Home Range Size and Habitat Usage of Hatchling and Juvenile Wood Turtles (Glyptemys insculpta) in Iowa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Sites
2.3. Radio-Telemetry and Head-Starting
2.4. Home Range Size and Habitat Usage
2.4.1. Categorical Habitat Usage
2.4.2. Distance from Water
2.5. Statistical Analyses
Comparison Groups
3. Results
3.1. Radiotelemetry
3.2. Home Range Size
3.3. Categorical Habitat Usage
3.4. Distance from Water
3.5. Statistical Analyses
3.5.1. Home Range Size
3.5.2. Categorical Habitat Usage
3.5.3. Distance from Water
4. Discussion
4.1. Comparisons with Other Hatchling and Juvenile Wood Turtle Studies
4.2. Comparisons with Adult Wood Turtle Studies
4.3. Conservation and Management Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iowa Natural Resource Commission. Chapter 77: Endangered and Threatened Plant and Animal Species. 2009. Available online: https://www.legis.iowa.gov/docs/ACO/chapter/571.77.pdf (accessed on 11 October 2025).
- van Dijk, P.P.; Harding, J. Glyptemys insculpta [Errata Version Published in 2016]; The International Union for the Conservation of Nature Red List of Threatened Species: Gland, Switzerland, 2011; Available online: www.IUCN.org (accessed on 11 October 2025).
- COSEWIC. COSEWIC Assessment and Status Report on the Wood Turtle Glyptemys insculpta in Canada; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2018; 51p, Available online: http://www.registrelepsararegistry.gc.ca/default.asp?lang=en&n=24F7211B-1 (accessed on 11 October 2025).
- Spradling, T.A.; Tamplin, J.W.; Dow, S.S.; Meyer, K.J. Conservation genetics of a peripherally isolated population of the Wood Turtle (Glyptemys insculpta) in Iowa. Conserv. Gen. 2010, 11, 1667–1677. [Google Scholar] [CrossRef]
- Otten, J.G.; Hulbert, A.C.; Berg, S.W.; Tamplin, J.W. Home range, site fidelity, and movement patterns of the Wood Turtle (Glyptemys insculpta) at the southwestern edge of its range. Chelonian Conserv. Biol. 2021, 20, 231–241. [Google Scholar] [CrossRef]
- Spaid, S.; Brown, D.J.; Mota, J.F.; Badje, A.F.; Crozier, G.E.; Lapin, C.N.; Lee, Y.M.; Moen, R.A.; Tamplin, J.W. Projected contemporary habitat distribution and quality for wood turtles across the Upper Midwest. J. Wildl. Manag. 2025, e70108. [Google Scholar] [CrossRef]
- Lapin, C.N.; Tamplin, J.W.; Cochrane, M.M.; Woodford, J.E.; Brown, D.J.; Moen, R.A. A regional analysis of Glyptemys insculpta (Wood Turtle) survival in the upper Midwest of the USA. Herpetol. Conserv. Biol. 2019, 14, 668–679. [Google Scholar]
- Tamplin, J.W. Upper Midwest Turtle Conservation—Phase 3; Final Report to the US Fish and Wildlife Service for Iowa State Wildlife; Grant No. F21AP01078; State of Iowa, Department of Natural Resources: Des Moines, IA, USA, 2024; 148p. [Google Scholar]
- Brewster, K.N.; Brewster, C.M. Movement and microhabitat use by juvenile Wood Turtles introduced into a riparian habitat. J. Herpetol. 1991, 25, 379–382. [Google Scholar] [CrossRef]
- Tuttle, S.E.; Carroll, D.M. Movements and behavior of hatchling wood turtles (Glyptemys insculpta). Northeast. Nat. 2005, 12, 331–348. [Google Scholar] [CrossRef]
- Castellano, C.M.; Behler, J.L.; Ultsch, G.R. Terrestrial movements of hatchling Wood Turtles (Glyptemys insculpta) in agricultural fields in New Jersey. Chelonian Conserv. Biol. 2008, 7, 113–118. [Google Scholar] [CrossRef]
- Paterson, J.E.; Steinberg, B.D.; Litzgus, J.D. Revealing a cryptic life-history stage: Differences in habitat selection and survivorship between hatchlings of two turtle species at risk (Glyptemys insculpta and Emydoidea blandingii). Wildl. Res. 2012, 39, 408–418. [Google Scholar] [CrossRef]
- Dragon, J. Habitat Selection, Movement, and Survival of Hatchling Wood Turtles (Glyptemys insculpta) in an Atypical Habitat. Master’s Thesis, George Mason University, Fairfax, VA, USA, 2014. [Google Scholar]
