Indigenous Bacterial Endophytes as Sustainable Alternatives for Management of Green Mould Disease in Agaricus bisporus
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushrooms Strains and Composts
2.2. Green Mould Strain Used in This Study
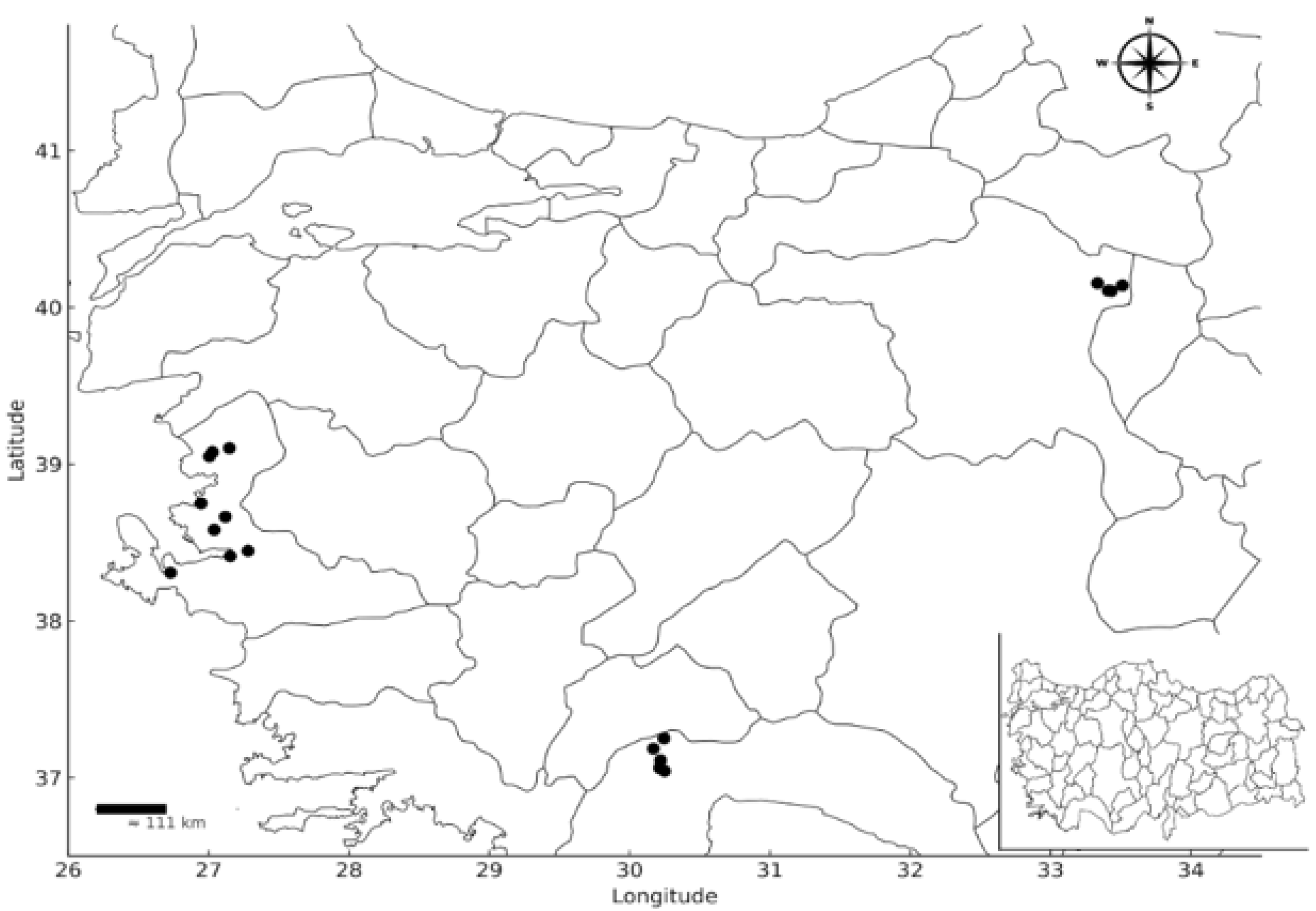
2.3. Isolation of Bacteria Used in This Study
2.4. Pre-Assessment Tests of Bacteria
2.5. Determination of Bacteria as Plant Growth-Promoting Aspects Under In Vitro Conditions
2.6. Determination of the Effects of Bacteria Against T. aggressivum f. aggressivum via In Vivo Bioassay
2.7. Assessment of the Efficacy of Bacteria Against T. aggressivum f. aggressivum Under Controlled Mushroom Cultivation Conditions
2.8. Molecular Identification of Bacterial Isolates
2.9. Statistical Analysis
3. Results
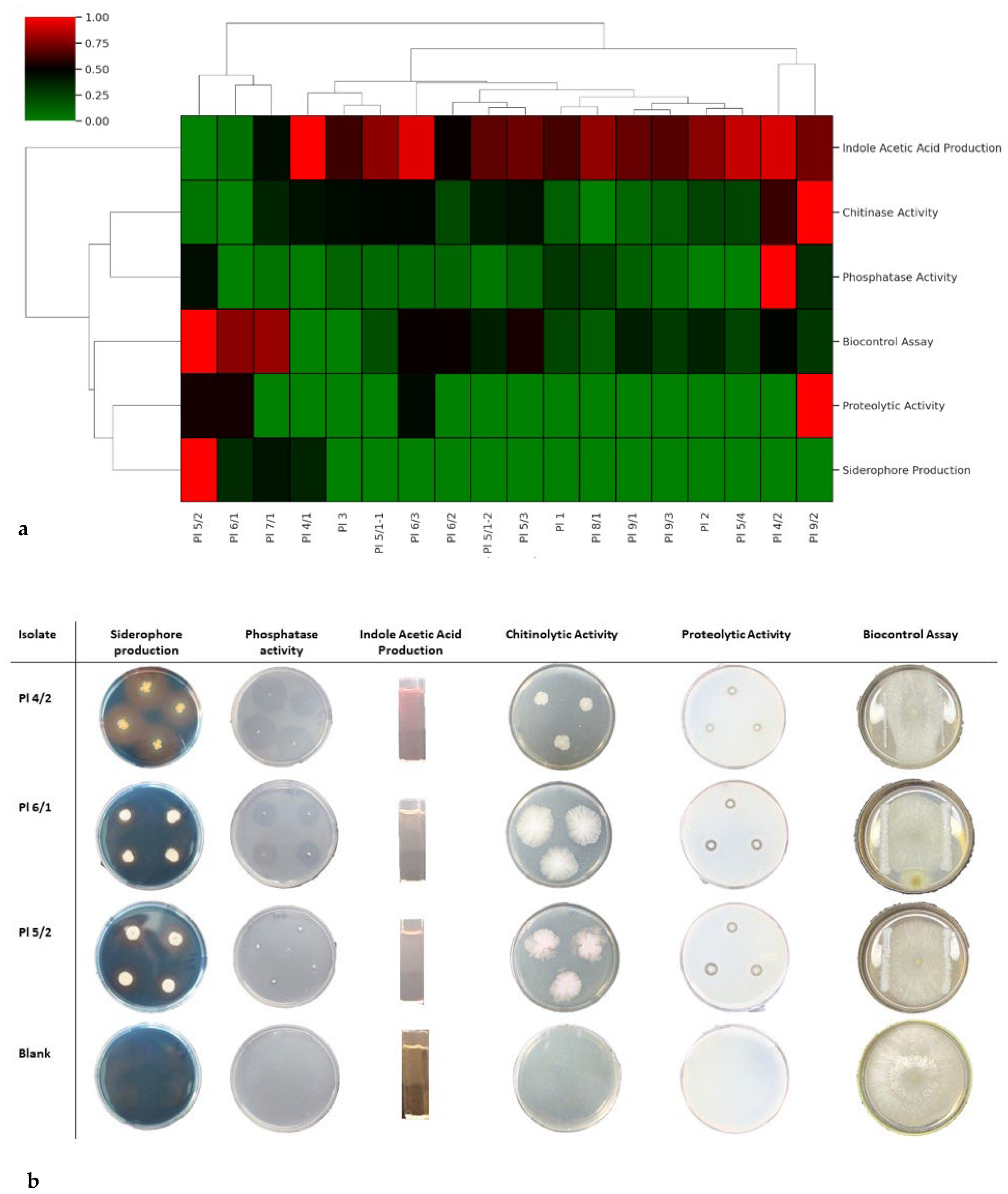
3.1. Results of In Vitro Assays
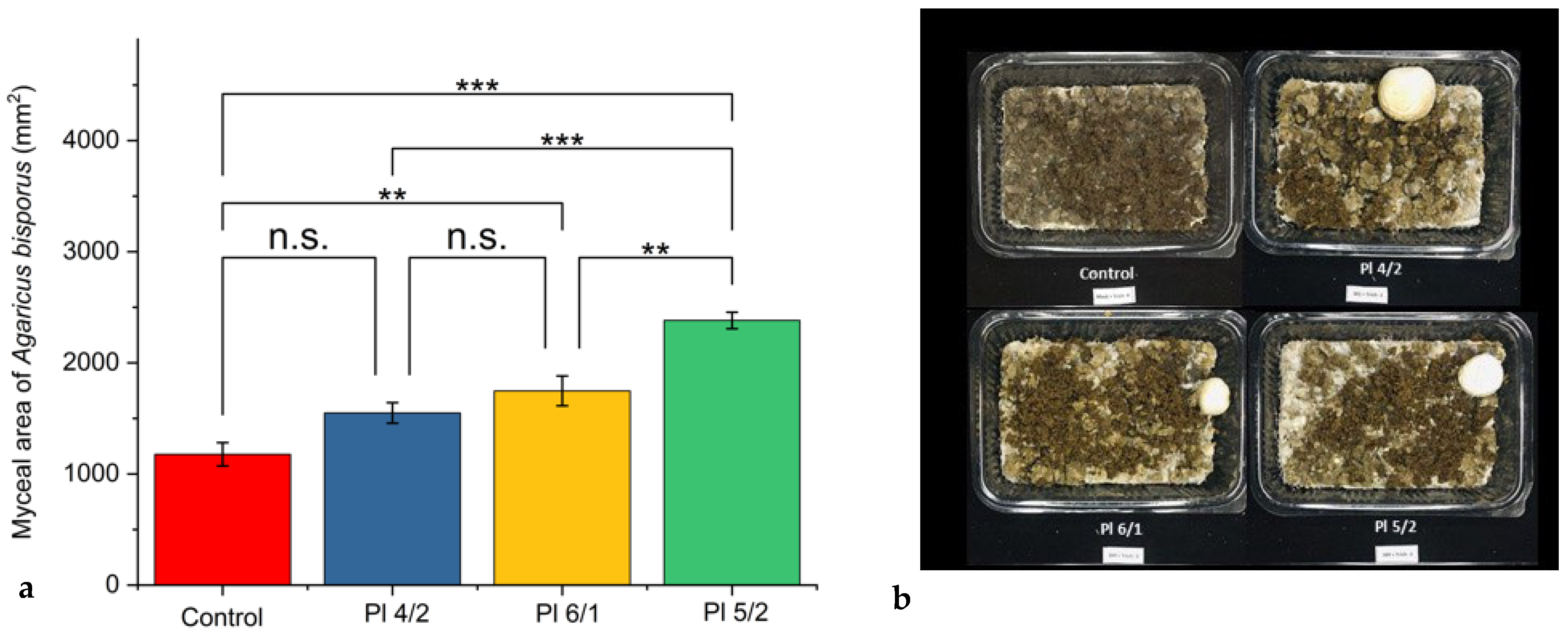
3.2. Results of In Vivo Assay
3.3. Results of Field Trials on Mushroom Cultivation Conditions
3.3.1. Results Related to the Effects on Disease Severity
3.3.2. Results Related to Their Impact on Yield
3.4. Results of Molecular Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BLAST | Basic Local Alignment Search Tool |
| CAS | Chrome Azurol S |
| cfu/mL | Colony forming unit per millilitre) |
| FAOSTAT | Food and Agriculture Organisation Statistical Database |
