Integrating Species Distribution Models to Identify Overlapping Predator–Prey Conservation Priorities in Misiones, Argentina
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Focal Species and Field Surveys
2.3. Genetic Analysis and Species Occurrence Data
2.4. Modeling the Ecological Niche
2.5. Predictor Variables for the Ecological Niche Model (ENM)
2.6. Potential Species Richness (PSR)
2.7. Identification of Regions in Need of Management Strategies
3. Results
3.1. Model Performance
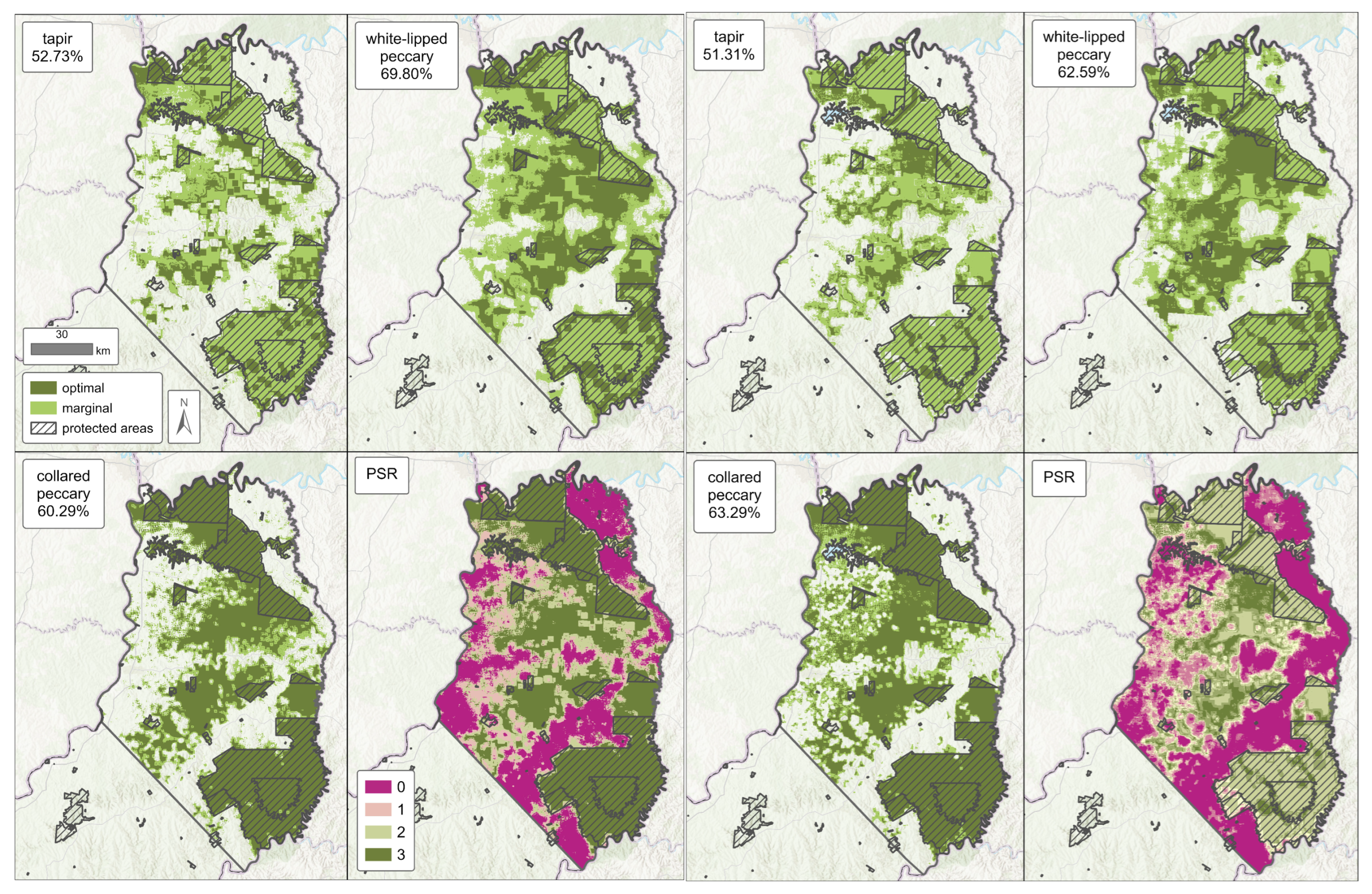
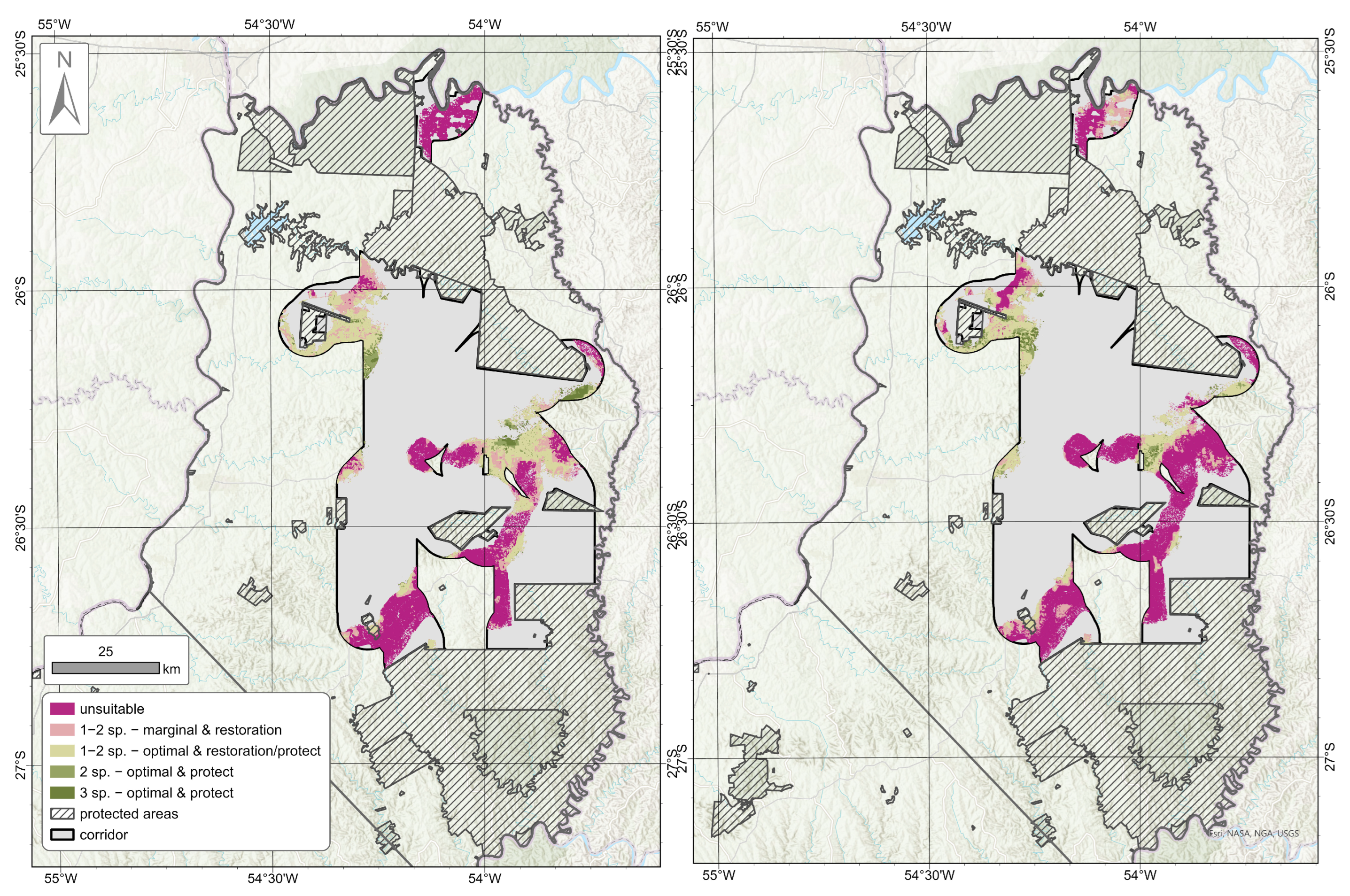
3.2. Predicted Habitat Suitability and Habitat Quality
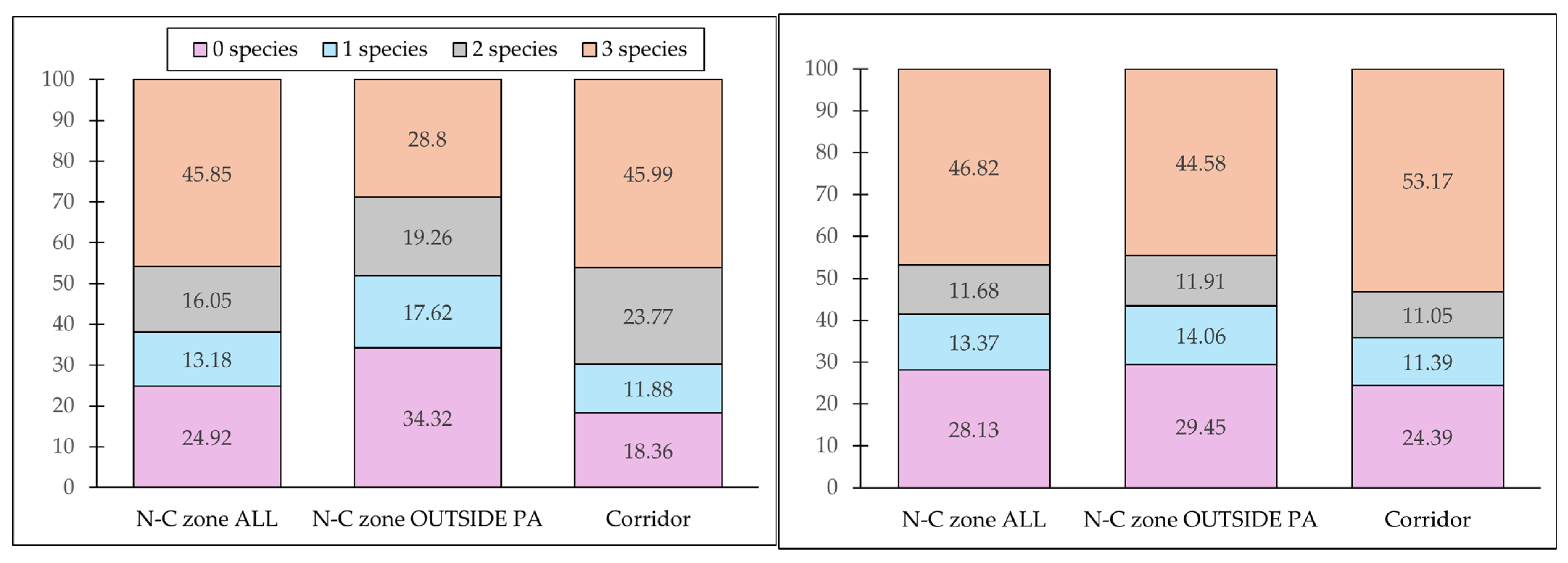
3.3. PSR
3.4. Comparison with DeMatteo et al. [52] Multispecies Corridor Model
3.5. Identification of Regions in Need of Management Strategies
4. Discussion
4.1. Predicted Habitat Suitability
4.2. PSR and Habitat Quality
4.3. Identification of Regions in Need of Management Strategies
4.4. Limitations of the Study
4.5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the ROC Curve |
| ENM | Ecological Niche Model |
| FCV1 | Fixed Cumulative Value 1 |
| MEyRNR | Ministerio de Ecología y Recursos Naturales Renovables of Misiones |
| MTP | Minimum Training Presence |
| MTSS | Minimum Training Sensitivity plus Specificity |
| N-C | Northern-Central |
| PAs | Protected Areas |
| PSR | Potential Species Richness |
| SD | Standard Deviation |
Appendix A
| Location | Zone | Tapir | WLP | CP |
|---|---|---|---|---|
| P.P. Urugua-í (84,000 ha) | N | 20 | 11 | |
| Refugio Privado Aguaraí-mi (3050 ha) | N | 1 | 7 | 9 |
| Reserva de Vida Silvestre Urugua-í (3243 ha) | N | --- | --- | 2 |
| P.P. Cruce Caballero (522 ha) & Valle del Arroyo Alegría (8000 ha) | C | --- | 1 | --- |
| P.P. Esmeralda (31,569 ha) & Reserva de Biósfera Yabotí (236,313 ha) | C | 2 | 13 | --- |
| Reserva Privada Yaguaroundí (400 ha) | C | --- | 2 | --- |
| outside protected areas | N | 11 | 74 | 8 |
| outside protected areas | C | 3 | 20 | 2 |
References
- Simberloff, D. Flagships, Umbrellas, and Keystones: Is Single-Species Management Passé in the Landscape Era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Martinez del Rio, C.; Dugelby, B.; Foreman, D.; Miller, B.; Noss, R.; Phillips, M. The Importance of Large Carnivores to Healthy Ecosystems. Endanger. Species Update 2001, 18, 202. [Google Scholar]
- Natsukawa, H.; Sergio, F. Top Predators as Biodiversity Indicators: A Meta-Analysis. Ecol. Lett. 2022, 25, 2062–2075. [Google Scholar] [CrossRef]
- Keuroghlian, A.; Eaton, D.P. Removal of Palm Fruits and Ecosystem Engineering in Palm Stands by White-Lipped Peccaries (Tayassu Pecari) and Other Frugivores in an Isolated Atlantic Forest Fragment. Biodivers. Conserv. 2009, 18, 1733–1750. [Google Scholar] [CrossRef]
- Altrichter, M.; Taber, A.; Beck, H.; Reyna-Hurtado, R.; Lizarraga, L.; Keuroghlian, A. Range-Wide Declines of a Key Neotropical Ecosystem Architect, the Near Threatened White-Lipped Peccary Tayassu pecari. Oryx 2012, 46, 87–98. [Google Scholar] [CrossRef]
- Medici, E.P.; Desbiez, A.L.J. Population Viability Analysis: Using a Modeling Tool to Assess the Viability of Tapir Populations in Fragmented Landscapes. Integr. Zool. 2012, 7, 356–372. [Google Scholar] [CrossRef]
- Keuroghlian, A.; Carmo Andrade Santos, M.; Eaton, D.P. The Effects of Deforestation on White-Lipped Peccary (Tayassu Pecari) Home Range in the Southern Pantanal. Mammalia 2014, 79, 491–497. [Google Scholar] [CrossRef]
- de Bustos, S. Dispersal and Herbivory of the Tapir (Tapirus terrestris) in a Yungas Forest [Dispersión y Herbivoría del Tapir (Tapirus terrestris) en un Bosque de Yungas]. Ph.D. Dissertation, Universidad Nacional de Tucumán, Tucumán, Argentina, 2018. [Google Scholar]
- Whitworth, A.; Beirne, C.; Basto, A.; Flatt, E.; Tobler, M.; Powell, G.; Terborgh, J.; Forsyth, A. Disappearance of an Ecosystem Engineer, the White-Lipped Peccary (Tayassu pecari), Leads to Density Compensation and Ecological Release. Oecologia 2022, 199, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.J. What Is the Future for Wild, Large Herbivores in Human-Modified Agricultural Landscapes? Wildl. Biol. 2009, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Goosem, M.; Laurance, S.G.W. Impacts of Roads and Linear Clearings on Tropical Forests. Trends Ecol. Evol. 2009, 24, 659–669. [Google Scholar] [CrossRef]
- Wolf, C.; Ripple, W.J. Prey Depletion as a Threat to the World’s Large Carnivores. R. Soc. Open Sci. 2016, 3, 160252. [Google Scholar] [CrossRef]
