Trichoderma in Sustainable Agriculture and the Challenges Related to Its Effectiveness
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Data Collection Process and Data Synthesis
2.3. Criteria for Selection
- Articles with titles and abstracts that really include the topics of interest.
- Data articles with strain, species, environmental conditions, soil type, host genotype, strain genotype, agrochemical dosages, and comparisons with other microorganisms.
- Review articles with relevant discussions.
- Articles with relevant experiments and data were added during the systematic review.
- We discarded duplicate articles.
3. Mechanisms of Action of Trichoderma
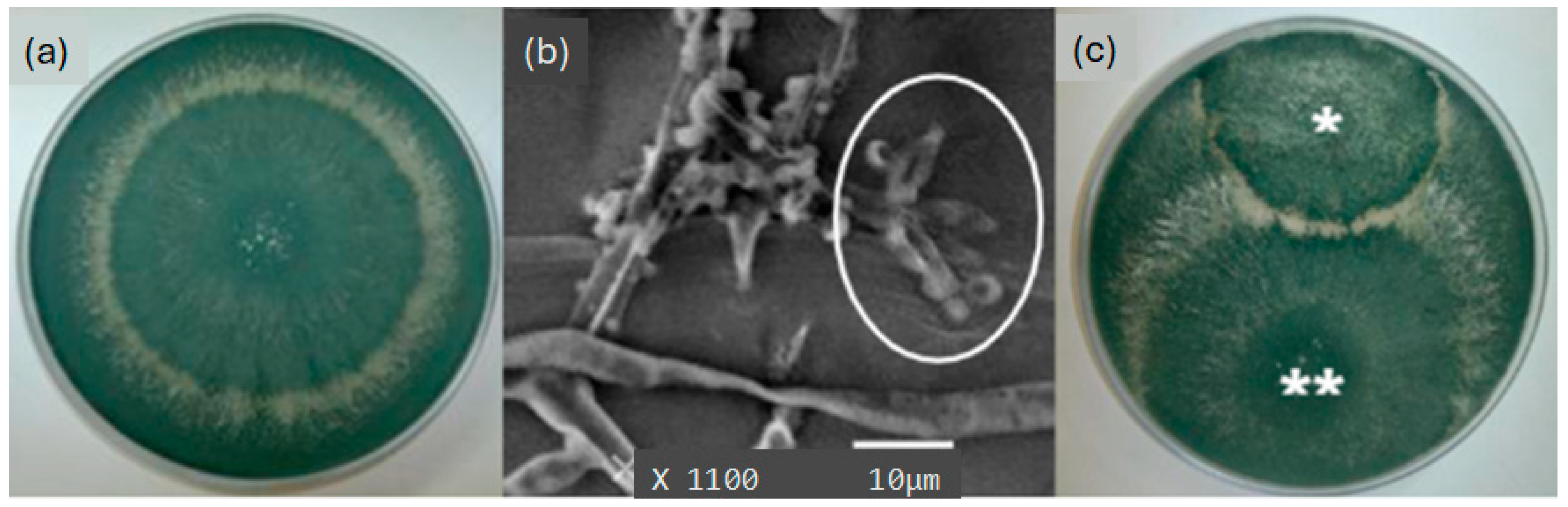
3.1. Mycoparasitism
3.2. Antibiosis
3.3. Induction of Systemic Resistance
3.4. Plant Growth Promotion
4. Applications of Trichoderma in Agriculture
- T. harzianum → 28
- T. asperellum → 17
- Trichoderma spp. → 13
- T. atroviride → 7
- T. viride → 5
- Trichoderma sp. → 5
- T. virens → 3
- T. longibrachiatum → 3
- T. afroharzianum → 3
- T. hamatum → 2
- T. gamsii → 2
- T. aureoviride → 1
- T. asperelloides → 1
- T. ghanense → 1
- T. koningiopsis → 1
- T. reesei → 1
- T. simmonsii → 1
- T. lignorum → 1
4.1. Most Used Trichoderma Species in Sustainable Agriculture
4.2. Cases of Success in Field Conditions
4.2.1. Disease Control in Field Conditions
4.2.2. Plant Growth Promotion and Nutrient Uptake
4.2.3. The Role of Trichoderma in Enhancing Plant Tolerance to Abiotic Stress
4.3. Implications for Field Applications
5. Context-Dependent Efficacy of Trichoderma
5.1. Influence of Strain-Specific Variability on Performance
5.2. Crop Type and Genotype Interactions
5.3. Soil Characteristics and Environmental Conditions
6. Challenges in the Application of Trichoderma
6.1. Variability in Field Performance vs. Laboratory Results
6.2. Compatibility with Agricultural Practices
6.3. Scalability and Cost-Effectiveness for Large-Scale Use
7. Advances in the Development of Trichoderma-Based Products
7.1. Formulation Technologies: Carrier Materials and Shelf-Life Improvements
7.2. Integration with Precision Agriculture and Biotechnological Tools
7.3. Molecular Identification and the Potential of Genetic Engineering to Enhance Strain Effectiveness
7.4. Market Trends and Consumer Acceptance
8. Knowledge Gaps and Future Perspectives
8.1. Need for Strain-Specific Studies Under Diverse Conditions
8.2. Development of Multi-Strain Consortia for Improved Resilience
8.3. Role of Microbial Ecology in Optimizing Trichoderma Performance
9. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCA | Biological control agent |
| ISR | Induced systemic resistance |
| 6-PP | 6-pentyl-α-pyrone |
| VOC | Volatile organic compound |
| PGPR | Plant growth-promoting rhizobacteria |
| PR | Pathogenesis-related |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| ROS | Reactive oxygen species |
| IAA | indole-3-acetic acid |
References
