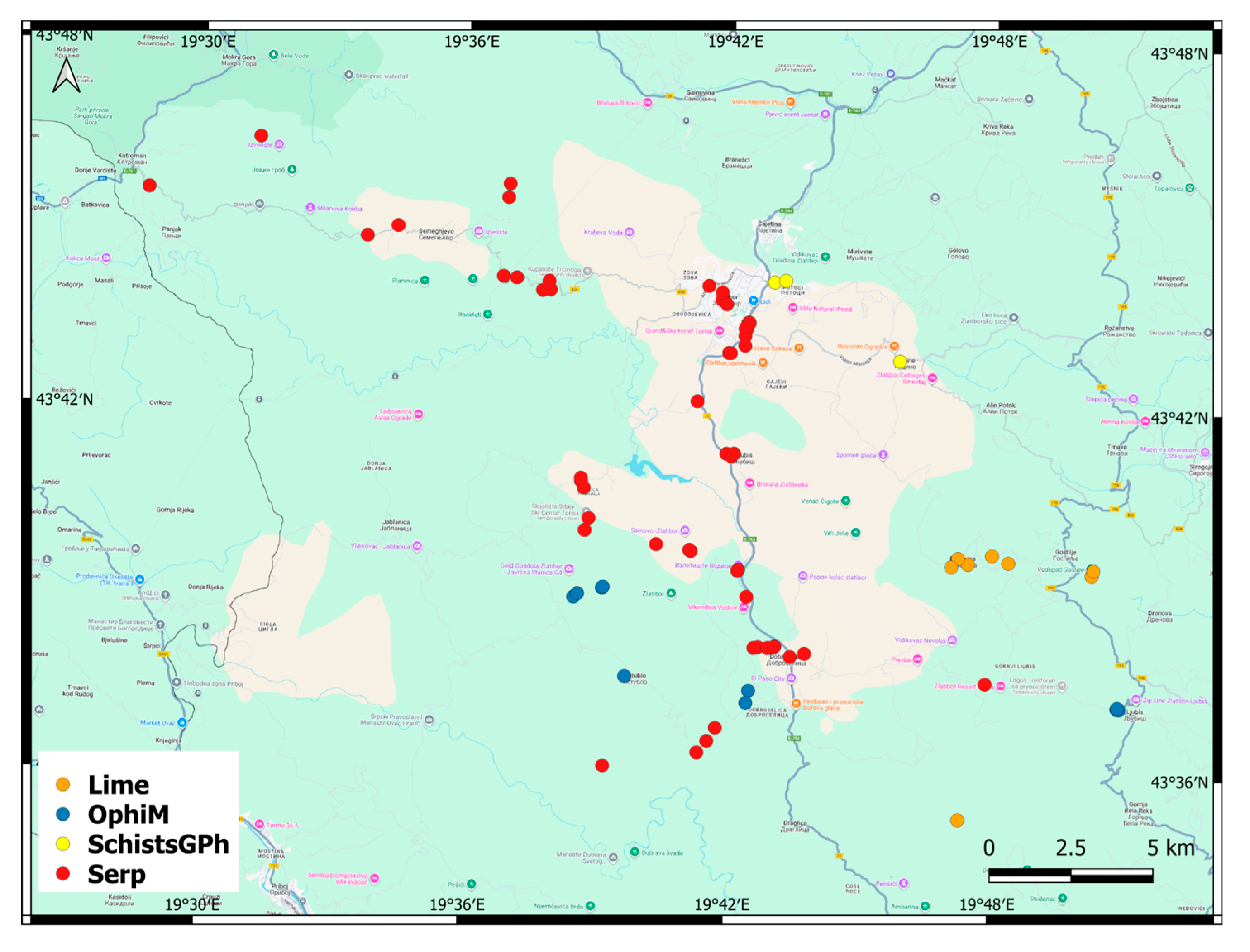
3.3. Factors Affecting the Distribution, Abundance and Composition of Alien Species
4. Discussion
4.1. Floristic Richness and Diversity Indices
4.2. Factors Affecting the Abundance and Composition of Alien Plant Species
4.3. Implications for Management Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Mountain Biodiversity, Its Causes and Function. Ambio 2004, 33, 11–17. [Google Scholar] [CrossRef]
- Perrigo, A.; Hoorn, C.; Antonelli, A. Why mountains matter for biodiversity. J. Biogeogr. 2020, 47, 315–325. [Google Scholar] [CrossRef]
- Beza, B.B. The aesthetic value of a mountain landscape: A study of the Mt. Everest Trek. Landsc. Urban Plan. 2010, 97, 306–317. [Google Scholar] [CrossRef]
- Schirpke, U.; Timmermann, F.; Tappeiner, U.; Tasser, E. Cultural ecosystem services of mountain regions: Modelling the aesthetic value. Ecol. Indic. 2016, 69, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Price, M.F. Why do mountains matter? In Mountains: A Very Short Introduction; Oxford Academic: Oxford, UK, 2015; pp. 1–17. [Google Scholar] [CrossRef]
- Theurillat, J.P.; Guisan, A. Potential Impact of Climate Change on Vegetation in the European Alps: A Review. Clim. Change 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Klanderud, K.; Totland, Ø. The relative role of dispersal and local interactions for alpine plant community diversity under simulated climate warming. Oikos 2007, 116, 1279–1288. [Google Scholar] [CrossRef]
- Spehn, E.M.; Libermann, M.; Körner, C. (Eds.) Land Use Change and Mountain Biodiversity, 1st ed.; CRC Press: Andover, UK, 2006. [Google Scholar]
- Inouye, D.W. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 2008, 89, 353–362. [Google Scholar] [CrossRef]
- Kueffer, C.; McDougall, K.; Alexander, J.; Daehler, C.; Edwards, P.; Haider, S.; Milbau, A.; Parks, C.; Pauchard, A.; Reshi, Z.A.; et al. Plant invasions into mountain protected areas: Assessment, prevention and control at multiple spatial scales. In Plant Invasions in Protected Areas; Invading Nature—Springer Series in Invasion Ecology; Foxcroft, L., Pyšek, P., Richardson, D., Genovesi, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 7, pp. 89–113. [Google Scholar] [CrossRef]
- Pyšek, P.; Bacher, S.; Chytrý, M.; Jarošík, V.; Wild, J.; Celesti-Grapow, L.; Gassó, N.; Kenis, M.; Lambdon, P.W.; Nentwig, W.; et al. Contrasting patterns in the invasions of European terrestrial and freshwater habitats by alien plants, insects and vertebrates. Glob. Ecol. Biogeogr. 2010, 19, 317–331. [Google Scholar] [CrossRef]
- Kuebbing, S.E.; Nuñez, M.A. Invasive non-native plants have a greater effect on neighbouring natives than other non-natives. Nat. Plants 2016, 2, 16134. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Merou, T. First inventory of the invasive alien plant species along Nestos River (East Macedonia, NE Greece). Phyton-Ann. Rei Bot. 2023, 62–63, 75–86. [Google Scholar] [CrossRef]
