Abstract
Ecological restoration of mine wastelands is central to biodiversity conservation and ecosystem recovery worldwide. However, the long-term ecological consequences of active restoration versus natural regeneration remain debated, particularly in mountainous karst landscapes. Using a space-for-time substitution, we established a five-stage chronosequence—recently abandoned, 10 years, 20 years, 30 years, and a late-successional forest (>35 years)—in a typical underground coal-mine wasteland in eastern Yunnan, southwest China. Each age class contained paired active restoration and natural regeneration sites; the late-successional forest served as a reference. We surveyed nested vegetation plots (20 × 20 m with shrub and herb subplots) in summer and autumn, recorded vertebrate species with camera traps, and quantified α-diversity (species richness, Shannon–Wiener diversity, Simpson’s diversity, Pielou’s evenness) and β-diversity (Bray–Curtis dissimilarity, non-metric multidimensional scaling). Overall plant α-diversity was highest in natural regeneration and lowest in active restoration, whereas tree-layer diversity was highest in active restoration and shrub and herb layers were richer under natural regeneration. Preliminary data from our camera traps suggested that animal species richness ranked late-successional forest > natural regeneration > active restoration, but evenness peaked in active restoration, suggesting early-stage homogenization. Plant β-diversity indicated stronger compositional divergence among active restoration sites and greater similarity between natural regeneration and the reference forest; both modes converged toward the reference forest over time but followed distinct patterns. These findings suggest that active restoration accelerates structural development yet increases between-site heterogeneity, whereas natural regeneration maintains higher overall diversity and compositional similarity to reference communities. Our results provide preliminary empirical guidance for selecting restoration strategies in similar karst coal-mine landscapes.
1. Introduction
Coal mining has long supported global economic development and energy security but has also produced extensive ecological debt. Worldwide, more than 6.7 million hectares of coal-mine wastelands require remediation, with nearly one quarter located in China [1]. Mining activities remove vegetation, cause acid mine drainage and heavy metal pollution, and generate subsidence and spoil heaps that fragment habitats and reduce soil fauna and carbon sequestration [2,3]. Consequently, coal-mine restoration has become a critical nexus for achieving carbon neutrality and biodiversity conservation, and it is a core task within the post-2020 Global Biodiversity Framework [4].
Restoration trajectories in mined landscapes essentially represent progressive succession [5,6], with biodiversity serving as the key indicator for tracking that succession [3,7]. However, most studies emphasize short-term (<15 years) plant-community dynamics, and systematic assessments spanning ≥ 20 years remain rare [8,9,10,11]. Critically, within-region comparisons between active restoration and natural regeneration seldom include long time series, compromising temporal evaluations of mode-specific effectiveness [12]. Moreover, the broad movement ranges of animals can confound local-scale diversity assessments [13], which has led to an underrepresentation of animal monitoring in related studies [14] and limited our ability to disentangle how animal communities couple with vegetation succession in vulnerable ecosystems. We contend that spatial variation in animal diversity better captures animals’ habitat-use preferences across microhabitats [15].
Southwestern China lies at the intersection of several global biodiversity hotspots, harboring exceptionally high biodiversity while facing substantial environmental pressures [16]. In particular, the karst mountains of eastern Yunnan have undergone intensive underground coal mining, leaving a legacy of disturbance [17]. Following industrial upgrading and transition, abandoned coal-mine wastelands continue to influence the region’s ecological security and sustainability agenda [18,19]. However, research on China’s coal-mine wastelands has focused largely on the contiguous, large-scale open-pit mines in the north [20,21], whereas the scattered underground mines in eastern Yunnan’s karst terrain have received far less attention; existing work there concentrates on surface deformation–geohazards and water-quality risk monitoring and process identification [22,23,24]. This imbalance hampers restoration and sustainable-development planning for a typical ecologically fragile zone. There is an urgent need to move beyond a narrow focus on pollution control/tailings management toward long-term comparative studies aligned with community- and biodiversity-succession processes and to embed biodiversity into mine restoration standards and performance evaluation [18].
The aim of this study is to compare the long-term ecological outcomes of active restoration versus natural regeneration in underground coal-mine wastelands of karst landscapes, using plant and animal diversity as indicators of recovery success. Specifically, our objectives are to: (1) quantify how α- and β-diversity of plants and animals develop across a 35-year chronosequence under contrasting restoration modes; (2) identify mode-specific successional patterns and convergence patterns toward reference forest conditions; and (3) provide evidence-based recommendations for cost-effective restoration strategies in similar karst mining landscapes. Using the core mine area closed in 2020 as the anchor, we established a space-for-time chronosequence radiating to adjacent mines abandoned 10, 20, and 30 years earlier. Each age class comprised paired active restoration and natural regeneration sites, complemented by a well-preserved reference forest (late-successional, >35 years) along the landscape margin.
2. Materials and Methods
2.1. Study Area
We selected the Xiongbi underground coal-mine wasteland in Shizong County, Qujing City, Yunnan Province, as the study area. The mine ceased all operations in June 2020; prior to the final closure, some pits had been intermittently shut down at different times in response to national energy-transition policies, and active restoration (seedling planting) had been implemented in parts of the decommissioned areas. This mosaic provided an ideal setting for a space-for-time chronosequence to compare biodiversity succession under contrasting restoration modes [11]. Active restoration involved planting tree seedlings—primarily Pinus armandii with some Juniperus chinensis—at approximately 2 m spacing to balance restoration costs and seedling survival rates. After initial planting in active restoration areas and following mine closure in natural regeneration areas, no further management interventions were implemented beyond restricting human disturbance. The study area is situated in one of China’s 61 key coal-producing regions and is characterized by mountainous karst terrain. Soils are predominantly red soils and sandy loams. Based on meteorological data from Qujing Meteorological Bureau (2022–2024), the area has a mean annual temperature of 14.5 °C and mean annual precipitation of 883.3 mm, with a distinct monsoon pattern: the wet season (May–October) accounts for 767.5 mm, while the dry season (November–April) receives 115.8 mm. Mean wind speed is 2.0 m/s, with prevailing southerly winds. All sampling plots were located at elevations between 2050 and 2160 m on south-facing slopes with gradients of 6–15°. The reference forest, dominated by native pines (Pinus armandii, P. yunnanensis) and diverse understory vegetation, represents the typical late-successional community for this elevation and aspect in karst mountain ecosystems of the region.
From 12 to 23 September 2023, we worked with five local guides familiar with the site’s environmental history—one former principal manager (59 years old), two former mine workers (55 and 57), and two community residents (42 and 48)—to delineate plots differing in restoration mode and time since closure. A plot was included only if at least two guides agreed on its closure time within ±3 years; otherwise, it was excluded from the survey plan. We ultimately identified five time classes: a recently restored class (2020), ~10 years (around 2014), ~20 years (around 2002), ~30 years (around 1994), and a reference forest (>35 years)—confirmed through field surveys and interviews to have remained undisturbed for at least 35 years, though precise dating beyond this threshold was not possible; this forest represents the late-successional climax stage for the study region (Figure 1). In all active restoration plots, tree seedlings had been planted at approximately 2 m spacing at the time of each pit’s closure, following standard local reforestation practices to balance cost and seedling survival.