- Bauni, V.; Anfuso, J.; Schivo, F. Wildlife Roadkill Mortality in the Upper Paraná Atlantic Forest, Argentina [Mortalidad de Fauna Silvestre por Atropellamientos en el Bosque Atlántico del Alto Paraná, Argentina]. Ecosistemas 2017, 26, 54–66. [Google Scholar] [CrossRef]
- de Bustos, S.; Varela, D.; Lizárraga, L.; Camino, M.; Quiroga, V. Tayassu pecari. In Categorización del Estado de Conservación de Mamíferos de Argentina. Lista Roja de los Mamíferos de Argentina; SAYDS–SAREM, Ed.; SAYDS–SAREM: Buenos Aires, Argentina, 2019; pp. 1–10. [Google Scholar]
- Fort, J.L.; Suriyamongkol, T.; Nielsen, C.K.; Carver, A.D.; Moreno, R.; Meyer, N.F.V. Large Felid and Peccary Habitat Use in Isolated and Contiguous Forest in Panamá: Implications for Conservation. Trop. Conserv. Sci. 2022, 15, 1–18. [Google Scholar] [CrossRef]
- Piña-Covarrubias, E.; Chávez, C.; Chapman, M.A.; Morales, M.; Elizalde-ArellanaArellano, C.; Doncaster, C. Ecology of Large Felids and Their Prey in Small Reserves of the Yucatán Peninsula of Mexico. J. Mammal. 2022, 104, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Falconi-Briones, F.A.; Bolom-Huet, R.; Naranjo, E.J.; Reyna-Hurtado, R.; Enríquez-Rocha, P.L.; Moreira-Ramírez, J.F. Connectivity at Risk: A Critical Scenario for the Endangered Baird’s Tapir and the Vulnerable White-Lipped Peccary in the Maya Forest. Biodivers. Conserv. 2025, 34, 235–254. [Google Scholar] [CrossRef]
- Snider, M.H.; Athreya, V.R.; Balme, G.A.; Bidner, L.R.; Farhadinia, M.S.; Fattebert, J.; Gompper, M.; Hunter, L.; Isbell, L.; Macdonald, D.; et al. Home Range Variation in Leopards Living across the Human Density Gradient. J. Mammal. 2021, 102, 1138–1148. [Google Scholar] [CrossRef]
- Thompson, J.J.; Morato, R.G.; Niebuhr, B.B.; Alegre, V.B.; Oshima, J.E.F.; Barros, A.E. Environmental and Anthropogenic Factors Synergistically Affect Space Use of Jaguars. Curr. Biol. 2021, 31, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Bice, S.M.; Gable, T.D.; Roth, J.D.; Bump, J.K. Patchy Indirect Effects of Predation: Predators Contribute to Landscape Heterogeneity and Ecosystem Function via Localized Pathways. Oikos 2023, 2023, 10065. [Google Scholar] [CrossRef]
- Zanón Martínez, J.; Iranzo, E.; Travaini, A.; McNitt, D.; Mansilla, A.; Llanos, R.; Kelly, M. Puma Density, Habitat Use, and Activity Patterns across a Mosaic Landscape of Ranches, Game Reserves, and a Protected Area in Central Argentina. Eur. J. Wildl. Res. 2023, 69, 89. [Google Scholar] [CrossRef]
- Fragoso, J.M.V. Chapter 18: A Long-Term Study of White-Lipped Peccary (Tayassu pecari) Population Fluctuations in Northern Amazonia—Anthropogenic Versus “Natural” Causes. In People in Natures: Wildlife Conservation in South and Central America; Silvius, K.M., Bodmer, R.E., Fragoso, J.M.V., Eds.; Columbia University Press: New York, NY, USA, 2004; pp. 286–296. [Google Scholar]
- Moreno, R.S.; Kays, R.W.; Samudio, R., Jr. Competitive Release in Diets of Ocelot (Leopardus pardalis) and Puma (Puma concolor) after Jaguar (Panthera onca) Decline. J. Mammal. 2006, 87, 808–816. [Google Scholar] [CrossRef]
- Winnie, J., Jr.; Creel, S. The Many Effects of Carnivores on Their Prey and Their Implications for Trophic Cascades, and Ecosystem Structure and Function. Food Webs 2017, 12, 88–94. [Google Scholar] [CrossRef]
- Fragoso, J.M.V.; Antunes, A.P.; Silvius, K.M.; Constantino, P.A.L.; Zapata-Ríos, G.; El Bizri, H.R. Large-Scale Population Disappearance and Cycling in the White-Lipped Peccary, a Tropical Forest Mammal. PLoS ONE 2022, 17, 0276297. [Google Scholar] [CrossRef]
- Northrup, J.; Vander Wal, E.; Bonar, M.; Fieberg, J.; Laforge, M.; Leclerc, M.; Prokopenko, C.; Gerber, B. Conceptual and Methodological Advances in Habitat-Selection Modeling: Guidelines for Ecology and Evolution. Ecol. Appl. 2022, 32, e02470. [Google Scholar] [CrossRef] [PubMed]
- Huey, R. Physiological Consequences of Habitat Selection. Am. Nat. 1991, 137, S91–S115. [Google Scholar] [CrossRef]
- Lennox, R.; Kambestad, M.; Berhe, S.; Birnie-Gauvin, K.; Cooke, S.; Dahlmo, L.; Eldoy, S.; Sindre, H.; Davidsen, J.; Hanssen, E.; et al. The Role of Habitat in Predator-Prey Dynamics with Applications to Restoration. Restor. Ecol. 2025, 33, e14354. [Google Scholar] [CrossRef]
- Ripari, L.; Premier, J.; Belotti, E.; Bluhm, H.; Breitenmoser-Wursten, C.; Bufka, L.; Cerveny, J.; Drouet-Hoguet, N.; Fuxjager, C.; Jedrzejewski, W.; et al. Human Disturbance Is the Most Limiting Factor Driving Habitat Selection of a Large Carnivore throughout Continental Europe. Biol. Conserv. 2022, 266, 109446. [Google Scholar] [CrossRef]