- Dragon, J.; Akre, T.; McShea, W.; Kleopfer, J.D. Post Emergence Behavior, Habitat Selection, and Survival of Neonatal Wood Turtles (Glyptemys Insculpta) in an Unusual Landscape; Abstract; Northeast Partners in Amphibian and Reptile Conservation: Branchville, NJ, USA, 2013. [Google Scholar]
- Paterson, J.E.; Steinberg, B.D.; Litzgus, J.D. Effects of body size, habitat selection and exposure on hatchling turtle survival. J. Zool. 2014, 294, 278–285. [Google Scholar] [CrossRef]
- Bernstein, N.P.; Fendrich, R.H.; McCollum, S.A. Do home range, movement patterns, and habitat use of Ornate Box Turtles (Terrepene ornata ornata) differ among age classes? J. Herpetol. 2023, 57, 1–10. [Google Scholar] [CrossRef]
- Macdonald, S.L.; Llewelyn, J.; Moritz, C.; Phillips, B.L. Peripheral isolates as sources of adaptive diversity under climate change. Front. Ecol. Evol. 2017, 5, 88. [Google Scholar] [CrossRef]
- Arvisais, M.; Bourgeois, J.-C.; Levesque, E.; Daigle, C.; Masse, D.; Jutras, J. Home range and movements of a wood turtle (Clemmys insculpta) population at the northern limit of its range. Can. J. Zool. 2002, 80, 402–408. [Google Scholar] [CrossRef]
- Jones, M.T.; Saumure, R.A.; Willey, L.L.; Roberts, H.P. Chapter 1, Introduction. In Biology and Conservation of the Wood Turtle; Jones, M.T., Willey, L.L., Eds.; Northeast Association of Fish and Wildlife Agencies: Petersburgh, NY, USA, 2021; pp. 1–20. [Google Scholar]
- Willey, L.L.; Akre, T.S.B.; Jones, M.T.; Brown, D.J.; Tamplin, J.W. Chapter 6, Spatial ecology and seasonal behavior. In Biology and Conservation of the Wood Turtle; Jones, M.T., Willey, L.L., Eds.; Northeast Association of Fish and Wildlife Agencies: Petersburgh, NY, USA, 2021; pp. 113–136. [Google Scholar]
- Row, J.R.; Blouin-Demers, G. Kernels are not accurate estimators of home-range size for herpetofauna. Copeia 2006, 2006, 797–802. [Google Scholar] [CrossRef]
- Boyle, S.A.; Lourenço, W.C.; da Silva, L.R.; Smith, A.T. Home range estimates vary with sample size and methods. Folia Primatol. 2009, 80, 33–42. [Google Scholar] [CrossRef]
- Boyle, S.A. Chapter 7, Home Range Analysis: Why the Methods Matter. In Spatial Analysis in Field Primatology: Applying GIS at Varying Scales; Dolins, F.L., Shaffer, C.A., Porter, L.M., Hickey, J.R., Nibbelink, N.P., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 129–151. [Google Scholar] [CrossRef]
- Weaver, R.E.; Suriyamongkol, T.; Shoemaker, S.N.; Gonzales, J.T.; Mali, I. Spatial ecology and movement of Ornate Box Turtles in the escalating drought conditions of the Great Plains ecoregion. Fire 2025, 8, 24. [Google Scholar] [CrossRef]
- Hayne, D.W. Calculation of size of home range. J. Mammal. 1949, 30, 1–18. [Google Scholar] [CrossRef]
- Powell, R.A. Animal home ranges and territories and home range estimators. In Research Techniques in Animal Ecology: Controversies and Consequences; Boitani, L., Fuller, T.K., Eds.; Columbia University Press: New York, NY, USA, 2000; pp. 65–100. [Google Scholar]
- Saumure, R.A. Spatial Ecology and Conservation of the North American Wood Turtle (Glyptemys insculpta) in a Fragmented Agri-Forested Landscape. Ph.D. Dissertation, McGill University, Montreal, QC, Canada, 2004. [Google Scholar]
- White, G.C.; Garrott, R.A. Analysis of Wildlife Radiotracking Data; Academic Press: San Diego, CA, USA, 1990; 382p. [Google Scholar]
- Silva, I.; Crane, M.; Marshall, B.M.; Strine, C.T. Reptiles on the wrong track? Moving beyond traditional estimators with dynamic Brownian bridge movement models. Mov. Ecol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Anderson, D.J. The home range: A new nonparametric estimation technique. Ecology 1982, 63, 103–112. [Google Scholar] [CrossRef]
