1. Introduction
Acropora palmata (elkhorn coral) was historically one of the most important reef-building coral species in the Caribbean. With fast growth rates (5–10 cm/year) and a branching morphology, it forms dense thickets that create natural reef crests in shallow waters (<10 m), dissipating wave energy and reducing coastal erosion and flooding while providing critical habitat for reef biodiversity [
1].
This species is a simultaneous hermaphrodite that reproduces sexually through synchronized broadcast spawning, releasing gamete bundles 2–10 days after the full moon in August or September [
2]. Gametes are released in bundles during a synchronized broadcast spawning, ensuring high larval dispersal and genetic connectivity. Successful fertilization depends on colony density, synchrony, and favorable environmental conditions.
Ecologically,
A. palmata plays a key role in coastal protection, dissipating wave energy and reducing erosion and flooding. It also provides habitat for numerous reef species. Since the 1980s, populations have declined by over 95% due to White Band Disease, hurricanes, rising sea temperatures, and pollution. As a result, it is listed as Critically Endangered by the IUCN and protected under various national and international frameworks [
3].
In Cozumel, particularly at the public beach south of the API pier,
A. palmata once formed natural coastal defenses. However, from 2018 to 2025, long-term monitoring revealed no synchronized spawning, minimal growth (<10 cm/year), and frequent mechanical damage. In contrast, colonies along the mainland (Cancún–Punta Venado) continue to spawn annually in a north-to-south gradient (pers. comm., Red Mexicana de Restauración de Corales). Although not measured in this study, water quality may represent a critical driver of reproductive impairment. Pollutants, sediment resuspension, and coastal eutrophication have been shown to disrupt gametogenesis and spawning synchrony in Caribbean corals [
4,
5] and thus merit further investigation in the context of the observed island–mainland differences.
Our long-term monitoring suggests that these Cozumel colonies may be allocating most of their energy simply to survival. Observed stressors include prolonged sea surface temperature anomalies, seasonal sea level drops that expose colonies, mechanical damage from shore-based fishing (e.g., entangled fishing lines, spark plugs), and frequent trampling. Colonies are often used as ‘steps’ to enter the water despite educational signage in the area. These combined physical and anthropogenic pressures may be inhibiting growth and suppressing reproductive behavior, further compromising this keystone species’ role in reef recovery and coastal resilience.
3. Results and Discussion
Across eight consecutive years of monitoring (2018–2025), no synchronized spawning of
Acropora palmata was observed in Cozumel. Only three isolated spawning events were recorded: a single colony at Tikila in 2019, two colonies at the public beach in 2021 (on different nights), and two colonies at La Caletita and Playa Corona in 2025 (
Figure 2). All other colonies either showed “setting” behavior or no reproductive activity. In contrast, mainland sites from Cancún to Punta Venado exhibited predictable annual synchronized spawning, consistent with the well-documented north–south gradient of reproductive activity in the Mexican Caribbean [
9,
10].