- Smith, B.; MacNulty, D.; Stahler, D.; Smith, D.; Avgar, T. Density-Dependent Habitat Selection Alters Drivers of Population Distribution in Northern Yellowstone Elk. Ecol. Lett. 2023, 26, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Grenier-Potvin, A.; Clermont, J.; Gauthier, G.; Berteaux, D. Prey and Habitat Distribution Are Not Enough to Explain Predator Habitat Selection: Addressing Intraspecific Interactions, Behavioural State and Time. Mov. Ecol. 2021, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Lathouwers, M.; Dendoncker, N.; Artois, T.; Meenaerts, N.; Conway, G.; Henderson, I.; Shewring, M.; Cross, T.; Ulenaers, E.; Eens, R. Multi-Scale Habitat Selection throughout the Annual Cycle of a Long-Distance Avian Migrant. Ecol. Indic. 2023, 156, 111099. [Google Scholar] [CrossRef]
- Meyer, N.; Esser, H.J.; Moreno, R.; va Langevelde, F.; Liefting, Y.; Oller, D.R.; Vogels, C.; Carver, A.; Nielsen, C.; Jansen, P. An Assessment of the Terrestrial Mammal Communities in Forest of Central Panama Using Camera-Trap Surveys. J. Nat. Conserv. 2015, 26, 28–35. [Google Scholar] [CrossRef]
- Núñez-Reguerio, M.; Branch, L.; Fletcher, R., Jr.; Marás, G.A.; Derlindata, E.; Tálamo, A. Spatial Patterns of Mammal Occurrence in Forest Strips Surrounded by Agricultural Crops of the Chaco Region, Argentina. Biol. Conserv. 2015, 187, 19–26. [Google Scholar] [CrossRef]
- Malakoutikhah, S.; Fakheran, S.; Hemami, M.R.; Tarkesh, M.; Senn, J. Assessing Future Distribution, Suitability Corridors and Efficiency of Protected Areas to Conserve Vulnerable Ungulates under Climate Change. Divers. Distrib. 2020, 26, 1383–1396. [Google Scholar] [CrossRef]
- Riggio, J.; Foreman, K.; Freedman, E.; Gottlieb, B.; Hendler, D.; Radomille, D. Predicting Wildlife Corridors for Multiple Species in an East African Ungulate Community. PLoS ONE 2022, 17, 0265136. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, E.; Macdonald, D.; Cushman, S. Using Simulation Modelling to Evaluate the Relative Efficacy of Core Area and Corridor-Based Conservation Designs for Biodiversity Conservation. Landsc. Ecol. 2025, 40, 171. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Iwamura, T.; Butt, N. Mapping Vulnerability and Conservation Adaptation Strategies under Climate Change. Nat. Clim. Change 2013, 3, 989–994. [Google Scholar] [CrossRef]
- Siddig, A.A.H.; Ellison, A.M.; Ochs, A.; Villar-Leeman, C.; Lau, M.K. How Do Ecologists Select and Use Indicator Species to Monitor Ecological Change? Insights from 14 Years of Publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef]
- Schuster, R.; Buxton, R.; Hanson, J.O.; Binley, A.D.; Pittman, J.; Tulloch, V. Protected Area Planning to Conserve Biodiversity in an Uncertain Future. Conserv. Biol. 2023, 37, 14048. [Google Scholar] [CrossRef]
- Chanove-Manrique, A.; Cárdenas-Pillco, B. Landscape Fragmentation and Loss of Connectivity in Peru’s Queñua (Polylepis) Forests and Their Vulnerability to Climate Change [Fragmentación del Paisaje y Pérdida de Conectividad en los Bosque de Queñua (Polylepis) en Perú y su Vulnerabilidad ante el Cambio Climático]. Madera Bosques 2024, 30, 3032593. [Google Scholar] [CrossRef]
- Galindo-Leal, C.; Gusmão Câmara, I. Chapter 1: The Atlantic Forest Hotspot: An Overview. In The Atlantic Forest of South America: Biodiversity, Status, Threats, and Outlooks (State of the Hotspots); Island Press: Washington, DC, USA, 2003; pp. 3–11. ISBN 1-55963-989-X. [Google Scholar]
- Zuleta, G.A.; Gauto, O.A.; Varela, D.M.; Angelo, C.; Johnson, B.G.; Lorán, D. Strategic Environmental Assessments and Biodiversity Monitoring Program in the Mesopotamia and Paraná Delta Regions [Evaluaciones Ambientales Estratégicas y Programa de Monitoreo de la Biodiversidad en las Regiones de Mesopotamia y Delta de Paraná]. In Final Report (Informe Final). 2016. Ministerio de Agroindustria de la Nación; Universidad Maimónides: Buenos Aires, Argentina; Universidad Nacional de Misiones—CONICET: Posadas, Argentina, 2016. [Google Scholar]
- Iezzi, M.E.; Cruz, P.; Diego, V.; Bitetti, M.S.; Angelo, C. Fragment Configuration or Environmental Quality? Understanding What Really Matters for the Conservation of Native Mammals in the Atlantic Forest of Argentina. J. Nat. Conserv. 2019, 52, 125751. [Google Scholar] [CrossRef]
- Marques, M.; Trindade, W.; Bohn, A.; Grelle, C. Chapter 1: The Atlantic Forest: History, Biodiversity, Threats and Opportunities of the Megadiverse Forest of South America. In The Atlantic Forest: History, Biodiversity, Threats and Opportunities of Mega-diverse Forest; Springer Nature: Cham, Switzerland, 2021; pp. 3–23. ISBN 978-3-030-55322-7. [Google Scholar]