- Ernst, C.H.; Lovich, J.E. Turtles of the United States and Canada; Johns Hopkins University Press: Baltimore, MD, USA, 2009; 840p. [Google Scholar]
- Jones, M.T.; Willey, L.L.; Crowley, J.; Akre, T.S.B.; deMaynadier, P.; Yorks, D.T.; Tamplin, J.W.; Zarate, B.; Saumure, R.A.; Badje, A.; et al. Chapter 4, Distribution of the Wood Turtle. In Biology and Conservation of the Wood Turtle; Jones, M.T., Willey, L.L., Eds.; Northeast Association of Fish and Wildlife Agencies: Petersburgh, NY, USA, 2021; pp. 61–80. [Google Scholar]
- Jones, M.T.; Willey, L.L.; Mays, J.D.; Akre, T.S.B.; Tamplin, J.W.; Gipe, K.D.; Burne, M.R.; Kleopfer, J.D.; Badje, A. Chapter 5, Habitat. In Biology and Conservation of the Wood Turtle; Jones, M.T., Willey, L.L., Eds.; Northeast Association of Fish and Wildlife Agencies: Petersburgh, NY, USA, 2021; pp. 81–111. [Google Scholar]
- Arvisais, M.; Levesque, E.; Bourgeois, J.-C.; Daigle, C.; Masse, D.; Jutras, J. Habitat selection by the wood turtle (Clemmys insculpta) at the northern limit of its range. Can. J. Zool. 2004, 82, 391–398. [Google Scholar] [CrossRef]
- Niederberger, A.J.; Seidel, M.E. Ecology and status of a wood turtle (Clemmys insculpta) population in West Virginia. Chelonian Conserv. Biol. 1999, 3, 414–418. [Google Scholar]
- Tamplin, J.; Haugen, J.; Anderson, T.; Berg, S.; Burtch, J.; Hayes, A.; Hobbs, G. Water depth selection by Iowa Wood turtles (Glyptemys insculpta) during seasonal activity periods. Northeast. Nat. 2024, 31, G1–G17. [Google Scholar] [CrossRef]
- Akre, T.S.B.; Ernst, C.H. Population Dynamics, Habitat Use, and Home Range of the Wood Turtle, Glyptemys (=Clemmys) insculpta, in Virginia; Unpublished Report; Virginia Department of Game and Inland Fisheries: Richmond, VA, USA, 2006; 257p. [Google Scholar]
- Parren, S.G. A twenty-five-year study of the Wood Turtle (Glyptemys insculpta) in Vermont: Movements, behavior, injuries, and death. Herpetol. Conserv. Biol. 2013, 8, 176–190. [Google Scholar]
- Tamplin, J.W. Upper Midwest Turtle Conservation—Phase 2; Final Report to the US Fish and Wildlife Service for Iowa State Wildlife; Grant No. F21AP01078; State of Iowa, Department of Natural Resources: Des Moines, IA, USA, 2019; 40p. [Google Scholar]
- Mitchell, J.C.; Paton, P.W.C.; Raithel, C.J. Chapter 9, The importance of vernal pools to reptiles, birds, and mammals. In Science and Conservation of Vernal Pools in Northeastern North America; Calhoun, A.K., de Maynadier, P., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 169–190. [Google Scholar] [CrossRef]
- Hagani, J.S.; Macey, S.K.; Foley, J.D.; Seewagen, C.L. Movement ecology of the imperiled Wood Turtle (Glyptemys insculpta) in a lower Hudson River watershed. Chelonian Conserv. Biol. 2021, 20, 281–289. [Google Scholar] [CrossRef]
- McCoard, K.R.P.; Billings, A.A.; Anderson, J.T. Wood turtle home range and habitat use in the Central Appalachians. Chelonian Conserv. Biol. 2016, 15, 173–180. [Google Scholar] [CrossRef]
- Roberts, H.P.; Willey, L.L.; Jones, M.T.; Milam, J.; King, D.I. Long-term spatial ecology of three long-lived turtles. Northeast. Nat. 2024, 31, C92–C118. [Google Scholar] [CrossRef]
- Thompson, D.G.; Swystun, T.; Cross, J.; Cross, R.L.; Chartrand, D.; Edge, C.B. Fine and coarse-scale movements and habitat use by Wood Turtles (Glyptemys insculpta) based on probabilistic modeling of radio- and GPS-telemetry data. Can. J. Zool. 2018, 96, 1153–1164. [Google Scholar] [CrossRef]
- Cagle, F.R. A system of marking turtles for future identification. Copeia 1939, 1939, 170–173. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 26 March 2025).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. lme4: Linear Mixed-Efffects Models Using “Eigen” and S4. Version 1.1-37. 2025. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on 15 June 2025).