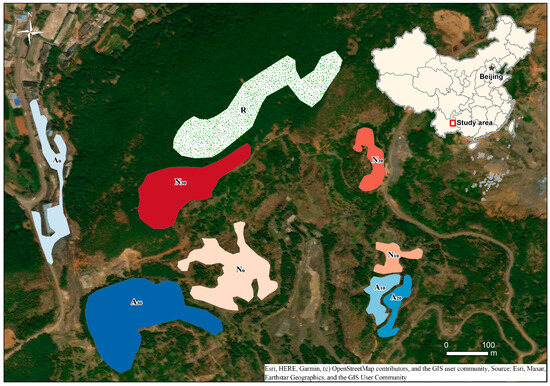
Figure 1.
Study area and sampling sites. Site codes combine restoration mode and time since closure: A = active restoration, N = natural regeneration, R = reference forest (>35 years); 0, 10, 20, 30 denote recent, ~10 years, ~20 years, and ~30 years, respectively (e.g., A10 = active restoration for ~10 years). Sites are located in the Xiongbi underground coal-mine wasteland (Shizong County, Qujing, Yunnan, China).
2.2. Survey Design and Field Sampling
We delineated five time classes: a recently restored class (2020), ~10 years (around 2014), ~20 years (around 2002), ~30 years (around 1994), and a reference forest (>35 years, representing the late-successional stage). For each time class except the reference forest, we established paired plots representing active restoration and natural regeneration. This yielded nine sites in total across restoration mode × time combinations.
From November 2023 to February 2025, we installed one infrared camera per site to survey animal diversity. We acknowledge that this single-camera-per-site design provides a preliminary, point-based assessment of species presence and activity rather than a spatially replicated measure of community diversity, and thus the resulting animal data should be interpreted with caution. Cameras (Ltl Acorn 6210) were configured to record three consecutive photographs plus a 20 s video per trigger, with a 1 s inter-trigger delay; units operated 24 h a day and were mounted at <0.5 m above ground. Data were retrieved monthly, and camera positions were adjusted within each site based on animal sign and activity [25].
In November 2023 (autumn), we conducted the first vegetation survey. For each site (i.e., each restoration mode × successional stage combination), we randomly established three 20 × 20 m plots. In July 2024 (summer), we conducted the second survey following the same protocol, establishing a new set of three randomly located 20 × 20 m plots per site. In every plot, we placed five 5 × 5 m shrub subplots and five 1 × 1 m herb subplots at the center and the four corners. Across both surveys, this yielded 54 vegetation plots in total (3 plots × 9 sites × 2 seasons).
Our study employed a space-for-time substitution (SFT) approach to construct a 35-year chronosequence of ecosystem development under two different restoration modes. This approach assumes that the spatial variation among sites selected to represent different successional ages reflects the temporal changes that would occur at a single site over time. To meet the assumptions of the SFT approach, we selected sites with similar initial environmental conditions, including parent material, topography, slope, and pre-disturbance land use history, to minimize the influence of confounding factors.
2.3. Data Analysis
We processed camera data using a manual screening workflow to identify species and activity times. Species identification was conducted using the reference books Mammals of China and Field Guide to the Birds of China. Each 24 h period of continuous camera operation was treated as one valid camera day. We defined independent photographs (IPs) as records of the same species separated by ≥30 min; within <30 min, records were also considered independent if they involved different species or distinct individuals of the same species [25]. Using R software (version 4.5.1), we generated interpolation/extrapolation (rarefaction) curves for animal species and evaluated saturation from the slope of the extrapolated curves [26]. Relative abundance index (RAI) is expressed as the proportion of independent records by species and is used for relative comparisons among sites without explicit camera-day normalization.
For plants, we calculated the importance value (IV) to identify dominant species under different restoration modes [27]. For animals, we quantified RAI based on independent records, which we used to identify dominant animal species in each restoration mode [28]. We assessed biodiversity using species richness, α-diversity, and β-diversity. α-Diversity included the Shannon–Wiener index (H), Simpson’s diversity (D), and Pielou’s evenness (J) [29]. β-diversity was measured by the Bray–Curtis dissimilarity index (BCd) (Table 1), where larger values indicate lower compositional similarity and thus higher β-diversity [30]. We used the Kruskal–Wallis test to compare overall α- and β-diversity among active restoration, natural regeneration, and reference forest, followed by Mann–Whitney U tests for pairwise differences [31]. We then fitted linear regressions of α-diversity against restoration age to infer successional trends. Finally, we used the vegan and ecodist packages in R to perform non-metric multidimensional scaling (NMDS) and evaluate β-diversity relationships among the three modes [32] and applied permutational multivariate analysis of variance (PERMANOVA) to test for group differences.

Table 1.
Indices and formulas (animal relative abundance, species richness, α- and β-diversity).
3. Results
3.1. Species Composition of Plants and Animals in a Typical Coal-Mine Wasteland of Eastern Yunnan
Plants. Because separate sets of randomly located plots were established for the summer and autumn surveys (see Materials and Methods), the observed differences in species counts between seasons reflect both phenological variation and spatial sampling variation. We recorded 2311 trees across 8 families, 12 genera, and 15 species in the study area. In summer, we counted 1213 trees belonging to 6 families, 7 genera, and 10 species; in autumn, 1098 trees belonging to 6 families, 9 genera, and 12 species. For shrubs, we recorded 3524 individuals, comprising 60 species from 50 genera and 33 families (summer: 1187 individuals, 33 species, 29 genera, 22 families; autumn: 2337 individuals, 53 species, 45 genera, 30 families). Herbaceous plants comprised 80 species from 75 genera and 37 families (summer: 53 species from 48 genera and 24 families; autumn: 53 species from 52 genera and 27 families). According to the importance value analysis (Table 2), the dominant species were as follows. In active restoration plots the dominant trees were Juniperus chinensis, Cryptomeria japonica, and Juniperus rigida. The dominant shrubs were Hypericum monogynum, Morella rubra, and Rhododendron myrsinifolium. The dominant herbs were Miscanthus sinensis, Imperata cylindrica, and Bidens pilosa. In natural regeneration plots the dominant trees were Pinus armandii, Pinus yunnanensis, and Alnus nepalensis. The dominant shrubs were Hypericum monogynum, Rhododendron myrsinifolium, and Morella rubra. The dominant herbs were Pteridium aquilinum, Miscanthus sinensis, and Imperata cylindrica. In the reference forest the dominant trees were Pinus armandii, Pinus yunnanensis, and Cerasus serrulata. The dominant shrubs were Eurya japonica, Rhododendron myrsinifolium, and Morella rubra. The dominant herbs were Lophatherum gracile, Cibotium barometz, and Viola philippica. Among the recorded species, only one invasive species was identified: Bidens pilosa, which occurred as a dominant herb in active restoration plots but was absent from natural regeneration and reference forest sites. Overall, the species composition data reveal that active restoration sites were characterized by planted exotic/cultivated tree species and lower overall species richness, whereas natural regeneration and reference forest communities were dominated by native species with higher diversity, particularly in shrub and herb layers.

