Improving the Knowledge on the Distribution and Ecology of the Protected Echinoid Centrostephanus longispinus (Philippi, 1845) in the Alboran Sea
Abstract
1. Introduction
2. Materials and Methods
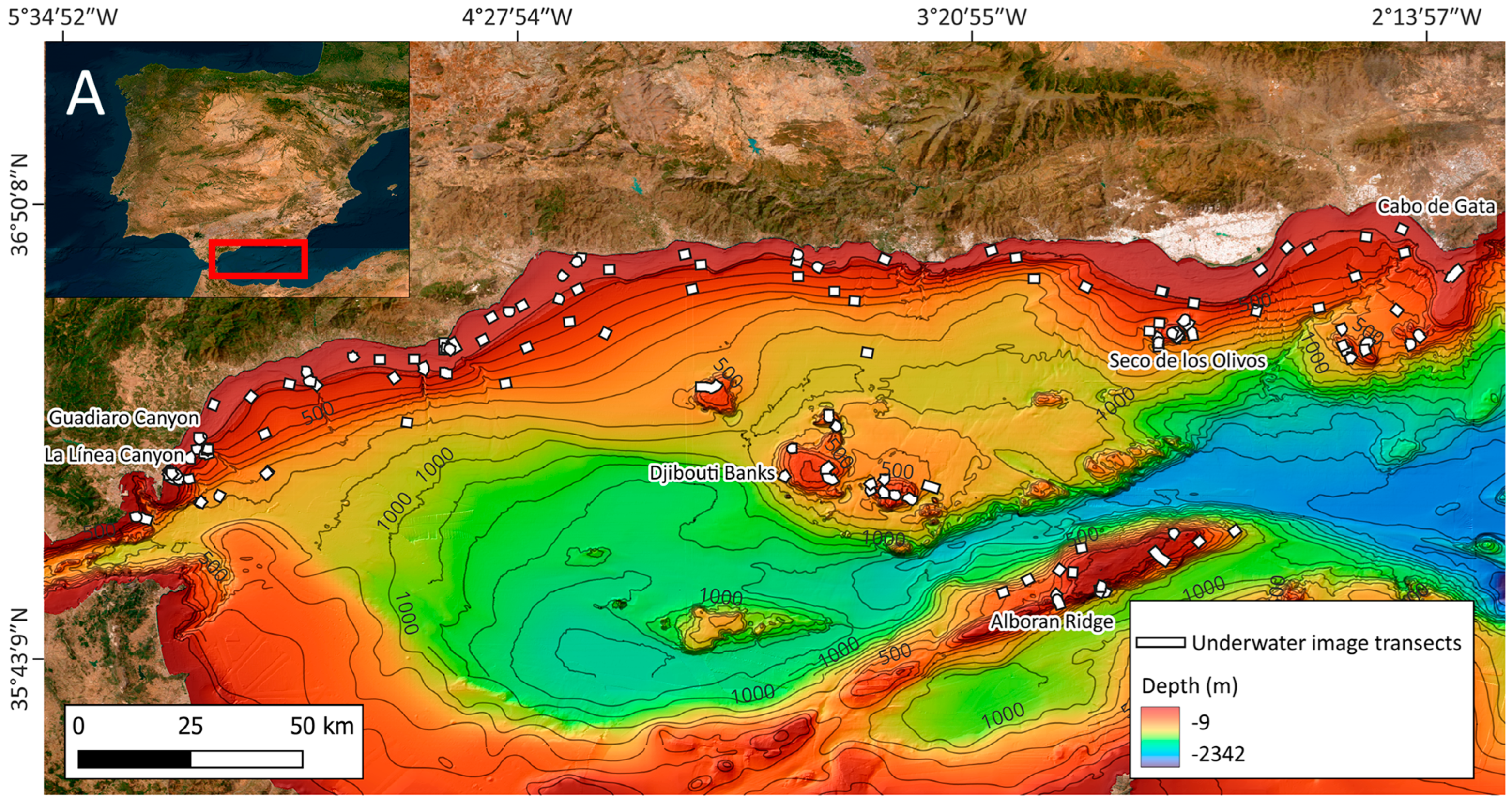
2.1. Study Area
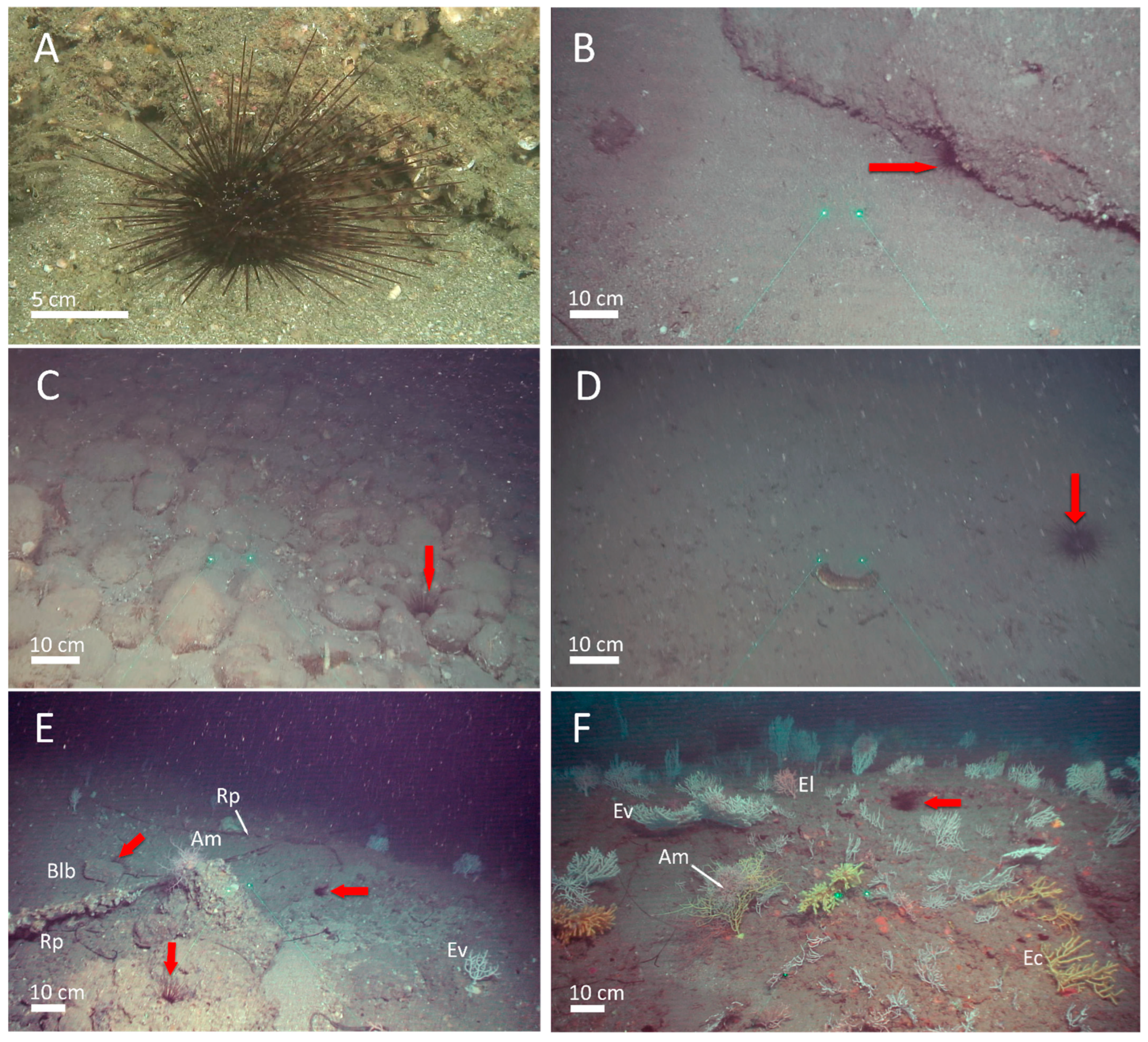
2.2. Underwater Image Acquisition
2.3. Underwater Image Processing
3. Results
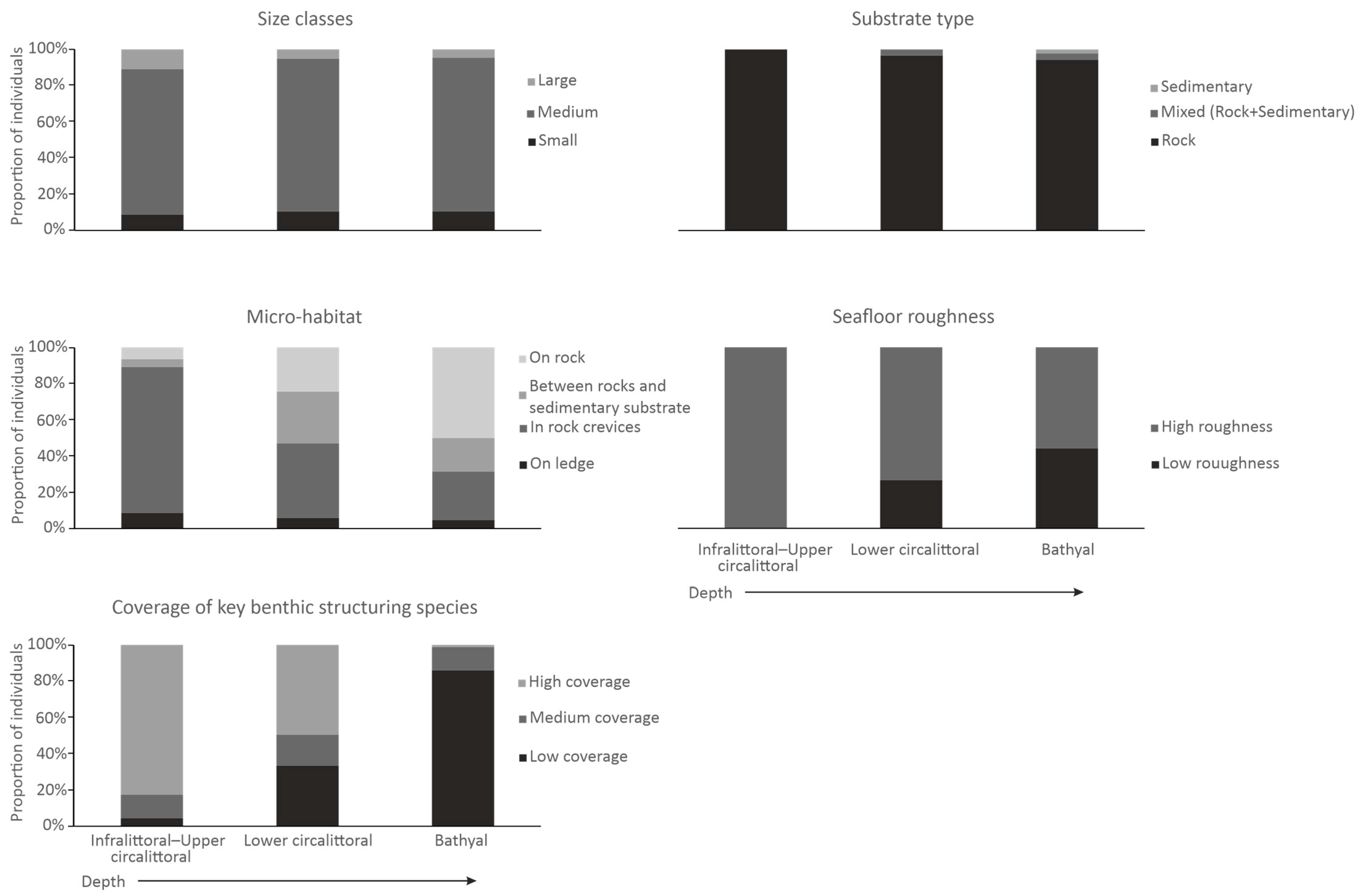
3.1. General Results and Comparissons of Proportions in Relation to Depth and Seafloor Features