- Glasnović, P.; Cernich, S.; Peroš, J.; Tišler, M.; Fišer, Ž.; Surina, B. Diversity and Typology of Land-Use Explain the Occurrence of Alien Plants in a Protected Area. Plants 2022, 11, 2358. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Pyšek, P.; van Kleunen, M.; Kinlock, N.L.; Lučanová, M.; Leitch, I.J.; Pierce, S.; Dawson, W.; Essl, F.; Kreft, H.; et al. Plant invasion and naturalization are influenced by genome size, ecology and economic use globally. Nat. Commun. 2024, 15, 1330. [Google Scholar] [CrossRef]
- Šipek, M.; Šajna, N. Lowland forest fragment characteristics and anthropogenic disturbances determine alien plant species richness and composition. Biol. Invasions 2024, 26, 1595–1614. [Google Scholar] [CrossRef]
- McDougall, K.L.; Khuroo, A.A.; Loope, L.L.; Parks, C.G.; Pauchard, A.; Reshi, Z.A.; Rushworth, I.; Kueffer, C. Plant invasions in mountains: Global lessons for better management. Mt. Res. Dev. 2011, 31, 380–387. [Google Scholar] [CrossRef]
- Tecco, P.A.; Pais-Bosch, A.I.; Funes, G.; Marcora, P.I.; Zeballos, S.R.; Cabido, M.; Urcelay, C. Mountain invasions on the way: Are there climatic constraints for the expansion of alien woody species along an elevation gradient in Argentina? J. Plant Ecol. 2016, 9, 380–392. [Google Scholar] [CrossRef]
- Iseli, E.; Chisholm, C.; Lenoir, J.; Haider, S.; Seipel, T.; Barros, A.; Hargreaves, A.L.; Kardol, P.; Lembrechts, J.J.; McDougall, K.; et al. Rapid upwards spread of non-native plants in mountains across continents. Nat. Ecol. Evol. 2023, 7, 405–413. [Google Scholar] [CrossRef]
- Kitayama, K.; Mueller-Dombois, D. Biological invasion on an oceanic island mountain: Do alien plant species have wider ecological ranges than native species? J. Veg. Sci. 1995, 6, 667–674. [Google Scholar] [CrossRef]
- Pauchard, A.; Kueffer, C.; Dietz, H.; Daehler, C.C.; Alexander, J.; Edwards, P.J.; Arévalo, J.R.; Cavieres, L.A.; Guisan, A.; Haider, S.; et al. Ain’t no mountain high enough: Plant invasions reaching new elevations. Front. Ecol. Environ. 2009, 7, 479–486. [Google Scholar] [CrossRef]
- Alexander, J.M.; Kueffer, C.; Daehler, C.C.; Edwards, P.J.; Pauchard, A.; Seipel, T.; Consortium, M. Assembly of non-native floras along elevational gradients explained by directional ecological filtering. Proc. Natl. Acad. Sci. USA 2011, 108, 656–661. [Google Scholar] [CrossRef]
- Kosaka, Y.; Saikia, B.; Mingki, T.; Tag, H.; Riba, T.; Ando, K. Roadside distribution patterns of invasive alien plants along an altitudinal gradient in Arunachal Himalaya, India. Mt. Res. Dev. 2010, 30, 252–258. [Google Scholar] [CrossRef]
- Seipel, T.; Kueffer, C.; Rew, L.J.; Daehler, C.C.; Pauchard, A.; Naylor, B.J.; Alexander, J.M.; Edwards, P.J.; Parks, C.G.; Arevalo, J.R.; et al. Processes at multiple spatial scales determine non-native plant species richness and similarity in mountain regions around the world. Glob. Ecol. Biogeogr. 2012, 21, 236–246. [Google Scholar] [CrossRef]
- Alexander, J.M.; Lembrechts, J.J.; Cavieres, L.A.; Daehler, C.; Haider, S.; Kueffer, C.; Liu, G.; McDougall, K.; Milbau, A.; Pauchard, A.; et al. Plant invasions into mountains and alpine ecosystems: Current status and future challenges. Alp. Bot. 2016, 126, 89–103. [Google Scholar] [CrossRef]
- McDougall, K.L.; Alexander, J.M.; Haider, S.; Pauchard, A.; Walsh, N.G.; Kueffer, C. Alien flora of mountains: Global comparisons for the development of local preventive measures against plant invasions. Divers. Distrib. 2011, 17, 103–111. [Google Scholar] [CrossRef]
