Abstract
The Mystras UNESCO World Heritage Site (MUWHS) is a medieval historical area located on a small hill facing Sparta in the Mediterranean hotspot of the Peloponnese and receives a high number of visitors annually. The main aim of this study is the inventory and analysis of plant species composition and diversity of the Mystras archaeological area, with emphasis on different aspects of its flora, on the specialist endemic plants, and on the generalist ruderal and alien taxa. A high plant species richness was observed, and 321 vascular plant taxa were registered. Most of the taxa are Mediterranean or have a more widespread distribution, and half of them are ruderals. Concerning endemism, 14 Greek and 7 Balkan endemic taxa were registered. As anticipated, the most species-rich plant families recorded in the study area are Asteraceae, Fabaceae, and Poaceae. The total flora is predominantly composed of therophytes, reflecting the site’s Mediterranean climate and disturbance-adapted ecological conditions while the endemic flora is mostly composed of hemicryptophytes. Comparisons of MUWHS plant diversity with four other archaeological sites of the same floristic region of Greece, the Peloponnese, highlighted its high α-diversity on all aspects of its flora and its floristic dissimilarity from the other areas and, additionally, the high plant species richness that is comprised in all five of them. Comparisons of the flora of MUWHS with other Greek and Mediterranean archaeological areas showed significant similarities in the floristic elements considered as deteriogenic for the protected walls and monuments. The findings of our study underscore the urgent need to prioritise the sustainable conservation of archaeological sites such as Mystras. These landscapes are not only cultural monuments but also reservoirs of biodiversity and ecological value. Effective management must, therefore, adopt an integrated approach that balances the preservation of historical structures with the protection of native flora and ecological processes.
1. Introduction
Cultural and natural heritage conservation serves as a vital driver of sustainable development, particularly in the face of growing pressures from tourism, urban expansion, and climate change [1]. Through the successful execution of the 1972 World Heritage Convention, nature conservation and the preservation of cultural properties are among the main actions that recognise people–nature interactions, according to the Recommendation on the Historic Urban Landscape [2], incorporating detailed assessments and spatial documentation of the natural, cultural, and human assets within historic urban areas [3]. Credibility, conservation, capacity building, communication, and communities are the five strategic objectives of the World Heritage Convention (https://whc.unesco.org/en/convention, accessed on 10 September 2025). The landscape–heritage nexus [4] is evident in archaeological areas that are habitat islands, which offer a critical understanding of historical relationships between humans and their environments and host a mosaic of plant communities and rich biodiversity [3,5,6,7,8,9,10,11,12]; emphasis must be given on nature as a valuable component of the cultural environment [13].
The number of studies concerning archaeological and historical sites is rather limited [14], and this is also the case for studies concerning plant composition and diversity in archaeological sites, mainly concerning medieval castles, archaeological parks, and the impacts of diverse heritage site stewardship strategies on their flora (see, among others, [9,10,11,15,16,17,18,19,20]). Archaeological areas as habitat islands often give refuge to a high amount of plant species diversity and endemism [10,11,12,21], but the growth of some plant taxa on ancient walls and monuments, mainly depending on specific functional traits, may cause stone biodeterioration [22,23,24,25,26,27,28,29].
Greece has been characterised as a regional plant diversity hotspot in the Mediterranean, a globally significant centre of biodiversity [30,31,32]. The Peloponnese, the southern peninsular part of Greece, is a small ‘Cape region’ belonging to the biodiversity hotspot of Central and Southern Greece, characterised by a complex landscape of natural, semi-natural, and anthropogenic habitats with great overall species diversity and the highest number of Greek endemic plant species and taxa among the 13 phytogeographical regions of Greece [33,34,35,36,37]. Together with its rich plant diversity, the Peloponnese is also distinguished by its archaeological richness since archaeological sites, ancient ruins, and medieval monuments are more prevalent in the Peloponnese than anywhere else in Greece [33].
A historic urban landscape reflects the accumulated layers of cultural and natural values shaped over time in a wider urban context (a site’s physical and spatial characteristics, including its topography, geomorphological structure, natural elements, architectural fabric, infrastructure systems, open public spaces, land uses, etc.). This necessitates a holistic approach to documenting, safeguarding, and administering historic urban landscapes aligned with long-term sustainability goals [38]. In the Middle Ages, landscapes were influenced by the built environment and socio-economic activities rooted in human history [39]. The medieval castle city of Mystras is among the 19 UNESCO World Heritage Sites in Greece. Its layered history is vividly reflected in the enduring presence of fortifications, palatial structures, religious institutions, residential buildings, urban pathways, and civic squares. It served as a strategic asset within the broader political landscape of its time, a political and administrative provincial centre of the Byzantine state, and underwent significant development and aesthetic enhancement as befitting its dual legacy as a hub of state power and a vibrant centre of cultural heritage. Its design followed an amphitheatre-like configuration, radiating outward from the fortified core that was erected in 1249 by the Franks, reconquered by the Byzantines in 1262 (initially established as the base of the military governor, the site gained prominence in 1348 when it became the administrative seat of the Despotate of Morea—a semi-autonomous province of the Byzantine Empire governed by imperial family members), occupied by the Turks in 1460, then the Venetians, and finally abandoned in 1832, leaving only the awe-inspiring remnants of its medieval architecture (https://whc.unesco.org/en/list/511; http://odysseus.culture.gr/h/3/eh351.jsp?obj_id=2397, accessed on 30 August 2024). Mystras is under constant maintenance; many churches and palaces have been restored, attracting thousands of visitors every year.
The UNESCO World Heritage Site of Mystras is included within the context of the floristic inventories of the archaeological areas of the Peloponnese [10,11]. Archaeological areas as habitat islands are fragmented habitats enclosed by a matrix of markedly different terrestrial ecosystems [40,41,42] and often comprise refugia for plants under sustainable management [8,10,11,18,43,44]. This study aims to explore and assess the composition and diversity of total, endemic, ruderal, and alien plant species, as well as plants considered to have a deteriogenic role, within the archaeological site of Mystras (Figure 1). By comparing the floristic profile of Mystras with other archaeological landscapes across Greece and the broader Mediterranean region, one more aim is to highlight the ecological role of such sites—not merely as cultural monuments, but as active contributors to biodiversity conservation.
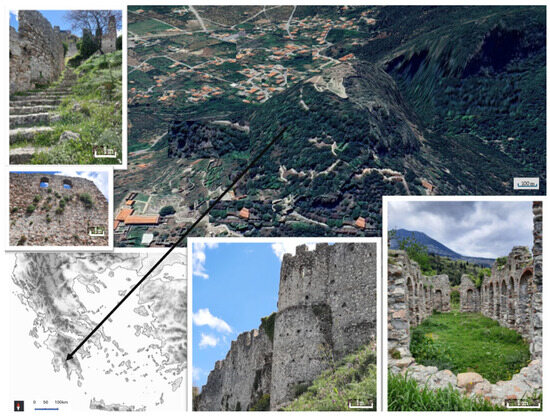
Figure 1.
Study area. The arrow shows the location of the UNESCO World Heritage Site of Mystras in Greece.
2. Materials and Methods
2.1. Study Area
The archaeological site of Mystras (Figure 1) was inscribed in the UNESCO World Heritage Sites in 1989 (https://whc.unesco.org/en/list/511, accessed on 30 August 2024). It is located on a steep foothill, mainly composed of limestone, on the northern slopes of Mt. Taygetos (geographical coordinates N37 4 50.016 E22 22 0.012), 6 km NW of Sparta (Prefecture of Laconia, Peloponnese). Xerothermic climatic conditions prevail at the site, which has an elevation of 620 m; its core area totals 0.544 km2 and its buffer zone totals 12.025 km2.
Mystras, as the centre of Byzantine power, quickly attracted inhabitants and institutions, and it was under the Despots from 1348, when Mystras reached its zenith. The medieval city of Mystras is a unique example of a well-preserved, fortified, late-Byzantine city with elaborate spatial planning organisation, fortifications with a citadel on top of the hill, and two fortified precincts at the lower level (https://whc.unesco.org/en/list/511; http://odysseus.culture.gr/h/3/eh351.jsp?obj_id=2397, accessed on 30 August 2024). Mystras receives many thousands of visitors annually.
2.2. Data Collection, Database, and Floristic Analysis
Field work for the botanical exploration of the archaeological site of Mystras (MUWHS) was carried out between 2019 and 2024, during autumn and spring, with at least two days’ worth of field work on every visit. The site includes ruderal habitats, cliffs, rocks and walls, phrygana, woodlands, and scrubs. The sampling scheme included the full collection and observation of vascular plants across the whole of the archaeological site so that the registered data were representative of the local plant heterogeneity. During this period, we did not observe significant changes concerning the climate.