- de Lima, R.; Dauby, G.; Gasper, A.; Fernandez, E.; Vibrans, A.; Oliveira, A.; Prado, P.; Souza, V.; Siqueira, M.; Steege, H. Comprehensive Conservation Assessments Reveal High Extinction Risks across Atlantic Forest Trees. Science 2024, 383, 219–255. [Google Scholar] [CrossRef]
- Izquierdo, A.E.; Angelo, C.D.; Aide, T.M. Thirty Years of Human Demography and Land-Use Change in the Atlantic Forest of Misiones, Argentina: An Evaluation of the Forest Transition Model. Ecol. Soc. 2008, 13, 3. [Google Scholar] [CrossRef]
- Haag, T.; Santos, A.; Sana, D.; Morato, R.; Cullen, L., Jr.; Crawshaw, P., Jr.; De Angelo, C.; Di Bitetti, M.; Salzano, F.; Eizirik, E. The Effect of Habitat Fragmentation on the Genetic Structure of a Top Predator: Loss of Diversity and High Differentiation among Remnant Populations of Atlantic Forest Jaguars (Panthera onca). Mol. Ecol. 2010, 19, 4906–4921. [Google Scholar] [CrossRef]
- Sancha, N.U.; Higgins, C.L.; Presley, S.J.; Strauss, R.E. Metacommunity Structure in a Highly Fragmented Forest: Has Deforestation in the Atlantic Forest Altered Historic Biogeographic Patterns? Divers. Distrib. 2014, 20, 1058–1070. [Google Scholar] [CrossRef]
- Galetti, M.; Brocardo, C.R.; Begotti, R.; Hortenci, L.; Rocha-Mendes, F.; Bernardo, C.; Bueno, R.; Nobre, R.; Bovendorp, R.; Marques, R.; et al. Defaunation and Biomass Collapse of Mammals in the Largest Atlantic Forest Remnant. Anim. Conserv. 2017, 20, 270–281. [Google Scholar] [CrossRef]
- Iezzi, M.E.; Cruz, P.; Varela, D.; Angelo, C.; Bitetti, M.S. Tree Monocultures in a Biodiversity Hotspot: Impact of Pine Plantations on Mammal and Bird Assemblages in the Atlantic Forest. For. Ecol. Manag. 2018, 424, 216–227. [Google Scholar] [CrossRef]
- DeMatteo, K.E.; Rinas, M.A.; Zurano, J.P.; Selleski, N.; Schneider, R.G.; Argüelles, C.F. Using Niche-Modelling and Species-Specific Cost Analyses to Determine a Multispecies Corridor in a Fragmented Landscape. PLoS ONE 2017, 12, 0183648. [Google Scholar] [CrossRef]
- Lambeck, R.J. Focal Species: A Multi-Species Umbrella for Nature Conservation. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Beier, P.; Majka, D.R.; Newell, S.L. Uncertainty Analysis of Least-Cost Modelling for Designing Wildlife Linkages. Ecol. Appl. 2009, 19, 2067–2077. [Google Scholar] [CrossRef]
- Brodie, J.F.; Giordana, A.J.; Dickson, B.; Hebblewhite, M.; Bernard, H.; Mohd-Azlan, J. Evaluating Multispecies Landscape Connectivity in a Threatened Tropical Mammal Community. Conserv. Biol. 2014, 29, 122–132. [Google Scholar] [CrossRef]
- Wood, S.L.R.; Martins, K.T.; Dumais-Lalonde, V.; Tangua, O.; Maure, F.; St-Denis, A.; Rayfield, B.; Martin, A.; Gonzalez, A. Missing Interactions: The Current State of Multispecies Connectivity Analysis. Front. Ecol. Evol. 2022, 10, 830822. [Google Scholar] [CrossRef]
- Bohnett, E.; Oetting, J.; Noss, R.; O’Brien, M.; Frakes, R.; Smith, D. Consolidating Diverse Modeling Methods and Spatial Prioritization for Multispecies Connectivity Planning. Front. Conserv. Sci. 2024, 5, 1406944. [Google Scholar] [CrossRef]
- DeMatteo, K.E.; Rinas, M.A.; Escalante, O.M.; Sotorres, D.; Ibañez Alegre, D.M.; Argüelles, C.F. A Multispecies Corridor in a Fragmented Landscape: Evaluating Effectiveness and Identifying High-Priority Target Areas. PLoS ONE 2023, 18, 0283258. [Google Scholar] [CrossRef]
- Galetti, M.; Keuroghlian, A.; Hanada, L.; Morato, M.I. Frugivory and Seed Dispersal by the Lowland Tapir (Tapirus terrestris) in Southeast Brazil. Biotropica 2001, 33, 723–726. [Google Scholar] [CrossRef]
- Beck, H.; Thebpanya, P.; Filiaggi, M. Do Neotropical Peccary Species (Tayassuidae) Function as Ecosystem Engineers for Anurans? J. Trop. Ecol. 2010, 26, 407–414. [Google Scholar] [CrossRef]
- Simpson, B.K.; Shukor, M.N.; Magintan, D.; Ling, W.S. Food Selection of the Maylayan Tapir (Tapirus indicus) under Semi-Wild Conditions. In Proceedings of the 2013 UKM FST Postgraduate Colloquium—AIP Conference Proceedings, Selangor Kembangan, Malaysia, 3–4 July 2013; Murad, A.M.H.A., Yen, C.C., Ismail, E.S., Maskat, M.Y., Noorani, M.S.M., Ibrahim, N., Eds.; AIP Publishing: Melville, NY, USA, 2013; pp. 317–324. [Google Scholar]
- Giombini, M.I.; Bravo, S.P.; Tosto, D.S. The Key Role of the Largest Extant Neotropical Frugivore (Tapirus terrestris) in Promoting Admixture of Plant Genotypes across the Landscape. Biotropica 2016, 48, 499–508. [Google Scholar] [CrossRef]
