Dinoflagellates and Saudi Marine Borders: A Special Consideration for Ballast Water, Invasive Species and BWM Convention
Abstract
1. Introduction
1.1. General Characteristics of Dinoflagellates
1.1.1. Nuclear Organization and Internal Cellular Structures
1.1.2. The Cell Envelope and Thecal Plates
1.2. Taxonomy and Life Cycle
1.3. Nutritional Strategies and Mutualistic Relationships
1.4. Toxin Production and Implications
1.5. Dinoflagellates Distribution, Abundance and Formation of Harmful Algal Blooms
1.6. Dinoflagellates and Climate Change
1.7. The Dispersal of Dinoflagellates and Their Cysts by Ballast Water and Biofouling
1.8. The Kingdom of Saudi Arabia and the BWM Convention Concerning Regulations of Ballast Water
2. Methods of Monitoring and Management of Dinoflagellates
2.1. Traditional Microscopy
2.2. Molecular Detection
2.2.1. Molecular Detection of Some Toxic Dinoflagellates
2.2.2. Molecular Detections of Dinoflagellates Cysts
2.2.3. Molecular Detection of Dinoflagellates Using DNA Metbarcoding in Saudi Arabia
2.3. Remote Sensing as a Monitoring and Forecasting Tool for Bloom Formation and Distribution
2.4. Remote Sensing Application in Saudi Arabia
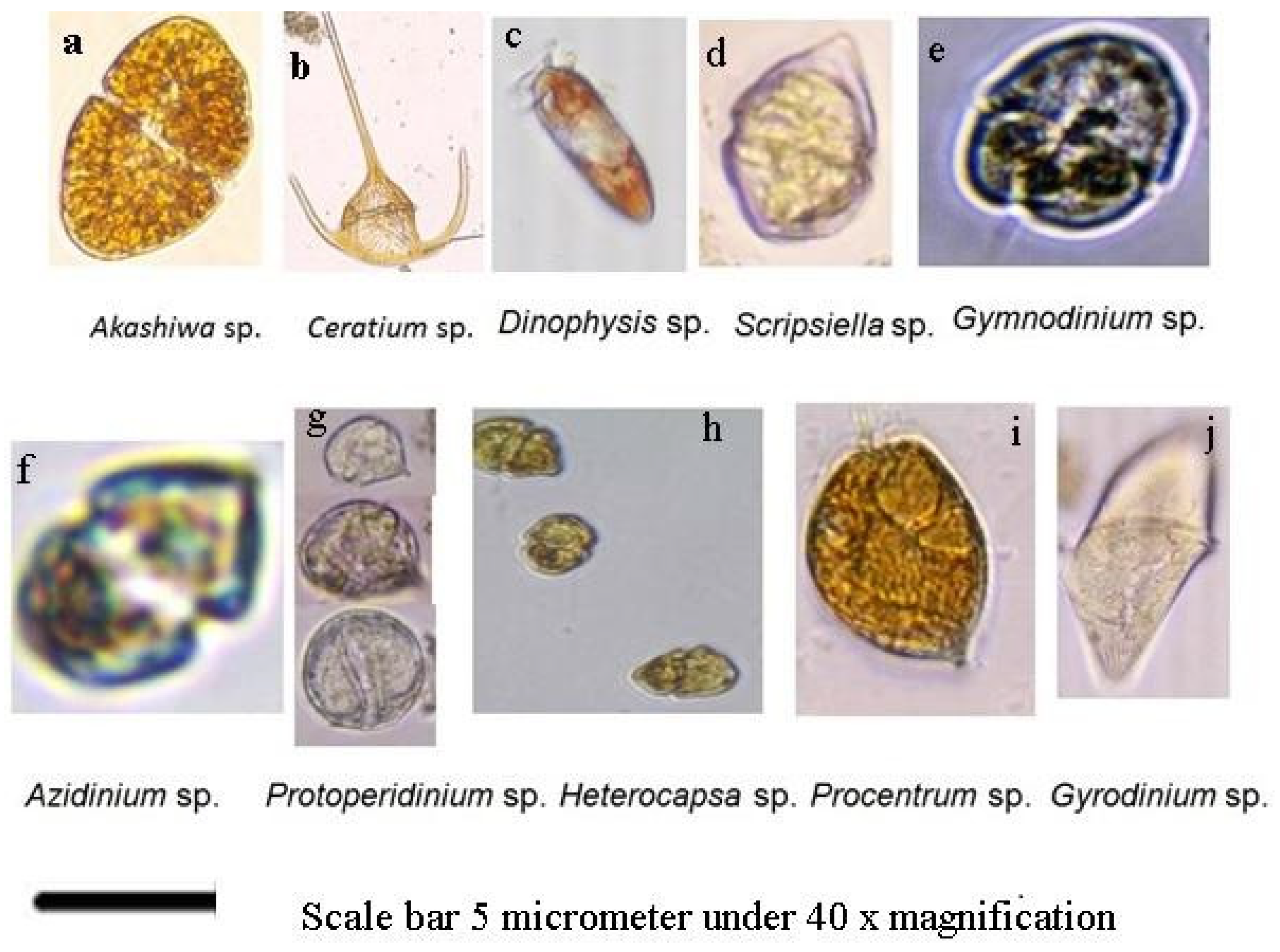
3. Results of Previous Studies Regarding Some Dinoflagellates in Saudi Arabian Marine Borders Including Some Invasive Species
- a—The Red Sea research
- b—The Arabian Gulf research
3.1. Factors That Were Found to Contribute to the Prevalence of Concerning Dinoflagellates
3.2. Engineering and Regulatory Considerations for Ballast Water Management
- Internal loads: the emptying of ballast tanks can induce free surface effects and elevate the vessel’s vertical center of gravity, so dynamic loads must be managed.
- Vessel operations: ensuring an unobstructed sea view (minimum of two ship lengths or 500 m) is vital for navigation safety.
- Pump and piping integrity: frequent use of the ballast system can lead to wear on components, necessitating regular maintenance to avoid over-pumping through air pipes.
3.3. Exemptions from BWM
4. Discussion
5. Conclusions and Recommendations
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dipper, F. Open water lifestyles: Marine plankton. In Elements of Marine Ecology, 5th ed.; Dipper, F., Ed.; Butterworth-Heinemann: Oxford, UK, 2022; pp. 193–228. [Google Scholar] [CrossRef]
- Not, F.; Siano, R.; Kooistra, W.; Simon, N.; Vaulot, R.; Probert, I. Diversity and ecology of eukaryotic marine phytoplankton. In Advances in Botanical Research; Piganeau, G., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 64, pp. 1–53. [Google Scholar]
- Stoecker, D.K. Mixotrophy among dinoflagellates. J. Eukaryot. Microbiol. 2007, 46, 397–401. [Google Scholar] [CrossRef]
- Spector, D.L. Dinoflagellates (Cell Biology); Academic Press: Cambridge, MA, USA, 2012; ISBN 9780323138130. [Google Scholar]
- Carty, S.; Parrow, M. Dinoflagellates. In Freshwater Algae of North America: Ecology and Classification, 2nd ed.; Wehr, J.D., Sheath, R.G., Kociolek, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 773–807. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Sielicki, M.; Haxo, F.T. Chloroplast pigment patterns in dinoflagellates. J. Phycol. 2008, 11, 374–384. [Google Scholar] [CrossRef]