The plant specimens collected were determined mainly by following Flora Europaea and Flora Hellenica [45,46,47,48,49,50,51], and they are deposited at the Herbarium of the University of Patras (Herbarium UPA). Taxa are ordered alphabetically. All information about the plant taxa, including valid names, status, biological types, and chorological categories, follows the checklist of the Flora of Greece [36,52,53]. The biological types used are geophytes, therophytes, chamaephytes, hemicryptophytes, and phanerophytes. The registered taxa were characterised according to their geographical distribution (widespread; Mediterranean, Balkan, and Greek endemics; and established alien taxa of various origins). Information about the deteriogenic status of taxa was derived from [22,23,24,25,26,27,28,54].
The participation percentages of botanical families and biological and chorological types were calculated. The extinction risk assessment of the plant taxa according to criteria A and B of the IUCN and the values of the Evolutionary Distinct and Globally Endangered index (EDGE) followed [55].
2.3. Comparison of MUWHS’s Flora with Those of Other Archaeological Sites
Using available data, the similarities and dissimilarities between the flora of Mystras and other archaeological areas in Greece, like the Epidaurus UNESCO World Heritage Site and other sites of the same phytogeographical area of Greece (the Peloponnese), were examined to assess the status of Mystras’s plant richness and diversity. The data included in the floristic studies included (a) data from the Peloponnese archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN), and Monemvasia (MON) [10,11,12]; (b) data provided by related publications, where plant lists were available, concerning the flora of eight other Greek archaeological areas [56,57], as well as those in other countries of the Mediterranean, such as the site of the Neapolis of Syracuse (NEA) [58,59], the UNESCO World Heritage Site of the Etruscan Necropolis of Tarquinia (TAR) [44], 8 archaeological sites in Alexandria in Egypt (ALE) [21], 40 Croatian Historic Structures (CRO) [27], “Bosco di Santo Pietro” (South-Eastern Sicily) [60], and the following archaeological parks in Italy (POM): Pompeii, Herculaneum, Paestum, and Velia [22]. These data were used to check the correlation of different aspects of vascular plant diversity, where available, including of plants considered deteriogenic, and to conduct an overall assessment of all flora and registered endemic plant taxa, as well as the significance of their conservation.
For the estimation of “α-diversity”, we used the species richness per unit area, the simplest metric, counting the number of species present in a sample [61]. The free software Past 4.04 [62] was used for analysis. Correlation (Pearson correlation coefficient r), hierarchical clustering (paired group UPGMA, Euclidean distance), and the Shannon diversity index were used for the comparisons of the different aspects of the flora of MUWHS with those of the other archaeological areas of Greece and the Mediterranean area.
3. Results
3.1. Floristic Composition and Local Distribution of the Taxa
The floristic study of the Mystras archaeological site showed that it includes 321 taxa (246 species and 75 subspecies), of which 4 are Pteridophytes, 3 Gymnosperms, and 314 Angiosperms, belonging to 236 genera and 81 families. The floristic composition of the studied castle is presented in Supplementary Table S1. Asteraceae (32 genera, 43 taxa), Fabaceae (16, 35), Poaceae (17, 21), Brassicaceae (9, 9), Caryophyllaceae (5, 12), Apiaceae (18, 21), Lamiaceae (15, 18), Boraginaceae (5, 6), Orchidaceae (5, 6), Ranunculaceae (5, 11), and Rosaceae (6, 7) are the richest families. A total of 56.3% of the genera and 58.9% of the taxa of the total flora belong to these families. The genera richest in taxa are Trifolium (10), Silene (7); Crepis, Euphorbia, and Medicago (6 taxa each); Ranunculus (5); and Campanula and Vicia (4 taxa each). Melia azedarach has been registered but not included in the plant lists, since it is not an established alien.
The complicated landscape of MUWHS permits the analysis of different aspects of its floristic composition, starting with the life form spectra (Figure 2). Therophytes predominate when total, ruderal, and wall flora are examined, followed by hemicryptophytes. Hemicryptophytes, therophytes, and chamaephytes represent the highest proportions of endemic and chasmophytic plant groups, while phanerophytes represent the majority of naturalised (established) alien taxa, followed by therophytes and hemicryptophytes. Therophytes belong to 28 families, and it should be noted that (a) 66% of them belong to the families of Asteraceae, Fabaceae, Poaceae, Brassicaceae, Caryophyllaceae, and Apiaceae, and that (b) 78% of the Fabaceae, 66.7% of the Poaceae, and 51% of the Asteraceae are therophytes (Figure 2).
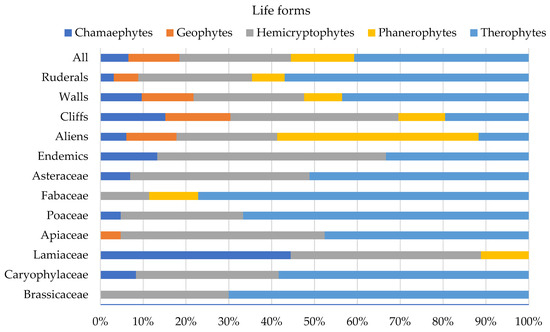
Figure 2.
Life form spectra of different aspects of the floristic composition of the UNESCO World Heritage Site of Mystras (MUWHS). Abbreviations: All = all taxa registered at MUWHS.
Regarding the chorology of the castle’s total, ruderal, wall, and chasmophytic floras, Mediterranean and widespread taxa dominate (Figure 3). Chasmophytic and wall floras represent the highest proportions of Greek and Balkan endemics, while ruderal flora represent the highest proportion of alien taxa (9.5%). Mediterranean and widespread taxa also dominate among the taxa of the families of Asteraceae, Fabaceae, Poaceae, Brassicaceae, Caryophyllaceae, and Apiaceae. Endemic taxa represent the highest proportions among the taxa of the families of Caryophyllaceae and Lamiaceae, while the families of Fabaceae and Poaceae do not include endemics (Figure 3).
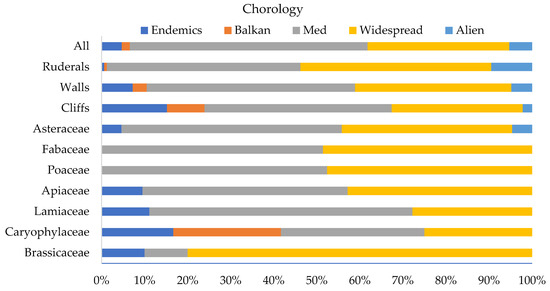
Figure 3.
Chorological analysis of different aspects of the floristic composition of the UNESCO World Heritage Site of Mystras (MUWHS). Abbreviations: All = all taxa registered at MUWHS.
The endemic flora of the studied castle consist of 14 taxa (Table 1), all range-restricted, belonging to 9 families and 12 genera. More specifically, three of them are Peloponnesian endemics, three are also distributed to Sterea Ellas (the Greek mainland), and three also spread to the Ionian area. Concerning their life forms, 13.3% are geophytes, 53.4% hemicryptophytes, and 33.3% therophytes (Figure 2). A total of 46.7% of the endemics are found on cliffs, rocks, and/or walls, of which 21% exclusively belong to their respective habitat. Five of the endemic taxa registered have been assessed as Endangered and eight as Vulnerable, while the Evolutionary Distinct and Globally Endangered index (EDGE) for them has values from 2.85 to 4.74 [55].

Table 1.
The Greek endemic plant taxa registered at the UNESCO World Heritage Site of Mystras (MUWHS), their extinction risk status for both IUCN Criteria A and B, and their distribution at the archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN), and Monemvasia (MON). Abbreviations: ERAB: extinction risk based on both the IUCN Criteria A and B; EDGE: Evolutionary Distinct and Globally Endangered index [55].
Balkan endemics are represented by seven taxa, namely Campanula spatulata subsp. spatulata, Centranthus ruber subsp. sibthorpii, Malcolmia graeca subsp. hydraea, Petrorhagia glumacea, Saxifraga rotundifolia subsp. chrysospleniifolia, Silene congesta, and S. vulgaris subsp. megalosperma. Many of the Balkan endemics are hemicryptophytes (57.1%), while chamaephytes, geophytes, and therophytes comprise low proportions (14.3% each). Plants preferably growing on cliffs and walls constitute 57.1% of the Balkan endemics.
About one third of the plant taxa (31.1%) registered at MUWHS were found on its walls and rocks. Therophytes predominate on the castle walls (43.5%), followed by hemicryptophytes (25.8%), geophytes (12.1%), chamaephytes (9.7%), and phanerophytes (8.9%) (Figure 2). Most wall plants have a widespread (43.5%) or Mediterranean (36.3%) distribution, followed by taxa of the eastern Mediterranean (9.7%), Greek endemics (7.3%), taxa of alien origin (4.8%), and Balkan endemics (3.2%) (Figure 3).
Cliff plants represent 14.3% of the castle’s total flora (46 taxa, of which 17 are obligate chasmophytes). Greek endemic taxa represent 15.2% of the cliff plants, the rest being widespread (21.8%), Mediterranean (41.3%), east Mediterranean (10.8%), alien (2.2%), and Balkan (8.7%) taxa. Hemicryptophytes predominate (39.2%), followed by chamaephytes (15.2%), therophytes (19.6%), geophytes (15.2%), and phanerophytes (10.8%) (Figure 2).