- Ranzani de Luca, J.; Pardini, R. Use of Early and Late Successional Forest Patches by the Endangered Lowland Tapir Tapirus terrestris (Perissodactyla: Tapiridae). Mamm. Biol. 2017, 86, 107–114. [Google Scholar] [CrossRef]
- Vélez, J.; Espelta, J.M.; Rivera, O.; Armenteras, D. Effects of Seasonality and Habitat on the Browsing and Frugivory Preferences of Tapirus terrestris in North-Western Amazonia. J. Trop. Ecol. 2017, 33, 395–406. [Google Scholar] [CrossRef]
- Cruz, L.; Muylaert, R.; Galetti, M.; Pires, M. The Geography of Diet Variation in Neotropical Carnivora. Mammal Rev. 2022, 52, 112–128. [Google Scholar] [CrossRef]
- King, R.; Braun, J.; Westphal, M.; Lortie, C. Prey Distribution Mapping to Support Conservation Planning. Divers. Distrib. 2025, 31, e70048. [Google Scholar] [CrossRef]
- de Gabriel Hernando, M.; Karamanlidis, A.; Grivas, K.; Krambokoukis, L.; Papakostas, G.; Beecham, J. Habitat Use and Selection Patterns Inform Habitat Conservation Priorities of an Endangered Large Carnivore in Southern Europe. Endanger. Species Res. 2021, 44, 203–215. [Google Scholar] [CrossRef]
- de Bustos, S.; Varela, D.; Lizárraga, L.; Cirignoli, S.; Quiroga, V.; Chalukián, S.; Giombini, M. Tapirus terrestris. In Categorización del Estado de Conservación de Mamíferos de Argentina—Lista Roja de los Mamíferos de Argentina; SAYDS–SAREM, Ed.; SAYDS–SAREM: Buenos Aires, Argentina, 2019; pp. 1–18. [Google Scholar]
- Vancine, M.H.; Muylaert, R.L.; Niebuhr, B.B.; Faria Oshima, J.E.; Tonetti, V.; Bernardo, R. The Atlantic Forest of South America: Spatiotemporal Dynamics of the Vegetation and Implications for Conservation. Biol. Conserv. 2024, 291, 110499. [Google Scholar] [CrossRef]
- Souza, C., Jr.; Shimbo, J.; Rosa, M.; Parente, L.; Alencar, A.; Rudorff, B. Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- Reyna-Hurtado, R.; Rojas-Flores, E.; Tanner, G.W. Home Range and Habitat Preferences of White-Lipped Peccaries (Tayassu Pecari) in the Calakmul Forest, Campeche, Mexico. J. Mammal. 2009, 90, 1199–1209. [Google Scholar] [CrossRef]
- Keuroghlian, A.; Desbiez, A.; Reyna-Hurtado, R.; Altrichter, M.; Beck, H.; Taber, A.; Fragoso, J.M.V. Tayassu Pecari. IUCN Red List. Threat. Species 2013, 2013, e.T41778A44051115. [Google Scholar] [CrossRef]
- Medici, E.P.; Desbiez, A.L.J.; Gatti, A.; Chalukian, S.C. Report of the Tapir Specialist Group. In IUCN SSC and Secretariate. 2023 Report of the IUCN Species Survival Commission and Secretariat; IUCN: Gland, Switzerland, 2024. [Google Scholar]
- Chalukian, S.; Bustos, S.; Bitetti, M.; Angelo, C.; Paviolo, A. Orden Perissodactyla. In Libro Rojo de los Mamíferos Amenazados de la Argentina; Ojeda, R., Chillo, V., Díaz Isenrath, G.B., Eds.; Sociedad Argentina para el Estudio de los Mamíferos (SAREM): Buenos Aires, Argentina, 2012; p. 116. [Google Scholar]
- Chalukian, S.; Bustos, S.; Lizárraga, L.; Varela, D.; Paviolo, A.; Quse, V. Plan de Acción para la Conservación del Tapir (Tapirus terrestris) en Argentina; Dirección de Fauna Silvestre de la Nación—Secretaría de Ambiente y Desarrollo Sustentable: Buenos Aires, Argentina, 2009. [Google Scholar]
- Gongora, J.; Reyna-Hurtado, R.; Beck, H.; Taber, A.; Altrichter, M.; Keuroghlian, A. Pecari Tajacu. IUCN Red List. Threat. Species 2011, 2011, e.T41777A10562361. [Google Scholar] [CrossRef]
- MAyDS. Listado de Especies de Fauna Silvestre en Riesgo de Extinción en Argentina; Secretaría de Ambiente: Buenos Aires, Argentina, 2020. [Google Scholar]
- Illia, G. Effects of Habitat Modification on Ecosystem Health: The Role of the Capuchin Monkey (Sapajus nigritus) in the Transmission of Infectious Diseases in Remnants of the Atlantic Forest of Argentina [Efectos de La Modificación de Habitat En La Salud Del Ecosistema: El Papel Del Mono Caí (Sapajus nigritus) En La Tranmisión de Enfermedades Infecciosas En Remanentes Del Bosque Atlántico Argentino]. Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2025. [Google Scholar]
- Martínez de Zorzi, V. Impact of Habitat Fragmentation on Capuchin Monkeys (Sapajus nigritus cucullatus) in Northeastern Argentina [Impacto de La Fragmentación de Habitat Sobre Los Monos Caí (Sapajus nigritus cucullatus) En El Noreste Argentino]. Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2025. [Google Scholar]
- DeMatteo, K.E.; Rinas, M.A.; Sede, M.M.; Davenport, B.; Argüelles, C.F.; Lovett, K. Detection Dogs: An Effective Technique for Bush Dog Surveys. J. Wildl. Manag. 2009, 73, 1436–1440. [Google Scholar] [CrossRef]