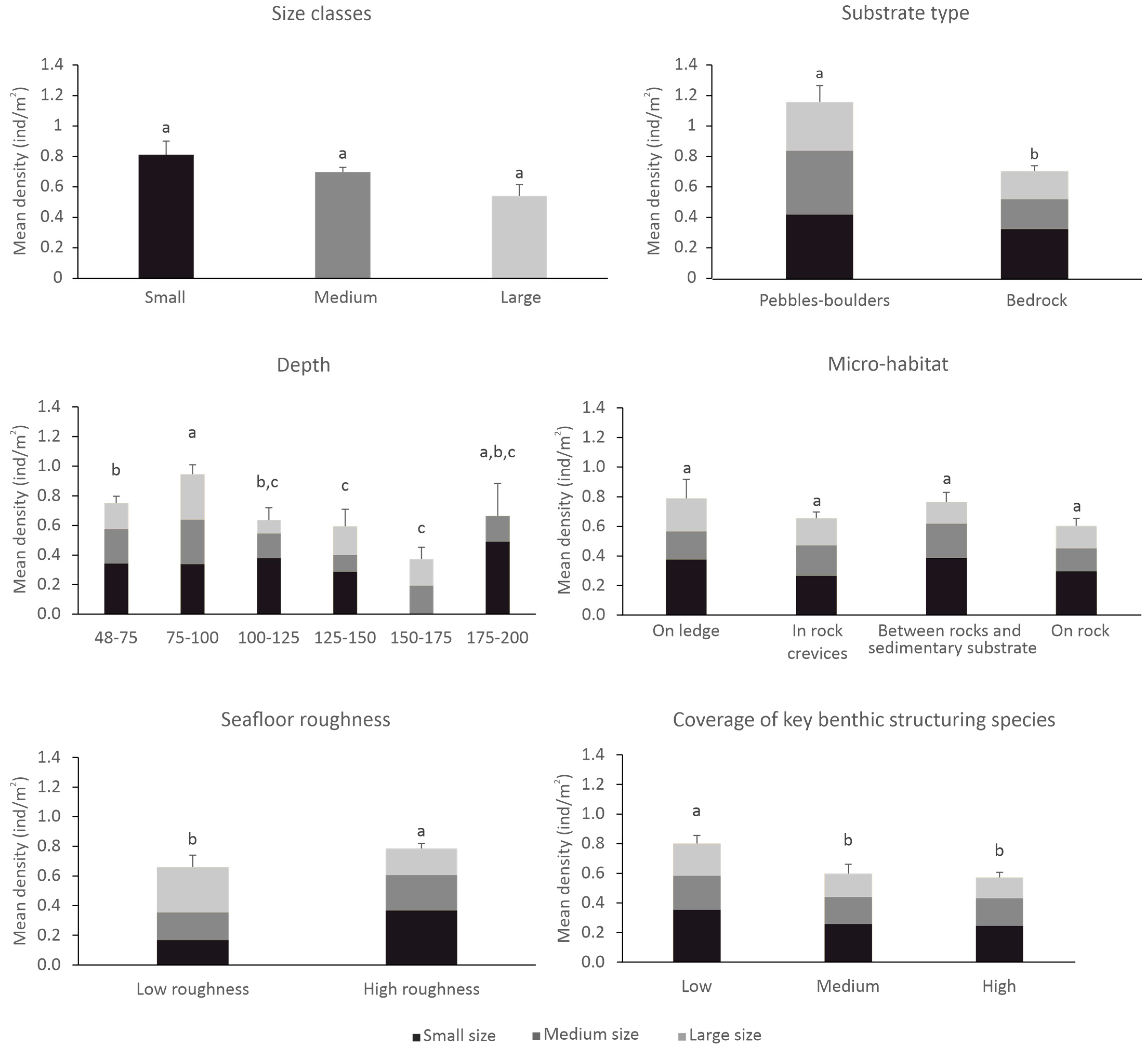
3.2. Density Estimations and Comparisons in Relation to Depth and Seafloor Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawson, D.L.; Miller, J.E. Systematics and Ecology of the Sea-Urchin Genus Centrostephanus (Echinodermata: Echinoidea) from the Atlantic and Eastern Pacific Oceans; Smithsonian Contributions to the Marine Sciences; Smithsonian Institution Press: Washington, DC, USA, 1983; Volume 20, pp. 1–14. [Google Scholar]
- Mortensen, T. A monograph of the Echinoidea Volume III 1: Aulodonta; C.A. Reitzel: Copenhagen, Denmark, 1940; pp. 154–196. [Google Scholar]
- Andrew, N.; Byrne, M. Ecology of Centrostephanus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 37, pp. 191–204. [Google Scholar]
- Kroh, A.; Mooi, R. Centrostephanus A. Agassiz, 1864. In World Echinoidea Database. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=123456 (accessed on 30 September 2025).