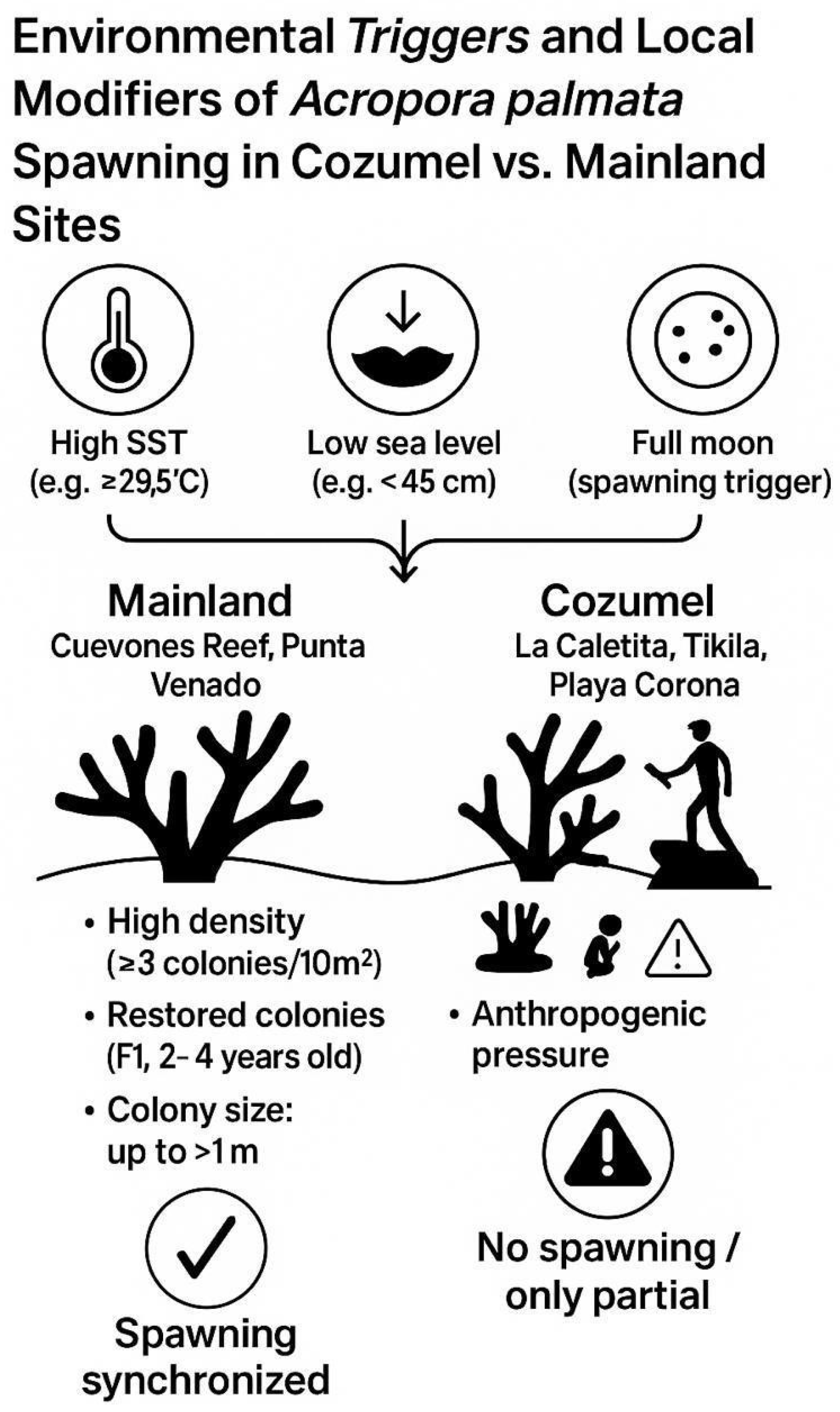
Mainland sites (Cuevones Reef and Punta Venado) were characterized by high colony density (≥3 colonies per 10 m
2), restored F
1 colonies aged 2–4 years, and large colony sizes (>1 m), which supported synchronized spawning. In contrast, Cozumel sites (La Caletita, Tikila, and Playa Corona) exhibited low colony density (≤1 colony per 10 m
2), smaller colonies (<50 cm), and chronic anthropogenic pressures, including trampling, fishing-gear entanglement, and artificial light at night. Consistent with these local stressors, most Cozumel colonies showed only “setting” behavior or no reproductive activity, whereas mainland colonies displayed predictable, annual synchronized spawning (
Figure 3).
Histological analyses confirmed that gametogenesis persisted in several colonies across all years, with gametes observed at different developmental stages even when spawning was not detected in situ. However, maturation rarely advanced to synchronized release, aligning with the recurrent observation of “setting” without spawning. In colonies where histology revealed the presence of mature gametes, collector traps occasionally recovered small numbers of bundles, yet fertilization success was negligible due to the absence of multi-colony synchrony.
Colony growth in Cozumel remained consistently low (<10 cm/year), and evidence of chronic mechanical disturbance was widespread, including broken branches, entanglement with fishing gear, spark plugs, and trampling. Despite the installation of educational signage, colonies in the public beach area were frequently used as physical steps to enter the sea, suggesting persistent anthropogenic pressure.