- Lin, S.; Wu, S.; He, J.; Wang, X.; Grossman, A.R. Shining light on dinoflagellate photosystem I. Nat. Commun. 2024, 15, 3337. [Google Scholar] [CrossRef]
- Wisecaver, J.H.; Hackett, J.D. Transcriptome analysis reveals nuclear-encoded proteins for the maintenance of temporary plastids in the dinoflagellate Dinophysis acuminata. BMC Genom. 2010, 11, 366. [Google Scholar] [CrossRef]
- Qiu, D.; Huang, L.; Liu, S.; Lin, S. Nuclear, mitochondrial and plastid gene phylogenies of Dinophysis miles (Dinophyceae): Evidence of variable types of chloroplasts. PLoS ONE 2011, 6, e29398. [Google Scholar] [CrossRef]
- Gómez, F. Diversity and classification of dinoflagellates. In Dinoflagellates: Classification, Evolution, Physiology and Ecological Significance; Nova Science Publishers: Hauppauge, NY, USA, 2020; pp. 1–38. [Google Scholar]
- Cavalier-Smith, T.; Chao, E.E. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). Eur. J. Protistol. 2004, 40, 185–212. [Google Scholar] [CrossRef]
- Gould, S.B.; Tham, W.-H.; Cowman, A.F.; McFadden, G.I.; Waller, R.F. Alveolins, a new family of cortical proteins that define the protist infrakingdom Alveolata. Mol. Biol. Evol. 2008, 25, 1219–1230. [Google Scholar] [CrossRef]
- El Semary, N.A. Benthic dinoflagellates from Red Sea, Egypt: Early records. Egypt. J. Aquat. Res. 2016, 42, 177–184. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Murray, S.A.; Chomérat, N.; Horiguchi, T. Marine Benthic Dinoflagellates–Unveiling Their Worldwide Biodiversity (Kleine Senckenberg-Reihe 54); E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 2015. [Google Scholar]
- Takano, Y.; Horiguchi, T. Surface ultrastructure and molecular phylogenetics of four unarmoured heterotrophic dinoflagellates, including the type species of the genus Gyrodinium (Dinophyceae). Phycol. Res. 2004, 52, 107–116. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Daugbjerg, N. On dinoflagellate phylogeny and classification. In Unravelling the Algae: The Past, Present and Future of Algal Systematics; Brodie, J., Lewis, J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 215–230. [Google Scholar]
- Hoppenrath, M.; Murray, S.; Sparmann, S.F.; Leander, B.S. Morphology and molecular phylogeny of Ankistrodinium gen. nov. (Dinophyceae), a new genus of marine sand-dwelling dinoflagellates formerly classified within Amphidinium. J. Phycol. 2012, 48, 1143–1152. [Google Scholar] [CrossRef]
- Takano, Y.; Horiguchi, T. Acquiring scanning microscopical, light microscopical and multiple gene sequence data from a single dinoflagellate cell. J. Phycol. 2005, 42, 251–256. [Google Scholar] [CrossRef]
- Zaheri, B.; Morse, D. An overview of transcription in dinoflagellates. Gene 2022, 829, 146505. [Google Scholar] [CrossRef]
- Perini, F.; Bastianini, M.; Capellacci, S.; Pugliese, L.; Di Poi, E.; Cabrini, M.; Buratti, S.; Marini, M.; Penna, A. Molecular methods for cost-efficient monitoring of HAB (harmful algal bloom) dinoflagellate resting cysts. Mar. Pollut. Bull. 2019, 147, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Flynn, K.J.; Burkholder, J.M.; Berge, T.; Calbet, A.; Raven, J.A.; Granéli, E.; Glibert, P.M.; Hansen, P.J.; Stoecker, D.K.; et al. The role of mixotrophic protists in the biological carbon pump. Biogeosciences 2014, 11, 995–1005. [Google Scholar] [CrossRef]