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Bowers, B.C.; Walkup, D.K.; Hibbitts, T.J.; Crump, P.S.; Ryberg, W.A.; Lawing, A.M.; Lopez, R.R. Should I stay or should I go? Spatial ecology of Western Chicken Turtles (Deirochelys reticularia miaria). Herpetol. Cons. Biol. 2021, 16, 594–611. [Google Scholar]
- Ross, D.A.; Brewster, K.N.; Anderson, R.K.; Ratner, N.; Brewster, C.M. Aspects of the ecology of wood turtles, Clemmys insculpta, in Wisconsin. Can. Field Nat. 1991, 105, 363–367. [Google Scholar] [CrossRef]
- Jones, M.T.; Willey, L.L. Cross-watershed dispersal and annual movement in adult Wood Turtles (Glyptemys insculpta). Herpetol. Rev. 2020, 51, 208–211. [Google Scholar]
- Tuttle, S.E.; Carroll, D.M. Home range and seasonal movements of the Wood Turtle (Glyptemys insculpta) in Southern New Hampshire. Chelonian Conserv. Biol. 2003, 4, 656–663. [Google Scholar]
- Brown, D.J.; Nelson, M.D.; Rugg, D.J.; Buech, R.R.; Donner, D.H. Spatial and temporal habitat-use patterns of Wood Turtles at the western edge of their distribution. J. Herpetol. 2016, 50, 347–356. [Google Scholar] [CrossRef]