| HDTMA | Hexadecyltrimethylammonium bromide |
| IAA | Indole-3-acetic acid |
| NBRIP | National Botanical Research Institute’s phosphate solubilising medium |
| NCBI | National Centre for Biotechnology Information |
| OD600 | Optic density 600 nm |
| PCR | Polymerase chain reaction |
| PDA | Potato dextrose agar |
References
- Hamza, A.; Mylarapu, A.; Krishna, K.; Biotechnology, D.K. An Insight into the Nutritional and Medicinal Value of Edible Mushrooms: A Natural Treasury for Human Health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Mowna Sundari, T.; Alwin Prem Anand, A.; Jenifer, P.; Shenbagarathai, R. Bioprospection of Basidiomycetes and Molecular Phylogenetic Analysis Using Internal Transcribed Spacer (ITS) and 5.8 S RRNA Gene Sequence. Sci. Rep. 2018, 8, 10720. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Study and Characterization of Selected Nutrients in Wild Mushrooms from Portugal by Gas Chromatography and High Performance Liquid Chromatography. Microchem. J. 2009, 93, 195–199. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom Nutraceuticals for Improved Nutrition and Better Human Health: A Review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Huang, K.; El-Seedi, H.R.; Xu, B. Critical Review on Chemical Compositions and Health-Promoting Effects of Mushroom Agaricus blazei Murill. Curr. Res. Food Sci. 2022, 5, 2190–2203. [Google Scholar] [CrossRef]