- El Semary, N.A.H. Algae and fishes: Benefits and hazards. In Climate Change Impacts on Agriculture and Food Security in Egypt; Ewis Omran, E.S., Negm, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Prabowo, D.; Agustí, S. Free-living dinoflagellates of the central Red Sea, Saudi Arabia: Variability, new records and potentially harmful species. Mar. Pollut. Bull. 2019, 141, 629–648. [Google Scholar] [CrossRef]
- Kem, W. Marine organisms. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Wang, D.Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef]
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–449. [Google Scholar] [CrossRef]
- Tebben, J.; Zurhelle, C.; Tubaro, A.; Samdal, I.; Krock, B.; Kilcoyne, J.; Sosa, S.; Trainer, V.; Deeds, J.; Tillmann, U. Structure and toxicity of AZA-59, an azaspiracid shellfish poisoning toxin produced by Azadinium poporum (Dinophyceae). Harmful Algae 2023, 124, 102388. [Google Scholar] [CrossRef]
- El Gammal, M.; Nageeb, M.; Al-Sabeb, S. Phytoplankton abundance in relation to the quality of the coastal water–Arabian Gulf, Saudi Arabia. Egypt. J. Aquat. Res. 2017, 43, 275–282. [Google Scholar] [CrossRef]
- Rajaneesh, K.M.; Joydas, T.V.; Heinle, M.; Pavithratha, S.; Asharaf, M.; Manikandan, K.P.; Qurban, M.A.; Al-Suwailem, A. Phytoplankton community dynamics in the western Arabian Gulf: Spatial and seasonal trends. In Coral Reefs and Associated Marine Fauna Around the Arabian Peninsula; CRC Press: Boca Raton, FL, USA, 2024; pp. 288–304. [Google Scholar] [CrossRef]
- Uddin, S.; Behbehani, M.; Al-Ghadban, A.N.; Sajid, S.; Kumar, V.V.; Al-Musallam, L.; Al-Zekri, W.; Ali, M.; Al-Julathi, S.-M.; Alam, F. 210Po concentration in selected diatoms and dinoflagellates in the northern Arabian Gulf. Mar. Pollut. Bull. 2018, 129, 343–346. [Google Scholar] [CrossRef]
- Manning, T.; Thilagaraj, A.; Mouradov, D.; Piola, R.; Grandison, C.; Gordon, M.; Shimeta, J.; Mouradov, A. Diversity of dinoflagellate assemblages in coastal temperate and offshore tropical waters of Australia. BMC Ecol. Evol. 2021, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Siegel, D.A.; Brzezinski, M.A.; Guillocheau, N. Controls on temporal patterns in phytoplankton community structure in the Santa Barbara Channel, California. J. Geophys. Res. Ocean. 2008, 113, C04038. [Google Scholar] [CrossRef]
- Hanley, K.; Widder, E. Bioluminescence in dinoflagellates: Evidence that the adaptive value of bioluminescence in dinoflagellates is concentration dependent. Photochem. Photobiol. 2017, 93, 519–530. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Hoppenrath, M. Dinoflagellate diversity and distribution. Biodivers. Conserv. 2008, 17, 407–418. [Google Scholar] [CrossRef]
- Kibler, S.R.; Tester, P.A.; Kunkel, K.E.; Moore, S.K.; Litaker, R.W. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Model. 2015, 316, 194–210. [Google Scholar] [CrossRef]
- Wei, Y.; Luan, Q.; Shan, X.; Cui, H.; Qu, K.; Cui, Z.; Sun, J. Temperature and nutrients drive distinct successions between diatoms and dinoflagellates over the past 40 years: Implications for climate warming and eutrophication. Sci. Total Environ. 2024, 931, 172997. [Google Scholar] [CrossRef]