- Dietz, H.; Kueffer, C.; Parks, C.G. MIREN: A New Research Network Concerned with Plant Invasion into Mountain Areas. Mt. Res. Dev. 2006, 26, 80–81. [Google Scholar] [CrossRef]
- Kalusová, V.; Čeplová, N.; Danihelka, J.; Večeřa, M.; Pyšek, P.; Albert, A.; Anastasiu, P.; Biurrun, I.; Boch, S.; Cottaz, C.; et al. Alien plants of Europe: An overview of national and regional inventories. Preslia 2024, 96, 149–182. [Google Scholar] [CrossRef]
- Haziri, A. Invasive alien plant species (IAPS), identified in the Polog region–Republic of North Macedonia. Analele Univ. Oradea Fasc. Biol. 2024, 31, 40–43. [Google Scholar]
- Stojanović, V.; Bjedov, I.; Jovanović, I.; Jelić, I.; Obratov-Petković, D.; Nešić, M.; Nedeljković, D. Selected Invasive Alien Plant Species in the Flora of Serbia [Odabrane Invazivne Strane Vrste u Flori Srbije]; Zavod za Zaštitu Prirode Srbije: Belgrade, Serbia, 2021; ISBN 978-86-80877-73-0. [Google Scholar]
- Rat, M.M.; Gavrilović, M.T.; Radak, B.Đ.; Bokić, B.S.; Jovanović, S.D.; Božin, B.N.; Boža, P.P.; Anačkov, G.T. Urban flora in the Southeast Europe and its correlation with urbanization. Urban Ecosyst. 2017, 20, 811–822. [Google Scholar] [CrossRef]
- Jovanović, S.; Glišić, M. An analysis of research into urban flora and vegetation in Southeast Europe. Acta Bot. Croat. 2021, 80, 74–81. [Google Scholar] [CrossRef]
- Glišić, M.; Jakovljević, K.; Lakušić, D.; Šinžar-Sekulić, J.; Vukojičić, S.; Tabašević, M.; Jovanović, S. Influence of Habitat Types on Diversity and Species Composition of Urban Flora—A Case Study in Serbia. Plants 2021, 10, 2572. [Google Scholar] [CrossRef]
- Tabašević, M.; Jovanović, S.; Lakušić, D.; Vukojičić, S.; Kuzmanović, N. Diversity of Ruderal Communities in Urban Environments—A Case Study from Serbia (SE Europe). Diversity 2021, 13, 638. [Google Scholar] [CrossRef]
- Stanković, V.; Kabaš, E.; Kuzmanović, N.; Vukojičić, S.; Lakušić, D.; Jovanović, S. A suitable method for assessing invasibility of habitats in the Ramsar sites—An example of the southern part of the Pannonian Plain. Wetlands 2020, 40, 745–755. [Google Scholar] [CrossRef]
- Stanković, V.; Kuzmanović, N.; Kabaš, E.; Vukojičić, S.; Lakušić, D.; Jovanović, S. Established stands of the highly invasive Echinocystis lobata on the Ramsar sites of the southern part of the Pannonian Plain. Bot. Serbica 2022, 46, 197–207. [Google Scholar] [CrossRef]
- Anđelković, A.A.; Pavlović, D.M.; Marisavljević, D.P.; Živković, M.M.; Novković, M.Z.; Popović, S.S.; Cvijanović, D.L.; Radulović, S.B. Plant invasions in riparian areas of the Middle Danube Basin in Serbia. NeoBiota 2022, 71, 23–48. [Google Scholar] [CrossRef]
- Jovanović, S.; Jakovljević, K.; Djordjević, V.; Vukojičić, S. Ruderal flora and vegetation of the town of Žabljak (Montenegro)—An overview for the period 1990–1998. Bot. Serbica 2013, 37, 55–69. [Google Scholar]
- Marković, M. Flora of the Vidlič Mt (Southeastern Serbia). Ethnobotany 2022, 2, 45–128. [Google Scholar] [CrossRef]