- Ajith, T.A.; Janardhanan, K.K. Indian Medicinal Mushrooms as a Source of Antioxidant and Antitumor Agents. J. Clin. Biochem. Nutr. 2007, 40, 157–162. [Google Scholar] [CrossRef]
- Heng, H.H.Q.; Stevens, J.B.; Liu, G.; Bremer, S.W.; Ye, K.J.; Reddy, P.; Wu, G.S.; Wang, Y.A.; Tainsky, M.A.; Ye, C.J. Stochastic Cancer Progression Driven by Non-clonal Chromosome Aberrations. J. Cell. Physiol. 2006, 208, 461–472. [Google Scholar] [CrossRef]
- Singh, I.; Thakur, P. Impact of Fungi on the World Economy and Its Sustainability: Current Status and Potentials. In Fungal Resources for Sustainable Economy: Current Status and Future Perspectives; Springer: Singapore, 2023; pp. 3–37. ISBN 9789811991035. [Google Scholar]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms: Technology and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Baars, J.J.P.; Scholtmeijer, K.; Sonnenberg, A.S.M.; van Peer, A. Critical Factors Involved in Primordia Building in Agaricus bisporus: A Review. Molecules 2020, 25, 2984. [Google Scholar] [CrossRef]
- Anonymous Concerning the Marketing and Commercial Quality Control of Cultivated Mushrooms. Available online: https://www.unece.org/fileadmin/DAM/trade/agr/standard/standard/fresh/FFV-Std/English/24_CultivatedMushrooms.pdf (accessed on 1 May 2025).