- DeMatteo, K.E.; Rinas, M.A.; Argüelles, C.F.; Holman, B.E.; Bitetti, M.S.; Davenport, B. Using Detection Dogs and Genetic Analyses of Scat to Expand Knowledge and Assist Felid Conservation in Misiones, Argentina. Integr. Zool. 2014, 9, 623–639. [Google Scholar] [CrossRef]
- DeMatteo, K.E.; Rinas, M.A.; Argüelles, C.F.; Zurano, J.P.; Selleski, N.; Bitetti, M.S. Noninvasive techniques provide novel insight for the elusive bush dog (Speothos venaticus). Wildl. Soc. Bull. 2014, 38, 862–873. [Google Scholar] [CrossRef]
- Cusack, J.J.; Dickman, A.J.; Rowcliffe, J.M.; Carbone, C.; Macdonald, D.W.; Coulson, T. Random versus Game Trail-Based Camera Trap Placement Strategy for Monitoring Terrestrial Mammal Communities. PLoS ONE 2015, 10, 0126373. [Google Scholar] [CrossRef]
- Kolowski, J.M.; Forrester, T.D. Camera Trap Placement and the Potential for Bias Due to Trails and Other Features. PLoS ONE 2017, 12, 0186679. [Google Scholar] [CrossRef]
- Foster, R.; Harmsen, B. A Critique of Density Estimation from Camera-Trap Data. J. Wildl. Manag. 2012, 76, 224–236. [Google Scholar] [CrossRef]
- Wearn, O.R.; Glover-Kapfer, P. Camera-Trapping for Conservation: A Guide to Best-Practices; WWW Conservation Technology Series; WWF-UK: Woking, UK, 2017. [Google Scholar]
- Nussear, K.; Esque, T.; Heaton, J.; Cablk, M.; Drake, K.; Valentin, C.; Yee, J.; Medica, P. Are Wildlife Detector Dogs or People Better at Finding Desert Tortoises (Gopherus agassizii). Herpetol. Conserv. Biol. 2008, 3, 103–115. [Google Scholar]
- Delgado, P.M.; Argüelles, C.F.; DeMatteo, K.E. Using Noninvasive Techniques to Monitor Game Species Targeted by Poaching in Misiones, Argentina. Neotrop. Biodivers. 2021, 7, 78–85. [Google Scholar] [CrossRef]
- Sotorres, D. Forensic Identification and Analysis of Distribution of Species in the Proposed Biological Corridor for Misiones (Argentina) [Identificación Forense y Análisis de Distribución de Especies Presa a Lo Largo Del Corredor Biológico Propuesto Para Misiones (Argentina)]. Bachelor’s Thesis, Universidad Nacional de Misiones—Facultad de Ciencias Exactas, Químicas y Naturales, Posadas, Misiones, Argentina, 2019. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Vynne, C. Landscape Use by Wide-Ranging Mammals of the Brazilian Cerrado. Ph.D. Dissertation, University of Washington, Seattle, WA, USA, 2010. [Google Scholar]
- Farrell, L.E.; Roman, J.; Sunquist, M.E. Dietary Separation of Sympatric Carnivores Identified by Molecular Analysis of Scats. Mol. Ecol. 2000, 9, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Miotto, R.; Rodrigues, F.; Ciocheti, G.; Galetti, P., Jr. Determination of the Minimum Population Size of Pumas (Puma concolor) through Fecal DNA Analysis in Two Protected Cerrado Areas in the Brazilian Southeast. Biotropica 2007, 39, 647–654. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lopman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Fan, W.-Y.; Wang, Z.-G. The Predictive Performance and Stability of Six Species Distribution Models. PLoS ONE 2014, 9, 112764. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.; Anderson, R.; Dudík, M.; Ferrier, S.; Guisan, A. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hemami, M.-R.; Kaboli, M.; Shabani, F. MaxEnt Brings Comparable Results When the Input Data Are Being Completed; Model Parameterization of Four Species Distribution Models. Ecol. Evol. 2023, 13, e9827. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological Niche Modeling in Maxent: The Importance of Model Complexity and the Performance of Model Selection Criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions. What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.; Rodder, D.; Secondi, J. Mapping Species Distribution with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Trisurat, Y.; Bhumpakphan, N.; Reed, D.H.; Kanchanasaka, B. Using Species Distribution Modeling to Set Management Priorities for Mammals in Northern Thailand. J. Nat. Conserv. 2012, 20, 264–273. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G.; Pearson, R. Selecting Thresholds for the Prediction of Species Occurrence with Presence-Only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the Selection of Thresholds for Predicting Species Occurrence with Presence-Only Data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-Niche-Factor Analysis: How to Computer Habitat-Suitability Maps without Absences Data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Medici, E.P.; Mezzini, S.; Fleming, C.; CalabreseCH, J.; Noonan, M. Movement Ecology of Vulnerable Lowland Tapirs between Areas of Varying Human Disturbance. Mov. Ecol. 2022, 10, 14. [Google Scholar] [CrossRef]