- McCoard, K.R.P.; McCoard, N.S.; Turk, P.J.; Anderson, J.T. Habitat characteristics that influence the occurrence of Wood Turtles at the southern limits of their range in the central Appalachians. J. Herpetol. 2016, 50, 381–387. [Google Scholar] [CrossRef]
- Bougie, T.A.; Peery, M.Z.; Lapin, C.N.; Woodford, J.E.; Pauli, J.N. Not all management is equal: A comparison of methods to increase wood turtle population viability. J. Wildl. Manag. 2022, 86, e22234. [Google Scholar] [CrossRef]
- McKnight, D.T.; Bower, D.S.; Ariel, E.; Beatty, S.; Clulow, S.; Connell, M.; Deppe, A.R.; Doody, S.; Freeman, A.; Georges, A.; et al. Does a lack of juveniles indicate a threat? Understanding body size distributions in a group of long-lived vertebrates. J. Anim. Ecol. 2025, 94, 1962–1982. [Google Scholar] [CrossRef] [PubMed]
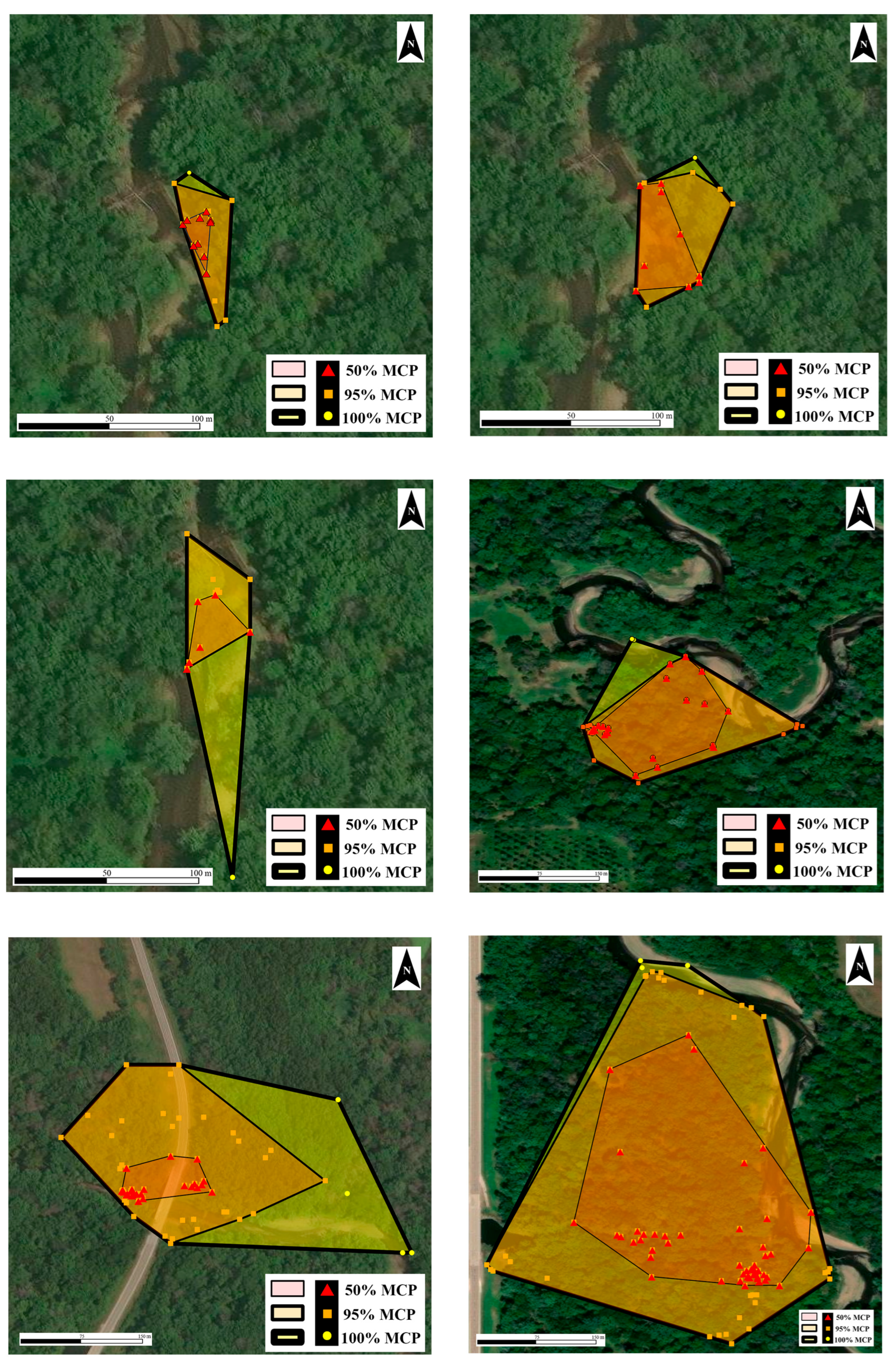


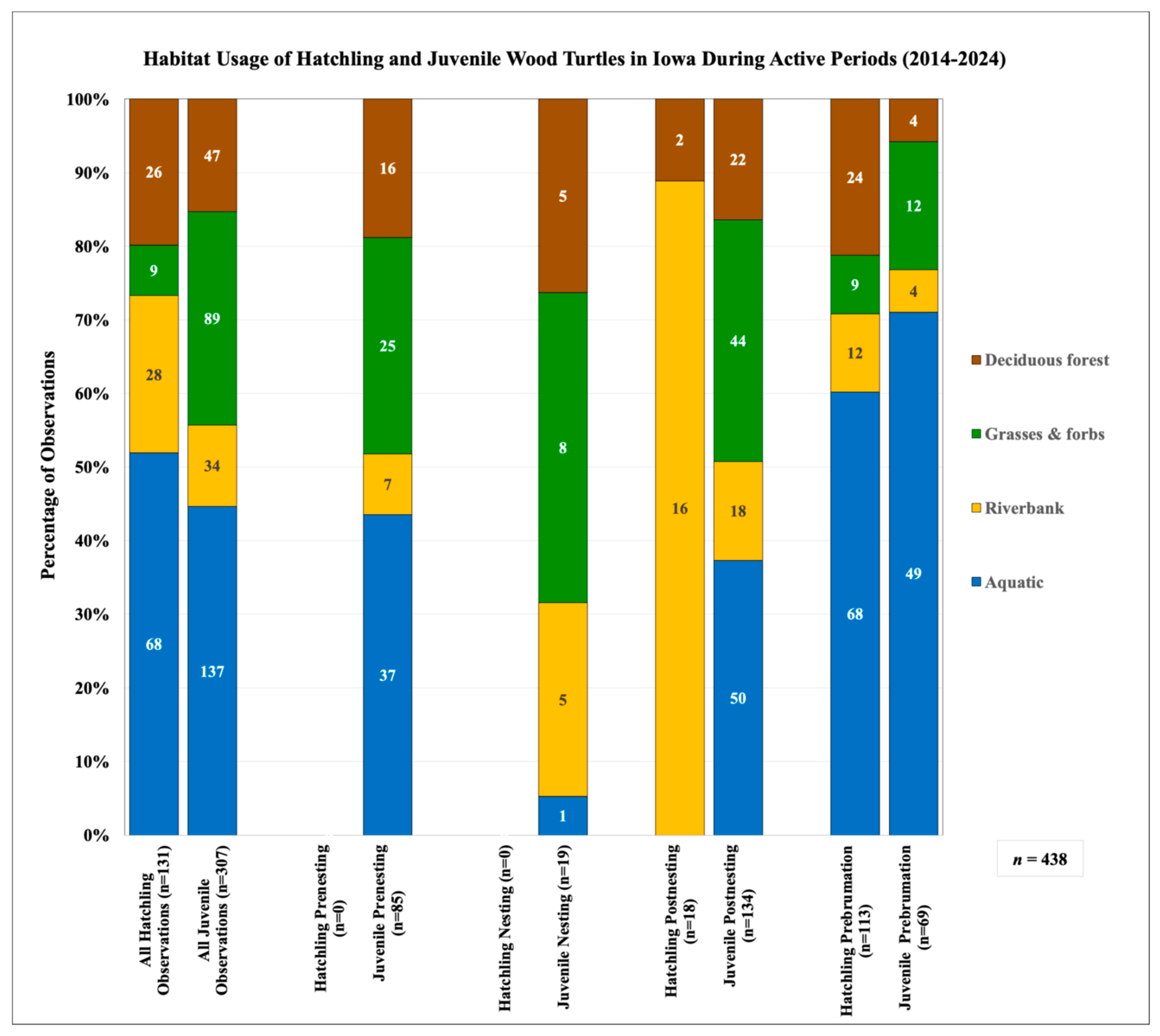
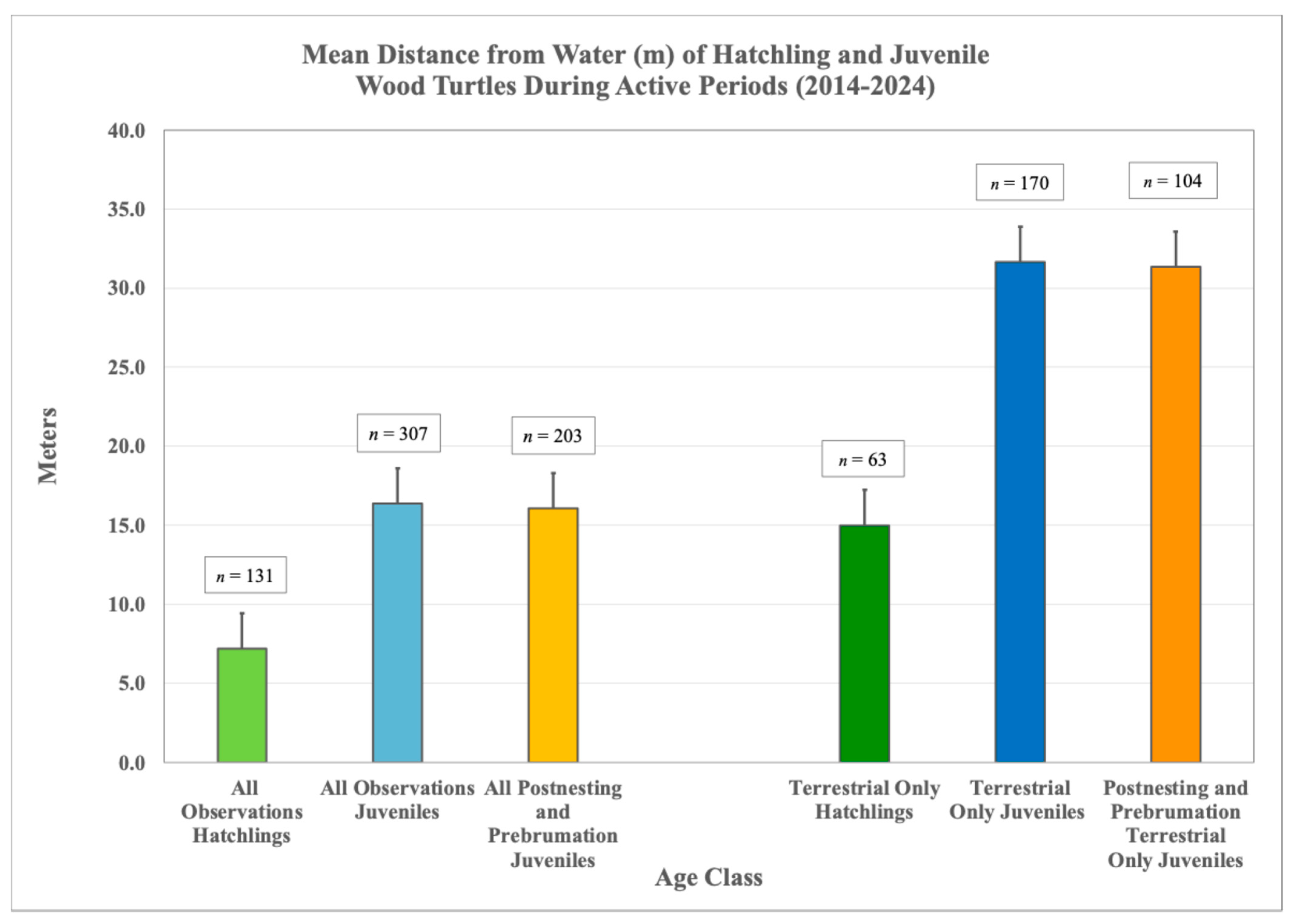



| Age Class | 100% MCP (ha) | 95% MCP (ha) | 50% MCP (ha) | 95% KDE (ha) | 50% KDE (ha) | LHR (m) | SHR (m) | n Locations | n Weeks | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 0.18 | 0.16 | 0.06 | 0.92 | 0.22 | 100.62 | 39.38 | 21.83 | 6.00 | |