- Gondim, A.I.; Bendayan, R.M.; Lindsey, C.M.; Dias, T.L.P. Rediscovery of the poorly known sea urchin Centrostephanus longispinus rubicingulus (H.L. Clark, 1921) (Echinodermata, Echinoidea, Diadematidae) on the continental shelf of Brazil, with notes on its morphology and synonymy. Mar. Biol. Res. 2018, 14, 778–789. [Google Scholar] [CrossRef]
- Guallart, J.; Templado, J. Centrostephanus longispinus . In Bases Ecológicas Preliminares Para la Conservación de las Especies de Interés Comunitario en España: Invertebrados; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2012; p. 58. [Google Scholar]
- Yokes, B.; Galil, B.S. The first record of the needle-spined urchin Diadema setosum (Leske, 1778) (Echinodermata: Echinoidea: Diadematidae) from the Mediterranean Sea. Aquat. Invasions 2006, 1, 188–190. [Google Scholar] [CrossRef]
- Marchesi, V.; Cerrano, C.; Gambardella, C.; Pulido Mantas, T.; Roveta, C.; Santana Mendonça de Oliveira, L.J.; Turicchia, E.; Ponti, M.; Di Camillo, C.G. A Baseline for the Conservation of the Native and Protected Centrostephanus longispinus (Philippi, 1845) and the Management of the Invasive Diadema setosum (Leske, 1778) (Echinoidea: Diadematidae) in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2025, 35, e70155. [Google Scholar] [CrossRef]
- Öndes, F.; Alan, V.; Kaiser, M.J.; Güçlüsoy, H. Spatial distribution and density of the invasive sea urchin Diadema setosum in Turkey (eastern Mediterranean). Mar. Ecol. 2022, 43, e12724. [Google Scholar] [CrossRef]
- Sanahuja, M.J.G. Contribución al conocimiento de Diadema antillarum hilippi 1845, en Canarias. Ph.D. Thesis, Las Palmas de Gran Canaria, Las Palmas, Spain, 2003. [Google Scholar]
- Fletcher, W.J. Interactions Among Subtidal Australian Sea Urchins, Gastropods, and Algae: Effects of Experimental Removals. Ecol. Monogr. 1987, 57, 89–109. [Google Scholar] [CrossRef]
- Przeslawski, R.; Chick, R.C.; Davis, T.; Day, J.K.; Glasby, T.M.; Knott, N.; Byrne, M. A review of urchin barrens and the longspined sea urchin (Centrostephanus rodgersii) in New South Wales, Australia. Mar. Freshw. Res. 2025, 76, MF24149. [Google Scholar] [CrossRef]
- Ling, S.D.; Keane, J.P. Climate-driven invasion and incipient warnings of kelp ecosystem collapse. Nat. Commun. 2024, 15, 400. [Google Scholar] [CrossRef] [PubMed]
- Andrew, N.L. Spatial Heterogeneity, Sea Urchin Grazing, and Habitat Structure on Reefs in Temperate Australia. Ecology 1993, 74, 292–302. [Google Scholar] [CrossRef]
- Caley, A.; Marzinelli, E.M.; Byrne, M.; Mayer-Pinto, M. Artificial light at night and warming impact grazing rates and gonad index of the sea urchin Centrostephanus rodgersii. Proc. R. Soc. B 2024, 291, 20240415. [Google Scholar] [CrossRef]
- Smith, J.E.; Dietz, C.; Keane, J.; Mundy, C.; Oellermann, M.; Gardner, C. Trophic discrimination factors and stable isotope variability in a captive feeding trial of the southern rock lobster Jasus edwardsii (Hutton, 1875) (Decapoda: Palinuridae) in Tasmania, Australia. J. Crustacean Biol. 2023, 43, ruad055. [Google Scholar] [CrossRef]
- Day, J.K.; Huggett, M.J.; Gaston, T.F. Suspected Key Predators of Long-Spined Urchins Fail to Show Signs of Significant Predation in Gut Contents Analyses. Estuaries Coasts 2025, 48, 88. [Google Scholar] [CrossRef]
- Francour, P. L’ourchin Centrostephanus longispinus en Méditerranée occidentale: Résultats d’une enquête sur la répartition et son écologie. Vie Mar. 1989, 10, 138–147. [Google Scholar]
- Francour, P. Statut de Centrostephanus longispinus en Méditerranée. In Les Espèces Marines à Proteger en Méditerranée; Boudouresque, C.F., Avon, M., Gravez, V., Eds.; GIS Posidonie Publishers: Marseille, France, 1991; pp. 187–202. [Google Scholar]
- Rivera, V. Dos equínidos interesantes, Centrostephanus longispinus y Genocidaris maculata. Notas Resúmenes Inst. Esp. Oceanogr. 1928, 2, 1–9. [Google Scholar]
- Cherbonnier, G. Les échinodermes de Tunisie. Bull. INSTM Mar. Freshw. Sci. 1956, 53, 1–77. [Google Scholar]
- Francour, P. Nouvelles données sur la biologie et l’ecologie de l’oursin Centrostephanus longispinus en Mediterranée nord-occidentale. GIS Posidonie 1996, 1, 1–21. [Google Scholar]
- Bonhomme, P.; Goujard, A.; Javel, F.; Grondin, J.; Boudouresque, C. Unexpected artificial-reef-like effect due to a Mediterranean pipeline and the conservation of two circalittoral emblematic species: Centrostephanus longispinus and Cystoseira zosteroides. In Proceedings of the 2nd Mediterranean Sumposium on the Conservation of Coralligenous and Other Calcareous Bio-Concretions, Portorož, Slovenia, 29–30 October 2014. [Google Scholar]
- Pergent-Martini, C.; Bulteel, P.; Francour, P.; Gambi, M.C.; Harmelin-Vivien, M.; Lorenti, M.; Mazzella, L.; Pergent, G.; Momero, J.; Russo, G. Signalisations de Centrostephanus longispinus autour de l’île d’Ischia (Italie). In Les Espèces Marines à Protéger En Méditerranée; GIS Posidonie Publishers: Marseille, France, 1991; pp. 203–207. [Google Scholar]
- González-Irusta, J.M.; González-Porto, M.; Sarralde, R.; Arrese, B.; Almón, B.; Martín-Sosa, P. Comparing species distribution models: A case study of four deep sea urchin species. Hydrobiologia 2015, 745, 43–57. [Google Scholar] [CrossRef]