- Casas-Monroy, O.; Roy, S.; Rochon, A. Ballast sediment-mediated transport of non-indigenous species of dinoflagellates on the East Coast of Canada. Aquat. Invasions 2011, 6, 231–248. [Google Scholar] [CrossRef]
- Richlen, M.L.; Morton, S.L.; Jamali, E.A.; Rajan, A.; Anderson, D.M. The catastrophic 2008–2009 red tide in the Arabian Gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 2010, 9, 163–172. [Google Scholar] [CrossRef]
- International Maritime Organization. International Convention for the Control and Management of Ships’ Ballast Water and Sediments (BWM). 2017. Available online: https://www.imo.org/en/about/conventions/pages/international-convention-for-the-control-and-management-of-ships’-ballast-water-and-sediments-(bwm).aspx (accessed on 1 October 2025).
- Buck, E.H. Ballast Water Management to Combat Invasive Species; Report No. RL32344; U.S. Congressional Research Service: Washington, DC, USA, 2010.
- Kotb, M.A.; Hassan, A.A.; Ghurab, H.H. Ballast water pollution in Saudi Arabia ports. Int. J. Multidiscip. Curr. Res. 2015, 3, 1230–1234. Available online: http://ijmcr.com/ballast-water-pollution-in-saudi-arabia-ports/ (accessed on 1 October 2025).
- Zakariaand, M.; Abdulrahman, A. Occurrence and germination of dinoflagellate cysts in surface sediments from the Red Sea off the coasts of Saudi Arabia. Oceanologia 2011, 53, 121–136. [Google Scholar] [CrossRef]
- Penna, A.; Galluzzi, L. PCR techniques as diagnostic tools for the identification and enumeration of toxic marine phytoplankton species. In Algal Toxins: Nature, Occurrence, Effect and Detection; Evangelista, V., Barsanti, L., Frassanito, A.M., Passarelli, V., Gualtieri, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–12. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Presting, G.G. Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J. Phycol. 2007, 43, 605–608. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Hou, Y.; Zhuang, Y.; Miranda, L. High-level diversity of dinoflagellates in the natural environment revealed by assessment of mitochondrial cox1 and cob genes for dinoflagellate DNA barcoding. Appl. Environ. Microbiol. 2009, 75, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, R.; Tomlinson, M. Remote sensing of harmful algal blooms. In Remote Sensing of Coastal Aquatic Environments; Springer: Berlin/Heidelberg, Germany, 2008; pp. 277–296. [Google Scholar] [CrossRef]
- Rodriguez Benito, C.; Navarro, G.; Caballero, I. Using Copernicus Sentinel-2 and Sentinel-3 data to monitor harmful algal blooms in Southern Chile during the COVID-19 lockdown. Mar. Pollut. Bull. 2020, 161, 111722. [Google Scholar] [CrossRef] [PubMed]
- Detoni, A.M.S.; Navarro, G.; Garrido, J.L.; Rodríguez, F.; Hernández-Urcera, J.; Caballero, I. Mapping dinoflagellate blooms (Noctiluca and Alexandrium) in aquaculture production areas in the NW Iberian Peninsula with the Sentinel-2/3 satellites. Sci. Total Environ. 2023, 868, 161579. [Google Scholar] [CrossRef]
- Kahru, M.; Anderson, C.; Barton, A.D.; Carter, M.L.; Catlett, D.; Send, U.; Sosik, H.M.; Weiss, E.L.; Mitchell, B.G. Satellite detection of dinoflagellate blooms off California by UV reflectance ratios. Elem. Sci. Anthr. 2021, 9, 00157. [Google Scholar] [CrossRef]