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and principles of environmentally sustainable food and agriculture systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological Control of Plant Diseases—What Has Been Achieved and What Is the Direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef]
- Thomine, E.; Mumford, J.; Rusch, A.; Desneux, N. Using Crop Diversity to Lower Pesticide Use: Socio-Ecological Approaches. Sci. Total Environ. 2022, 804, 150156. [Google Scholar] [CrossRef]
- Villavicencio-Vásquez, M.; Espinoza-Lozano, F.; Espinoza-Lozano, L.; Coronel-León, J. Biological Control Agents: Mechanisms of Action, Selection, Formulation and Challenges in Agriculture. Front. Agron. 2025, 7, 1578915. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.P.; Knox, O.G.G.; Forsyth, L.M. Biological Control Agents: The Importance for Specific and Targeted Screening Techniques to Enable Their Effective and Successful Implementation. Eur. J. Plant Pathol. 2025, 172, 713–728. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as Biological Control Agent: Scope and Prospects to Improve Efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A Multipurpose, Plant-Beneficial Microorganism for Eco-Sustainable Agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Li, W.-C.; Lin, T.-C.; Chen, C.-L.; Liu, H.-C.; Lin, H.-N.; Chao, J.-L.; Hsieh, C.-H.; Ni, H.-F.; Chen, R.-S.; Wang, T.-F. Complete Genome Sequences and Genome-Wide Characterization of Trichoderma Biocontrol Agents Provide New Insights into Their Evolution and Variation in Genome Organization, Sexual Development, and Fungal-Plant Interactions. Microbiol. Spectr. 2021, 9, e0066321. [Google Scholar] [CrossRef]
- Rush, T.A.; Shrestha, H.K.; Gopalakrishnan Meena, M.; Spangler, M.K.; Ellis, J.C.; Labbé, J.L.; Abraham, P.E. Bioprospecting Trichoderma: A Systematic Roadmap to Screen Genomes and Natural Products for Biocontrol Applications. Front. Fungal Biol. 2021, 2, 716511. [Google Scholar] [CrossRef] [PubMed]
- Akladious, S.A.; Abbas, S.M. Application of Trichoderma harzianum T22 as a biofertilizer potential in maize growth. J. Plant Nutr. 2014, 37, 30–49. [Google Scholar] [CrossRef]
- Das, B.C.; Das, B.K.; Dutta, P.; Sarmah, D.K. Bioformulation of Trichoderma harzianum Rifai for Management of Soybean Stem-Rot Caused by Rhizoctonia solani Kuhn. J. Biol. Control 2006, 20, 57–64. [Google Scholar]
- Júnior, F.W.R.; Mulinari, J.; Camargo, A.F.; Scapini, T.; Chiomento, J.L.T.; Pritsch, E.J.P.; Berto, C.; dos Santos, L.H.; Fongaro, G.; Mossi, A.J.; et al. Trichoderma sp.: Bioproducts and Their Main Uses in Agriculture. In Trichoderma: Taxonomy, Biodiversity and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2023. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, n71. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Shaanker, R.U. Inhibition of Plant Pathogenic Fungi by Endophytic Trichoderma spp. through Mycoparasitism and Volatile Organic Compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef] [PubMed]