Figure 3.
Environmental triggers and local conditions of Acropora palmata spawning in the Mexican Caribbean. Mainland sites (Cuevones Reef and Punta Venado) show high colony density, restored F1 individuals, and large colonies (>1 m) that support synchronized spawning. In Cozumel (La Caletita, Tikila, and Playa Corona), low colony density, smaller remnant colonies, and chronic anthropogenic stressors—including artificial light at night (ALAN), trampling, and fishing gear entanglement—appear to suppress reproductive synchrony.
Figure 3.
Environmental triggers and local conditions of Acropora palmata spawning in the Mexican Caribbean. Mainland sites (Cuevones Reef and Punta Venado) show high colony density, restored F1 individuals, and large colonies (>1 m) that support synchronized spawning. In Cozumel (La Caletita, Tikila, and Playa Corona), low colony density, smaller remnant colonies, and chronic anthropogenic stressors—including artificial light at night (ALAN), trampling, and fishing gear entanglement—appear to suppress reproductive synchrony.
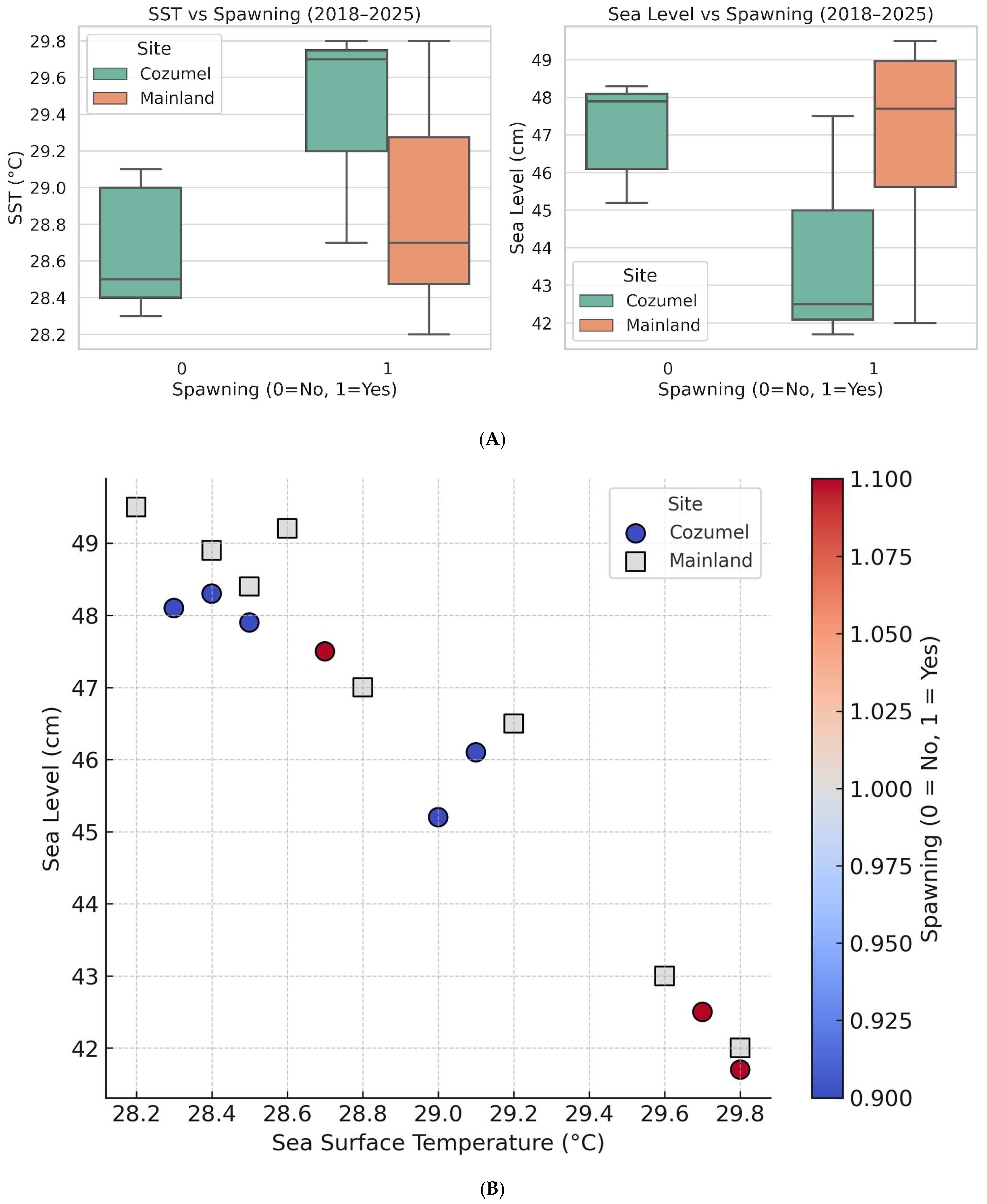
Environmental variables further highlight the vulnerability of these colonies. Spawning events in 2019, 2021, and 2025 coincided with elevated SST (≥29.7 °C) and unusually low sea levels (~42–43 cm). In contrast, years without spawning were characterized by lower SST (~28.6 °C) and higher mean sea levels (~47 cm) (
Figure 4A,B). A logistic regression model confirmed this association: each 1 °C increase in SST increased the odds of spawning nearly sixfold (
p = 0.04), while low sea level exerted a marginally negative effect (
p = 0.08). These results are shown in
Figure 4. The location factor (Cozumel vs. mainland) was not statistically significant, suggesting that environmental triggers remain universal but their expression may be suppressed by local stressors. This pattern suggests that when colonies experience heightened heat stress, a specific physiological, molecular or genetic mechanism may be triggered, prompting them to reproduce, possibly enhancing their survival prospects.
Comparable reproductive collapses have been reported in the Florida Keys, where
A. palmata populations show minimal recruitment due to low colony density and compounded environmental stress [