- Alrehili, B. Remote Sensing Techniques for Monitoring Algal Blooms in the Area Between Jeddah and Rabigh on the Red Sea Coast. Remote Sens. Appl. Soc. Environ. 2023, 30, 100935. [Google Scholar]
- Wafar, M.; Ashraf, M.; Kandan, M.; Qurban, M.; Kattan, Y. Propagation of Gulf of Aden Intermediate Water (GAIW) in the Red Sea during autumn and its importance to biological production. J. Mar. Syst. 2015, 154, 243–251. [Google Scholar] [CrossRef]
- Devassy, R.; Al-Aidaroos, A.; El-Sherbiny, M.; Al-Sofyani, A. Spatial variation in the phytoplankton standing stock and diversity in relation to the prevailing environmental conditions along the Saudi Arabian coast of the northern Red Sea. Mar. Biodivers. 2017, 47, 995–1008. [Google Scholar] [CrossRef]
- Devlin, M.J.; Breckels, M.; Graves, C.A.; Barry, J.; Capuzzo, E.; Huerta, F.P.; Al Ajmi, F.; Al-Hussain, M.M.; Le Quesne, W.J.F.; Lyons, B.P. Seasonal and temporal drivers influencing phytoplankton community in Kuwait marine waters: Documenting a changing landscape in the Gulf. Front. Mar. Sci. 2019, 6, 141. [Google Scholar] [CrossRef]
- Sathish, T.; Cicily, L.; Purushothaman, A.; Benny, N.; Padmakumar, K.B. First recorded bloom of Akashiwo sanguinea (Dinophyceae) from the Cochin backwaters, a tropical estuarine system along the south-eastern Arabian Sea. Oceanol. Hydrobiol. Stud. 2020, 49, 391–397. [Google Scholar] [CrossRef]
- Fagín, E.; Bravo, I.; Garrido, J.L.; Rodríguez, F.; Figueroa, R.I. Scrippsiella acuminata versus Scrippsiella ramonii: A physiological comparison. Cytom. A 2019, 95, 985–996. [Google Scholar] [CrossRef]
- AlKhawari, A. Seasonal variation in composition and abundance of harmful dinoflagellates in Yemeni waters, southern Red Sea. Mar. Pollut. Bull. 2016, 112, 225–234. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, H.J.; You, J.H.; Kim, S. Morphological and genetic characterization and the nationwide distribution of the phototrophic dinoflagellate Scrippsiella lachrymosa in the Korean waters. Algae 2018, 33, 21–35. [Google Scholar] [CrossRef]
- Tillmann, U.; Soehner, S.; Nézan, E.; Krock, B. First record of the genus Azadinium (Dinophyceae) from the Shetland Islands, including the description of Azadinium polongum sp. nov. Harmful Algae 2012, 20, 142–155. [Google Scholar] [CrossRef]
- Polikarpov, I.; Saburova, M.; Al-Yamani, F. Diversity and distribution of winter phytoplankton in the Arabian Gulf and the Sea of Oman. Cont. Shelf Res. 2016, 119, 85–99. [Google Scholar] [CrossRef]
- Abadie, E.; Chiantella, C.; Crottier, A.; Rhodes, L.; Masseret, E.; Berteaux, T.; Laabir, M. What are the main environmental factors driving the development of the neurotoxic dinoflagellate Vulcanodinium rugosum in a Mediterranean ecosystem (Ingril lagoon, France)? Harmful Algae 2018, 75, 75–86. [Google Scholar] [CrossRef]
- International Maritime Organization (IMO). Resolution MEPC.149(55): Guidelines for Ballast Water Exchange Design and Construction Standards (G11) and Resolution MEPC.151(55): Guidelines on Designation of Areas for Ballast Water Exchange (G14). 2006. Available online: https://www.imorules.com/MEPCRES_151.55_ANN.html (accessed on 1 October 2025).