Table 2.
Dominant species and their importance values (IVs) or relative abundance indices (RAIs) in the study area.
Animals. Camera traps documented 53 vertebrate species across 22 families and 44 genera, including 10 mammals and 43 birds. Predominant mammals included Rattus tanezumi, Melogale moschata, and Tupaia belangeri; predominant birds included Chrysolophus amherstiae, Turdus dissimilis, and Streptopelia orientalis (Table 2).
Rarefaction–extrapolation curves (calculated using the iNEXT package) showed that the number of independent records approached an asymptote after >500 records when all sites were pooled across the entire study area (“Overall” in Figure 2); the same pattern was observed when data were pooled separately for each restoration mode—active restoration, natural regeneration, and reference forest (Figure 2). This indicates that our sampling effort was sufficient to capture the majority of detectable species at the level of restoration modes, though individual camera sites (n = 9) were not independently saturated. The curves represent species accumulation across sites within each restoration mode, encompassing the full range of successional stages (0–30 years) sampled in this chronosequence.
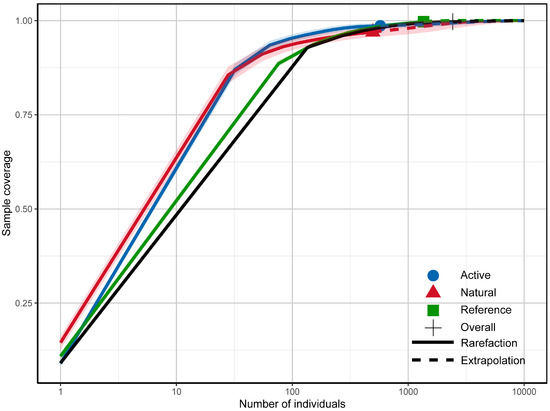
Figure 2.
Rarefaction and extrapolation curves of camera-trap survey in the study area.
3.2. α-Diversity Progression Under Contrasting Restoration Modes
All plants (whole community). Species richness and diversity indices were highest in natural regeneration (S = 20.92 ± 5.82; H = 2.30 ± 0.60; D = 0.78 ± 0.18; J = 0.77 ± 0.19), intermediate in the reference forest (S = 19.00 ± 6.75; H = 2.07 ± 0.25; D = 0.74 ± 0.05; J = 0.72 ± 0.05), and lowest in active restoration (S = 12.92 ± 4.99; H = 1.65 ± 0.38; D = 0.67 ± 0.13; J = 0.69 ± 0.17). Kruskal–Wallis tests detected significant differences among modes for species richness (H_KW = 17.30, df = 2, p < 0.001), Shannon (H_KW = 13.77, df = 2, p = 0.001), and Simpson (H_KW = 6.31, df = 2, p = 0.043), but Pielou’s evenness was not significant (H_KW = 3.38, df = 2, p = 0.185) (Figure 3).
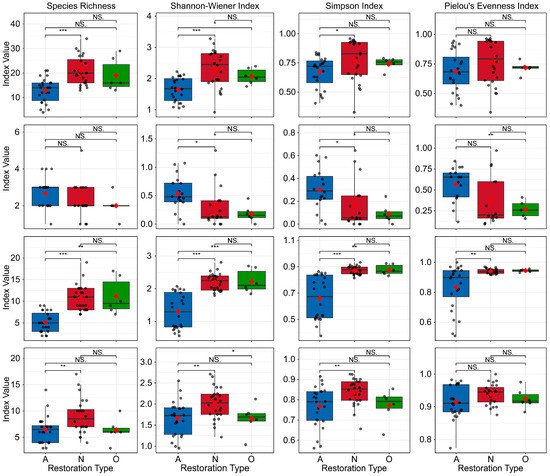
Figure 3.
Plant species richness and α-diversity across restoration modes. Rows indicate (1) whole community, (2) trees, (3) shrubs, and (4) herbs. Restoration codes: A = active restoration, N = natural regeneration, R = reference forest. Red diamonds denote means. Asterisks indicate significance levels: * p < 0.05; ** p < 0.01; *** p < 0.001; NS, not significant (p ≥ 0.05).
Trees (upper layer). All indices were highest in active restoration (S = 2.67 ± 0.84; H = 0.54 ± 0.30; D = 0.30 ± 0.18; J = 0.57 ± 0.21), followed by natural regeneration (S = 2.29 ± 0.99; H = 0.30 ± 0.35; D = 0.16 ± 0.21; J = 0.38 ± 0.31) and the reference forest (S = 2.00 ± 0.63; H = 0.18 ± 0.15; D = 0.09 ± 0.08; J = 0.27 ± 0.10). Shannon (H_KW = 8.77, df = 2, p = 0.012), Simpson (H_KW = 7.80, df = 2, p = 0.020), and Pielou (H_KW = 6.48, df = 2, p = 0.039) differed significantly, whereas species richness did not (H_KW = 3.69, df = 2, p = 0.158) (Figure 3).
Shrubs (middle layer). Natural regeneration (S = 10.83 ± 3.02; H = 2.21 ± 0.27; D = 0.87 ± 0.03; J = 0.94 ± 0.02) and the reference forest (S = 11.17 ± 4.26; H = 2.23 ± 0.36; D = 0.88 ± 0.04; J = 0.95 ± 0.01) were similar and both higher than active restoration (S = 5.21 ± 2.28; H = 1.31 ± 0.54; D = 0.66 ± 0.17; J = 0.83 ± 0.15). Differences among modes were significant for richness (H_KW = 29.73, df = 2, p < 0.001), Shannon (H_KW = 28.57, df = 2, p < 0.001), Simpson (H_KW = 28.11, df = 2, p < 0.001), and Pielou (H_KW = 8.28, df = 2, p = 0.016) (Figure 3).
Herbs (lower layer). Active restoration (S = 6.50 ± 2.54; H = 1.66 ± 0.41; D = 0.77 ± 0.10; J = 0.92 ± 0.05) and the reference forest (S = 6.33 ± 2.25; H = 1.65 ± 0.35; D = 0.77 ± 0.08; J = 0.92 ± 0.03) were similar and both lower than natural regeneration (S = 8.96 ± 3.30; H = 2.01 ± 0.38; D = 0.84 ± 0.07; J = 0.94 ± 0.03). Differences among modes were significant for richness (H_KW = 8.61, df = 2, p = 0.014), Shannon (H_KW = 8.57, df = 2, p = 0.014), and Simpson (H_KW = 8.44, df = 2, p = 0.015) but not for Pielou (H_KW = 2.89, df = 2, p = 0.235) (Figure 3).