- Muszynska, B.; Kala, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and Biological Properties of Agaricus bisporus Fruiting Bodies—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef]
- Eswari, S.; Bhavan, P.S.; Kalpana, R.; Dharani, C.; Manjula, T.; Sarumathi, K.; Rajkumar, G. Phytochemical Characterization of the Mushroom Agaricus bisporus and Assessment of Its Nutritional Ability in the Place of Fishmeal for Survival and Growth of the Freshwater Prawn Macrobrachium rosenbergii Post-Larvae. Integrative Food. Nutr. Metab. 2019, 6, 1–11. [Google Scholar]
- Tirta Ismaya, W.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules 2020, 25, 2368. [Google Scholar] [CrossRef] [PubMed]
- An, X.-Y.; Cheng, G.-H.; Gao, H.-X.; Li, X.-F.; Yang, Y.; Li, D.; Li, Y. Phylogenetic Analysis of Trichoderma Species Associated with Green Mold Disease on Mushrooms and Two New Pathogens on Ganoderma sichuanense. J. Fungi 2022, 8, 704. [Google Scholar] [CrossRef] [PubMed]
- Samuels, G.J.; Dodd, S.L.; Gams, W.; Castlebury, L.A.; Petrini, O. Trichoderma Species Associated with the Green Mold Epidemic of Commercially Grown Agaricus bisporus. Mycologia 2002, 94, 146–170. [Google Scholar] [CrossRef] [PubMed]
- Goltapeh, E.M.; Danesh, Y.R. Studies on Interaction between Trichoderma Species and Agaricus bisporus Mycelium. In Science and Cultivation of Edible Fungi, Proceedings of the 15th International Congress on the Science and Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; CRC Press: Boca Raton, FL, USA, 2000; pp. 661–666. [Google Scholar]
- Fletcher, J.T.; Gaze, R.H. Mushroom Pest and Disease Control: A Color Handbook; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 0123739845. [Google Scholar]
- Largeteau, M.L.; Savoie, J.-M. Microbially Induced Diseases of Agaricus bisporus: Biochemical Mechanisms and Impact on Commercial Mushroom Production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef]
- Savoie, J.-M.; Iapicco, R.; Largeteau-Mamoun, M.L. Factors Influencing the Competitive Saprophytic Ability of Trichoderma harzianum Th2 in Mushroom (Agaricus bisporus) Compost. Mycol. Res. 2001, 105, 1348–1356. [Google Scholar] [CrossRef]
- Guthrie, J.L.; Castle, A.J. Chitinase Production during Interaction of Trichoderma aggressivum and Agaricus bisporus. Can. J. Microbiol. 2006, 52, 961–967. [Google Scholar] [CrossRef]
- Rinker, D.L.; Alm, G. Management of Green Mould Disease in Canada. In Science and Cultivation of Edible Fungi: Proceedings of the 15th International Congress on the Science and Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; A. A. Balkema Publisher: Rotterdam, The Netherlands, 2000; Volume 15, p. 617. [Google Scholar]
- Romaine, C.P.; Royse, D.J.; Schlagnhaufer, C. Emergence of Benzimidazole-Resistant Green Mould, Trichoderma aggressivum, on Cultivated Agaricus bisporus in North America. Mushroom Sci. 2008, 17, 510–523. [Google Scholar]
- Stanojević, O.; Milijašević-Marčić, S.; Potočnik, I.; Stepanović, M.; Dimkić, I.; Stanković, S.; Berić, T. Isolation and Identification of Bacillus spp. from Compost Material, Compost and Mushroom Casing Soil Active against Trichoderma spp. Arch. Biol. Sci. 2016, 68, 845–852. [Google Scholar]
- Saubenova, M.; Oleinikova, Y.; Sadanov, A.; Yermekbay, Z.; Bokenov, D.; Shorabaev, Y. The Input of Microorganisms to the Cultivation of Mushrooms on Lignocellulosic Waste. AIMS Agric. Food 2023, 8, 239–277. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Bahmani, K.; Hasanzadeh, N.; Harighi, B.; Marefat, A. Isolation and Identification of Endophytic Bacteria from Potato Tissues and Their Effects as Biological Control Agents against Bacterial Wilt. Physiol. Mol. Plant Pathol. 2021, 116, 101692. [Google Scholar] [CrossRef]
- Suslow, T.V.; Schroth, M.N.; Isaka, M. Application of a Rapid Method for Gram Differentiation of Plant Pathogenic and Saprophytic Bacteria Without Staining. 1982. Available online: https://www.apsnet.org/publications/phytopathology/backissues/Documents/1982Articles/Phyto72n07 (accessed on 1 May 2025).