- Chapman, G. Aspects of the fauna and flora of the Azores. IV. Echinodermata. Ann. Mag. Nat. Hist. 1955, 8, 398–400. [Google Scholar] [CrossRef]
- Zavodnik, D. Echinodermata of the marine National park Kornati (Adriatic Sea). Period. Biol. 1997, 99, 367–380. [Google Scholar]
- Templado, J.; Moreno, D. Nuevos datos sobre la distribución de Centrostephanus longispinus (Echinodermata: Echinoidea) en las costas españolas. Graellsia 1996, 52, 107–113. [Google Scholar] [CrossRef]
- Öztoprak, B.; Doğan, A.; Dagli, E. Checklist of Echinodermata from the coasts of Turkey. Turk. J. Zool. 2014, 38, 892–900. [Google Scholar] [CrossRef]
- Hernández, J.C.; Clemente, S.; Tuya, F.; Pérez-Ruzafa, A.; Sangil, C.; Moro-Abad, L.; Bacallado-Aránega, J.J. Echinoderms of the Canary Islands, Spain. In Echinoderm Research and Diversity in Latin America; Alvarado, J.J., Solis-Marin, F.A., Eds.; Springer: Berlin, Germany, 2013; pp. 471–510. [Google Scholar]
- Mironov, A.N. Echinoids from seamounts of the north-eastern Atlantic; onshore/offshore gradients in species distribution. In Biogeography of the North Atlantic Seamounts; Mironov, A.N., Gebruk, A.V., Southward, A.J., Eds.; KMK Scientific Press Ltd.: Moscow, Russia, 2006; pp. 96–133. [Google Scholar]
- Bernal-Ibáñez, A.; Cacabelos, E.; Melo, R.; Gestoso, I. The Role of Sea-Urchins in Marine Forests From Azores, Webbnesia, and Cabo Verde: Human Pressures, Climate-Change Effects and Restoration Opportunities. Front. Mar. Sci. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Anadón, R. Equinodermos recogidos durante la campaña “Altor VII” en las costas noroccidentales de Africa (noviembre 1975). Res. Esp. Cient. B/O Cornide 1977, 6, 165–168. [Google Scholar]
- Tortonese, E. Echinodermata. In Fauna d’Italia; Accademia Nazionale Italiana di Entomologia e Unione Zoologica Italiana, Ed.; Edizioni Calderini: Bologna, Italy, 1965; Volume 6, p. 422. [Google Scholar]
- Leonard, C.; Evans, J.; Knittweis, L.; Aguilar, R.; Alvarez, H.; Borg, J.A.; Garcia, S.; Schembri, P.J. Diversity, Distribution, and Habitat Associations of Deep-Water Echinoderms in the Central Mediterranean. Mar. Biodivers. 2020, 50, 69. [Google Scholar] [CrossRef]
- Moya-Urbano, E.; Urra Recuero, J.; Manjón-Cabeza, M.E.; Gallardo-Núñez, M.; Mateo-Ramírez, Á.; Farias Rapallo, C.; Ordinas Cerdá, F.; Moya, F.; Bárcenas Gascón, P.; García-Ruiz, C.; et al. Echinoderm Assemblages From Circalittoral and Bathyal Sedimentary Habitats of the Northern Alboran Sea (Western Mediterranean): Diversity, Distribution and Relationships with Environmental Variables. Thalassas 2025, 41, 1–28. [Google Scholar] [CrossRef]
- Lawrence, J.M. Sea Urchins: Biology and Ecology; Elsevier Science & Technology: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Regis, M.B. Adaptations morphofonctionnelles de la microstructure des radioles d’échinoides réguliers. Téthys 1981, 10, 177–184. [Google Scholar]
- Paul, O.; Boudouresque, C.F.; Robert, P. Présence de Centrostephanus longispinus (Echinoderme) dans l’herbier à Posidonia oceanica de l’Ile de Port-Cros. Etude des contenus digestifs. Trav. Sci. Parc. Natl. Port-Cros 1983, 9, 189–193. [Google Scholar]
- Barea-Azcón, J.M.; Ballesteros-Duperón, E.; Moreno, D. Libro Rojo de los Invertebrados de Andalucía; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2008. [Google Scholar]
- González-Irusta, J.; Punzón, A.; Serrano, A. Environmental and fisheries effects on Gracilechinus acutus (Echinodermata: Echinoidea) distribution: Is it a suitable bioindicator of trawling disturbance? ICES J. Mar. Sci. 2012, 69, 1457–1465. [Google Scholar] [CrossRef]
- Kurihara, H.; Shirayama, Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 2004, 274, 161–169. [Google Scholar] [CrossRef]
- La Mesa, G.; Zingaro, M.; Tunesi, L. Application of a national monitoring approach for an initial assessment of the current distribution of marine invertebrate protected by the Habitat Directive (92/43/EEC) along the Italian coasts. J. Mar. Biol. Assoc. UK 2025, 105, e38. [Google Scholar] [CrossRef]
- Poyales, F. Ficogeografía del mar de Alborán en el contexto del meditteráneo occidental. An. Jard. Bot. Madr. 1989, 46, 21–26. [Google Scholar]
- Templado, J.; Calvo, M.; Moreno, A.; Flores-Moya, A.; Conde, F.; Abad, R.; Rubio, J.; López-Fé, C.M.; Ortiz, M. Flora y Fauna de la Reserva Marina y Reserva de Pesca de la isla de Alborán; Secretaría General de Pesca Marítima; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2006. [Google Scholar]
- Templado, J.; Luque, Á.A.; Moreno, D.; Tierno de Figueroa, J.M.; Sánchez Tocino, L.; Aguilar, R.; de la Torriente, A. Invertebrates: The Realm of Diversity. In Alboran Sea—Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.-T., Camiñas, J.A., Malouli Idrissi, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 359–430. [Google Scholar]
- García Raso, J.E.; Gofas, S.; Salas, C.; Manjón-Cabeza, M.E.; Urra, J.; García Muñoz, E. El mar más rico de Europa: Biodiversidad del litoral occidental de Málaga entre Calaburras y Calahonda; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2010. [Google Scholar]
- Aguilar, R.; Akissou, M.; Templado, J.; Romani, M. Scientific rationale for the proposed CIESM Near Atlantic Marine Peace Park (zone 1). CIESM Workshop Moniographs 2011, 41, 43–49. [Google Scholar]