About half of the taxa (161 taxa) are ruderals, of which 85 (26.5% of the taxa registered) are exclusive ruderals occurring chiefly or exclusively in ruderal and arable habitats. Figure 4 showcases the representation of ruderal taxa in the families at MUWHS that are the richest in taxa. Among ruderals, therophytes predominate (57%), followed by hemicryptophytes (26.6%) (Figure 2). Widespread and Mediterranean taxa represent 81.6% of the ruderal flora of the MUWHS and alien taxa 9.5%. Ruderal taxa represent 45.9% (57 taxa) of the MUWHS’s wall flora, of which 50.9% are exclusive ruderals.
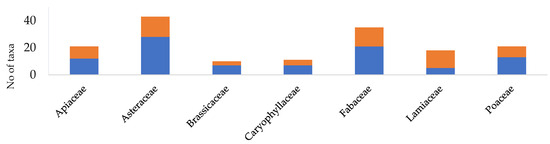
Figure 4.
Representation of ruderal (blue bars) and non-ruderal (orange bars) taxa among the richest-in-taxa families of the UNESCO World Heritage Site of Mystras.
A total of 17 established alien taxa were registered at the MUWHS, belonging to 15 families and 16 genera. Among the alien taxa, phanerophytes predominate (47%) followed by hemicryptophytes (23.5%), geophytes (11.7%), and therophytes (11.7%). Most alien taxa (88.2%) are ruderals. Melia azedarach, an unestablished alien phanerophyte, is also present as separate individuals.
The vascular flora considered as deteriogenic comprise more than 17% (55 taxa) of the taxa registered at the MUWHS. These taxa belong to 31 families and 47 genera, of which 49.1% are ruderals, 12.7% are chasmophytes, 27.3% prefer forest habitats, and 10.9% prefer mainly phryganic communities. Among the taxa characterised as deteriogenic, 29.1% are therophytes, 25.5% are phanerophytes, 27.3% are hemicryptophytes, 10.9% are chamaephytes, and 7.2% are geophytes. The rather common taxa of Adiantum capillus-veneris, Asparagus acutifolius, Asplenium ceterach, Capparis sicula, Ficus carica, Hedera helix, and Smilax aspera, as well as the alien taxa Oxalis pes-caprae, Cymbalaria muralis, Erigeron bonariensis, and Euphorbia prostrata, are among the taxa considered as deteriogenic that are registered at the MUWHS.
The presence of the widespread taxon Hedera helix at the MUWHS is remarkable since it covers large parts of the walls at the MUWHS (Figure 5).

Figure 5.
Extensive presence of the widespread taxon Hedera helix (white arrows) on the walls of the UNESCO World Heritage Site of Mystras.
3.2. Comparison of Different Aspects of the UNESCO World Heritage Site of Mystras’s Flora with Those of Other Archaeological Areas in Greece and the Mediterranean
Comparisons of the distribution of endemic and alien taxa registered at the MUWHS and four other archaeological sites of the Peloponnese for which there is available information are presented in Table 1 and Table 2, respectively.

Table 2.
Alien taxa registered at the UNESCO World Heritage Site of Mystras (MUWHS) and their distribution in the archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN), and Monemvasia (MON). Abbreviations: ERAB: extinction risk based on both the IUCN Criteria A and B; EDGE: Evolutionary Distinct and Globally Endangered index [55].
Figure 6 presents the correlation of the total floras between the MUWHS and the archaeological areas EPD, AKK, AKN, and MON. Figure 7 shows the Shannon diversity index values for five aspects of the floras of the five archaeological sites: all taxa, ruderal, alien, chasmophytic, and endemic taxa.
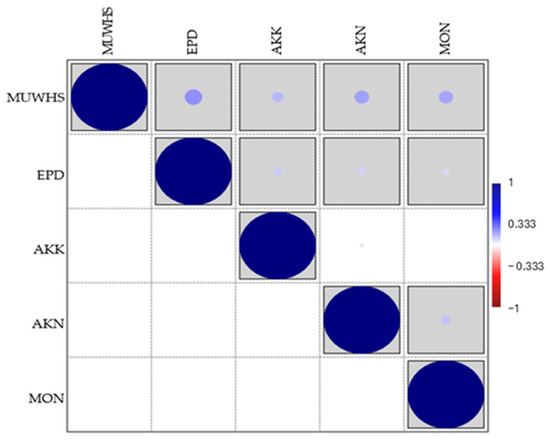
Figure 6.
Diagrams of correlation (Pearson correlation coefficient (r), grey boxed cells p < 0.05) the plant diversity of the UNESCO World Heritage Site of Mystras (MUWHS) and the archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN), and Monemvasia (MON). Different sizes of circles reflect Pearson correlation coefficents’s values (from 1 to −1, as shown to the column on the right side of the diagram).
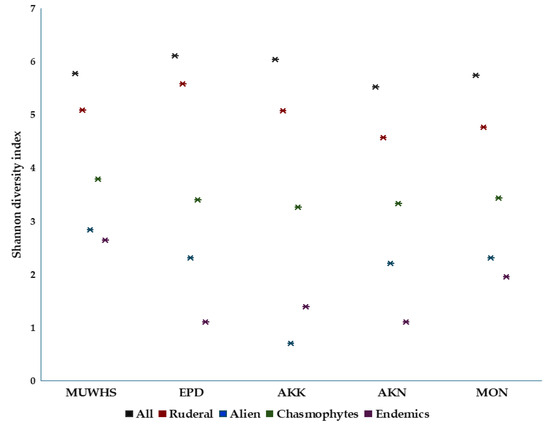
Figure 7.
Shannon diversity index values for different aspects of the floras of the UNESCO World Heritage Site of Mystras (MUWHS) and the archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN), and Monemvasia (MON).
The results of hierarchical clustering (UPGMA-Euclidean distance) among all the floras (and their aspects) of the UNESCO World Heritage Site of Mystras (MUWHS) and the Greek archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN) and Monemvasia (MON) are presented in Figure 8, and Figure 9 presents those concerning hierarchical clustering (UPGMA-Euclidean) for the deteriogenic floras of the MUWHS; EPD, AKK, AKN, and MON; the Italian heritage site of the Etruscan Necropolis of Tarquinia (TAR); archaeological site of Alexandria city in Egypt (ALE); Croatian Historic Structures (CRO); and the Pompeii, Herculaneum, Paestum, and Velia archaeological parks in Italy (POM).
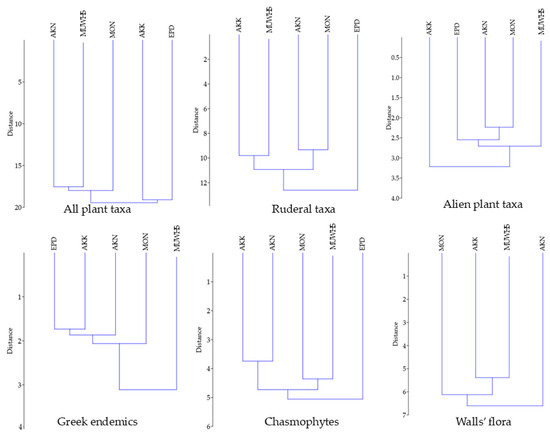
Figure 8.
Hierarchical clustering (UPGMA-Euclidean) for different aspects of plant diversity at the UNESCO World Heritage Site of Mystras (MUWHS) and the archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN), and Monemvasia (MON).
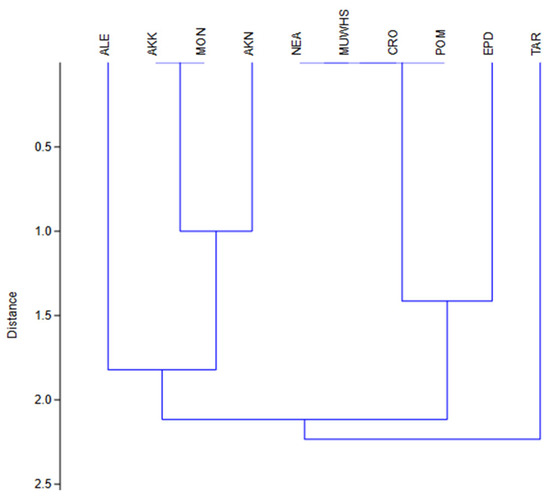
Figure 9.
Hierarchical clustering (UPGMA-Euclidean) for the deteriogenic floras at the UNESCO World Heritage Site of Mystras (MUWHS); the Greek archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN), and Monemvasia (MON); the Italian heritage site of the Etruscan Necropolis of Tarquinia (TAR); the archaeological site of Alexandria city in Egypt (ALE); Croatian Historic Structures (CRO); and the Pompeii, Herculaneum, Paestum, and Velia archaeological parks of Italy (POM).