Animals (cross-mode differences). Species richness differed significantly among restoration modes (H_KW = 8.39, df = 2, p = 0.015), whereas Shannon (H_KW = 4.62, df = 2, p = 0.099), Simpson (H_KW = 3.38, df = 2, p = 0.185), and Pielou (H_KW = 1.12, df = 2, p = 0.571) showed no overall differences. Pairwise tests indicated that species richness in the reference forest differed from active restoration (U = 202.50, p = 0.009) and from natural regeneration (U = 116.00, p = 0.028); Shannon also differed between the reference forest and active restoration (U = 215.50, p = 0.040) (Figure 4).
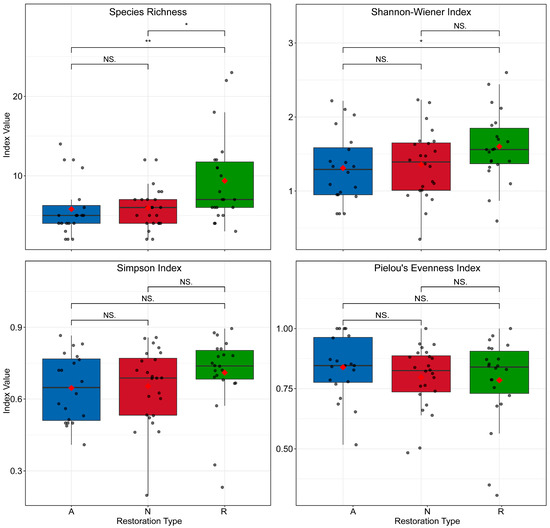
Figure 4.
Animal species richness and α-diversity across restoration modes. Restoration codes: A = active restoration, N = natural regeneration, R = reference forest. Red diamonds denote means. Asterisks indicate significance levels: * p < 0.05; ** p < 0.01; *** p < 0.001; NS, not significant (p ≥ 0.05).
Temporal trends of plant α-diversity. Contrasting active restoration with natural regeneration revealed distinct patterns. Under active restoration, overall plant diversity, shrub diversity, and herb diversity increased with restoration age, whereas tree diversity decreased; nevertheless, all layers converged toward the reference forest. Under natural regeneration, overall α-diversity, tree diversity, and herb diversity declined while approaching the reference forest, and shrub α-diversity remained stable (Figure 5).
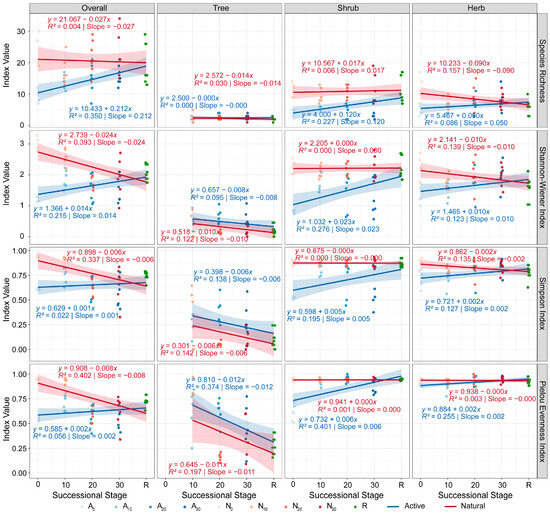
Figure 5.
Temporal patterns of plant α-diversity under active restoration and natural regeneration. Blue lines indicate active restoration, red lines indicate natural regeneration, and green symbols indicate the reference forest. Time codes: 0 = recent, 10 = 10 years, 20 = 20 years, 30 = 30 years.
Temporal trends of animal α-diversity. Under active restoration, species richness (S), Shannon (H), and Simpson (D) increased toward the reference forest with restoration age, while Pielou’s evenness (J) decreased. Under natural regeneration, α-diversity indices showed similar directions but flatter trends (Figure 6). Taken together, these α-diversity patterns indicate that active restoration and natural regeneration follow divergent successional pathways: active restoration shows increasing diversity from a low initial state, whereas natural regeneration exhibits declining diversity from an initially high baseline, with both modes converging toward reference forest conditions over time.
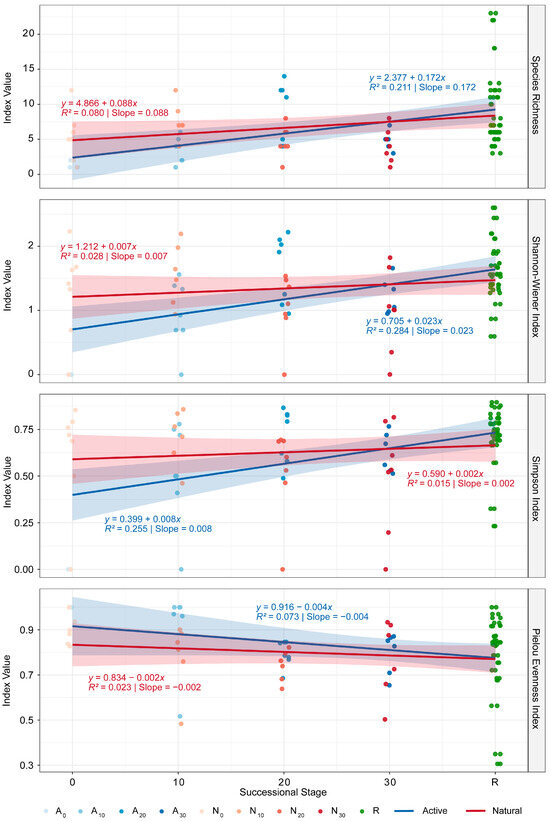
Figure 6.
Temporal trends of animal α-diversity under active restoration and natural regeneration. Restoration codes: Blue lines indicate active restoration, red lines indicate natural regeneration, and green symbols indicate the reference forest. Time codes: 0 = recent, 10 = 10 years, 20 = 20 years, 30 = 30 years.
3.3. β-Diversity Patterns Under Contrasting Restoration Modes
For plants, Bray–Curtis dissimilarity index was similar between active restoration (BCd = 0.71 ± 0.20) and the reference forest (BCd = 0.71 ± 0.19) and higher than in natural regeneration (BCd = 0.63 ± 0.17). Differences among modes were significant (H_KW = 176.54, df = 2, p < 0.001). Pairwise tests showed that natural regeneration differed from both the reference forest (U = 145,138.50, p < 0.001) and active restoration (U = 1,074,966.50, p < 0.001), indicating greater between-site heterogeneity in active restoration and the reference forest (Figure 7a). For animals, Bray–Curtis ranked active restoration (BCd = 0.47 ± 0.24) > reference forest (BCd = 0.42 ± 0.19) > natural regeneration (BCd = 0.39 ± 0.17), implying the largest among-site differences under active restoration; however, differences were not significant (H_KW = 3.17, df = 2, p = 0.205) (Figure 7b).