- Niketić, M.; Tomović, G.; Anačkov, G.; Djordjević, V.; Đurović, S.; Duraki, Š.; Kabaš, E.; Lakušić, D.; Petkovski, G.; Petrović, S.; et al. Material on the annotated checklist of vascular flora of Serbia. Nomenclatural, taxonomic and floristic notes IV. Bull. Nat. Hist. Mus. Belgr. 2022, 15, 27–96. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D. Niche analysis and conservation of the orchids of east Macedonia (NE Greece). Acta Oecol. 2008, 33, 27–35. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Stevanović, V. Factors affecting the distribution and abundance of orchids in grasslands and herbaceous wetlands. Syst. Biodivers. 2016, 14, 355–370. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Harrison, S.; Safford, H.D.; Grace, J.B.; Viers, J.H.; Davies, K.F. Regional and local species richness in an insular environment: Serpentine plants in California. Ecol. Monogr. 2006, 76, 41–56. [Google Scholar] [CrossRef]
- Harrison, S.; Rice, K.; Maron, J. Habitat patchiness promotes invasion by alien grasses on serpentine soil. Biol. Conserv. 2001, 100, 45–53. [Google Scholar] [CrossRef]
- Going, B.M.; Hillerislambers, J.; Levine, J.M. Abiotic and biotic resistance to grass invasion in serpentine annual plant communities. Oecologia 2009, 159, 839–847. [Google Scholar] [CrossRef]
- Chiari, M.; Djerić, N.; Garfagnoli, F.; Hrvatović, H.; Krstić, M.; Levi, N.; Malasoma, A.; Marroni, M.; Menna, F.; Nirta, G.; et al. The geology of the Zlatibor-Maljen area (western Serbia): A geotraverse accross the ophiolites of the Dinaric-Hellenic collisional belt. Ofioliti 2011, 36, 139–166. [Google Scholar]
- Gawlick, H.J.; Sudar, M.; Missoni, S.; Suzuki, H.; Lein, R.; Jovanović, D. Triassic-Jurassic geodynamic evolution of the Dinaridic Ophiolite Belt (Inner Dinarides, SW Serbia). J. Alp. Geol. 2017, 55, 1–167. [Google Scholar]
- Gavrilović, D. (Ed.) Statistical Yearbook of the Republic of Serbia; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2024. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Jakovljević, K.; Stevanović, V. Patterns of distribution, abundance and composition of forest terrestrial orchids. Biodivers. Conserv. 2020, 29, 4111–4134. [Google Scholar] [CrossRef]
- Kojić, M.; Popović, R.; Karadžić, B. Vascular Plants of Serbia as Indicators of Habitats; Institut za Biološka Istraživanja ‘Siniša Stanković’: Belgrade, Serbia, 1997. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geol. 2001, 18, 1–262. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Vienna, Austria, 1964. [Google Scholar]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet approach. In Handbook of Vegetation Science V; Whittaker, R.H., Ed.; Junk: The Hague, The Netherlands, 1973; pp. 617–726. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4, 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 16 May 2025). [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.3.3; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Kochjarová, J.; Blanár, D.; Jarolímek, I.; Slezák, M. Wildlife supplementary feeding facilitates spread of alien plants in forested mountainous areas: A case study from the Western Carpathians. Biologia 2023, 78, 1381–1399. [Google Scholar] [CrossRef]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on invasive Tree of Heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef]