4. Discussion
The insularity of fragmented habitat patches—habitat islands—may bring positive as well as negative effects, and the significance of habitat fragmentation as a driver of species loss has been subject to debate [63]. Preserving habitat islands is crucial due to their role in supporting unique microenvironments and enabling species dispersal between larger ecosystems [64,65]. The fragmentation of habitat islands is both natural and human-caused [65]; it seems that maintaining “several small” islands and not “a single large” island is more useful for conserving biodiversity in them [63,65,66,67] and that their unique characteristics could demand tailored management approaches, unlike those typically employed in expansive landscapes [65].
UNESCO’s World Heritage Sites represent the combined interaction of nature and people [1]. Viewing nature and humanity as an inseparable unity [68] offers valuable insights and expands the role of culture in both influencing and being influenced by the natural world [69]. Protecting cultural and biological diversity in archaeological areas, especially in UNESCO’s World Heritage Sites, is very important for future generations [8,16,17,70] and an important issue for their management [71,72].
Mystras, a UNESCO World Heritage Site, is regarded as well preserved and carefully maintained, especially in relation to its notable monuments and architectural heritage (https://whc.unesco.org/en/list/511). It is characterised by the provision of several cultural ecosystem function indicators, among which are cultural heritage and environmental assets, like the area’s ecological integrity and biodiversity, including species richness and the presence of key species, complemented by environmental education features—such as vegetated zones, flower-viewing opportunities, designated conservation areas, scientific monitoring sites, scenic landscapes, and both natural and cultural heritage landmarks [71,73,74]. The MUWHS attracts a substantial annual influx of visitors and the pressures on its natural environment are many and rather intense. The pressures and management of this archaeological area are very different to those of other areas, and their species pool demonstrates the cumulative influence of sustained human activity [75].
4.1. Floristic Composition, Local Distribution of Taxa, and Natural Evaluation
The complex history of the UNESCO World Heritage Site of Mystras (MUWHS) and the continuous and extensive human activity has significantly altered the existing natural vegetation preserved within the internal site area. The MUWHS is characterised by rich flora consisting mainly of (a) widespread and mediterranean plants adapted to ruderal and urbanised areas; (b) therophytes adapted to Mediterranean environmental conditions and human interference, most of which belong to the richest-in-taxa families of Asteraceae, Fabaceae, and Poaceae, as is the case in numerous floristic studies carried out in Greece and other Mediterranean areas, archaeological or not, at low elevations [10,11,56,58,59,76,77,78,79,80].
Beyond its historical prominence, Mystras also serves as a reservoir of botanical diversity. The high number of plant taxa is due to the habitat heterogeneity (cliffs, walls, ruins, shrubland, degraded areas, managed areas, etc.) and different plant communities (chasmophytic, ruderals, aliens, etc.) observed on MUWHS. The rather high number of endemic species (14 Greek and 7 Balkan endemics) that have been found mainly on cliffs and walls punctuates the potential of the UNESCO World Heritage Site of Mystras (MUWHS) as a refuge for rare plant taxa, as is the case for other areas in the Mediterranean [60]. The Greek endemic taxa registered, of which three are regional endemics and 13 have been assessed as Vulnerable or Endangered and which present significant EDGE values, are range-restricted and threatened, occurring mainly on cliffs, rocks or walls where rather extreme conditions for plants occur [81,82]. The wall’s flora is usually related to very dry environments because of the lack of water and sediments on these walls and usually represents very different plant communities. The populations of the endemic and range-restricted taxa found on the walls are represented by a very small number of individuals, and the monitoring of their conservation status is important [6,7,83,84].
On the other hand, the high number of widespread ruderal taxa is attributable to the area’s topographical characteristics and the continuous anthropogenic influence. The same factors, together with the ecological structure of the landscape, drive the dominance of ruderals and therophytes that have a rapid life cycle [85]. Most of the ruderals are therophytes that can survive in the soil seed bank during summer and after periodic cutting and other management practices [9,10,86,87] in archaeological areas. It is important to mention that ruderal taxa are early colonisers of ecologically degraded or abandoned areas and play a vital role in ecosystem functionality, acting as one of the drivers for urban green space sustainability [88]. Among ruderal habitats, a rather low number of alien taxa have been registered, most of which are phanerophytes like Agave americana, Punica granatum, Lantana camara, Opuntia ficus-indica and Carpobrotus edulis. The latter three taxa are invasive, presenting negative effects on Mediterranean ecosystems [89], and are among the most strongly influential plant communities of the Mediterranean [90,91].
Τhe natural significance of the UNESCO World Heritage Site of Mystras (321 plant taxa in total, 14 Greek endemics, 7 Balkan endemics, 161 ruderal and 17 established alien plant taxa) is as high as its historical significance (the medieval city of MUWHS). Although the plant diversity of the MUWHS is high and it is important that it is preserved—together with the archaeological and historical monuments—the role of a few plants, mainly those growing on the ancient walls, is controversial [16,17,28,44,92,93,94], and it is important that this is considered in the conservation evaluation of archaeological areas [8]. Hedera helix on the MUWHS’s walls is a perfect example (Figure 5), since it has a double protective and deteriogenic role [92,95]. Hedera helix can shield surfaces from direct weathering; it also contributes to physical and chemical deterioration, causing structural instability and fragmentation over time [92]. Maintaining both cultural heritage and the intricate diversity of wall surfaces is made possible through expert restoration practices [93,96,97,98].
4.2. Comparison of Different Aspects of the UNESCO World Heritage Site of Mystras’s Flora with Those of Other Archaeological Areas in Greece and the Mediterranean
A total of 818 plant taxa have been registered at the UNESCO World Heritage Site of Mystras (MUWHS) and the other four archaeological sites of the same phytogeographical area of Greece, the Peloponnese (Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN) and Monemvasia (MON)), of which 53 (6.5%) are endemics, 298 (36.4%) are ruderals and 40 (4.9%) are aliens.
The comparison of the MUWHS with the other four archaeological areas of the Peloponnese (AKK, AKN, MON and EPD), which altogether cover an area of 72.38 km2, revealed their high total and endemic plant diversity that represents 20.2% of the total flora or 25.8% of the autochthonous spermatophyte flora and 11.1% of the Greek endemics found on the Peloponnese. The high number of endemic taxa registered in these archaeological areas highlights their importance as refuges for rare and endangered or vulnerable plants. Among the 53 Greek endemic taxa registered in these five archaeological areas, 22 have been assessed as Vulnerable according to the IUCN criteria A and B (8 at the MUWHS), 21 as Endangered (5 at the MUWHS) and 3 as Critically Endangered, while their EDGE values are from 2.19 to 6.59 [55].
Comparing the endemism of the UNESCO World Heritage Site of Mystras (MUWHS) with that of the other four archaeological sites of the Peloponnese for which there is available information, namely, Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN) and Monemvasia (MON), six endemic taxa were found at the MUWHS but not at EPD or the three castles of the East Peloponnese (AKK, AKN and MON), while one, Campanula drabifolia, was common to the MUWHS, EPD, AKK, AKN and MON (Table 1). Inula verbascifolia subsp. methanaea was registered at the MUWHS and all three castles of the East Peloponnese (AKK, AKN, MON), while Campanula topaliana subsp. topaliana, Delphinium hellenicum, Scaligeria moreana, Silene integripetala, Silene italica subsp. peloponnesiaca and Stachys candida were found only at the MUWHS (Table 1).
When comparing their alien flora, Oxalis pes-caprae is the most common alien taxon at the MUWHS and all four archaeological sites, followed by Agave americana subsp. americana, Erigeron bonariensis, Lantana camara, and Prunus dulcis (Table 2). Oxalis pes-caprae, Euphorbia prostrata and Cymbalaria muralis are among the alien taxa that are also considered deteriogenic.
The α-diversity of the total plant flora and of the endemic flora of the UNESCO World Heritage Site of Mystras (MUWHS) is 25.5 and 1.1, respectively. The α-diversity of the total plant flora (818 taxa) and of the endemic flora (53 taxa) of the MUWHS together with EPD, AKK, AKN and MON (total surface area 72.38 km2) is 11.3 and 0.73, respectively. The α-diversity of the total plant flora and of the endemic flora of the five archaeological sites (11.3 and 0.73) is much higher than that of the Peloponnese (0.18 and 0.02, respectively), highlighting the importance of the conservation of their plant diversity and communities. All areas present high values of Shannon diversity for their total, ruderal (generalist plant taxa) and chasmophytic (specialised on cliffs) flora, and the MUWHS presents the highest values for endemic (specialists thriving in areas with limited anthropogenic pressure and management influence) and alien (generalists) flora, underlying the need for sustainable landscape management that considers the site’s cultural heritage and environmental characteristics [8,16,44,80,82,85,86,99,100].
The correlation between the total floras of the UNESCO World Heritage Site of Mystras (MUWHS) and the archaeological areas EPD, AKK, AKN and MON is rather weak (Figure 6) since the Pearson correlation coefficient (r) values among the flora of the MUWHS and those of EPD, AKK, AKN and MON are 0.219, 0.141, 0.184, and 0.178, respectively. The Shannon diversity index values for five aspects of their floras, namely, all taxa, ruderal, chasmophytic, alien, and endemic taxa, and especially the higher values for the first three aspects (Figure 7), indicate their high diversity at these five archaeological sites.