| Hatchlings | SD | 0.05 | 0.06 | 0.04 | 0.41 | 0.08 | 38.96 | 17.34 | 0.41 | 0.00 |
| (n = 6) | SE | 0.02 | 0.02 | 0.02 | 0.17 | 0.03 | 15.91 | 7.08 | 0.17 | 0.00 |
| All Obs. | Min | 0.10 | 0.08 | 0.01 | 0.48 | 0.13 | 75.80 | 15.00 | 21.00 | 6.00 |
| Max | 0.26 | 0.26 | 0.10 | 1.60 | 0.32 | 176.00 | 68.80 | 22.00 | 6.00 | |
| Mean | 3.96 | 2.61 | 0.82 | 14.06 | 3.41 | 440.00 | 445.86 | 22.00 | 22.00 | |
| Juveniles | SD | 2.16 | 2.59 | 0.87 | 10.78 | 3.03 | 125.44 | 189.52 | 0.00 | 0.00 |
| (n = 7) | SE | 0.82 | 0.98 | 0.33 | 4.07 | 1.15 | 47.41 | 71.63 | 0.00 | 0.00 |
| Reduced | Min | 1.41 | 0.45 | 0.06 | 4.26 | 1.12 | 247.00 | 285.00 | 22 (12) | 22 (12) |
| Obs. | Max | 8.24 | 8.21 | 2.47 | 36.43 | 9.91 | 630.00 | 828.00 | 22 (12) | 22 (12) |
| Mean | 5.76 | 4.44 | 1.16 | 13.17 | 2.87 | 459.57 | 487.29 | 47.00 | 46.43 | |
| Juveniles | SD | 3.19 | 3.50 | 0.99 | 8.12 | 1.79 | 129.96 | 175.97 | 23.90 | 24.25 |
| (n = 7) | SE | 1.21 | 1.32 | 0.37 | 3.07 | 0.68 | 49.12 | 66.51 | 9.03 | 9.17 |