11]. Nevertheless, successful synchronized spawning has been documented in nursery-raised and outplanted colonies, both in Florida and Mexico, demonstrating that reproductive capacity can be restored under controlled or less-disturbed conditions [
10].
Recent studies further indicate that coral reproductive synchrony is increasingly disrupted at global scales. Artificial light at night (ALAN), for instance, has been identified elsewhere as a key driver delaying gametogenesis and hindering synchronization of gamete release, in some cases leading to a complete collapse of reproductive cycles [
12,
13]. Although we did not measure ALAN directly in this study, we highlight it here as a candidate hypothesis for Cozumel, where coastal infrastructure provides persistent light exposure during spawning windows. Similarly, thermal stress has also been shown to override lunar and tidal spawning cues in corals, as reported in cases from the Indian Ocean and the Maldives [
14].
In Cozumel, we hypothesize that several of these stressors may be acting simultaneously. Persistent exposure to artificial light from coastal infrastructure, recurrent thermal anomalies during the spawning season, and continuous mechanical damage from trampling and fishing gear likely compound the physiological stress of colonies, disrupting gamete maturation and reproductive synchrony despite the presence of environmental cues.
At the physiological level, published transcriptomic analyses of
A. palmata under abiotic stress provide insights into potential mechanisms underlying these observations [
15,
16]. Although gene expression was not measured in this study, previous work suggests that chronic stress may trigger molecular pathways associated with heat response, oxidative stress, apoptosis, and extracellular matrix (ECM) remodeling—core processes that reflect the sensitivity of this species to elevated temperature and acidification. The activation of these pathways can represent compensatory responses linked to bleaching, calcification loss, and tissue repair, ultimately diverting energetic resources away from gametogenesis and reproductive synchronization. This chronic energetic imbalance may therefore contribute to the reproductive collapse observed in Cozumel colonies.