The results of hierarchical clustering (UPGMA-Euclidean distance) show that, on the one hand, all floras of the archaeological areas of the MUWHS, EPD, AKK, AKN and MON are rather similar, and on the other hand, underline the significant differences in different aspects of their plant diversity, especially concerning Greek endemics (Figure 8). The low correlation and the high dissimilarity of most of the different aspects of plant diversity among the MUWHS, EPD, AKK, AKN and MON reflect the high floristic independence of the MUWHS. Human impact seems to act as a key driver enhancing the existing high plant species richness of these archaeological areas [89,99,101,102,103] since their elevation is rather low and their surface area is not the dominant factor affecting their species richness [10].
The comparison of the deteriogenic flora of the UNESCO World Heritage Site of Mystras (MUWHS) with those of the Greek archaeological areas of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN) and Monemvasia (MON) showed that 43% of the taxa registered at the MUWHS were also found at these four sites (45.4% of the taxa are therophytes, chamaephytes and hemicryptophytes are both represented by 18.2% each, and phanerophytes and geophytes both by 9.1%) and 33% at the MUWHS at three of the sites. Among the most common taxa found on the walls and the surfaces of the monuments of MUWHS and at EPD, AKK, AKN and MON, as well as eight more Greek archaeological areas studied by [56,57], are Asplenium ceterach, Avena barbata, Capparis spp., Dactylis glomerata subsp. hispanica, Ficus carica, Galium aparine, Mercurialis annua, Oxalis pes-caprae, Papaver rhoeas, Parietaria cretica, Piptatherum miiaceum, Reicharia picroides, Sanguisorba minor, Sonchus oleraceus, Trifolium campestre and Umbilicus horizontalis.
When the deteriorating flora of the UNESCO World Heritage Site of Mystras (MUWHS) is compared with that of the Greek sites of Epidaurus (EPD), Akrokorinthos (AKK), Akronafplia (AKN) and Monemvasia (MON) and the Mediterranean areas of the Italian heritage site of the Etruscan Necropolis of Tarquinia (TAR), archaeological sites of Alexandria city of Egypt (ALE), Croatian Historic Structures (CRO), and Pompeii, Herculaneum, Paestum, and Velia archaeological Parks of Italy (POM) (Figure 9), significant-to-very-high similarities are revealed, especially among MUWHS, NEA, CRO and POM. A proportion of 27.3% of the deteriogenic taxa registered at the MUWHS were also found at all or most of NEA, TAR, ALE, CRO and POM. A total of 60% of these taxa are therophytes, 26.7% are hemicryptophytes, followed by 6.7% chamaephytes and geophytes. The most common deteriogenic taxa at the archaeological areas are Avena barbata, Anagalis arvensis, Asplenium ceterach (obligate chasmophyte), Bromus madritensis subsp. madritensis, Capparis spp., Catapodium rigidum, Dactylis glomerata subsp. hispanica, Ficus carica, Hedera helix, Malva sylvestris, Mercurialis annua, Oxalis pes-caprae, Papaver rhoeas, Parietaria spp., Piptatherum miliaceum, Pistacia lentiscus, Reicharia picroides, Sonchus spp., Trifolium spp. and Verbascum sinuatum.
The life forms of the taxa, the type of roots, their habitat preferences and specific functional traits are good indicators, since the growth of pioneer forests’ phanerophytes (trees, shrubs and lianas) with woody roots on ancient walls and monuments strongly increases hazards, while hemicryptophytes, chamaephytes and geophytes, ruderals and chasmophytes can cause very-low-to-medium hazards, and therophytes with fasciculate roots can cause very low to low [13,22,23,26,98]. At the UNESCO World Heritage Site of Mystras (MUWHS), among the taxa considered deteriogenic, phanerophytes are represented by 25%, indicating a rather high hazard risk for the walls and buildings, and therophytes predominate (29%), indicating a low one. Woody species like trees, shrubs, and climbing vines develop deep, powerful roots that can penetrate cracks and joints in stone, brick, or mortar [104] and they are much more destructive than herbaceous plants [23]. Woody invasive species can reach the upper sections of monuments via wind or bird dispersal, and when established, they are harder to detect and manage, especially in tall or inaccessible ruins [104].
Depending on the risk of deterioration [13,24], specific management measures and practices can be used with respect to both natural and cultural environments [22,93,94,96,98]. For example, for Ficus carica and perennial Parietaria spp., as shown by [28], regular plant control is needed when they are present on vertical middle and upper parts of walls or in inclined cavities. A management plan should first focus on aggressive deteriogenic woody plant taxa, identify monuments with existing or potential infestations, and schedule (a) careful removal techniques (e.g., the removal of roots and stems during dormancy to reduce regrowth), (b) post-removal monitoring, (c) preventive measures (buffer zones, visitor education and native planting) and (d) collaboration with local authorities and community engagement [105].
5. Conclusions
The effective and sustainable conservation of UNESCO World Heritage Sites, such as the medieval city of Mystras, and all archaeological areas must be based on in-depth knowledge of their history, human interactions with ecosystems, the landscape, land uses, biodiversity, and all cultural and natural aspects [8,9,10,11,17,44,54,92,93,94,106,107,108]. The necessity for combined measures to maintain cultural landscapes and to conserve the biodiversity within the archaeological areas is urgent and substantial [21].
The current study highlights the substantial richness of plant taxa of the habitat island of the archaeological area of Mystras, and the need for inventorying and investigations concerning the interactions and co-existence of the historical elements and biodiversity (plant species diversity) within the framework of climate change, as well as the continuous influence of the numerous visitors, for devising the best case-specific management approach.
This research underscores the importance of integrating ecological awareness into the sustainable management of heritage landscapes. Archaeological areas, often rich in microhabitats, can function as reservoirs for native flora and as models for balancing conservation with cultural preservation. In habitat islands, as with archaeological areas, the sustainable management and conservation of both monuments and plant diversity presuppose a deep knowledge of all aspects of species diversity, ecology and functional traits and different actions depending on the possible danger of biodeterioration of the monuments and on the ecosystem services that plants and plant diversity provide [7,17,18,24,28,44,56,92,109,110]. Species-rich areas and ecosystems are typically found in landscapes characterised by structural complexity and reduced anthropogenic pressure [111]. While changes in the extent of habitat islands offer limited insight into shifts in species richness, strategic efforts to improve habitat conditions and regulate resident populations can significantly boost ecological diversity [112].
People consumed resources and transformed or ‘resculpted’ natural landscapes and ecosystems in the Mediterranean to their own ends [89]. The dynamic interplay between cultural identity and ecological context has been highlighted by the Nature as Culture perspective [68,69]. The often-overlooked role of cultural areas concerning their contribution to plant diversity conservation and the promotion of sustainable ecological management is underscored through this research. It is essential to investigate all aspects of plant diversity, focusing on the conservation of endemic taxa and on controlling the populations of the plants considered deteriogenic, using nature-based solutions and techniques. Conservation strategies should include additional actions to address climate change’s primary and cascading effects, together with non-climate impacts, based on an understanding of the primary risks associated with climate change or capitalising on climate-induced shifts for strategic long-term benefits [111]. Strategies for the sustainable conservation of archaeological landscapes should include site-specific conservation plans tailored to the unique historical, ecological, and cultural attributes of each location [113]. These plans must integrate detailed assessments of the site’s physical condition, biodiversity, visitor impact, and surrounding land use. As emphasised in international guidelines such as UNESCO’s Management Plans for World Heritage Sites [114], such frameworks serve as essential tools for balancing preservation with adaptive reuse, community engagement, and environmental resilience.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17110749/s1, Table S1. Plant taxa registered at the Mystras UNESCO World Heritage Site.