- Keuroghlian, A.; Eaton, D.P.; Longland, W.S. Area Use by White-Lipped and Collared Peccaries (Tayassu Pecari and Tayassu Tajacu) in a Tropical Forest Fragment. Biol. Conserv. 2004, 120, 411–425. [Google Scholar] [CrossRef]
- Manuel, J.; Fragoso, V. Perception of Scale and Resource Partitioning by Peccaries: Behavioral Causes and Ecological Implications. J. Mammal. 1999, 80, 993–1003. [Google Scholar] [CrossRef]
- Carrillo, E.; Saenz, J.; Fuller, T. Movements and Activities of White-Lipped Peccaries in Corcovado National Park, Costa Rica. Biol. Conserv. 2002, 108, 317–324. [Google Scholar] [CrossRef]
- Judas, J.; Henry, O. Seasonal Variation of Home Range of Collared Peccary in Tropical Rain Forests of French Guiana. J. Wildl. Manag. 1999, 63, 546–552. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. J. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Skov, F.; Borchsenius, F. Predicting Plant Species Distribution Patterns Using Simple Climatic Parameters: A Case Study of Ecuadorian Palms. Ecography 1997, 20, 347–355. [Google Scholar] [CrossRef]
- Swets, K.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Ripple, W.; Estes, J.; Beschta, R.; Wilmers, C.; Ritchie, E.; Hebblewhite, M. Status and Ecological Effects of the World’s Largest Carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [PubMed]
- Flesher, K.M.; Medici, E.P. The Distribution and Conservation Status of Tapirus Terrestris in the South American Atlantic Forest. Neotrop. Biol. Conserv. 2022, 17, 1–19. [Google Scholar] [CrossRef]
- Kiltie, R.A.; Terborgh, J. Observations on the Behavior of Rain-Forest Peccaries in Peru—Why Do White-Lipped Peccaries Form Herds. Z. Tierpsychol. 1983, 62, 241–255. [Google Scholar] [CrossRef]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Powell, G. Habitat Use, Activity Patterns and Use of Mineral Licks by Five Species of Ungulate in South-Eastern Peru. J. Trop. Ecol. 2009, 25, 261–270. [Google Scholar] [CrossRef]
- Goulart, F.; Cáceres, N.; Graipel, M.; Tortato, M.; Ghizoni, I., Jr.; Oliveira-Santos, L. Habitat Selection by Large Mammals in a Southern Brazilian Atlantic Forest. Mamm. Biol. 2009, 74, 182–190. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Copeland, H.; Pocewicz, A.; McKenney, B. Development by Design: Blending Landscape-Level Planning with the Mitigation Hierarchy. Front. Ecol. Environ. 2010, 8, 261–266. [Google Scholar] [CrossRef]
- Sandker, M.; Campbell, B.M.; Ruiz-Pérez, M.; Sayer, J.A.; Cowling, R.; Habtemariam, K.; Knight, A. The Role of Participatory Modeling in Landscape Approaches to Reconcile Conservation and Development. Ecol. Soc. 2010, 15, 13. [Google Scholar] [CrossRef]
- Young, J.C.; Marzano, M.; White, R.M.; McCracken, D.I.; Redpath, S.; Carss, D.; Quine, C.; Watt, A. The Emergence of Biodiversity Conflicts from Biodiversity Impacts: Characteristics and Management Strategies. Biodivers. Conserv. 2010, 19, 3973–3990. [Google Scholar] [CrossRef]
- McShane, T.O.; Hirsch, P.D.; Trung, T.C.; Songorwa, A.N.; Kinzig, A.; Monteferri, B. Hard Choices: Making Trade-Offs between Biodiversity Conservation and Human Well-Being. Biol. Conserv. 2011, 144, 966–972. [Google Scholar] [CrossRef]
- Veíssimo, D. Influencing Human Behaviour: An Underutilized Tool for Biodiversity Management. Conserv. Evid. 2013, 10, 29–31. [Google Scholar]
- Young, J.C.; Jordan, A.; Searle, K.R.; Butler, A.; Chapman, D.S.; Simmons, P. Does Stakeholder Involvement Really Benefit Biodiversity Conservation. Biol. Conserv. 2013, 158, 359–370. [Google Scholar] [CrossRef]
- Young, J.C.; Jordan, A.; Searle, K.R.; Butler, A.; Simmons, P.; Watt, A.D. Framing Scale in Participatory Biodiversity Management May Contribute to More Sustainable Solutions. Conserv. Lett. 2013, 6, 333–340. [Google Scholar] [CrossRef]
- Dilkina, B.; Houtman, R.; Gomes, C.P.; Montgomery, C.A.; McKelvey, K.S.; Kendall, K. Trade-Offs and Efficiencies in Optimal Budget-Constrained Multispecies Corridor Networks. Conserv. Biol. 2017, 31, 192–202. [Google Scholar] [CrossRef]
- Keesstra, S.; Nunes, J.; Novara, A.; Finger, D.; Avelar, D.; Kalantari, C. The Superior Effect of Nature Based Solutions in Land Management for Enhancing Ecosystem Services. Sci. Total Environ. 2018, 610–611, 997–1009. [Google Scholar] [CrossRef]