- Selvi, F.; Bettarini, I.; Cabrucci, M.; Colzi, I.; Coppi, A.; Lazzaro, L.; Mugnai, M.; Gonnelli, C. Metal concentrations in invasive Ailanthus altissima vs native Fraxinus ornus on ultramafic soils: Evidence for higher efficiency in Ni exclusion and adjustments to Mg and Ca imbalance. Ecol. Res. 2024, 39, 479–491. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef]
- Song, U.; Son, D.; Kang, C.; Lee, E.J.; Lee, K.; Park, J.S. Mowing: A cause of invasion, but also a potential solution for management of the invasive, alien plant species Erigeron annuus (L.) Pers. J. Environ. Manag. 2018, 223, 530–536. [Google Scholar] [CrossRef]
- Jovanović, S.; Hlavati-Širka, V.; Lakušić, D.; Jogan, N.; Nikolić, T.; Anastasiu, P.; Vladimirov, V.; Šinžar-Sekulić, J. Reynoutria niche modelling and protected area prioritization for restoration and protection from invasion: A Southeastern Europe case study. J. Nat. Conserv. 2018, 41, 1–15. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Zhu, C.; Qu, Y.; Wang, H. Predicting the potential distribution of an invasive species, Erigeron canadensis L., in China with a maximum entropy model. Glob. Ecol. Conserv. 2020, 21, e00822. [Google Scholar] [CrossRef]
- Stanković, V.; Djordjević, V.; Lazarević, P.; Verloove, F.; Kabaš, E. Cotoneaster bullatus Bois. (Rosaceae), a new non-native species to the flora of the Balkan Peninsula. BioInvasions Rec. 2025, 14, 487–499. [Google Scholar] [CrossRef]
- McDougall, K.L.; Morgan, J.W.; Walsh, N.G.; Williams, R.J. Plant invasions in treeless vegetation of the Australian Alps. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 159–171. [Google Scholar] [CrossRef]
- Anderson, L.G.; Rocliffe, S.; Haddaway, N.R.; Dunn, A.M. The Role of Tourism and Recreation in the Spread of Non-Native Species: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140833. [Google Scholar] [CrossRef]
- Hall, C.M. Biological invasion, biosecurity, tourism, and globalisation. In Handbook of Globalisation and Tourism; Timothy., D.J., Ed.; Edward Elgar Publishing: Cheltenham, UK, 2019; pp. 114–125. [Google Scholar]
- Mount, A.; Pickering, C.M. Testing the capacity of clothing to act as a vector for non-native seed in protected areas. J. Environ. Manag. 2009, 91, 168–179. [Google Scholar] [CrossRef]
- Alexander, J.M.; Naylor, B.; Poll, M.; Edwards, P.J.; Dietz, H. Plant invasions along mountain roads: The altitudinal amplitude of alien Asteraceae forbs in their native and introduced ranges. Ecography 2009, 32, 334–344. [Google Scholar] [CrossRef]
- McDougall, K.L.; Lembrechts, J.; Rew, L.J.; Haider, S.; Cavieres, L.A.; Kueffer, C.; Milbau, A.; Naylor, B.J.; Nuñez, M.A.; Pauchard, A.; et al. Running off the road: Roadside non-native plants invading mountain vegetation. Biol. Invasions 2018, 20, 3461–3473. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Milbau, A.; Nijs, I. Alien Roadside Species More Easily Invade Alpine than Lowland Plant Communities in a Subarctic Mountain Ecosystem. PLoS ONE 2014, 9, e89664. [Google Scholar] [CrossRef]
- Taylor, K.; Brummer, T.; Taper, M.L.; Wing, A.; Rew, L.J. Human-mediated long-distance dispersal: An empirical evaluation of seed dispersal by vehicles. Divers. Distrib. 2012, 18, 942–951. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Maxianová, A.; Winkler, J.; Adamcová, D.; Podlasek, A. Environmental consequences and the role of illegal waste dumps and their impact on land degradation. Land Use Policy 2019, 89, 104234. [Google Scholar] [CrossRef]