Beyond these physiological responses, demographic constraints and colony density may further exacerbate the loss of reproductive synchrony in Cozumel. We did not conduct density transects in this study; instead, we infer low density from repeated in situ observations of scattered, fragmented colonies. The prolonged absence of synchronized spawning in Cozumel may reflect a demographic Allee effect, where low colony density undermines fertilization success and disrupts reproductive recovery. Experimental work in Palau demonstrated that fertilization rates plummet as distances between conspecific colonies increase beyond a few meters, underscoring the importance of proximally aggregated colonies for successful reproduction [
17]. In contrast, mainland outplanting efforts at Cuevones Reef yielded gravid F
1 colonies with measurable fecundity and successful spawning two years post-transplantation, allowing viable gamete fertilization and larval development [
10]. This contrast highlights that, while environmental triggers remain active, reproductive failure in Cozumel is likely exacerbated by both ecological (low density, fragmentation) and chronic stress barriers. These results demonstrate the potential of sexual restoration interventions to enhance colony density and connectivity, supporting the functional recovery and resilience of Caribbean
Acropora spp. populations [
18].
Our findings show that although gametogenesis continues in Cozumel colonies, synchrony has been lost. Environmental cues (SST and tidal cycles) remain active but are insufficient under conditions of low colony density, chronic disturbance, and heightened stress. Evidence from other Caribbean reefs indicates that water quality and sediment-associated toxicity can further suppress coral reproduction [
4,
5], suggesting that pollutants and turbidity may exacerbate reproductive failure in Cozumel. Mainland sites, on the other hand, still display synchronized spawning and successful fertilization, emphasizing the impact of local stressors in causing site-specific reproductive failure. These findings emphasize the urgent need for targeted management strategies to reduce human pressures in coastal areas, thereby restoring the reproductive function and long-term viability of
A. palmata populations.
4. Conclusions
Eight years of observations reveal a persistent collapse of reproductive synchrony in Acropora palmata at Cozumel. Despite three isolated spawning events (2019, 2021, 2025), colonies did not spawn in concert, effectively preventing fertilization and recruitment. In contrast, nearby mainland reefs continue to exhibit predictable, annual synchronized spawning, indicating that regional environmental cues remain intact.
Multiple chronic local stressors, artificial light at night, trampling, and entanglement or damage from shore-based activities, combined with shallow exposure during seasonal sea-level drops, likely drive colonies into a persistent “survival mode.” This interpretation is consistent with a physiological trade-off: stress-response pathways (heat/oxidative stress, HSPs/chaperones, ECM restructuring) are upregulated at the expense of reproductive processes, aligning with our conceptual model of energy reallocation under repeated disturbance.
Demographically, the system shows signs of an Allee effect: fragmented and isolated colonies reduce the probability of gamete encounter even when individual colonies retain the capacity to develop gametes and, sporadically, to spawn. The contrast with mainland sites and the documented sexual reproduction of restored F1 colonies there demonstrates that reproductive function can be rebuilt when density, connectivity, and local conditions are favorable.
Management should therefore focus on restoring the conditions for synchrony, not only the condition of individual colonies. Immediate, site-specific measures are warranted: (i) strict controls on coastal lighting (shielding, spectrum shifts, curfews) during the spawning window; (ii) physical exclusion and dedicated entry points to eliminate trampling and “coral-as-steps” behavior; (iii) no-gear shoreline buffers to stop snagging and entanglement; and (iv) targeted reinforcement of colony density and spacing (sexual restoration/assisted fertilization) to overcome mate limitation and re-establish local synchrony.
Finally, we identify priority tests to accelerate recovery and reduce uncertainty: coordinated ALAN mitigation experiments (before/after at Cozumel sites), continuous in situ thermal/tidal logging aligned to gametogenesis, periodic histology of colonies to track reproductive development, light-environment mapping, and multi-site comparisons of density, spacing, and synchrony indices. Equally important, we highlight water quality monitoring (e.g., turbidity, pollutants, sediment toxicity) as a priority for future work, since evidence from other Caribbean systems indicates it may critically constrain coral reproduction.
Together, these interventions can move Cozumel from isolated, energetically costly spawning to recurrent, synchronized reproduction, safeguarding the coastal protection and ecological functions uniquely provided by A. palmata in this high-use shoreline.