| All Obs. | Min | 2.44 | 0.45 | 0.16 | 6.37 | 1.12 | 247.00 | 294.00 | 22.00 | 22.00 |
| Max | 11.98 | 11.53 | 3.10 | 24.99 | 5.73 | 630.00 | 853.00 | 88.00 | 88.00 |
| Hatchlings (n = 6) | Juveniles (n = 7) | ||||
|---|---|---|---|---|---|
| All locations (ha ± SD) | 22 locations only (ha ± SD) | t | df | p-value | |
| 100% MCP | 0.1764 ± 0.0542 | 3.9568 ± 2.1583 | −11.96 | 11 | <0.0001 |
| 95% MCP | 0.1620 ± 0.0578 | 2.6010 ± 2.5863 | −6.41 | 11 | 0.0001 |
| 50% MCP | 0.0575 ± 0.0399 | 0.8221 ± 0.8687 | −3.68 | 11 | 0.0036 |
| 95% KDE | 0.9150 ± 0.4052 | 14.0557 ± 10.7822 | −7.92 | 11 | <0.0001 |
| 50% KDE | 0.2224 ± 0.0819 | 3.4144 ± 3.0306 | −7.50 | 11 | <0.0001 |
| All locations (ha ± SD) | All locations (ha ± SD) | t | df | p-value | |
| 100% MCP | 0.1764 ± 0.0542 | 5.7616 ± 3.1944 | −13.39 | 11 | <0.0001 |
| 95% MCP | 0.1620 ± 0.0578 | 4.4445 ± 3.5025 | −6.91 | 11 | <0.0001 |
| 50% MCP | 0.0575 ± 0.0399 | 1.1612 ± 0.9869 | −5.30 | 11 | 0.0003 |
| 95% KDE | 0.9150 ± 0.4052 | 13.1651 ± 8.1184 | −8.97 | 11 | <0.0001 |