- Damalas, C.A. Distribution, biology, and agricultural importance of Galinsoga parviflora (Asteraceae). Weed Biol. Manag. 2008, 8, 147–153. [Google Scholar] [CrossRef]
- Juárez-Escario, A.; Conesa, J.A.; Solé-Senan, X.O. Management as a driver of functional patterns and alien species prominence in weed communities of irrigated orchards in Mediterranean areas. Agric. Ecosyst. Environ. 2017, 249, 247–255. [Google Scholar] [CrossRef]
- Alexander, E.B.; Coleman, R.G.; Keeler-Wolf, T.; Harrison, P.S. Serpentine Geoecology of Western North America: Geology, Soils, and Vegetation; Oxford University Press: New York, NY, USA, 2007; 512p. [Google Scholar] [CrossRef]
- Kazakou, E.; Dimitrakopoulos, P.G.; Baker, A.J.M.; Reeves, R.D.; Troumbis, A.Y. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: From species to ecosystem level. Biol. Rev. 2008, 83, 495–508. [Google Scholar] [CrossRef]
- Proctor, J.; Wooddell, S.R.J. The Plant Ecology of Serpentine: I. Serpentine Vegetation of England and Scotland. J. Ecol. 1971, 59, 375–395. [Google Scholar] [CrossRef]
- D’Amico, M.E.; Previtali, F. Edaphic influences of ophiolitic substrates on vegetation in the Western Italian Alps. Plant Soil 2012, 351, 73–95. [Google Scholar] [CrossRef]
- Morri, C.; Montefalcone, M.; Gatti, G.; Vassallo, P.; Paoli, C.; Bianchi, C.N. An Alien Invader is the Cause of Homogenization in the Recipient Ecosystem: A Simulation-Like Approach. Diversity 2019, 11, 146. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Schlaepfer, D.; Jeschke, J.M.; Fischer, M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 2010, 13, 947–958. [Google Scholar] [CrossRef]
- Sol, D.; Maspons, J.; Vall-Llosera, M.; Bartomeus, I.; García-Peña, G.E.; Piñol, J.; Freckleton, R.P. Unraveling the life history of successful invaders. Science 2012, 337, 580–583. [Google Scholar] [CrossRef]
- Kallimanis, A.S.; Mazaris, A.D.; Tzanopoulos, J.; Halley, J.M.; Pantis, J.D.; Sgardelis, S.P. How does habitat diversity affect the species-area relationship? Glob. Ecol. Biogeogr. 2008, 17, 532–538. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Tichý, L.; Danihelka, J.; Fajmon, K.; Hájek, O.; Kintrová, K.; Láníková, D.; Otýpková, Z.; Řehořek, V. Biotic homogenization of Central European urban floras depends on residence time of alien species and habitat types. Biol. Conserv. 2012, 145, 179–184. [Google Scholar] [CrossRef]
- Chytrý, M.; Maskell, L.C.; Pino, J.; Pyšek, P.; Vilà, M.; Font, X.; Smart, S.M. Habitat invasions by alien plants: A quantitative comparison among Mediterranean, subcontinental and oceanic regions of Europe. J. Appl. Ecol. 2008, 45, 448–458. [Google Scholar] [CrossRef]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Chytrý, M.; Pyšek, P.; Wild, J.; Pino, J.; Maskell, L.C.; Vilà, M. European map of alien plant invasions based on the quantitative assessment across habitats. Divers. Distrib. 2009, 15, 98–107. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Freese, D. Black locust (Robinia pseudoacacia L.) ecophysiological and morphological adaptations to drought and their consequence on biomass production and water-use efficiency. N. Z. J. For. Sci. 2014, 44, 29. [Google Scholar] [CrossRef]