- Schaad, N.W. Laboratory Guide for Identification of Plant Pathogenic Bacteria; Amer Phytopathological Society: Saint Paul, MN, USA, 1981; ISBN 0890540284. [Google Scholar]
- Sun, Y.; Wu, J.; Shang, X.; Xue, L.; Ji, G.; Chang, S.; Niu, J.; Emaneghemi, B. Screening of Siderophore-Producing Bacteria and Their Effects on Promoting the Growth of Plants. Curr. Microbiol. 2022, 79, 150. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Janati, W.; Mikou, K.; El Ghadraoui, L.; Errachidi, F. Isolation and Characterization of Phosphate Solubilizing Bacteria Naturally Colonizing Legumes Rhizosphere in Morocco. Front. Microbiol. 2022, 13, 958300. [Google Scholar] [CrossRef]
- Rangel, F.; Santos, R.A.; Monteiro, M.; Lavrador, A.S.; Gasco, L.; Gai, F.; Oliva-Teles, A.; Enes, P.; Serra, C.R. Isolation of Chitinolytic Bacteria from European Sea Bass Gut Microbiota Fed Diets with Distinct Insect Meals. Biology 2022, 11, 964. [Google Scholar] [CrossRef]
- Chung, D.; Yu, W.J.; Lim, J.Y.; Kang, N.S.; Kwon, Y.M.; Choi, G.; Bae, S.S.; Cho, K.; Lee, D.S. Characterization of the Proteolytic Activity of a Halophilic Aspergillus reticulatus Strain Sk1-1 Isolated from a Solar Saltern. Microorganisms 2022, 10, 29. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Bacterial Indole-3-Acetic Acid Influences Soil Nitrogen Acquisition in Barley and Chickpea. Plants 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Aydoğdu, M.; Sülü, S.M.; Kurbetli, İ.; Sülü, G. In vitro and in vivo Biological Control of the Green Mold Using Different Bacteria in Button Mushroom Cultivation. Egypt. J. Biol. Pest Control 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Babier, Y.; Akköprü, A. Çeşitli Kültür Bitkilerinden İzole Edilen Endofitik Bakterilerin Karakterizasyonu ve Bitki Patojeni Bakterilere Karşı Antagonistik Etkilerinin Belirlenmesi. Yuz. Yıl Univ. J. Agric. Sci. 2020, 30, 521–534. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Cetin, M.; Atila, F.; Eren, E. Valorization of Olive Press Cake as a Sustainable Alternative to Peat in White Button Mushroom (Agaricus bisporus) Cultivation. Biomass Convers. Biorefinery 2025, 15, 20589–20599. [Google Scholar] [CrossRef]
- Roberti, R.; Di Francesco, A.; Innocenti, G.; Mari, M. Potential for Biocontrol of Pleurotus ostreatus Green Mould Disease by Aureobasidium pullulans De Bary (Arnaud). Biol. Control 2019, 135, 9–15. [Google Scholar] [CrossRef]
- Townsend, G.R.; Heuberger, J.W.; Townsend, G.; Heuberger, J. Methods for Estimating Losses Caused by Diseases in Fungicidal Experiments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.C.; Navarro, M.J. Efficacy and Effects on Yield of Different Fungicides for Control of Wet Bubble Disease of Mushroom Caused by the Mycoparasite Mycogone perniciosa. Crop Prot. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Kadaikunnan, S.; Rejiniemon, S.S.; Khaled, J.M.; Alharbi, N.S.; Mothana, R. In vitro Antibacterial, Antifungal, Antioxidant and Functional Properties of Bacillus amyloliquefaciens. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 1–11. [Google Scholar] [CrossRef]
- De Mendiburu, F.; Simon, R. Agricolae—Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology. PeerJ 2015, 3, 1–18. [Google Scholar] [CrossRef]