| 50% KDE | 0.2224 ± 0.0819 | 2.8690 ± 1.7861 | −8.43 | 11 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamplin, J.W.; Otten, J.G.; Berg, S.W.; Patel, N.E.; Tipton, J.B.; Radunzel, J.M.R. Home Range Size and Habitat Usage of Hatchling and Juvenile Wood Turtles (Glyptemys insculpta) in Iowa. Diversity 2025, 17, 733. https://doi.org/10.3390/d17100733
Tamplin JW, Otten JG, Berg SW, Patel NE, Tipton JB, Radunzel JMR. Home Range Size and Habitat Usage of Hatchling and Juvenile Wood Turtles (Glyptemys insculpta) in Iowa. Diversity. 2025; 17(10):733. https://doi.org/10.3390/d17100733
Chicago/Turabian StyleTamplin, Jeffrey W., Joshua G. Otten, Samuel W. Berg, Nadia E. Patel, Jacob B. Tipton, and Justine M. R. Radunzel. 2025. "Home Range Size and Habitat Usage of Hatchling and Juvenile Wood Turtles (Glyptemys insculpta) in Iowa" Diversity 17, no. 10: 733. https://doi.org/10.3390/d17100733
APA StyleTamplin, J. W., Otten, J. G., Berg, S. W., Patel, N. E., Tipton, J. B., & Radunzel, J. M. R. (2025). Home Range Size and Habitat Usage of Hatchling and Juvenile Wood Turtles (Glyptemys insculpta) in Iowa. Diversity, 17(10), 733. https://doi.org/10.3390/d17100733