- Trifilò, P.; Raimondo, F.; Nardini, A.; Lo Gullo, M.A.; Salleo, S. Drought resistance of Ailanthus altissima: Root hydraulics and water relations. Tree Physiol. 2004, 24, 107–114. [Google Scholar] [CrossRef]
- Knüsel, S.; Conedera, M.; Zweifel, R.; Bugmann, H.; Etzold, S.; Wunder, J. High growth potential of Ailanthus altissima in warm and dry weather conditions in novel forests of southern Switzerland. Trees 2019, 33, 395–409. [Google Scholar] [CrossRef]
- Becker, T.; Dietz, H.; Billeter, R.; Buschmann, H.; Edwards, P.J. Altitudinal distribution of alien plant species in the Swiss Alps. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 173–183. [Google Scholar] [CrossRef]
- Giorgis, M.A.; Tecco, P.A.; Cingolani, A.M.; Renison, D.; Marcora, P.; Paiaro, V. Factors associated with woody alien species distribution in a newly invaded mountain system of central Argentina. Biol. Invasions 2011, 13, 1423–1434. [Google Scholar] [CrossRef]
- McNeely, J.A.; Mooney, H.A.; Neville, L.E.; Schei, P.; Waage, J.K. A Global Strategy on Invasive Alien Species; IUCN: Gland, Switzerland; Cambridge, UK, 2001. [Google Scholar]
- Wittenberg, R.; Cock, M.J.W. (Eds.) Invasive Alien Species: A Toolkit of Best Prevention and Management Practices; CAB International: Wallingford, UK, 2001. [Google Scholar]
- Hatcher, P.E.; Melander, B. Combining physical, cultural and biological methods: Prospects for integrated non-chemical weed management strategies. Weed Res. 2003, 43, 303–322. [Google Scholar] [CrossRef]
- Melander, B.; Rasmussen, I.A.; Bàrberi, P. Integrating physical and cultural methods of weed control—Examples from European research. Weed Sci. 2005, 53, 369–381. [Google Scholar] [CrossRef]
- Miller, T.W. Integrated strategies for management of perennial weeds. Invasive Plant Sci. Manag. 2016, 9, 148–158. [Google Scholar] [CrossRef]
- Soler, J.; Izquierdo, J. The Invasive Ailanthus altissima: A Biology, Ecology, and Control Review. Plants 2024, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Milakovic, I.; Fiedler, K.; Karrer, G. Management of roadside populations of invasive Ambrosia artemisiifolia by mowing. Weed Res. 2014, 54, 256–264. [Google Scholar] [CrossRef]
- Canavan, K.; Canavan, S.; Clark, V.R.; Gwate, O.; Richardson, D.M.; Sutton, G.F.; Martin, G.D. The Alien Plants That Threaten South Africa’s Mountain Ecosystems. Land 2021, 10, 1393. [Google Scholar] [CrossRef]
- Pocock, M.J.; Adriaens, T.; Bertolino, S.; Eschen, R.; Essl, F.; Hulme, P.E.; Jeschke, J.M.; Roy, H.E.; Teixeira, H.; de Groot, M. Citizen science is a vital partnership for invasive alien species management and research. iScience 2024, 27, 108623. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, K.M.; Hauser, C.E.; Williams, N.S.; Moore, J.L. Optimizing invasive species control across space: Willow invasion management in the Australian Alps. J. Appl. Ecol. 2011, 48, 1286–1294. [Google Scholar] [CrossRef]


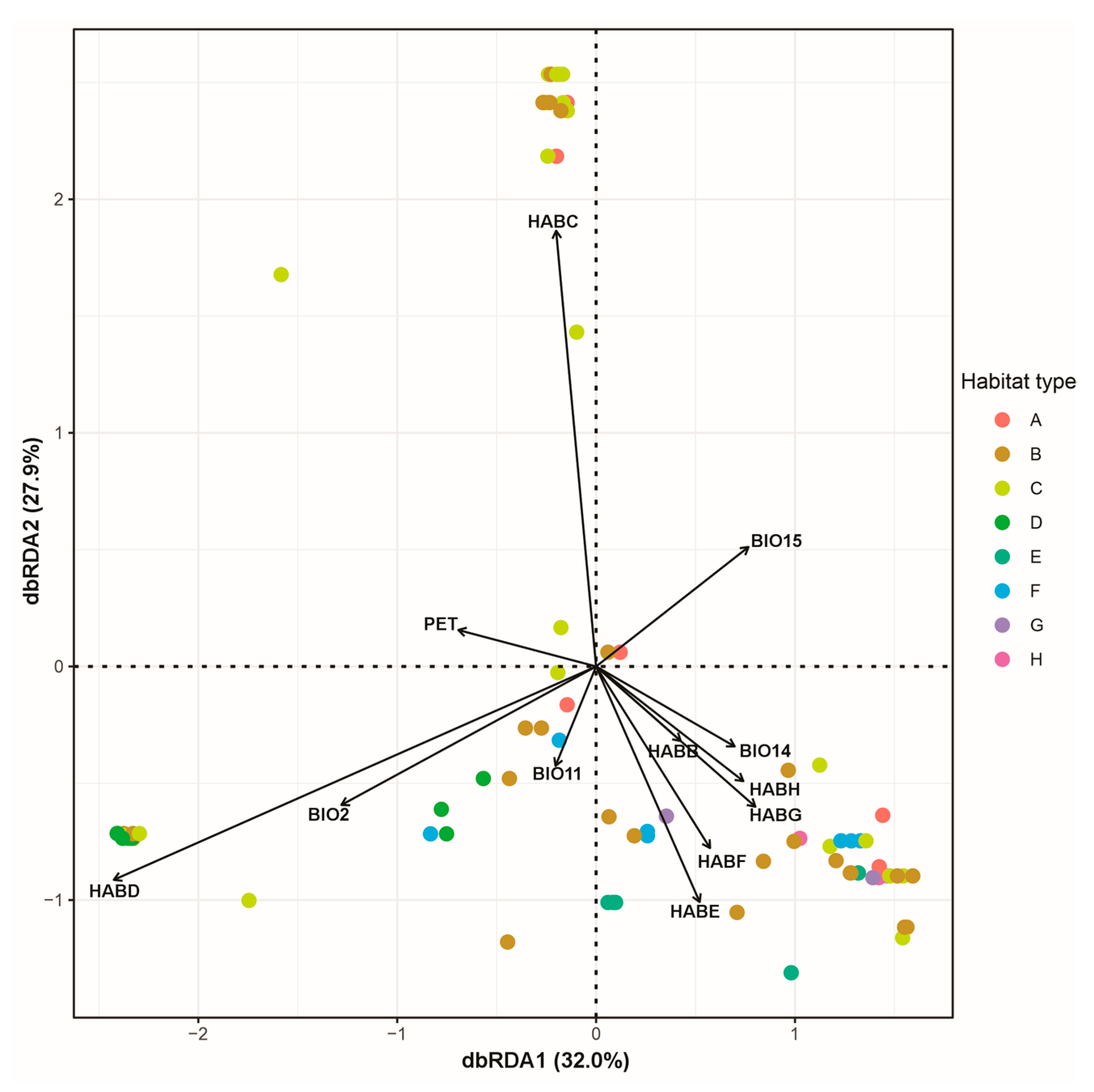
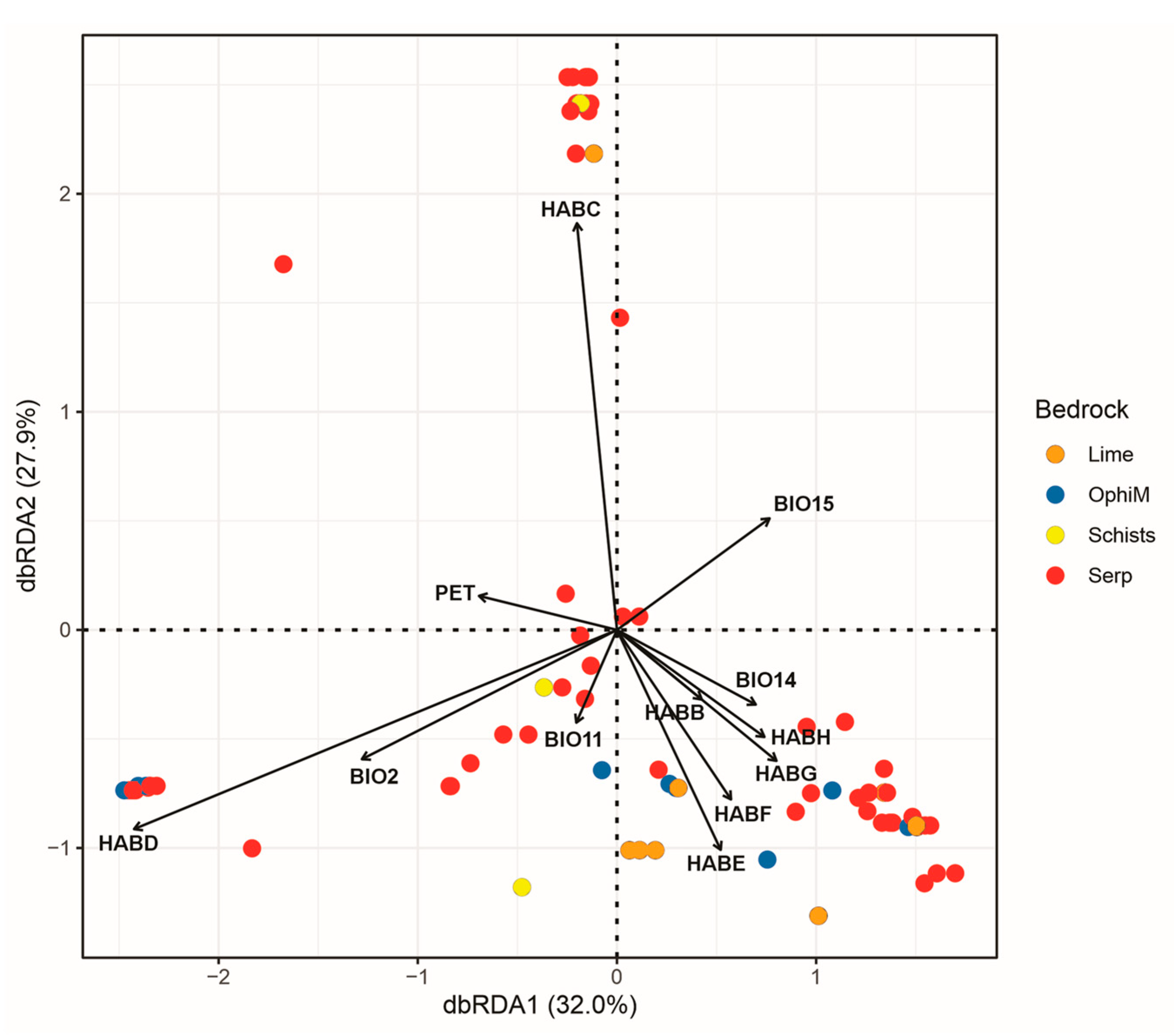