Author Contributions
Conceptualization, M.P.; methodology, M.P.; validation, M.P., M.T. and I.C.; formal analysis, M.P., M.T., I.C. and I.N.; investigation, M.P., M.T., I.C. and I.N.; resources, M.P., M.T., I.C. and I.N.; data curation, M.T., I.C. and I.N.; writing—original draft preparation, M.P.; writing—review and editing, M.P., M.T. and I.C.; visualisation, M.P. and M.T.; supervision, M.P.; project administration, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
This research was supported in part by ongoing research funded by the Natural Environment and Climate Change Agency (NECCA) of Greece, in the framework of the project “Biodiversity and Archaeological areas: Inventory of flora and fauna of archaeological areas”. Fieldwork was carried out through special permits issued by the Ministry of Culture and Sports (Protocol Number: 70512-24/2/2023) and the Ministry of Environment and Energy (A∆A: ΨΥΓP4653Π8-ΦEP), both supporting the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wiktor-Mach, D. What Role for Culture in the Age of Sustainable Development? UNESCO’s Advocacy in the 2030 Agenda Negotiations. Int. J. Cult. Policy 2020, 26, 312–327. [Google Scholar] [CrossRef]
- UNESCO. Recommendation on the Historic Urban Landscape. In Proceedings of the Records of the General Conference 31st Session, Paris, France, 15 October–3 November 2011; Volume 1. [Google Scholar]
- Turner, S.; Kinnaird, T.; Koparal, E.; Lekakis, S.; Sevara, C. Landscape Archaeology, Sustainability and the Necessity of Change. World Archaeol. 2020, 52, 589–606. [Google Scholar] [CrossRef]
- Fairclough, G. Landscape and Heritage: Ideas from Europe for Culturally Based Solutions in Rural Environments. J. Environ. Plan. Manag. 2019, 62, 1149–1165. [Google Scholar] [CrossRef]
- Capotorti, G.; Del Vico, E.; Lattanzi, E.; Tilia, A.; Celesti-Grapow, L. Exploring Biodiversity in a Metropolitan Area in the Mediterranean Region: The Urban and Suburban Flora of Rome (Italy). Plant Biosyst. 2013, 147, 174–185. [Google Scholar] [CrossRef]
- Ceschin, S.; Caneva, G. Plants as Bioindicators for Archaeological Prospection: A Case of Study from Domitian’s Stadium in the Palatine (Rome, Italy). Environ. Monit. Assess 2013, 185, 5317–5326. [Google Scholar] [CrossRef] [PubMed]
- Ceschin, S.; Bartoli, F.; Salerno, G.; Zuccarello, V.; Caneva, G. Natural Habitats of Typical Plants Growing on Ruins of Roman Archaeological Sites (Rome, Italy). Plant Biosyst. 2016, 150, 866–875. [Google Scholar] [CrossRef]
- Cicinelli, E.; Salerno, G.; Caneva, G. An Assessment Methodology to Combine the Preservation of Biodiversity and Cultural Heritage: The San Vincenzo al Volturno Historical Site (Molise, Italy). Biodivers Conserv. 2018, 27, 1073–1093. [Google Scholar] [CrossRef]
- Cicinelli, E.; Benelli, F.; Bartoli, F.; Traversetti, L.; Caneva, G. Trends of Plant Communities Growing on the Etruscan Tombs (Cerveteri, Italy) Related to Different Management Practices. Plant Biosyst. 2020, 154, 158–164. [Google Scholar] [CrossRef]
- Panitsa, M.; Tsakiri, M.; Kampiti, D.; Skotadi, M. Archaeological Areas as Habitat Islands: Plant Diversity of Epidaurus UNESCO World Heritage Site (Greece). Diversity 2024, 16, 403. [Google Scholar] [CrossRef]
- Panitsa, M.; Trigas, P.; Kontakos, D.; Valli, A.T.; Iatrou, G. Natural and Cultural Heritage Interaction: Aspects of Plant Diversity in Three East Peloponnesian Castles (Greece) and Conservation Evaluation. Plant Biosyst. 2022, 156, 538–552. [Google Scholar] [CrossRef]
- Iatrou, G.; Trigas, P.; Pettas, N. The Vascular Flora of Akrokorinthos Castle and Its Surrounding Area (NE Peloponnese, Greece). Phytol. Balc. 2007, 13, 83–93. [Google Scholar]
- Caneva, G.; Hosseini, Z.; Bartoli, F.; Capotorti, G.; Attorre, F.; Blasi, C. Revaluating CUNA Places (CUltural Place of High Relevance for NAture): Rome as a Multifaced Example of Outstanding Values and Potentials. Land 2025, 14, 226. [Google Scholar] [CrossRef]
- Vlami, V.; Kokkoris, I.P.; Zogaris, S.; Cartalis, C.; Kehayias, G.; Dimopoulos, P. Cultural Landscapes and Attributes of “Culturalness” in Protected Areas: An Exploratory Assessment in Greece. Sci. Total Environ. 2017, 595, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Cutini, M.; Pacini, A.; Vinci, M. Analysis of the Colosseum’s Floristic Changes during the Last Four Centuries. Plant Biosyst. 2002, 136, 291–311. [Google Scholar] [CrossRef]
- Caneva, G.; Benelli, F.; Bartoli, F.; Cicinelli, E. Safeguarding Natural and Cultural Heritage on Etruscan Tombs (La Banditaccia, Cerveteri, Italy). Rend Lincei-Sci. Fis. 2018, 29, 891–907. [Google Scholar] [CrossRef]
- Cicinelli, E.; Zangari, G.; Bartoli, F.; Isola, D.; Lucchese, F.; Caneva, G. Protecting Monuments and Plant Biodiversity in Archaeological Sites: The Case of the Etruscan Necropolis of “Monterozzi” (Tarquinia, Central Italy). In Proceedings of the Acta Horticulturae, VIII International Conference on Landscape and Urban Horticulture, Catania, Italy, 15–17 December 2022; Volume 1345. [Google Scholar]
- Hosseini, Z.; Bartoli, F.; Cicinelli, E.; Lucchese, F. First Floristic Investigation in Archaeological Sites of Iran: Features and Plant Richness of the Pasargadae World Heritage Site. Plant Biosyst. 2023, 157, 605–621. [Google Scholar] [CrossRef]
- Scafidi, F.; Raimondo, F.M. Contribution to the Vascular Flora of the Archaeological Park of Selinunte and Cave of Cusa (South-Western Sicily, Italy): Preliminary Results. Flora Mediterr. 2018, 28, 371–390. [Google Scholar] [CrossRef]
- Dehnen-Schmutz, K. Alien Species Reflecting History: Medieval Castles in Germany. Divers Distrib. 2004, 10, 147–151. [Google Scholar] [CrossRef]
- Heneidy, S.Z.; Al-Sodany, Y.M.; Bidak, L.M.; Fakhry, A.M.; Hamouda, S.K.; Halmy, M.W.A.; Alrumman, S.A.; Al-Bakre, D.A.; Eid, E.M.; Toto, S.M. Archeological Sites and Relict Landscapes as Refuge for Biodiversity: Case Study of Alexandria City, Egypt. Sustainability 2022, 14, 2416. [Google Scholar] [CrossRef]
- Cozzolino, A.; Bonanomi, G.; Motti, R. The Role of Stone Materials, Environmental Factors, and Management Practices in Vascular Plant-Induced Deterioration: Case Studies from Pompeii, Herculaneum, Paestum, and Velia Archaeological Parks (Italy). Plants 2025, 14, 514. [Google Scholar] [CrossRef]
- Cozzolino, A.; Adamo, P.; Bonanomi, G.; Motti, R. The Role of Lichens, Mosses, and Vascular Plants in the Biodeterioration of Historic Buildings: A Review. Plants 2022, 11, 3429. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Stinca, A. Analysis of the Biodeteriogenic Vascular Flora at the Royal Palace of Portici in Southern Italy. Int. Biodeterior. Biodegrad. 2011, 65, 1256–1265. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Stinca, A. Biodeteriogens at a Southern Italian Heritage Site: Analysis and Management of Vascular Flora on the Walls of Villa Rufolo. Int. Biodeterior. Biodegrad. 2021, 162, 105252. [Google Scholar] [CrossRef]
- Gunasdi, Y.; Aksakal, O.; Kemaloglu, L. Biodeterioration of Some Historical Monuments in Erzurum by Vascular Plants. Int Biodeterior Biodegrad. 2023, 176, 105530. [Google Scholar] [CrossRef]
- Cozzolino, A.; Motti, R.; Vitasovic-Kosic, I. Vascular Flora on Croatian Historic Structures: Drivers of Biodeterioration and Conservation Implications. Plants 2025, 14, 1773. [Google Scholar] [CrossRef]
- Caneva, G.; Hosseini, Z.; Bartoli, F. Risk, Hazard, and Vulnerability for Stone Biodeterioration Due to Higher Plants: The Methodological Proposal of a Multifactorial Index (RHV). J. Cult. Herit. 2023, 62, 217–229. [Google Scholar] [CrossRef]
- Hosseini, Z.; Caneva, G. Evaluating Hazard Conditions of Plant Colonization in Pasargadae World Heritage Site (Iran) as a Tool of Biodeterioration Assessment. Int. Biodeterior. Biodegrad. 2021, 160, 105216. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Hot-Spots Analysis for Conservation of Plant Biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial Refugia Influence Plant Diversity Patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Monnet, A.C.; Cilleros, K.; Médail, F.; Albassatneh, M.C.; Arroyo, J.; Bacchetta, G.; Bagnoli, F.; Barina, Z.; Cartereau, M.; Casajus, N.; et al. WOODIV, a Database of Occurrences, Functional Traits, and Phylogenetic Data for All Euro-Mediterranean Trees. Sci. Data 2021, 8, 89. [Google Scholar] [CrossRef]