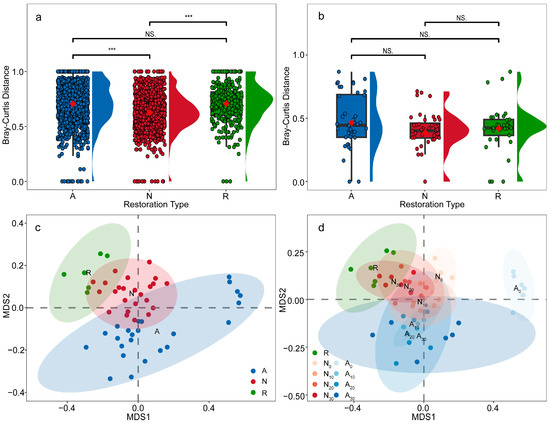
Figure 7.
Differences in Bray–Curtis dissimilarity index across restoration modes and NMDS ordinations for plant communities. (a) Plant Bray–Curtis dissimilarity index by restoration mode; (b) Animal Bray–Curtis dissimilarity index by restoration mode; (c) NMDS ordination of plant communities by restoration mode (based on Bray–Curtis dissimilarity); (d) NMDS ordination of plant communities across mode × time classes. Blue indicates active restoration, red indicates natural regeneration, and green indicates reference forest. Time codes: 0 = recent, 10 = 10 years, 20 = 20 years, 30 = 30 years. Red diamonds denote means (in panels (a,b)). Asterisks indicate significance levels: * p < 0.05; ** p < 0.01; *** p < 0.001; NS, not significant (p ≥ 0.05).
NMDS and PERMANOVA. NMDS based on Bray–Curtis distances showed clear separation among plant communities across restoration modes (stress = 0.18): natural regeneration clustered near the reference forest, whereas active restoration was farther from the reference (Figure 7c). Examining time classes revealed that communities moved toward the reference forest with increasing restoration age under both modes (Figure 7d). PERMANOVA detected significant differences among restoration modes (R2 = 0.20, p = 0.001) and among mode × successional stage combinations (R2 = 0.56, p = 0.001). Because one camera was deployed per mode × stage combination, the animal dataset was too limited for NMDS. In summary, β-diversity analyses demonstrate that natural regeneration maintains lower between-site heterogeneity and closer compositional similarity to the reference forest, whereas active restoration exhibits higher spatial heterogeneity and greater compositional divergence from natural forest conditions, though both modes show temporal convergence along distinct trajectories.
4. Discussion
4.1. Synthesis of Key Findings
Together, our findings depict a two-phase recovery dynamic: rapid early gains in herbaceous richness under active interventions, followed by a slower accrual of structural complexity that tends to be more pronounced under passive natural regeneration. These mode-specific patterns help explain why short-term indicators may overestimate recovery under active restoration while underrepresenting longer-term habitat development.
Our 35-year chronosequence reveals that natural regeneration and active restoration follow distinct successional patterns in coal-mine wastelands of karst landscapes. Natural regeneration maintained higher overall plant α-diversity, exhibited compositional similarity to late-successional reference forest, and showed lower between-site heterogeneity; patterns for animals are detailed below. In contrast, active restoration accelerated structural development but resulted in lower initial diversity, greater compositional divergence from the reference forest, and higher spatial heterogeneity among sites. These contrasting patterns suggest fundamental differences in how community assembly proceeds under passive versus managed recovery. These diversity patterns are underpinned by differences in dominant species composition. Dominant plant species differed among modes; particularly in the tree layer where active restoration was dominated by planted species (Juniperus, Cryptomeria) while natural regeneration and the reference forest shared native pine dominance (Pinus armandii, P. yunnanensis; Table 2); shrub and herb layers showed greater overlap. Importantly, natural regeneration’s shared tree-layer dominants with the reference forest indicate better maintenance of native canopy dominance [33]. For animals, wider movement ranges and the environmental tolerance of dominant species likely attenuated cross-mode differences, yielding comparatively smaller contrasts among the three modes [34,35].
Considering whole-community and layer-specific α-diversity and their trends, natural regeneration had the highest α-diversity, the reference forest was intermediate, and active restoration was lowest. With succession, α-diversity increased under active restoration but declined under natural regeneration. These patterns are consistent with rapid recruitment from surrounding seed sources under passive recovery [36], followed by competitive sorting that elevates a subset of dominants and gradually lowers diversity. In contrast, active restoration initially imposes planted dominance that homogenizes structure, after which diversity increases as communities assemble [37,38]. The tree layer showed higher α-diversity under active restoration but declined over time, likely reflecting early, evenly distributed plantings of dominant trees [39] and subsequent competition that constrains native regeneration, reducing tree-layer diversity [33]. By contrast, shrub and herb α-diversity were highest in natural regeneration; shrub diversity remained stable whereas herb diversity declined, while both indices were lowest in active restoration yet increased with time. These trends suggest that removing direct disturbance under natural regeneration yields rapid short-term gains across layers, but canopy closure and increasing competition drive trees and herbs to converge more closely with the reference forest [40,41]. Similar patterns of lower spatial heterogeneity under natural regeneration compared to active restoration have been documented in post-mining landscapes elsewhere [42]. Sustained shrub diversity may reflect facilitation/retention processes that provide a reservoir for community development [43]. Overall, our findings support that natural regeneration is closer to natural states in function and composition, especially given shrub/herb signals of rebuilding community complexity and disturbance resistance/stability [8,44]. These contrasting patterns align with classical succession models. The initially high diversity followed by decline under natural regeneration is consistent with Egler’s (1954) initial floristic composition model [45], where early colonization from the regional species pool creates high diversity that subsequently declines through competitive sorting. In contrast, the gradual diversity increase under active restoration resembles relay floristics, where planted dominants initially constrain diversity but subsequent colonization progressively enriches communities. This divergence suggests that restoration mode fundamentally alters the mechanism of succession.
Animal α-diversity was highest in the reference forest, intermediate in natural regeneration, and lowest in active restoration. Given the limited spatial coverage of our camera-trap design (one camera per site), these findings are preliminary and indicative rather than comprehensive. The structural integrity and continuity of the reference forest likely underpin its higher diversity by providing complex spatial structure and rich resources [35,46]. Pielou’s evenness was highest in active restoration and lowest in the reference forest; together with the observed decline in evenness over time, this pattern is consistent with an early homogenization effect—actively restored sites initially host fewer species with more even abundances, whereas the reference forest is more dominance-skewed [47,48,49]. Through time, both modes showed increasing animal diversity approaching the reference forest, plausibly because recovery expands habitat space and food resources for birds and mammals [50,51].