- Rueda, J.L.; Gofas, S.; Aguilar, R.; de la Torriente, A.; García Raso, J.E.; lo Iacono, C.; Luque, Á.A.; Marina, P.; Mateo-Ramírez, Á.; Moya-Urbano, E.; et al. Benthic Fauna of Littoral and Deep-Sea Habitats of the Alboran Sea: A Hotspot of Biodiversity. In Alboran Sea—Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.-T., Camiñas, J.A., Malouli Idrissi, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 285–358. [Google Scholar]
- Gofas, S. Marine Molluscs with a Very Restricted Range in the Strait of Gibraltar. Divers. Distrib. 1998, 4, 255–266. [Google Scholar]
- Ercilla, G.; Vázquez, J.-T.; Alonso, B.; Bárcenas, P.; Casas, D.; d’Acremont, E.; Estrada, F.; Fernández-Salas, L.M.; Galindo-Zaldívar, J.; Juan, C.; et al. Seafloor Morphology and Processes in the Alboran Sea. In Alboran Sea—Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.-T., Camiñas, J.A., Malouli Idrissi, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 157–205. [Google Scholar]
- Watts, A.B.; Piatt, J.P.; Buhl, P. Tectonic evolution of the Alboran Sea basin. Basin Res. 1993, 5, 153–177. [Google Scholar] [CrossRef]
- Maurizio, W.; Rovere, M. Atlas of the Mediterranean Seamounts and Seamount-Like Structures; IUCN: Gland, Switzerland, 2015. [Google Scholar]
- Pérez-Belzuz, F.; Alonso, B.; Ercilla, G. History of mud diapirism and trigger mechanisms in the Western Alboran Sea. Tectonophysics 1997, 282, 399–422. [Google Scholar] [CrossRef]
- Palomino, D.; López-González, N.; Vázquez, J.-T.; Fernández-Salas, L.-M.; Rueda, J.-L.; Sánchez-Leal, R.; Díaz-del-Río, V. Multidisciplinary study of mud volcanoes and diapirs and their relationship to seepages and bottom currents in the Gulf of Cádiz continental slope (northeastern sector). Mar. Geol. 2016, 378, 196–212. [Google Scholar] [CrossRef]
- Ercilla, G.; Baraza, J.; Alonso, B.; Estrada, F.; Casas, D.; Farran, M. The Ceuta Drift, Alboran Sea, southwestern Mediterranean. Geol. Soc. Lond. Mem. 2002, 22, 155–170. [Google Scholar] [CrossRef]
- Palomino, D.; Vazquez, J.-T.; Ercilla, G.; Alonso, B.; Lopez-Gonzalez, N.; Díaz-del-Río, V. Interaction between seabed morphology and water masses around the seamounts on the Motril Marginal Plateau (Alboran Sea, Western Mediterranean). Geo-Mar. Lett. 2011, 31, 465–479. [Google Scholar] [CrossRef]
- Mutti, E.; Normark, W.R. An Integrated Approach to the Study of Turbidite Systems. In Proceedings of the Seismic Facies and Sedimentary Processes of Submarine Fans and Turbidite Systems; Weimer, P., Link, M.H., Eds.; Springer: New York, NY, USA, 1991; pp. 75–106. [Google Scholar]
- Vargas-Yáñez, M.; Plaza, F.; García-Lafuente, J.; Sarhan, T.; Vargas, J.M.; Vélez-Belchi, P. About the seasonal variability of the Alboran Sea circulation. J. Mar. Syst. 2002, 35, 229–248. [Google Scholar] [CrossRef]
- Parrilla, G.; Kinder, T.H. Oceanografía física del mar de Alborán. Bol. Inst. Esp. Oceanogr. 1987, 4, 133–165. [Google Scholar]
- Cano, N.; Lafuente, J.G.; Hernández-Guerra, A.; Blanco, J.M.; Escánez, J. Hidrología del mar de Alborán. Publ. Espec. Inst. Esp. Oceanogr. 1993, 24, 9–26. [Google Scholar]
- Lanoix, F. Projet Alboran. Etude hydrologique et dynamique de la mer d’Alboran; Tech. Rept. 66; North Atlantic Treaty Organization: Brussels, Belgium, 1974. [Google Scholar]
- Gil, J. Consideraciones acerca de un fenómeno de afloramiento de la zona noroccidental del mar de Alborán. Inf. Téc. Inst. Esp. Oceanogr. 1985, 35, 1–11. [Google Scholar]
- Bryden, H.L.; Candela, J.; Kinder, T.H. Exchange through the Strait of Gibraltar. Prog. Oceanogr. 1994, 33, 201–248. [Google Scholar] [CrossRef]
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Ramírez, Á.; Marina, P.; Moreno, D.; Alcántara Valero, A.F.; Aguilar, R.; Báez, J.C.; Bárcenas, P.; Baro, J.; Caballero-Herrera, J.A.; Camiñas, J.A.; et al. Marine Protected Areas and Key Biodiversity Areas of the Alboran Sea and Adjacent Areas. In Alboran Sea—Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.-T., Camiñas, J.A., Malouli Idrissi, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 819–923. [Google Scholar]
- Gubbay, S. Marine protected areas—Past, present and future. In Marine Protected Areas: Principles and Techniques for Management; Gubbay, S., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 1–14. [Google Scholar]
- Roberts, C.M.; Bohnsack, J.A.; Gell, F.; Hawkins, J.P.; Goodridge, R. Effects of marine reserves on adjacent fisheries. Science 2001, 294, 1920–1923. [Google Scholar] [CrossRef] [PubMed]
- Gell, F.R.; Roberts, C.M. Benefits beyond boundaries: The fishery effects of marine reserves. Trends Ecol. Evol. 2003, 8, 448–455. [Google Scholar] [CrossRef]
- Council Directive 92/43/EEC. On the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities 1992, 206, 7–50. [Google Scholar]
- Ling, S.D.; Mahon, I.; Marzloff, M.P.; Pizarro, O.; Johnson, C.R.; Williams, S.B. Stereo-imaging AUV detects trends in sea urchin abundance on deep overgrazed reefs. Limnol. Oceanogr. Methods 2016, 14, 293–304. [Google Scholar] [CrossRef]
- Templado, J.; Ballesteros, E.; Galparsoro, I.; Borja, A.; Serrano, A. Guía Interpretativa. Inventario Español de Hábitats Marinos; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2012; p. 231. [Google Scholar]