- Poletto, T.; Fantinel, V.S.; Muniz, M.F.B.; Quevedo, A.C.; Strahl, M.A.; Poletto, I.; Stefenon, V.M. Efficacy of Five Trichoderma Species Against Anthracnose Pathogens in Pecan Through Mycoparasitism and Antibiosis. J. Crop Health 2024, 76, 673–681. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Sehim, A.E.; Hewedy, O.A.; Altammar, K.A.; Alhumaidi, M.S.; Abd Elghaffar, R.Y. Trichoderma asperellum Empowers Tomato Plants and Suppresses Fusarium oxysporum through Priming Responses. Front. Microbiol. 2023, 14, 1140378. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a Mechanism of Trichoderma- Mediated Suppression of Plant Diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Moreno-Ruiz, D.; Lichius, A.; Turrà, D.; Di Pietro, A.; Zeilinger, S. Chemotropism Assays for Plant Symbiosis and Mycoparasitism Related Compound Screening in Trichoderma atroviride. Front. Microbiol. 2020, 11, 601251. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Sanz, L.; Rey, M.; Monte, E.; Llobell, A. BGN16.3, a Novel Acidic β-1,6-Glucanase from Mycoparasitic Fungus Trichoderma harzianum CECT 2413. FEBS J. 2005, 272, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Aghcheh, R.K.; Druzhinina, I.S.; Kubicek, C.P. The Putative Protein Methyltransferase LAE1 of Trichoderma atroviride Is a Key Regulator of Asexual Development and Mycoparasitism. PLoS ONE 2013, 8, e67144. [Google Scholar] [CrossRef]
- Landeis, A.; Schmidt-Heydt, M. Sequencing and Analysis of the Entire Genome of the Mycoparasitic Fungus Trichoderma afroharzianum. Microbiol. Resour. Announc. 2021, 10, e00211-21. [Google Scholar] [CrossRef]
- Stange, P.; Kersting, J.; Sivaprakasam Padmanaban, P.B.; Schnitzler, J.P.; Rosenkranz, M.; Karl, T.; Benz, J.P. The Decision for or against Mycoparasitic Attack by Trichoderma spp. Is Taken Already at a Distance in a Prey-Specific Manner and Benefits Plant-Beneficial Interactions. Fungal Biol. Biotechnol. 2024, 11, 14. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; Guzmán-Ortiz, D.A.; Gómez-Lim, M.A.; Délano-Frier, J.P.; de-Folter, S.; Sánchez-García, P.; Peña-Cabriales, J.J. Potential Use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a Biological Control Agent against Anthracnose in Mango (Mangifera indica L.). Biol. Control 2013, 64, 37–44. [Google Scholar] [CrossRef]
- Hoa, P.T.B.; Tue, N.H.; Huyen, L.T.T.; Linh, L.H.; Nhan, N.T.; Tien, N.Q.D.; Luong, N.N.; Huy, N.X.; Loc, N.H. Overexpression of 42 KDa Chitinase Genes from Trichoderma asperellum SH16 in Peanut (Arachis hypogaea). J. Crop Improv. 2023, 37, 463–478. [Google Scholar] [CrossRef]
- De Marco, J.L.; Felix, C.R. Purification and Characterization of a β-Glucanase Produced by Trichoderma harzianum Showing Biocontrol Potential. Braz. Arch. Biol. Technol. 2007, 50, 21–29. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.K.; Pradhan, B.; Tripathi, S.; Kumar, K.S.; Chand, S.; Rout, P.R.; Shahid, M.K. Harnessing Trichoderma Mycoparasitism as a Tool in the Management of Soil Dwelling Plant Pathogens. Microb. Ecol. 2024, 87, 158. [Google Scholar] [CrossRef]