Combining Bray–Curtis dissimilarity with NMDS clarifies mode-dependent β-diversity. For plants, active restoration exhibited β-diversity comparable to the reference forest, indicating high between-site heterogeneity; yet NMDS placed active restoration communities farther from the reference forest, signaling greater compositional differences despite similar heterogeneity levels. In contrast, natural regeneration showed lower β-diversity (greater between-site homogeneity) but closer composition to the reference forest, with ordinations showing convergence toward natural forest along the chronosequence [32,52]. For animals, β-diversity ranked active restoration > reference forest > natural regeneration, suggesting stronger among-site differences under active restoration and greater homogenization under natural regeneration [53,54]. Chronosequence NMDS further indicated that communities under both modes moved toward the reference forest with increasing restoration age, albeit along different pathways and speeds [55,56]. We acknowledge that seed dispersal by animals may contribute to floristic homogenization across sites, particularly given the mobility of our recorded bird and mammal species. However, our β-diversity and ordination results demonstrate that restoration mode exerts stronger and more persistent effects on community trajectories than potential dispersal-mediated homogenization. This finding aligns with global assessments showing that management intensity fundamentally shapes plant and animal community responses over time [57]. The convergence toward the reference forest suggests that both restoration modes are progressing toward an assumed community that approximates the potential natural vegetation of the region, though at different rates and via different assembly pathways. The relatively low β-diversity under natural regeneration in this karst landscape may reflect recruitment from a shared regional species pool via similar dispersal pathways, leading to convergent community assembly despite environmental heterogeneity [58]. However, we cannot rule out that a regionally constrained species pool in this historically disturbed mining area contributes to the observed homogeneity. Further investigation of dispersal limitation and species pool composition would help clarify these mechanisms.
4.2. Limitations and Mitigation
We use a space-for-time (SFT) approach that assumes site comparability and spatial proxies for temporal change. In heterogeneous coal-mine wastelands, unmeasured starting conditions (soil compaction, microbiota, early revegetation) may confound age effects; thus our trends are landscape-level signals, not age-only responses. Permanent plots are needed to validate and separate age from site effects. Design caveats remain: single-camera mammal data are indicative; two seasons can conflate phenology with space; residual edaphic/microtopographic heterogeneity likely persists. These caveats mirror post-mining findings (fast early herb gains under active restoration, slower structural recovery than under passive natural regeneration). Mitigation: prioritize locally sourced natives plus structural enrichment and extend monitoring with season-aligned sampling.
5. Conclusions
Natural regeneration and active restoration can take different courses of succession in karst landscapes after mining. Natural regeneration showed higher plant α-diversity, closer composition to the reference forest, and lower between-site heterogeneity. Active restoration accelerated structural development but began with lower diversity and greater compositional divergence. Over time, plant α-diversity declined under natural regeneration but increased under active restoration. Shrub and herb α-diversity were highest under natural regeneration. Tree-layer α-diversity was initially higher under active restoration and then decreased with age. Animal diversity was highest in the reference forest and rose through time in both modes, whereas evenness was initially higher under active restoration. For plants, β-diversity was lower and communities were compositionally closer to the reference forest under natural regeneration. For animals, β-diversity ranked active restoration > reference forest > natural regeneration. Chronosequence ordinations indicated convergence toward the reference forest in both modes, but along different pathways and rates. These patterns are consistent with classical succession models: initial floristics under natural regeneration and relay floristics under active restoration. Management should prioritize locally sourced native species with structural enrichment and maintain season-aligned long-term monitoring to track recovery.
Author Contributions
Conceptualization, H.W. and Z.P.; methodology, H.W., D.H., L.X. and Y.C.; software, D.H. and Z.P.; validation, H.W., Q.L., X.Z. and H.C.; formal analysis, H.C. and L.X.; investigation, H.W., D.H., Q.L., L.X., H.C., Y.C. and X.Z.; resources, H.W. and Z.P.; data curation, H.W., D.H. and Z.P.; writing—original draft preparation, H.W., D.H. and Z.P.; writing—review and editing, H.W., D.H., Q.L., L.X. and Z.P.; visualization, H.W., D.H. and Y.C.; supervision, H.W. and Z.P.; project administration, Q.L. and Z.P.; funding acquisition, H.W. and Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (92255301, 42130209), the Youth Innovation Promotion Association CAS (2021070), the Open Research Program of the International Research Center of Big Data for Sustainable Development Goals (CBAS2023ORP01), the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities’ Association (202101BA070001-209, 202101BA070001-279), the Yunnan Province Young and Middle-Aged Academic and Technical Leaders Reserve Talents Program (202305AC350252), the Special Basic Cooperative Research Innovation Programs of Qujing Science and Technology Bureau & Qujing Normal University (KJLH2022YB03, KJLH2024ZD06).
Institutional Review Board Statement
This research was a non-invasive observational study of wildlife using remote camera traps. No animals were handled or disturbed. Therefore, formal ethical approval was not required for this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (Z.P., panzhaohui@ivpp.ac.cn) due to conservation and privacy restrictions associated with raw camera-trap images and precise plot coordinates (sensitive wildlife localities and land-access agreements for the closed underground coal-mine sites).
Acknowledgments
We thank Siwei Ruan, Jianming Tang, Tao Chen, and Enrui Guo from Qujing Normal University for their field investigation and data collation in our manuscript. We also thank Qujing Meteorological Bureau for providing meteorological data (2022–2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Energy Agency (IEA). Coal 2023: Analysis and Forecast to 2026; IEA Publications: Paris, France, 2023; Available online: www.iea.org/reports/coal-2023 (accessed on 22 September 2025).