- Hayat, R.; Ahmed, I.; Sheirdil, R.A. An Overview of Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture. In Crop Production for Agricultural Improvement; Springer: Dordrecht, The Netherlands, 2012; Volume 9789400741, pp. 557–579. ISBN 9789400741164. [Google Scholar]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric Organic Compounds in the Soil-Microorganism-Plant System: Their Role in Iron Availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Ajit, N.S.; Verma, R.; Shanmugam, V. Extracellular Chitinases of Fluorescent Pseudomonads Antifungal to Fusarium oxysporum f. sp. dianthi Causing Carnation Wilt. Curr. Microbiol. 2006, 52, 310–316. [Google Scholar] [CrossRef]
- Malik, M.S.; Haider, S.; Rehman, A.; Rehman, S.U.; Jamil, M.; Naz, I.; Anees, M. Biological Control of Fungal Pathogens of Tomato (Lycopersicon esculentum) by Chitinolytic Bacterial Strains. J. Basic Microbiol. 2022, 62, 48–62. [Google Scholar] [CrossRef]
- Luan, P.; Yi, Y.; Huang, Y.; Cui, L.; Hou, Z.; Zhu, L.; Ren, X.; Jia, S.; Liu, Y. Biocontrol Potential and Action Mechanism of Bacillus amyloliquefaciens DB2 on Bipolaris sorokiniana. Front. Microbiol. 2023, 14, 1149363. [Google Scholar] [CrossRef]
- Jabiri, S.; Legrifi, I.; Benhammou, M.; Laasli, S.E.; Mokrini, F.; Amraoui, M.B.; Lahlali, R. Screening of Rhizobacterial Isolates from Apple Rhizosphere for Their Biocontrol and Plant Growth Promotion Activity. Appl. Microbiol. 2023, 3, 948–967. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Raio, A.; Puopolo, G. Pseudomonas chlororaphis Metabolites as Biocontrol Promoters of Plant Health and Improved Crop Yield. World J. Microbiol. Biotechnol. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Yu, J.M.; Wang, D.; Pierson, L.S.; Pierson, E.A. Effect of Producing Different Phenazines on Bacterial Fitness and Biological Control in Pseudomonas chlororaphis 30–84. Plant Pathol. J. 2018, 34, 44–58. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Z.; Shao, J.; Xu, Z.; Liu, Y.; Xun, W.; Miao, Y.; Shen, Q.; Zhang, R. Biocontrol Mechanisms of Bacillus: Improving the Efficiency of Green Agriculture. Microb. Biotechnol. 2023, 16, 2250–2263. [Google Scholar] [CrossRef] [PubMed]
- Duke, K.A.; Becker, M.G.; Girard, I.J.; Millar, J.L.; Dilantha Fernando, W.G.; Belmonte, M.F.; de Kievit, T.R. The Biocontrol Agent Pseudomonas chlororaphis PA23 Primes Brassica napus Defenses through Distinct Gene Networks. BMC Genom. 2017, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, E.; Tienda, S.; Vida, C.; De Vicente, A.; Cazorla, F.M. Fitness Features Involved in the Biocontrol Interaction of Pseudomonas chlororaphis with Host Plants: The Case Study of PcPCL1606. Front. Microbiol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Tienda, S.; Vida, C.; Lagendijk, E.; de Weert, S.; Linares, I.; González-Fernández, J.; Guirado, E.; de Vicente, A.; Cazorla, F.M. Soil Application of a Formulated Biocontrol Rhizobacterium, Pseudomonas chlororaphis PCL1606, Induces Soil Suppressiveness by Impacting Specific Microbial Communities. Front. Microbiol. 2020, 11, 1874. [Google Scholar] [CrossRef]
- Liu, F.; Yang, S.; Xu, F.; Zhang, Z.; Lu, Y.; Zhang, J.; Wang, G. Characteristics of Biological Control and Mechanisms of Pseudomonas chlororaphis Zm-1 against Peanut Stem Rot. BMC Microbiol. 2022, 22, 9. [Google Scholar] [CrossRef]