| Taxon | Origin | Occ. | A | B | C | D | E | F | G | H | Lime | OphiM | SchistsGPh | Serp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ailanthus altissima (Mill.) Swingle | E-As | 3 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Alcea rosea L. | W-As | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Amaranthus retroflexus L. | N-Am | 6 | 0 | 1 | 1 | 0 | 4 | 0 | 0 | 0 | 4 | 1 | 0 | 1 |
| Ambrosia artemisiifolia L. | N-Am | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| Calendula officinalis L. | SW-Eur | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cosmos bipinnatus Cav. | N-Am | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Cotoneaster bullatus Bois | E-As | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Erigeron annuus (L.) Desf. | N-Am | 29 | 2 | 12 | 6 | 0 | 2 | 3 | 2 | 2 | 4 | 3 | 1 | 21 |
| Erigeron canadensis L. | N-Am | 10 | 0 | 4 | 2 | 0 | 0 | 2 | 1 | 1 | 1 | 3 | 0 | 6 |
| Galinsoga parviflora Cav. | N+S-Am | 5 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 1 | 0 | 0 |
| Helianthus tuberosus L. | N-Am | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 |
| Oenothera biennis L. | N-Am | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Oenothera glazioviana Micheli | N-Am | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Oxalis stricta L. | E-As, N-Am | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Reynoutria × bohemica Chrtek & Chrtková | E-As | 19 | 3 | 5 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 18 |
| Robinia pseudoacacia L. | N-Am | 14 | 0 | 4 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 5 | 1 | 8 |
| Solidago gigantea Aiton | N-Am | 4 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Xanthium orientale L. | N+S-Am | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| Df | Variance | F | Pr (>F) | |
|---|---|---|---|---|
| HAB | 7 | 1.625915 | 3.330558 | 0.001 |
| PET | 1 | 0.072604 | 1.041072 | 0.367 |
| BIO2 | 1 | 0.085582 | 1.22715 | 0.262 |
| BIO11 | 1 | 0.073387 | 1.052287 | 0.366 |
| BIO14 | 1 | 0.09936 | 1.424714 | 0.193 |
| BIO15 | 1 | 0.074909 | 1.07412 | 0.357 |
| Residual | 68 | 4.742329 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djordjević, V.; Stanković, V.; Kabaš, E.; Lazarević, P.; Verloove, F.; Šinžar-Sekulić, J. Diversity Patterns of Alien Plant Species in Mountainous Areas: A Case Study from the Central Balkans. Diversity 2025, 17, 774. https://doi.org/10.3390/d17110774
Djordjević V, Stanković V, Kabaš E, Lazarević P, Verloove F, Šinžar-Sekulić J. Diversity Patterns of Alien Plant Species in Mountainous Areas: A Case Study from the Central Balkans. Diversity. 2025; 17(11):774. https://doi.org/10.3390/d17110774
Chicago/Turabian StyleDjordjević, Vladan, Vera Stanković, Eva Kabaš, Predrag Lazarević, Filip Verloove, and Jasmina Šinžar-Sekulić. 2025. "Diversity Patterns of Alien Plant Species in Mountainous Areas: A Case Study from the Central Balkans" Diversity 17, no. 11: 774. https://doi.org/10.3390/d17110774
APA StyleDjordjević, V., Stanković, V., Kabaš, E., Lazarević, P., Verloove, F., & Šinžar-Sekulić, J. (2025). Diversity Patterns of Alien Plant Species in Mountainous Areas: A Case Study from the Central Balkans. Diversity, 17(11), 774. https://doi.org/10.3390/d17110774