- Xu, J.X.; Zhao, H.; Yin, P.C.; Wu, L.X.; Li, G. Landscape ecological quality assessment and its dynamic change in coal mining area: A case study of Peixian. Environ. Earth. Sci. 2019, 78, 708. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Alberdi, A.; Morriën, E.; van der Putten, W.H.; Rodríguez-Uña, A.; Montoya, D. The long-term restoration of ecosystem complexity. Nat. Ecol. Evol. 2020, 4, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Riechers, M.; Loos, J.; Martin-Lopez, B.; Temperton, V.M. Making the UN Decade on Ecosystem Restoration a Social-Ecological Endeavour. Trends Ecol. Evol. 2021, 36, 20–28. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Collins, S.L.; Armesto, J.J. Models, mechanisms and pathways of succession. Bot. Rev. 1987, 53, 335–371. [Google Scholar] [CrossRef]
- Craven, D.; Eisenhauer, N.; Pearse, W.D.; Hautier, Y.; Isbell, F.; Roscher, C.; Bahn, M.; Beierkuhnlein, C.; Bönisch, G.; Buchmann, N.; et al. Multiple facets of biodiversity drive the diversity–stability relationship. Nat. Ecol. Evol. 2018, 2, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Craven, D.; Jakovac, C.C.; Van der Sande, M.T.; Amissah, L.; Bongers, F.; Chazdon, R.L.; Farrior, C.E.; Kambach, S.; Meave, J.A.; et al. Multidimensional tropical forest recovery. Science 2021, 374, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.H.; Tang, S.Y.; Zhang, M.; Zhang, R.T.; Huang, Y.K.; Shang, Z. Slope vegetation characteristics and community stability at different restoration years of open-pit coal mine waste dump. Acta Ecol. Sin. 2021, 41, 5764–5774. (In Chinese) [Google Scholar] [CrossRef]
- Jin, W.J.; Bian, Z.X.; Wei, Z.Y.; Dong, Z.C. Damage mechanisms of small-to-medium-scale mines on ecological networks at watershed scale and systematic nature-based mine restoration pathways. Ecol. Eng. 2025, 216, 107638. [Google Scholar] [CrossRef]
- Kreyling, J. Space-for-time substitution misleads projections of plant community and stand-structure development after disturbance in a slow-growing environment. J. Ecol. 2024, 112, 2197–2208. [Google Scholar] [CrossRef]
- Abreu, R.C.R.; Hoffmann, W.A.; Vasconcelos, H.L.; Pilon, N.A.; Rossatto, D.R.; Durigan, G. The biodiversity cost of carbon sequestration in tropical savanna. Sci. Adv. 2017, 3, e1701284. [Google Scholar] [CrossRef]
- Jeltsch, F.; Bonte, D.; Pe’er, G.; Reineking, B.; Leimgruber, P.; Balkenhol, N.; Schröder, B.; Buchmann, C.M.; Mueller, T.; Blaum, N.; et al. Integrating movement ecology with biodiversity research—Exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Xu, F.J.; Lin, T.; Xu, Q.; Yu, P.J.; Wang, C.H.; Aili, A.S.J.; Zhao, X.F.; Zhao, W.Y.; Zhang, P.; et al. A systematic review and comprehensive analysis on ecological restoration of mining areas in the arid region of China: Challenge, capability and reconsideration. Ecol. Indic. 2023, 154, 110630. [Google Scholar] [CrossRef]
- Wrensford, K.C.; Angert, A.L.; Gaynor, K.M. Linking individual animal behavior to species range shifts under climate change. Trends Ecol. Evol. 2025, 40, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yang, C.; Qiao, H.J.; Hu, J.H. More than two-fifths of the protected land in a global biodiversity hotspot in southwest China is under intense human pressure. Sci. Total Environ. 2024, 906, 167283. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, C.; Xiong, K.N.; Rong, L.; Zhang, S.H. Quantifying the biodiversity and ecosystem service outcomes of karst ecological restoration: A meta-analysis of South China Karst. Catena 2024, 245, 108278. [Google Scholar] [CrossRef]
- Yang, H.; Gao, X.; Wu, J.H.; Thompson, J.R.; Flower, R.J. Ecological restoration for China’s mines. Science 2024, 385, 1052–1053. [Google Scholar] [CrossRef]
- Xu, H.L.; Waheed, A.; Kuerban, A.; Muhammad, M.; Ailishiang, A. Dynamic approaches to ecological restoration in China’s mining regions: A scientific review. Ecol. Eng. 2025, 214, 107577. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhao, S.; Zuo, H.T.; Hu, X.; Guo, Y.; Han, D.; Chang, Y.J. Tracking the Vegetation Change Trajectory over Large-Surface Coal Mines in the Jungar Coalfield Using Landsat Time-Series Data. Remote Sens. 2023, 15, 5667. [Google Scholar] [CrossRef]
- Xu, Y.L.; Guo, L.; Li, J.; Zhang, C.Y.; Ran, W.Y.; Hu, J.Y.; Mao, H.T. Automatically identifying the vegetation destruction and restoration of various open-pit mines utilizing remotely sensed images: Auto-VDR. J. Clean. Prod. 2023, 414, 137490. [Google Scholar] [CrossRef]
- Shi, X.Z.; Zhang, W.Q. Characteristics of an underground stope channel supplied by atmospheric precipitation and its water disaster prevention in the karst mining areas of Guizhou. Sci. Rep. 2023, 13, 15892. [Google Scholar] [CrossRef]
- Huang, G.C.; Dong, J.H.; Xi, W.F.; Zhao, Z.L.; Li, S.F.; Kuang, Z.; An, Q.; Wei, J.; Zhu, Y.H. Study on surface deformation pattern in mine closure area of complex karst mountainous region based on SBAS-InSAR technology. Front. Earth Sci. 2024, 11, 1353593. [Google Scholar] [CrossRef]
- Tian, X.W.; Yao, X.; Zhou, Z.K.; Tao, T. Surface Multi-Hazard Effects of Underground Coal Mining in Mountainous Regions. Remote Sens. 2025, 17, 122. [Google Scholar] [CrossRef]
- Tanwar, K.S.; Sadhu, A.; Jhala, Y.V. Camera trap placement for evaluating species richness, abundance, and activity. Sci. Rep. 2021, 11, 23050. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A.N. iNEXT: An R package for interpolation and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Li, H.; Chen, W.B.; Lin, J.T.; Zhang, C.; Liang, H.F. Coupling relationship between soil properties and plant diversity under different ecological restoration patterns in the abandoned coal mine area of southern China. Ecol. Evol. 2024, 14, e70686. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.G.; Kinnaird, M.F.; Wibisono, H.T. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 2003, 6, 131–139. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, Y.; Xie, H.Y.; Du, C.; Zhan, A.B.; Xu, J.; Giesy, J.P.; Wu, F.C.; Jin, X.W. Spatial distribution of benthic taxonomic and functional diversity in the Yellow River Basin: From ecological processes to associated determinant factors. Environ. Int. 2024, 188, 108745. [Google Scholar] [CrossRef]
- Becsei, Á.; Fuschi, A.; Otani, S.; Kant, R.; Weinstein, I.; Alba, P.; Stéger, J.; Visontai, D.; Brinch, C.; de Graaf, M.; et al. Time-series sewage metagenomics distinguishes seasonal, human-derived and environmental microbial communities potentially allowing source-attributed surveillance. Nat. Commun. 2024, 15, 7551. [Google Scholar] [CrossRef] [PubMed]
- Kremer, K.N.; Promis, Á.; Bauhus, J. Natural Advance Regeneration of Native Tree Species in Pinus radiata Plantations of South-Central Chile Suggests Potential for a Passive Restoration Approach. Ecosystems 2021, 24, 1215–1229. [Google Scholar] [CrossRef]