- Montefalcone, M.; Tunesi, L.; Ouerghi, A. A review of the classification systems for marine benthic habitats and the new updated Barcelona Convention classification for the Mediterranean. Mar. Environ. Res. 2021, 169, 105387. [Google Scholar] [CrossRef]
- European Environment Agency. EUNIS Marine Habitat Classification Review 2022; European Environment Agency: Copenhagen, Denmark, 2022. [Google Scholar]
- Weber, W.; Dambach, M. Light-sensitivity of isolated pigment cells of the sea urchin Centrostephanus longispinus. Cell Tissue Res. 1974, 148, 437–440. [Google Scholar] [CrossRef]
- Gras, H.; Weber, W. Light-induced alterations in cell shape and pigment displacement in chromatophores of the sea urchin Centrostephanus longispinus. Cell Tissue Res. 1977, 182, 165–176. [Google Scholar] [CrossRef]
- Gras, H.; Weber, W. Spectral light sensitivity of isolated chromatophores of the sea urchin, Centrostephanus longispinus. Comp. Biochem. Physiol. A 1983, 76, 279–281. [Google Scholar] [CrossRef]
- Gras, H. Local light stimulation of isolated chromatophores of the sea urchin Centrostephanus longispinus. Eur. J. Cell Biol. 1981, 23, 258–266. [Google Scholar]
- Templado, J. Centrostephanus longispinus . In Los invertebrados no insectos de la “Directiva Hábitat” en España; Ramos, M.A., Bragado, D., Fernández, J., Eds.; Serie Técnica; Ministerio de Medio Ambiente: Madrid, Spain, 2001; pp. 177–186. [Google Scholar]
- RAC/SPA; UNEP-MAP. Alboran Island (Spain): SPAMI Site Report; Regional Activity Centre for Specially Protected Areas: Tunis, Tunisia, 2003; pp. 1–36. [Google Scholar]
- Koehler, R. Echinides du Musée Indien á Calcutta. III. In Echinides Réguliers. Echinoderma of the Indian Museum. Part X. Echinoidea (III); Indian Museum: Calcutta, India, 1927; Volume 10, pp. 1–158. [Google Scholar]
- Perkins, N.R.; Hill, N.A.; Foster, S.D.; Barrett, N.S. Altered niche of an ecologically significant urchin species, Centrostephanus rodgersii, in its extended range revealed using an Autonomous Underwater Vehicle. Estuar. Coast. Shelf Sci. 2015, 155, 56–65. [Google Scholar] [CrossRef]
- Puig, P.; Palanques, A.; Martín, J. Contemporary Sediment-Transport Processes in Submarine Canyons. Annu. Rev. Mar. Sci. 2014, 6, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Tubau, X.; Canals, M.; Lastras, G.; Rayo, X.; Rivera, J.; Amblas, D. Marine litter on the floor of deep submarine canyons of the Northwestern Mediterranean Sea: The role of hydrodynamic processes. Prog. Oceanogr. 2015, 134, 379–403. [Google Scholar] [CrossRef]
- Amaro, T.; Huvenne, V.A.I.; Allcock, A.L.; Aslam, T.; Davies, J.S.; Danovaro, R.; de Stigter, H.C.; Duineveld, G.C.A.; Gambi, C.; Gooday, A.J.; et al. The Whittard Canyon—A case study of submarine canyon processes. Prog. Oceanogr. 2016, 146, 38–57. [Google Scholar] [CrossRef]
- Fernandez-Arcaya, U.; Ramirez-Llodra, E.; Aguzzi, J.; Allcock, A.L.; Davies, J.S.; Dissanayake, A.; Harris, P.; Howell, K.; Huvenne, V.A.I.; Macmillan-Lawler, M.; et al. Ecological Role of Submarine Canyons and Need for Canyon Conservation: A Review. Front. Mar. Sci. 2017, 4, 5. [Google Scholar] [CrossRef]
- Byrne, M.; Andrew, N.L. Chapter 22—Centrostephanus rodgersii and Centrostephanus tenuispinus. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 43, pp. 379–396. [Google Scholar]
- Nelson, B.V.; Vance, R.R. Diel foraging patterns of the sea urchin Centrostephanus coronatus as a predator avoidance strategy. Mar. Biol. 1979, 51, 251–258. [Google Scholar] [CrossRef]
- Candela, J. The Gibraltar Strait and its role in the dynamics of the Mediterranean Sea. Dyn. Atmos. Oceans 1991, 15, 267–299. [Google Scholar] [CrossRef]
- Millot, C.; Taupier-Letage, I. Circulation in the Mediterranean Sea. In The Mediterranean Sea; Saliot, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 29–66. [Google Scholar]
- González-Duarte, M.M.; Megina, C.; Piraino, S.; Cervera, J.L. Hydroid assemblages across the Atlantic–Mediterranean boundary: Is the Strait of Gibraltar a marine ecotone? Mar. Ecol. 2013, 34, 33–40. [Google Scholar] [CrossRef]
- Sarhan, T.; García Lafuente, J.; Vargas, M.; Vargas, J.M.; Plaza, F. Upwelling mechanisms in the northwestern Alboran Sea. J. Mar. Syst. 2000, 23, 317–331. [Google Scholar] [CrossRef]
- Rivera, A.; Weidberg, N.; Pardiñas, A.F.; González-Gil, R.; García-Flórez, L.; Acuña, J.L. Role of upwelling on larval dispersal and productivity of gooseneck barnacle populations in the Cantabrian Sea: Management implications. PLoS ONE 2013, 8, e78482. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.E. Growth of filter-feeding benthic invertebrates from a region with variable upwelling intensity. Mar. Ecol. Prog. Ser. 2005, 295, 79–89. [Google Scholar] [CrossRef][Green Version]
- Pedrotti, M.; Fenaux, L. Distribution of echinoderm larval populations in the geostrophic frontal jet of the eastern Alboran Sea. Oceanol. Acta 1996, 19, 385–395. [Google Scholar][Green Version]
- Byrne, M.; Andrew, N. Chapter 17—Centrostephanus rodgersii. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38, pp. 243–256. [Google Scholar][Green Version]
- Kintzing, M.D.; Butler, M.J. The Influence of Shelter, Conspecifics, and Threat of Predation on the Behavior of the Long-Spined Sea Urchin (Diadema antillarum). J. Shellfish Res. 2014, 33, 781–785. [Google Scholar] [CrossRef]