- Başer, K.H.C. Endemic Plants of Greece The Peloponnese. Turk. J. Bot. 2002, 26, 10. [Google Scholar]
- Trigas, P.; Tsiftsis, S.; Tsiripidis, I.; Iatrou, G. Distribution Patterns and Conservation Perspectives of the Endemic Flora of Peloponnese (Greece). Folia Geobot. 2012, 47, 421–439. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Strid, A. The Botanical Exploration of Greece. Plant Syst. Evol. 2020, 306, 27. [Google Scholar] [CrossRef]
- UNESCO. UNESCO Moving Forward the 2030 Agenda for Sustainable Development; The United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Hoffmann, R. Medieval Land Use and the Formation of Traditional European Landscapes. In An Environmental History of Medieval Europe; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Watson, D.M. A Conceptual Framework for Studying Species Composition in Fragments, Islands and Other Patchy Ecosystems. J. Biogeogr. 2002, 29, 823–834. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Millien, V. A Global Synthesis of the Small-Island Effect in Habitat Islands. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181868. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Kadakas, U. Archaeological Heritage as a Sustainer of Biodiversity. Internet Archaeol. 2022, 60, 1–15. [Google Scholar] [CrossRef]
- Zangari, G.; Bartoli, F.; Lucchese, F.; Caneva, G. Plant Diversity in Archaeological Sites and Its Bioindication Values for Nature Conservation: Assessments in the UNESCO Site Etruscan Necropolis of Tarquinia (Italy). Sustainability 2023, 15, 16469. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Volume 2; Cambridge University Press: Cambridge, UK, 1968. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Volume 3; Cambridge University Press: Cambridge, UK, 1972. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Volume 4; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Volume 5; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea 1 (2nd Edition); Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Strid, A.; Tan, K. Flora Hellenica. 1; Koeltz Scientific Books: Königstein, Germany, 1997. [Google Scholar]
- Strid, A.; Tan, K. Flora Hellenica. 2.; A. R. G. Gantner: Ruggell, Switzerland, 2002. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Englera; Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany, 2013; pp. 1–372. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Strid, A. Flora of Greece Web. In Vascular Plants of Greece, an Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany, 2013. [Google Scholar]
- Hosseini, Z.; Zangari, G.; Carboni, M.; Caneva, G. Substrate Preferences of Ruderal Plants in Colonizing Stone Monuments of the Pasargadae World Heritage Site, Iran. Sustainability 2021, 13, 9381. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Kanellou, E.; Papafotiou, M.; Saitanis, C.; Economou, G. Ecological Analysis and Opportunities for Enhancement of the Archaeological Landscape: The Vascular Flora of Seven Archaeological Sites in Greece. Environments 2024, 11, 16. [Google Scholar] [CrossRef]
- Krigas, N.; Lagiou, E.; Hanlidou, E.; Kokkini, S. The Vascular Flora of the Byzantine Walls of Thessaloniki (N Greece). Willdenowia 1999, 29, 77–94. [Google Scholar] [CrossRef]
- Minissale, P.; Sciandrello, S. The Wild Vascular Flora of the Archaeological Park of Neapolis in Syracuse and Surrounding Areas (Sicily, Italy). Biodivers. J. 2017, 8, 87–104. [Google Scholar]
- Minissale, P.; Sciandrello, S.; Trigilia, A.; Brogna, F. Plants and Vegetation in the Archaeological Park of Neapolis of Syracuse (Sicily, Italy): A Management Effort and also an Opportunity for Better Enjoyment of the Site. Conserv. Manag. Archaeol. Sites 2015, 17, 340–369. [Google Scholar] [CrossRef]
- Azzaro, D.; Cambria, S.; Porrovecchio, M.; Minissale, P. Diachronic Analysis of the Floristic Diversity of the Special Area of Conservation (SAC) “Bosco Di Santo Pietro” (South-Eastern Sicily): A Mediterranean Biodiversity Hotspot. Plants 2025, 14, 788. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, D.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Whittaker, R.; Fernandez-Palacios, J.M.; Matthews, T.J. Island Biogeography: Geo-Environmental Dynamics, Ecology, Evolutio, Human Impact, and Conservation; Oxford University Press, Ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Hannah, L.; Flint, L.; Syphard, A.D.; Moritz, M.A.; Buckley, L.B.; McCullough, I.M. Fine-Grain Modeling of Species’ Response to Climate Change: Holdouts, Stepping-Stones, and Microrefugia. Trends Ecol. Evol. 2014, 29, 390–397. [Google Scholar] [CrossRef]
- Cartwright, J. Ecological Islands: Conserving Biodiversity Hotspots in a Changing Climate. Front. Ecol. Environ. 2019, 17, 331–340. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Cross, M.; Rowland, E.; Joseph, L.N.; Rao, M.; Seimo, A. Planning for Species Conservation in a Time of Climate Change. In Climate Change—Research and Technology for Adaptation and Mitigation; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar][Green Version]
- Watson, J.E.M.; Dudley, N.; Segan, D.B.; Hockings, M. The Performance and Potential of Protected Areas. Nature 2014, 515, 67–73. [Google Scholar] [CrossRef]
- Himes, A.; Muraca, B. Relational Values: The Key to Pluralistic Valuation of Ecosystem Services. Curr. Opin. Environ. Sustain. 2018, 35, 1–7. [Google Scholar] [CrossRef]
- Kim, H.J.; Peterson, G.D.; Cheung, W.W.L.; Ferrier, S.; Alkemade, R.; Arneth, A.; Kuiper, J.J.; Okayasu, S.; Pereira, L.; Acosta, L.A.; et al. Towards a Better Future for Biodiversity and People: Modelling Nature Futures. Glob. Environ. Change 2023, 82, 102681. [Google Scholar] [CrossRef]
- UNESCO. UNESCO Global Report on Culture and Sustainable Urban Development. Concept Note. In Proceedings of the International Conference on “Culture for Sustainable Cities, Hangzhou, China, 10–12 December 2015. [Google Scholar]
- Hølleland, H.; Skrede, J.; Holmgaard, S.B. Cultural Heritage and Ecosystem Services: A Literature Review. Conserv. Manag. Archaeol. Sites 2017, 19, 210–237. [Google Scholar] [CrossRef]
- Ndoro, W. Conservation and Management of Archaeological Heritage Resources. I. In Sharing Conservation Decisions: Current Issues and Future Strategies; Heritage, A., Copithorne, J., Eds.; International Centre for the Study of the Preservation and Restoration of Cultural Property (ICCROM): Rome, Italy, 2018. [Google Scholar]
- López Sánchez, M.; Tejedor Cabrera, A.; Linares Gómez del Pulgar, M. The Potential Role of Cultural Ecosystem Services in Heritage Research through a Set of Indicators. Ecol. Indic. 2020, 117, 106670. [Google Scholar] [CrossRef]
- Maes, J.; Liquete, C.; Teller, A.; Erhard, M.; Paracchini, M.L.; Barredo, J.I.; Grizzetti, B.; Cardoso, A.; Somma, F.; Petersen, J.E.; et al. An Indicator Framework for Assessing Ecosystem Services in Support of the EU Biodiversity Strategy to 2020. Ecosyst. Serv. 2016, 17, 14–23. [Google Scholar] [CrossRef]
- Celka, Z.; Brzeg, A.; Sobczyński, A. Transformations of Vascular Flora of a Medieval Settlement Site: A Case Study of a Fortified Settlement in Giecz (Wielkopolska Region, Western Poland). Diversity 2023, 15, 35. [Google Scholar] [CrossRef]
- Massaad, M.; Merheb, J.; Chalak, L. Flora Diversity and Distribution across Some Lebanese Archeological Sites. Flora Mediterr. 2023, 33, 101–109. [Google Scholar] [CrossRef]
- Panitsa, M.; Tzanoudakis, D. Floristic Diversity on Small Islands and Islets: Leros Islets’ Group (East Aegean Area, Greece). Phytol. Balc. 2010, 16, 271–284. [Google Scholar]
- Iliadou, E.; Panitsa, M.; Raus, T.; Dimopoulos, P. Flora and Factors Affecting Species Diversity in the Islet Groups of the Protected “Natura 2000” Sites of the Amvrakikos Gulf and Mesolongi Lagoon (Ionian Area, Greece). Willdenowia 2014, 44, 439–450. [Google Scholar] [CrossRef]
- Bergmeier, E.; Ristow, M.; Krause, J.; Meyer, S.; Panitsa, M. Phytodiversity of Limnos (North Aegean, Greece)—An Update and Evaluation. Flora Medit. 2021, 31, 233–246. [Google Scholar]