- Nicolás Lazarte, J.; Lopez, R.P.; Daniel Ghiringhelli, P.; Berón, C.M. Bacillus wiedmannii biovar thuringiensis: A Specialized Mosquitocidal Pathogen with Plasmids from Diverse Origins. Genome Biol. Evol. 2018, 10, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Danial, A.W.; Hamdy, S.M.; Alrumman, S.A.; Gad El-Rab, S.M.F.; Shoreit, A.A.M.; Hesham, A.E.L. Bioplastic Production by Bacillus wiedmannii As-02 Ok576278 Using Different Agricultural Wastes. Microorganisms 2021, 9, 2395. [Google Scholar] [CrossRef] [PubMed]
- Boulahouat, S.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Biocontrol Efficiency of Rhizospheric Bacillus against the Plant Pathogen Fusarium oxysporum: A Promising Approach for Sustainable Agriculture. Microbiol. Res. 2023, 14, 892–908. [Google Scholar] [CrossRef]
- Lalloo, R.; Moonsamy, G.; Ramchuran, S.; Görgens, J.; Gardiner, N. Competitive Exclusion as a Mode of Action of a Novel Bacillus cereus Aquaculture Biological Agent. Lett. Appl. Microbiol. 2010, 50, 563–570. [Google Scholar] [CrossRef]
- Gao, T.; Foulston, L.; Chai, Y.; Wang, Q.; Losick, R. Alternative Modes of Biofilm Formation by Plant-Associated Bacillus cereus. MicrobiologyOpen 2015, 4, 452–464. [Google Scholar] [CrossRef]
- Huang, C.J.; Wang, T.K.; Chung, S.C.; Chen, C.Y. Identification of an Antifungal Chitinase from a Potential Biocontrol Agent, Bacillus cereus 28-9. J. Biochem. Mol. Biol. 2005, 38, 82–88. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Zhu, K.; Hou, S.; Chen, L.; Mi, D.; Gui, Y.; Qi, Y.; Jiang, C.; Guo, J.H. Plant Root Exudates Are Involved in Bacillus cereus AR156 Mediated Biocontrol against Ralstonia solanacearum. Front. Microbiol. 2019, 10, 98. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Z.H.; Zu, X.; Yu, X.Y.; Zhu, H.J.; Li, X.J.; Zhong, J.; Liu, E.M. Efficacy of Plant Growth-Promoting Bacteria Bacillus cereus YN917 for Biocontrol of Rice Blast. Front. Microbiol. 2021, 12, 684888. [Google Scholar] [CrossRef]
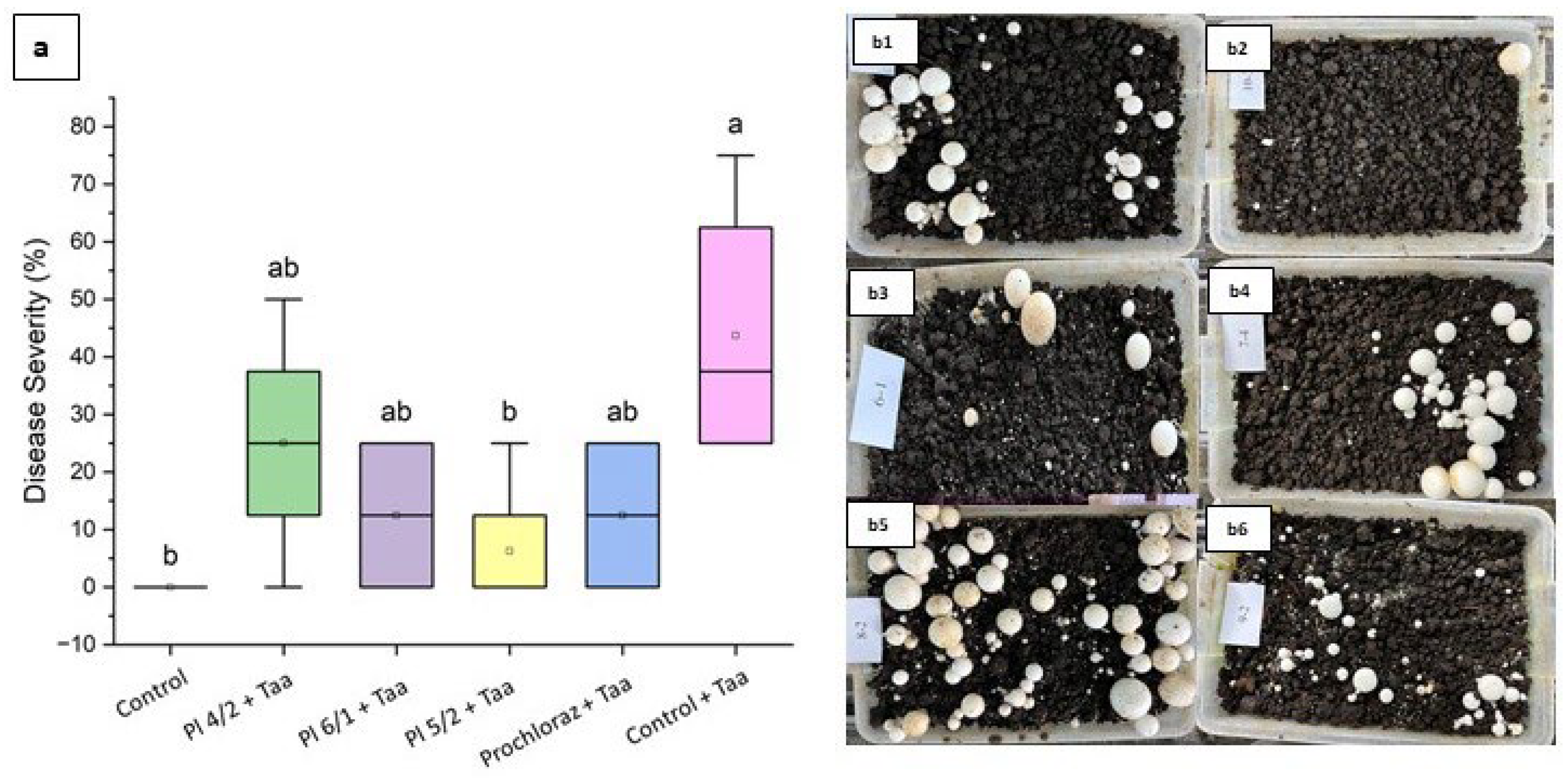
| Treatment Number | Description |
|---|---|
| 1 | Application of Pl 4/2 |
| 2 | Application of Pl 6/1 |
| 3 | Application of Pl 5/2 |
| 4 | Application of Prochloraz |
| 5 | Application of Control Treatment |
| 6 | Application of Pl 4/2 + T. aggressivum f. aggressivum |
| 7 | Application of Pl 6/1 + T. aggressivum f. aggressivum |
| 8 | Application of Pl 5/2 + T. aggressivum f. aggressivum |
| 9 | Application of Prochloraz + T. aggressivum f. aggressivum |
| 10 | Application of Control Treatment + T. aggresivum f. aggressivum |
| No. | Strain Code | Location | In Vitro Assay | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochemical Test | PGP Test | ||||||||||
| Gr 1 | Pec 2 | HR 3 | Sid 4 | PhA 5 | IAA 6 | CA 7 | PA 8 | BioCo 9 | |||
| 377 | Pl 1 | Antalya | − | − | − | 6.75 | 10.25 | 46.67 | 0 | 0.56 | 0 |
| 378 | Pl 2 | Antalya | − | − | − | 3 | 12.38 | 86.67 | 0 | 0.89 | 0 |
| 379 | Pl 3 | Antalya | − | − | − | 4.5 | 10 | 165.33 | 0 | 0 | 0 |
| 380 | Pl 4/1 | Antalya | + | − | − | 3.25 | 16.13 | 158 | 0 | 0 | 0 |
| 381 | Pl 4/2 | Antalya | − | − | − | 15.88 | 14.88 | 220.67 | 0 | 1.16 | 0.5 |