- De Almeida, C.; Reid, J.L.; Lima, R.A.F.; Pinto, L.F.G.; Viani, R.A.G. High-diversity Atlantic Forest restoration plantings fail to represent local floras. Perspect. Ecol. Conserv. 2024, 23, 6–11. [Google Scholar] [CrossRef]
- Zhu, H.L.; Zhang, J.L.; Cheuk, M.L.; Hau, B.C.H.; Fischer, G.A.; Gale, S.W. Monoculture plantations impede forest recovery: Evidence from the regeneration of lowland subtropical forest in Hong Kong. Front. For. Glob. Change 2023, 6, 1098666. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Liu, X.H.; Lv, Z.X.; Zhao, X.Y.; Yang, X.Z.; Jia, X.D.; Sun, W.L.; He, X.B.; He, B.S.; Cai, Q.; et al. Animal diversity responding to different forest restoration schemes in the Qinling Mountains, China. Ecol. Eng. 2019, 136, 23–29. [Google Scholar] [CrossRef]
- Burton, A.C.; Beirne, C.; Gaynor, K.M.; Sun, C.; Granados, A.; Allen, M.L.; Alston, J.M.; Alvarenga, G.C.; Calderón, F.S.Á.; Amir, Z.; et al. Mammal Responses to Global Changes in Human Activity Vary by Trophic Group and Landscape. Nat. Ecol. Evol. 2024, 8, 924–935. [Google Scholar] [CrossRef]
- Zivec, P.; Johnston-Bates, J. Seed rain as a propagule source for restoration of semi-arid floodplain old fields. Appl. Veg. Sci. 2024, 27, e70001. [Google Scholar] [CrossRef]
- Chen, S.B.; Hua, J.G.; Liu, W.T.; Yang, S.Y.; Wang, X.Q.; Ji, W.L. Effects of Artificial Restoration and Natural Recovery on Plant Communities and Soil Properties across Different Temporal Gradients after Landslides. Forests 2023, 14, 1974. [Google Scholar] [CrossRef]
- Houehanou, B.; Gaoue, O.G. Proximity to forests, fire and plantation characteristics influence understory plant species richness more than phylogenetic diversity in African mahogany plantations. Sci. Rep. 2025, 15, 12345. [Google Scholar] [CrossRef]
- Van der Sande, M.T.; Craven, D.; Ramirez, J.A.; Herrera, D.; Santini, L.; Chazdon, R.L.; Wright, S.J.; Finegan, B.; Martínez-Ramos, M.; Powers, J.S.; et al. Tropical forest succession increases tree taxonomic and functional richness but decreases evenness. Glob. Ecol. Biogeogr. 2024, 33, e13856. [Google Scholar] [CrossRef]
- Zheng, L.T.; Chen, H.Y.H.; Hautier, Y.; Bao, D.F.; Xu, M.S.; Yang, B.Y.; Zhao, Z.; Zhang, L.; Yan, E.R. Functionally diverse tree stands reduce herbaceous diversity and productivity via canopy packing. Funct. Ecol. 2022, 36, 950–961. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Norden, N.; Colwell, R.K.; Chao, A.N. Monitoring recovery of tree diversity during tropical forest restoration: Lessons from long-term trajectories of natural regeneration. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20210069. [Google Scholar] [CrossRef]
- Zaplata, M.K.; Dullau, S. Applying ecological succession theory to birds in Solar Parks: An approach to address protection and planning. Land 2022, 11, 718. [Google Scholar] [CrossRef]
- Velasco, N.; Soto-Agurto, C.; Carbone, L.; Massi, C.; Bustamante, R.; Smit, C. Large-scale facilitative effects for a single nurse shrub: Impact of the rainfall gradient, plant community and distribution across a geographical barrier. J. Ecol. 2024, 112, 233–245. [Google Scholar] [CrossRef]
- Hua, F.Y.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.R.; Wang, W.Y.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, eabl4649. [Google Scholar] [CrossRef]
- Egler, F.E. Vegetation science concepts I. Initial floristic composition, a factor in old-field vegetation development. Vegetatio 1954, 4, 412–417. [Google Scholar] [CrossRef]
- Martínez-Penados, A.L.; Arroyo-Rodríguez, V.; Morante-Filho, J.C.; Pinel-Ramos, E.J.; Schondube, J. Old-growth forests are critical to safeguard tropical birds in complex landscape mosaics exposed to slash-and-burn agriculture. Landsc. Ecol. 2024, 39, 118. [Google Scholar] [CrossRef]
- Mosanghini, D.; Oriolo, G.; Boscutti, F. Different ways to success: Plant community trajectories over time and a soil moisture gradient in restored wetlands. J. Appl. Ecol. 2023, 60, 29–40. [Google Scholar] [CrossRef]
- Weeks, J.M.; Miller, J.E.D.; Steel, Z.L.; Batzer, E.E.; Safford, H.D. High-severity fire drives persistent floristic homogenization in human-altered forests. Ecosphere 2023, 14, e4409. [Google Scholar] [CrossRef]
- Shaw, T.; Scherer-Lorenzen, M.; Müller, S. Forest structural heterogeneity positively affects bird richness and acoustic diversity in a temperate, central European forest. Front. Ecol. Evol. 2024, 12, 1387879. [Google Scholar] [CrossRef]
- Zhang, D.X.; Mao, R.R.; Liu, M.X.; Zhou, Q.; Wang, Y.Z.; Si, X.F.; Zhao, C.M.; Zhang, L.X. Impacts of forest restoration on multifaceted bird diversity and community assembly in the Loess Plateau of China. For. Ecol. Manag. 2024, 573, 122350. [Google Scholar] [CrossRef]
- Kortmann, M.; Chao, A.N.; Schaefer, H.M.; Blüthgen, N.; Gelis, R.; Tremlett, C.J.; Busse, A.; Püls, M.; Seibold, S.; Kriegel, P.; et al. Sample coverage affects diversity measures of bird communities along a natural recovery gradient of abandoned agriculture in tropical lowland forests. J. Appl. Ecol. 2025, 62, 480–491. [Google Scholar] [CrossRef]
- Guclu, C.; Luk, C.; Ashton, L.A.; Abbas, S.; Boyle, M.J. Beta diversity subcomponents of plant species turnover and nestedness reveal drivers of community assembly in a regenerating subtropical forest. Ecol. Evol. 2024, 14, e70233. [Google Scholar] [CrossRef]
- Joyce, F.H.; Rosales, J.A.; Holl, K.D.; Zahawi, R.A.; Bui, A.; Reid, J.L. Active restoration accelerates recovery of tropical forest bird assemblages over two decades. Biol. Conserv. 2024, 293, 110593. [Google Scholar] [CrossRef]
- Montes-Rojas, A.; Delgado-Morales, N.A.J.; Escucha, R.S.; Siabatto, L.C.; Link, A. Recovering connectivity through restoration corridors in a fragmented landscape in the magdalena river’s valley in Colombia. Biodivers. Conserv. 2024, 33, 3171–3185. [Google Scholar] [CrossRef]
- Noe, E.E.; Innes, J.; Barnes, A.D.; Joshi, C.; Clarkson, B.D. Habitat provision is a major driver of native bird communities in restored urban forests. J. Anim. Ecol. 2022, 91, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Haslem, A.; Clarke, R.H.; Maisey, A.C.; Stewart, A.; Radford, J.Q.; Bennett, A.F. Temporal dynamics in the composition of bird communities along a gradient of farmland restoration. Ecol. Appl. 2024, 34, e2947. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Veen, H.; Kuipers, K.; Schipper, A.; Marques, A.; Schelhaas, M.J.; Alkemade, R. A global assessment of plant and animal community responses to forest management over time. Glob. Change Biol. 2025, 31, e70279. [Google Scholar] [CrossRef] [PubMed]
- Zaplata, M.K.; Winter, S.; Fischer, A.; Kollmann, J.; Ulrich, W. Species-driven phases and increasing structure in early-successional plant communities. Am. Nat. 2013, 181, E17–E27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).