- Valli, A.T.; Kougioumoutzis, K.; Iliadou, E.; Panitsa, M.; Trigas, P. Determinants of Alpha and Beta Vascular Plant Diversity in Mediterranean Island Systems: The Ionian Islands, Greece. Nord. J. Bot. 2019, 37, e02156. [Google Scholar] [CrossRef]
- Kontopanou, A.; Panitsa, M. Habitat Islands on the Aegean Islands (Greece): Elevational Gradient of Chasmophytic Diversity, Endemism, Phytogeographical Patterns and Need for Monitoring and Conservation. Diversity 2020, 12, 33. [Google Scholar] [CrossRef]
- Panitsa, M.; Kontopanou, A. Diversity of Chasmophytes in the Vascular Flora of Greece: Floristic Analysis and Phytogeographical Patterns. Bot. Serb. 2017, 41, 199–211. [Google Scholar] [CrossRef]
- Cogoni, D.; Fenu, G.; Dessì, C.; Deidda, A.; Giotta, C.; Piccitto, M.; Bacchetta, G. Importance of Plants with Extremely Small Populations (Psesps) in Endemic-Rich Areas, Elements Often Forgotten in Conservation Strategies. Plants 2021, 10, 1504. [Google Scholar] [CrossRef]
- Ceschin, S.; Cancellieri, L.; Caneva, G.; Battisti, C. Size Area, Patch Heterogeneity and Plant Species Richness across Archaeological Sites of Rome: Different Patterns for Different Guilds. Vie Milieu 2012, 62, 165–171. [Google Scholar]
- Petersen, T.K.; Speed, J.D.M.; Grøtan, V.; Austrheim, G. Competitors and Ruderals Go to Town: Plant Community Composition and Function along an Urbanisation Gradient. Nord. J. Bot. 2021, 39, e03026. [Google Scholar] [CrossRef]
- Panitsa, M.; Iliadou, E.; Kokkoris, I.; Kallimanis, A.; Patelodimou, C.; Strid, A.; Raus, T.; Bergmeier, E.; Dimopoulos, P. Distribution Patterns of Ruderal Plant Diversity in Greece. Biodivers Conserv. 2020, 29, 869–891. [Google Scholar] [CrossRef]
- Torija, M.G.; Quintana, J.R.; Pino-Bodas, R.; Molina, J.A. Contribution of Ruderal Herbaceous Vegetation to Supporting Services in Mediterranean Urban Greenspaces. Biodivers Conserv. 2024, 34, 173–189. [Google Scholar] [CrossRef]
- Mogîldea, D.; Biță-Nicolae, C. Ruderal Plant Diversity as a Driver for Urban Green Space Sustainability. Urban Sci. 2024, 8, 159. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J.; Bodiou, J.-Y.; Boeuf, G. The Mediterranean Region: Biological Diversity in Space and Time; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Nentwig, W.; Bacher, S.; Kumschick, S.; Pyšek, P.; Vilà, M. More than “100 Worst” Alien Species in Europe. Biol. Invasions 2018, 20, 1611–1621. [Google Scholar] [CrossRef]
- Solomou, A.D.; Germani, R.; Galanis, C.; Kakara, S.; Vallianou, K.; Sarros, T. Alien Vascular Flora in Mediterranean Terrestrial Landscapes of Greece. In Advances in Science, Technology and Innovation; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Bartoli, F.; Romiti, F.; Caneva, G. Aggressiveness of Hedera helix L. Growing on Monuments: Evaluation in Roman Archaeological Sites and Guidelines for a General Methodological Approach. Plant Biosyst. 2017, 151, 866–877. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Gohlke, A.; Mahler, C.; Schmiedinger, A.; Beierkuhnlein, C. Quantification of Wall Surface Heterogeneity and Its Influence on Species Diversity at Medieval Castles—Implications for the Environmentally Friendly Preservation of Cultural Heritage. J. Cult. Herit. 2013, 14, 219–228. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural Biocides for the Conservation of Stone Cultural Heritage: A Review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Vercruysse, W.; Noppen, B.; Jozefczak, M.; Huybrechts, M.; Derveaux, E.; Vandecasteele, B.; Cuypers, A.; Marchal, W.; Vandamme, D. Common Ivy (Hedera helix L.) as a Novel Green Resource in an Urban Biorefinery Concept. ACS Sustain. Chem. Eng. 2023, 11, 14267–14286. [Google Scholar] [CrossRef]
- Arreche, R.; Vázquez, P. Green Biocides to Control Biodeterioration in Materials Science and the Example of Preserving World Heritage Monuments. Curr. Opin. Green Sustain. Chem. 2020, 25, 100359. [Google Scholar] [CrossRef]
- Leccese, F.; Leccisi, M.; Schirripa Spagnolo, G. Weed Control in Secondary Archaeological Sites by Means of Precision Agriculture Techniques. Acta IMECO 2024, 13, 1–9. [Google Scholar] [CrossRef]
- Carrari, E.; Aglietti, C.; Bellandi, A.; Dibari, C.; Ferrini, F.; Fineschi, S.; Galeotti, P.; Giuntoli, A.; Manganelli Del Fa, R.; Moriondo, M.; et al. The Management of Plants and Their Impact on Monuments in Historic Gardens: Current Threats and Solutions. Urban For. Urban Green 2022, 76, 127727. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean: Insights for Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Alanís-Méndez, J.L.; Martínez-Castillo, I.A.; Viveros-Valencia, J.; Sosa-Constantino, F.G.; Limón-Salvador, F. Archaeological Sites as a Safeguard for Orchid Diversity: A Study in El Tajin, Veracruz, Mexico. Nat. Areas J. 2023, 43, 179–184. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Bird, D.W.; Perry, G.H. Human Behaviour as a Long-Term Ecological Driver of Non-Human Evolution. Nat. Ecol. Evol. 2017, 1, 65. [Google Scholar] [CrossRef]
- Boivin, N.L.; Zeder, M.A.; Fuller, D.Q.; Crowther, A.; Larson, G.; Erlandson, J.M.; Denhami, T.; Petraglia, M.D. Ecological Consequences of Human Niche Construction: Examining Long-Term Anthropogenic Shaping of Global Species Distributions. Proc. Natl. Acad. Sci. USA 2016, 113, 6388–6396. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, B.M.; Louderback, L.A.; Vernon, K.B.; Yaworsky, P.M.; Wilson, C.; Clifford, A.; Codding, B.F. Plant Species Richness at Archaeological Sites Suggests Ecological Legacy of Indigenous Subsistence on the Colorado Plateau. Proc. Natl. Acad. Sci. USA 2021, 118, e2025047118. [Google Scholar] [CrossRef] [PubMed]
- Celesti-Grapow, L.; Ricotta, C. Plant Invasion as an Emerging Challenge for the Conservation of Heritage Sites: The Spread of Ornamental Trees on Ancient Monuments in Rome, Italy. Biol. Invasions 2021, 23, 1191–1206. [Google Scholar] [CrossRef]
- Roussos, O.; Kapetanopoulou, C.; Petza, D. Protecting Biodiversity from Invasive Alien Species by Improving Policy Instruments in Greece: The Invalis Project Action Plan. J. Mar. Sci. Eng. 2021, 9, 1205. [Google Scholar] [CrossRef]
- Batista, T.; de Mascarenhas, J.M.; Mendes, P. Guidelines for the Integration of Biological and Cultural Values in a Landscape Interpretation Centre: Application in Southern Portugal. Biodivers Conserv. 2015, 24, 3367–3386. [Google Scholar] [CrossRef]
- Bürgi, M.; Li, L.; Kizos, T. Exploring Links between Culture and Biodiversity: Studying Land Use Intensity from the Plot to the Landscape Level. Biodivers Conserv. 2015, 24, 3285–3303. [Google Scholar] [CrossRef]
- Domina, G. The Floristic Research in Italian Archaeological Sites. Flora Mediterr. 2018, 28, 377–383. [Google Scholar] [CrossRef]
- Katz, O. The Ecosystem Services Framework in Archaeology (and Vice Versa). People Nat. 2022, 4, 1450–1460. [Google Scholar] [CrossRef]
- Cheminal, A.; Kokkoris, I.P.; Zotos, A.; Strid, A.; Dimopoulos, P. Assessing the Ecosystem Services Potential of Endemic Floras: A Systematic Review on the Greek Endemics of Peloponnese. Sustainability 2022, 14, 5926. [Google Scholar] [CrossRef]
- Stein, B.A.; Staudt, A.; Cross, M.S.; Dubois, N.S.; Enquist, C.; Griffis, R.; Hansen, L.J.; Hellmann, J.J.; Lawler, J.J.; Nelson, E.J.; et al. Preparing for and Managing Change: Climate Adaptation for Biodiversity and Ecosystems. Front. Ecol. Environ. 2013, 11, 502–510. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Weiser, M.D. Towards a More General Species-Area Relationship: Diversity on All Islands, Great and Small. J. Biogeogr. 2001, 28, 431–445. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kanellou, E.; Economou, G. Integrated Design and Management of Vegetation at Archaeological Sites to Protect Monuments and Enhance the Historical Landscape. In Proceedings of the Acta Horticulturae, VI International Conference on Landscape and Urban Horticulture, Athens, Greece, 20–25 June 2017; Volume 1189. [Google Scholar]
- Ringbeck, B. Management Plans for World Heritage Sites—A Practical Guide; Brincks-Murmann, C., Fowler, A., Eds.; German Commission for UNESCO: Bonn, Germany, 2008. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).