- Peterson, D.L.; Miller, C.L.; Joyce, L.A.; Furniss, M.J.; Halofsky, J.E.; Neilson, R.P. Responding to Climate Change in National Forests: A Guidebook to Developing Adaptation Options; U.S. Department of Agriculture Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2011; p. 109.
- Schwartz, M.W. Using Niche Models with Climate Projections to Inform Conservation Management Decisions. Biol. Conserv. 2012, 155, 149–156. [Google Scholar] [CrossRef]
- Faleiro, F.V.; Machado, R.B.; Loyola, R.D. Defining Spatial Conservation Priorities in the Face of Land-Use and Climate Change. Biol. Conserv. 2013, 158, 248–257. [Google Scholar] [CrossRef]
- Di Minin, E.; Slotow, R.; Hunter, L.; Pouzols, F.; Toivonen, T.; Verburg, P. Global Priorities for National Carnivore Conservation under Land Use Change. Sci. Rep. 2016, 6, 23814. [Google Scholar] [CrossRef]
- Fick, S.E.; Higmans, R. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.; Beher, J.; Jones, K.; Possingham, H.; Laurance, W.; Wood, P.; Fekete, B.; et al. Global Terrestrial Human Footprint Maps for 1993 and 2009. Sci. Data 2016, 3, 160067. [Google Scholar] [CrossRef] [PubMed]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.; Beher, J.; Jones, K.; Possingham, H.; Laurance, W.; Wood, P.; Fekete, B.; et al. Sixteen Years of Change in the Global Terrestrial Human Footprint and Implications for Biodiversity Conservation. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef]
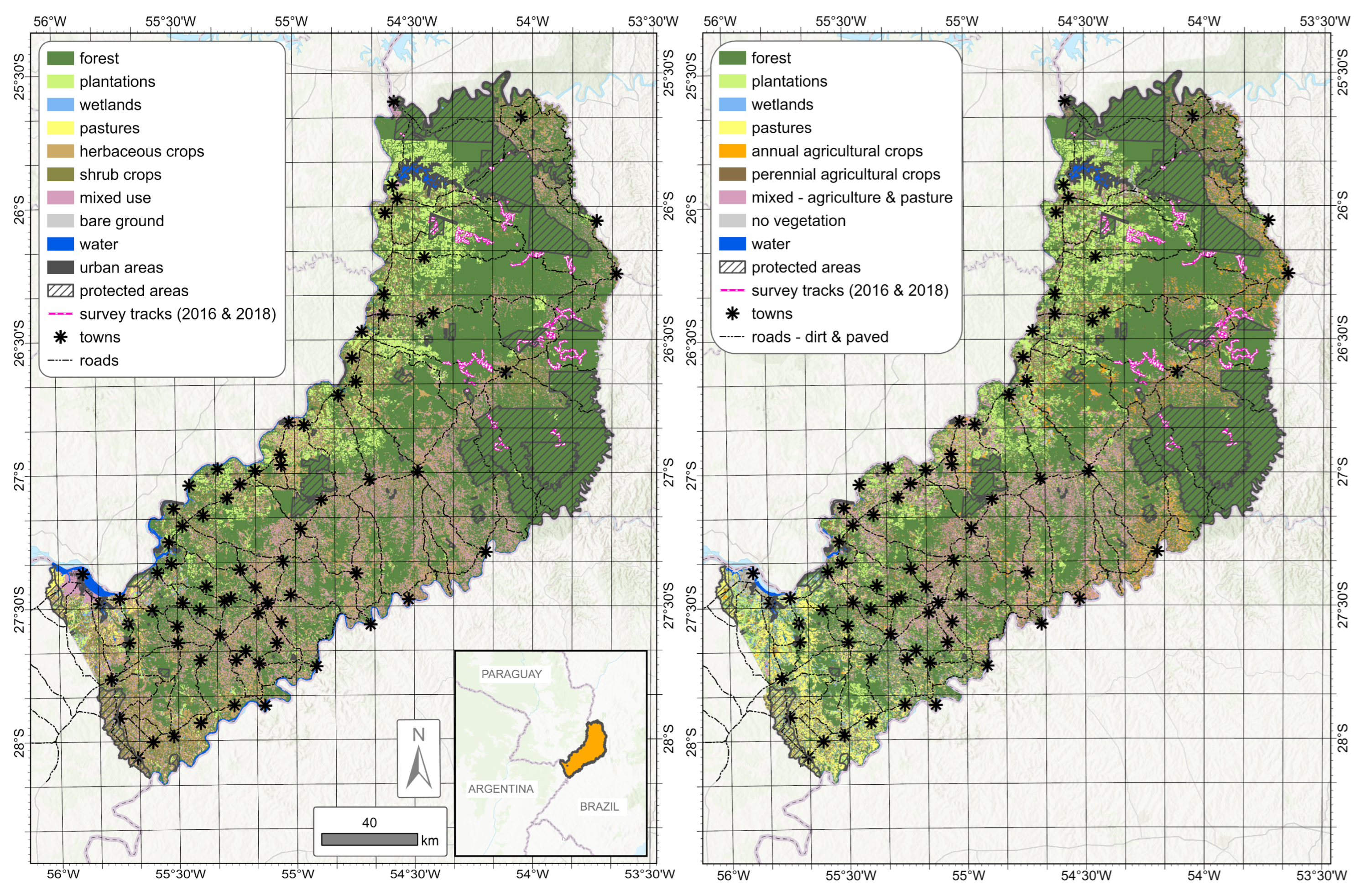
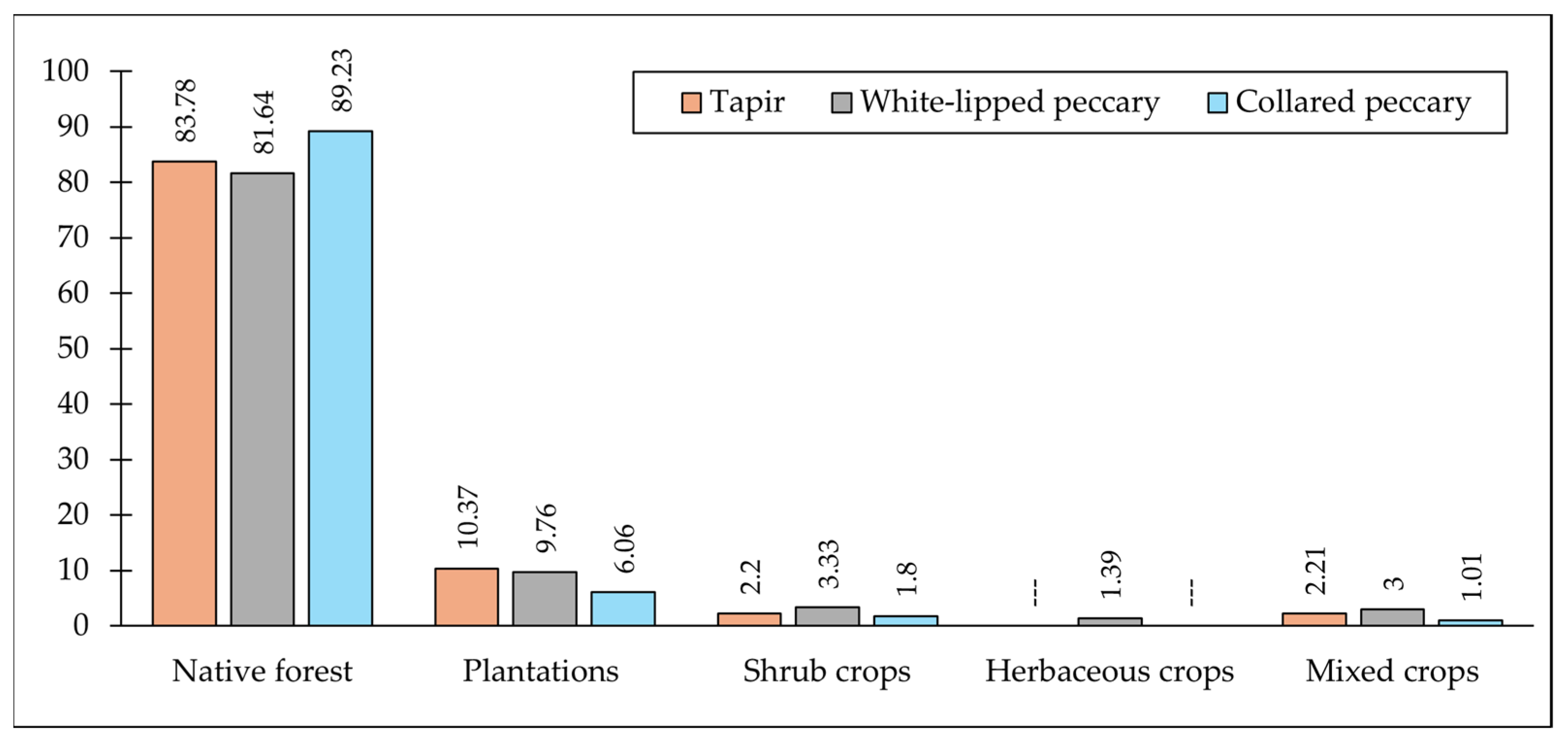
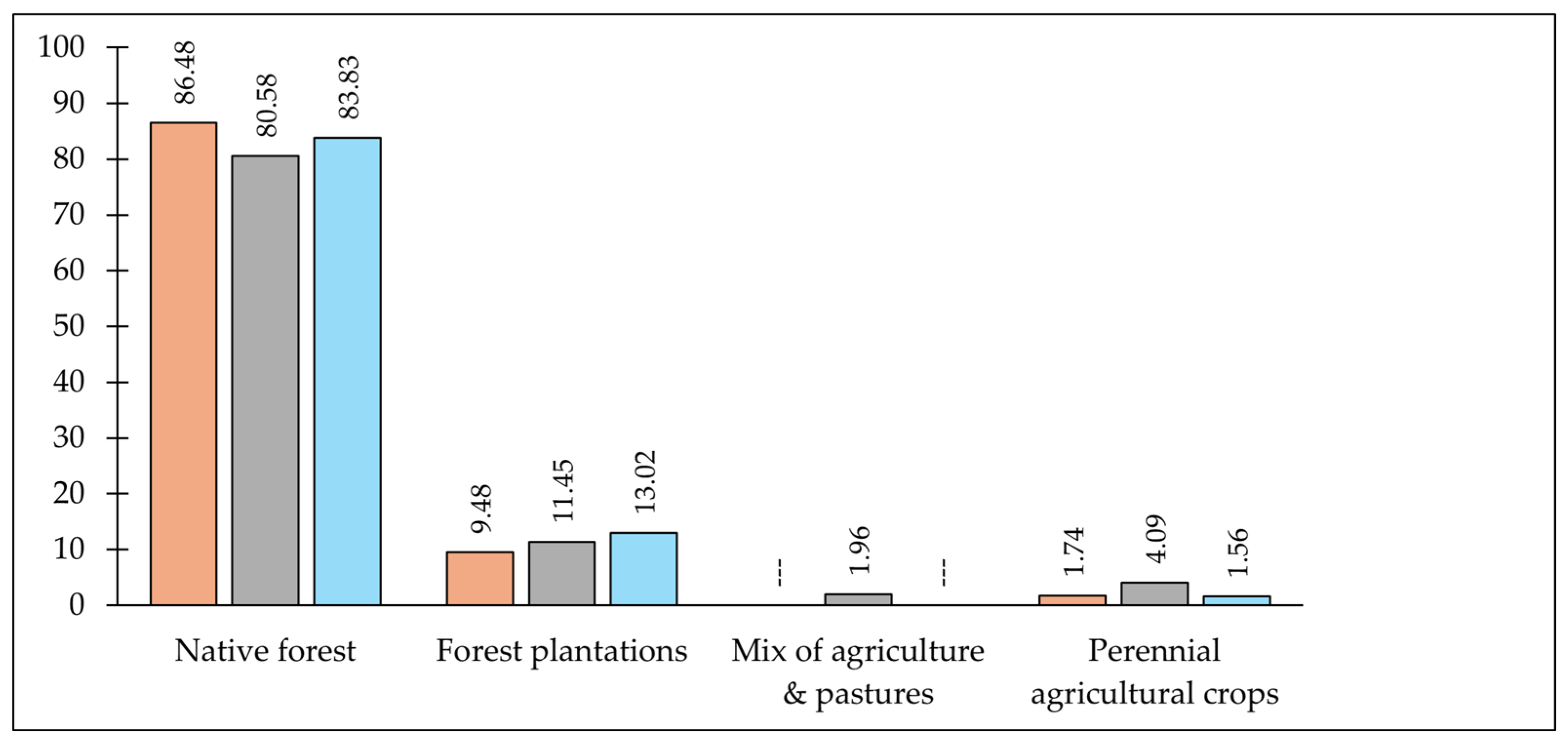
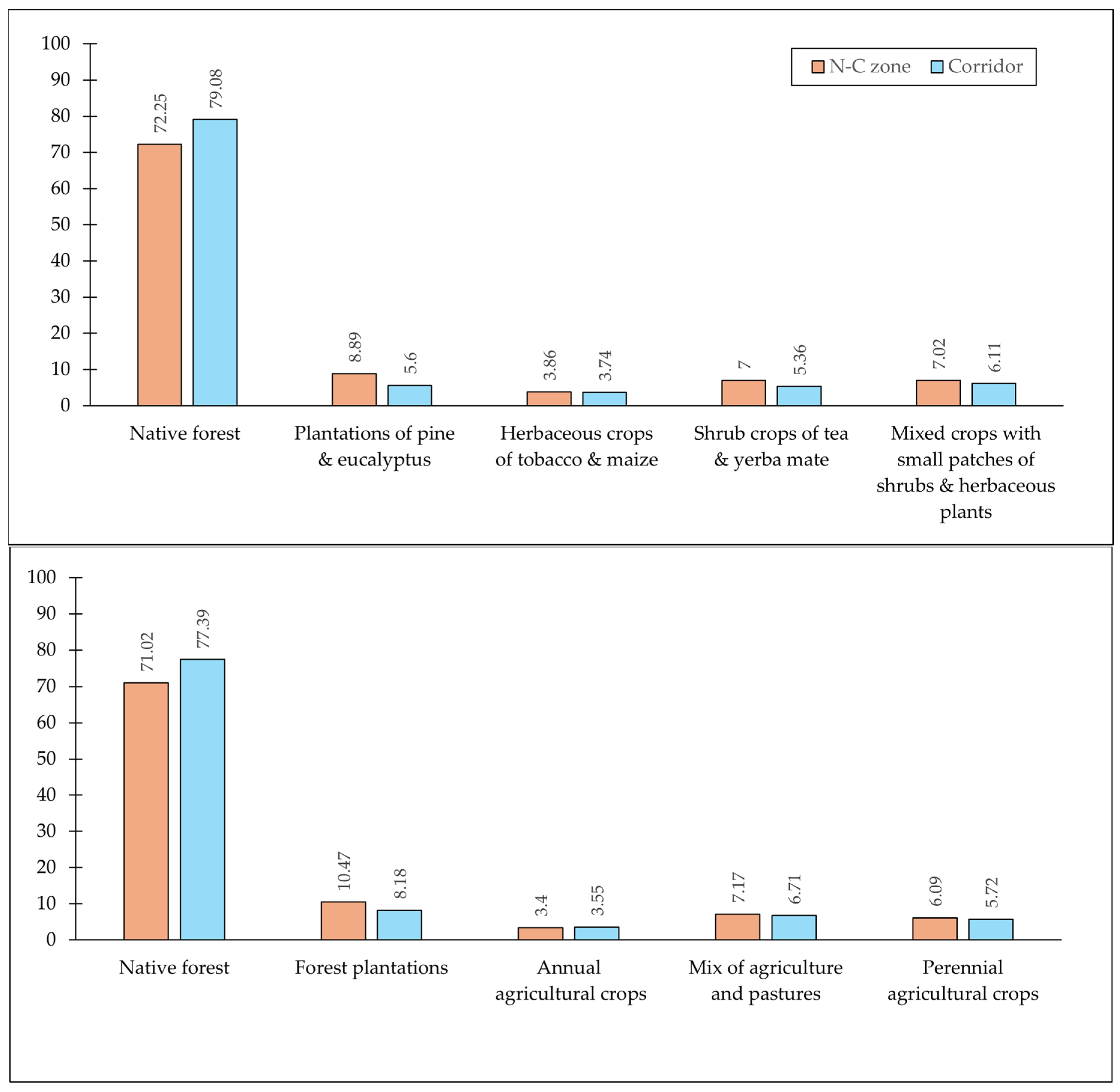


| Variable | Frequency | Final Model |
|---|---|---|
| Model 1: | ||
| Native forest | yes | yes |
| Plantations of pine & eucalyptus | yes | yes |
| Wetlands | yes | no |
| Pastures | yes | no |
| Herbaceous crops of tobacco & maize | yes | yes |
| Shrub crops of tea & yerba mate | yes | yes |
| Mixed crops with small patches of shrubs & herbaceous plants | yes | yes |
| Naturally bare ground | yes | no |
| Water bodies (natural & artificial) | yes | no |
| Urban and infrastructure | yes | yes |
| Landscape heterogeneity index | yes | yes |
| Land use type | no | yes |
| Model 2: | ||
| Native forest | yes | yes |
| Forest plantations | yes | yes |
| Wetlands | yes | no |
| Pastures | yes | yes |
| Annual agricultural crops | yes | yes |
| Mix of agriculture and pastures | yes | yes |
| No vegetation | yes | no |
| Water | yes | yes |
| Perennial agricultural crops | yes | yes |
| Landscape heterogeneity index | yes | yes |
| Land use type | no | yes |
| ENM | N-C Zone ALL | N-C Zone OUTSIDE PA | Corridor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Marginal | Optimal | Total | Marginal | Optimal | Total | Marginal | Optimal | ||
| Tapir | Model 1 | 52.73 | 36.17 | 16.56 | 37.91 | 25.16 | 12.75 | 51.52 | 31.00 | 20.52 |
| Model 2 | 51.31 | 38.15 | 13.16 | 37.11 | 25.26 | 11.85 | 59.34 | 38.46 | 20.88 | |
| White-lipped Peccary | Model 1 | 69.80 | 40.85 | 28.95 | 59.08 | 34.13 | 24.95 | 78.01 | 30.77 | 47.24 |
| Model 2 | 62.59 | 37.68 | 24.91 | 51.91 | 27.83 | 24.08 | 69.31 | 30.47 | 38.84 | |
| Collared Peccary | Model 1 | 60.29 | 14.17 | 46.12 | 45.55 | 17.58 | 27.97 | 67.86 | 20.24 | 47.62 |
| Model 2 | 63.29 | 16.08 | 47.21 | 50.89 | 20.05 | 30.84 | 64.36 | 17.70 | 46.66 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeMatteo, K.E.; Sotorres, D.; Escalante, O.M.; Ibañez Alegre, D.M.; Delgado, P.M.; Rinas, M.A.; Argüelles, C.F. Integrating Species Distribution Models to Identify Overlapping Predator–Prey Conservation Priorities in Misiones, Argentina. Diversity 2025, 17, 748. https://doi.org/10.3390/d17110748
DeMatteo KE, Sotorres D, Escalante OM, Ibañez Alegre DM, Delgado PM, Rinas MA, Argüelles CF. Integrating Species Distribution Models to Identify Overlapping Predator–Prey Conservation Priorities in Misiones, Argentina. Diversity. 2025; 17(11):748. https://doi.org/10.3390/d17110748
Chicago/Turabian StyleDeMatteo, Karen E., Delfina Sotorres, Orlando M. Escalante, Daiana M. Ibañez Alegre, Pryscilha M. Delgado, Miguel A. Rinas, and Carina F. Argüelles. 2025. "Integrating Species Distribution Models to Identify Overlapping Predator–Prey Conservation Priorities in Misiones, Argentina" Diversity 17, no. 11: 748. https://doi.org/10.3390/d17110748
APA StyleDeMatteo, K. E., Sotorres, D., Escalante, O. M., Ibañez Alegre, D. M., Delgado, P. M., Rinas, M. A., & Argüelles, C. F. (2025). Integrating Species Distribution Models to Identify Overlapping Predator–Prey Conservation Priorities in Misiones, Argentina. Diversity, 17(11), 748. https://doi.org/10.3390/d17110748