- Rahman, S. Implementation of ballast water management plan in ships through ballast water exchange system. Procedia Eng. 2017, 194, 323–329. [Google Scholar] [CrossRef]
- Rahman, A.; Karim, M. Green shipbuilding and recycling: Issues and challenges. Int. J. Environ. Sci. Dev. 2015, 6, 838–842. [Google Scholar] [CrossRef]
- Outinen, O.; Bailey, S.; Broeg, K.; Chassé, J.; Clarke, S.; Daigle, R.; Gollasch, S.; Kakkonen, J.; Lehtiniemi, M.; Normant-Saremba, M.; et al. Exceptions and exemptions under the ballast water management convention: Sustainable alternatives for ballast water management? J. Environ. Manag. 2021, 293, 112823. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M.; Bolch, C.J. Transport of diatom and dinoflagellate resting spores in ships’ ballast water: Implications for plankton biogeography and aquaculture. J. Plankton Res. 1992, 14, 1067–1084. [Google Scholar] [CrossRef]
- Hay, C.; Handley, S.; Dodgshun, T.; Taylor, M.; Gibbs, W. Cawthron’s Ballast Water Research: Final Report; Report No. 417; Cawthron Institute: Nelson, New Zealand, 1997. [Google Scholar]
- Harvey, M.; Gilbert, M.; Gauthier, D.; Reid, D.M. A preliminary assessment of risks for the ballast water-mediated introduction of nonindigenous marine organisms in the Estuary and Gulf of St. Lawrence. Can. Tech. Rep. Fish. Aquat. Sci. 1999. Available online: https://publications.gc.ca/site/eng/422080/publication.html (accessed on 1 October 2025).
- Hamer, J.P.; Lucas, I.; McCollin, T.A. Harmful dinoflagellate resting cysts in ships’ ballast tank sediments: Potential for introduction into English and Welsh waters. Phycologia 2001, 40, 246–255. [Google Scholar] [CrossRef]
- Pertola, S.; Faust, M.A.; Kuosa, H. Survey on germination and species composition of dinoflagellates from ballast tanks and recent sediments in ports on the south coast of Finland, north-eastern Baltic Sea. Mar. Pollut. Bull. 2006, 52, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Pereira, S.; Tavares, T.; Garcia, T.; Soares, M. Impacts of desalination discharges on phytoplankton and zooplankton: Perspectives on current knowledge. Sci. Total Environ. 2023, 863, 160671. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawajfeh, A.; Alzalabieh, E.; Bazedi, G.; Al-Mazaideh, G.; Shalayel, M. A review on harmful algae blooms in the Arabian Gulf: Causes and impacts on desalination plants. Desalination Water Treat. 2023, 290, 46–55. [Google Scholar] [CrossRef]
- Oemcke, D.J.; van Leeuwen, J.H. Ozonation of the marine dinoflagellate alga Amphidinium sp.—Implications for ballast water disinfection. Water Res. 2005, 39, 5119–5126. [Google Scholar] [CrossRef]
- De Lafontaine, Y.; Despatie, S. Performance of a biological deoxygenation process for ships’ ballast water treatment under very cold water conditions. Sci. Total Environ. 2013, 472, 1036–1043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Semary, N. Dinoflagellates and Saudi Marine Borders: A Special Consideration for Ballast Water, Invasive Species and BWM Convention. Diversity 2025, 17, 772. https://doi.org/10.3390/d17110772
El Semary N. Dinoflagellates and Saudi Marine Borders: A Special Consideration for Ballast Water, Invasive Species and BWM Convention. Diversity. 2025; 17(11):772. https://doi.org/10.3390/d17110772
Chicago/Turabian StyleEl Semary, Nermin. 2025. "Dinoflagellates and Saudi Marine Borders: A Special Consideration for Ballast Water, Invasive Species and BWM Convention" Diversity 17, no. 11: 772. https://doi.org/10.3390/d17110772
APA StyleEl Semary, N. (2025). Dinoflagellates and Saudi Marine Borders: A Special Consideration for Ballast Water, Invasive Species and BWM Convention. Diversity, 17(11), 772. https://doi.org/10.3390/d17110772