| 384 | Pl 6/1 | İzmir | + | − | − | 3 | 1 | 0 | 1.41 | 1.85 | 2.1 |
| 385 | Pl 6/2 | İzmir | − | − | − | 4.38 | 8.38 | 78 | 0 | 1.25 | 2.05 |
| 386 | Pl 6/3 | İzmir | − | − | − | 4.25 | 15.13 | 173.33 | 1.22 | 1.24 | 0.41 |
| 387 | Pl 5/1-1 | İzmir | − | − | − | 4.13 | 12.50 | 172 | 0 | 0.47 | 0 |
| 388 | Pl 5/1-2 | İzmir | − | − | − | 3.5 | 11 | 148.67 | 0 | 0.91 | 1.88 |
| 389 | Pl 5/2 | İzmir | + | − | − | 8.88 | 0 | 18 | 1.42 | 2.40 | 1.09 |
| 390 | Pl 5/3 | İzmir | − | − | − | 4.5 | 11.5 | 160 | 0 | 1.32 | 1.08 |
| 391 | Pl 5/4 | İzmir | − | − | − | 3 | 14.25 | 85.33 | 0 | 0.59 | 1.31 |
| 392 | Pl 7/1 | İzmir | + | − | − | 3.75 | 7.25 | 130 | 0 | 1.91 | 0 |
| 393 | Pl 8/1 | Ankara | − | − | − | 6.25 | 12.63 | 0 | 0 | 0.35 | 0.15 |
| 394 | Pl 9/1 | Ankara | − | − | − | 4.75 | 11.25 | 36.67 | 0 | 0.93 | 0 |
| 395 | Pl 9/2 | Ankara | − | − | − | 7.25 | 11.75 | 361.33 | 2.63 | 0.68 | 1.63 |
| 396 | Pl 9/3 | Ankara | − | − | − | 4 | 10.75 | 53 | 0 | 0.66 | 1.28 |
| Treatment | Number of Fruiting Bodies of Mushrooms | |||
|---|---|---|---|---|
| Piece | Weight of Mushrooms | |||
| g | E% | g | E% | |
| Pl 4/2 | 29.25 ± 22.86 a * | 24.47 | 473.25 ± 411.31 ab * | 16.78 |
| Pl 6/1 | 37.75 ± 8.99 a | 60.64 | 598.50 ± 186.87 ab | 47.69 |
| Pl 5/2 | 72.50 ± 26.91 a | 208.51 | 1268.00 ± 560.35 a | 212.89 |
| Prochloraz | 37.00 ± 25.91 a | 57.45 | 584.50 ± 388.66 ab | 44.23 |
| Control | 28.00 ± 4.76 a | 19.15 | 273.50 ± 76.30 b | −32.51 |
| Pl 4/2 + Taa | 33.25 ± 24.44 a | 41.49 | 354.50 ± 198.88 ab | −12.52 |
| Pl 6/1 + Taa | 32.50 ± 24.79 a | 38.30 | 485.50 ± 559.81 ab | 19.80 |
| Pl 5/2 + Taa | 57.00 ± 22.19 a | 142.55 | 854.50 ± 389.74 ab | 110.86 |
| Prochloraz + Taa | 44.00 ± 17.49 a | 87.23 | 675.50 ± 507.92 ab | 66.69 |
| Control + Taa | 23.50 ± 15.15 a | - | 405.25 ± 246.64 ab | - |
| No. | Strain Code | Bacteria Species | Reference Similarity Rate | Reference Access No. | NCBI Access No. |
|---|---|---|---|---|---|
| 381 | Pl 4/2 | Pseudomonas chlororaphis | 98.02 | CP027753 | PX069530 |
| 384 | Pl 6/1 | Bacillus wiedmannii | 98.26 | PV052600 | PX069532 |
| 389 | Pl 5/2 | Bacillus cereus | 98.01 | JX847613 | PX069538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şanver, U.; Ҫetin, M.; Güneş, N.; Atila, F.; Eren, E.; Özaktan, H. Indigenous Bacterial Endophytes as Sustainable Alternatives for Management of Green Mould Disease in Agaricus bisporus. Diversity 2025, 17, 757. https://doi.org/10.3390/d17110757
Şanver U, Ҫetin M, Güneş N, Atila F, Eren E, Özaktan H. Indigenous Bacterial Endophytes as Sustainable Alternatives for Management of Green Mould Disease in Agaricus bisporus. Diversity. 2025; 17(11):757. https://doi.org/10.3390/d17110757
Chicago/Turabian StyleŞanver, Utku, Mehmet Ҫetin, Nihan Güneş, Funda Atila, Erkan Eren, and Hatice Özaktan. 2025. "Indigenous Bacterial Endophytes as Sustainable Alternatives for Management of Green Mould Disease in Agaricus bisporus" Diversity 17, no. 11: 757. https://doi.org/10.3390/d17110757
APA StyleŞanver, U., Ҫetin, M., Güneş, N., Atila, F., Eren, E., & Özaktan, H. (2025). Indigenous Bacterial Endophytes as Sustainable Alternatives for Management of Green Mould Disease in Agaricus bisporus. Diversity, 17(11), 757. https://doi.org/10.3390/d17110757