- Vance, R.R.; Schmitt, R.J. The effect of the predator-avoidance behavior of the sea urchin, Centrostephanus coronatus, on the breadth of its diet. Oecologia 1979, 44, 21–25. [Google Scholar] [CrossRef]
- Jones, G.P.; Andrew, N.L. Herbivory and patch dynamics on rocky reefs in temperate Australasia: The roles of fish and sea urchins. Aust. J. Ecol. 1990, 15, 505–520. [Google Scholar] [CrossRef]
- Flukes, E.B.; Johnson, C.R.; Ling, S.D. Forming sea urchin barrens from the inside out: An alternative pattern of overgrazing. Mar. Ecol. Prog. Ser. 2012, 464, 179–194. [Google Scholar] [CrossRef]
- Smith, J.E.; Flukes, E.; Keane, J.P. The risky nightlife of undersized sea urchins. Mar. Freshw. Res. 2024, 75, 1–7. [Google Scholar] [CrossRef]
- Mayer, K. Grazing Impacts of the Sea Urchin Centrostephanus rodgersii on Rock Wall Habitats in Northeastern, Aotearoa New Zealand. Master’s Thesis, University of Auckland, Auckland, New Zealand, 2024. [Google Scholar]
- Boavida, J.; Assis, J.; Reed, J.; Serrão, E.A.; Gonçalves, J.M.S. Comparison of small remotely operated vehicles and diver-operated video of circalittoral benthos. Hydrobiologia 2016, 766, 247–260. [Google Scholar] [CrossRef]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. 2006, 44, 123–195. [Google Scholar]
- Sotelo-Casas, R.C.; Cupul-Magaña, A.L.; Rodríguez-Zaragoza, F.A.; Solís-Marín, F.A.; Rodríguez-Troncoso, A.P. Structural and environmental effects on an assemblage of echinoderms associated with a coral community. Mar. Biodivers. 2018, 48, 1401–1411. [Google Scholar] [CrossRef]
- Andrew, N.L. Survival of kelp adjacent to areas grazed by sea urchins in New South Wales, Australia. Aust. J. Ecol. 1994, 19, 466–472. [Google Scholar] [CrossRef]
- Parnell, P.E.; Fumo, J.T.; Lennert-Cody, C.E.; Schroeter, S.C.; Dayton, P.K. Sea Urchin Behavior in a Southern California Kelp Forest: Food, Fear, Behavioral Niches, and Scaling Up Individual Behavior. J. Shellfish Res. 2017, 36, 529–543. [Google Scholar] [CrossRef]
- Durán Muñoz, P.; Murillo, F.J.; Sayago-Gil, M.; Serrano, A.; Laporta, M.; Otero, I.; Gómez, C. Effects of deep-sea bottom longlining on the Hatton Bank fish communities and benthic ecosystem, north-east Atlantic. J. Mar. Biol. Assoc. UK 2011, 91, 939–952. [Google Scholar] [CrossRef]
- Rueda, J.L.; Mena-Torres, A.; Gallardo-Núñez, M.; González-García, E.; Martín-Arjona, A.; Valenzuela, J.; García-Ruiz, C.; González-Aguilar, M.; Mateo-Ramírez, Á.; García, M.; et al. Spatial Distribution and Potential Impact of Drifted Thalli of the Invasive Alga Rugulopteryx okamurae in Circalittoral and Bathyal Habitats of the Northern Strait of Gibraltar and the Alboran Sea. Diversity 2023, 15, 1206. [Google Scholar] [CrossRef]
- Roca, M.; Dunbar, M.B.; Román, A.; Caballero, I.; Zoffoli, M.L.; Gernez, P.; Navarro, G. Monitoring the marine invasive alien species Rugulopteryx okamurae using unmanned aerial vehicles and satellites. Front. Mar. Sci. 2022, 9, 1004012. [Google Scholar] [CrossRef]


| Directives, Conventions, and Conservation Lists | Annex/Category |
|---|---|
| Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora—consolidated version 01/01/2007 (EU Habitats Directive) | Annex IV |
| Convention on the conservation of European wildlife and natural habitats (Bern Convention) | Annex II |
| Barcelona Convention Protocol concerning Specially Protected Areas and Biological Diversity in the Mediterranean (SPA/BD Protocol) | Annex II |
| Listado de Especies Silvestres en Régimen de Protección Especial y del Catálogo Español de Especies Amenazadas * | Vulnerable |
| Libro Rojo de los Invertebrados de Andalucía * | Vulnerable |
| Catálogo Andaluz de Especies Amenazadas * | Species of special interest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, J.; González-García, E.; Mena-Torres, A.; Martín-Taboada, A.; Gallardo-Núñez, M.; García-Ledesma, A.; Barcenas, P.; Rueda, J.L.; Mateo-Ramírez, Á. Improving the Knowledge on the Distribution and Ecology of the Protected Echinoid Centrostephanus longispinus (Philippi, 1845) in the Alboran Sea. Diversity 2025, 17, 758. https://doi.org/10.3390/d17110758
Valenzuela J, González-García E, Mena-Torres A, Martín-Taboada A, Gallardo-Núñez M, García-Ledesma A, Barcenas P, Rueda JL, Mateo-Ramírez Á. Improving the Knowledge on the Distribution and Ecology of the Protected Echinoid Centrostephanus longispinus (Philippi, 1845) in the Alboran Sea. Diversity. 2025; 17(11):758. https://doi.org/10.3390/d17110758
Chicago/Turabian StyleValenzuela, Javier, Emilio González-García, Ana Mena-Torres, Adrián Martín-Taboada, Marina Gallardo-Núñez, Antonio García-Ledesma, Patricia Barcenas, José L. Rueda, and Ángel Mateo-Ramírez. 2025. "Improving the Knowledge on the Distribution and Ecology of the Protected Echinoid Centrostephanus longispinus (Philippi, 1845) in the Alboran Sea" Diversity 17, no. 11: 758. https://doi.org/10.3390/d17110758
APA StyleValenzuela, J., González-García, E., Mena-Torres, A., Martín-Taboada, A., Gallardo-Núñez, M., García-Ledesma, A., Barcenas, P., Rueda, J. L., & Mateo-Ramírez, Á. (2025). Improving the Knowledge on the Distribution and Ecology of the Protected Echinoid Centrostephanus longispinus (Philippi, 1845) in the Alboran Sea. Diversity, 17(11), 758. https://doi.org/10.3390/d17110758