- Zhang, S.W.; Xu, B.L.; Zhang, J.H.; Gan, Y.T. Identification of the Antifungal Activity of Trichoderma longibrachiatum T6 and Assessment of Bioactive Substances in Controlling Phytopathgens. Pestic. Biochem. Physiol. 2018, 147, 59–66. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, K.I.; Vázquez-Garcidueñas, M.S.; Herrera-Estrella, A.; Fernández-Pavía, S.P.; Salgado-Garciglia, R.; Larsen, J.; Ochoa-Ascencio, S.; Rodríguez-Alvarado, G.; Vazquez-Marrufo, G. Polyphasic Characterization of the Biocontrol Potential of a Novel Strain of Trichoderma atroviride Isolated from Central Mexico. J. Fungi 2024, 10, 758. [Google Scholar] [CrossRef]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Iftikhar, Y.; Sharma, S.; Kashyap, B.K.; Hussain, S.; et al. Trichoderma spp. Genes Involved in the Biocontrol Activity Against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar] [CrossRef]
- Tamandegani, P.R.; Marik, T.; Zafari, D.; Balázs, D.; Vágvölgyi, C.; Szekeres, A.; Kredics, L. Changes in Peptaibol Production of Trichoderma Species during in vitro Antagonistic Interactions with Fungal Plant Pathogens. Biomolecules 2020, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Zou, J.X.; Song, Y.P.; Ji, N.Y. Polyketides, Steroids, and Terpenoids from Trichoderma atroviride RR-Dl-3-9 and Their Occurrence in Coculture with Fusarium graminearum 4A1F. ACS Agric. Sci. Technol. 2025, 5, 1382–1391. [Google Scholar] [CrossRef]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-Pentyl-2H-Pyran-2-One (6-PAP) and Other Volatiles by Different Trichoderma Species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef]
- Hamrouni, R.; Molinet, J.; Dupuy, N.; Taieb, N.; Carboue, Q.; Masmoudi, A.; Roussos, S. The Effect of Aeration for 6-Pentyl-Alpha-Pyrone, Conidia and Lytic Enzymes Production by Trichoderma asperellum Strains Grown in Solid-State Fermentation. Waste Biomass Valorization 2020, 11, 5711–5720. [Google Scholar] [CrossRef]
- Hao, J.; Wuyun, D.; Xi, X.; Dong, B.; Wang, D.; Quan, W.; Zhang, Z.; Zhou, H. Application of 6-Pentyl-α-Pyrone in the Nutrient Solution Used in Tomato Soilless Cultivation to Inhibit Fusarium oxysporum HF-26 Growth and Development. Agronomy 2023, 13, 1210. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Jiang, Y.; Cui, S.; Hu, J.; Wei, Y.; Li, J.; Wu, Y. Volatile Organic Compounds Produced by Co-Culture of Burkholderia vietnamiensis B418 with Trichoderma harzianum T11-W Exhibits Improved Antagonistic Activities against Fungal Phytopathogens. Int. J. Mol. Sci. 2024, 25, 11097. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez de Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the Plant Heritable Priming Responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Behiry, S.; Soliman, S.A.; Massoud, M.A.; Abdelbary, M.; Kordy, A.M.; Abdelkhalek, A.; Heflish, A. Trichoderma pubescens Elicit Induced Systemic Resistance in Tomato Challenged by Rhizoctonia solani. J. Fungi 2023, 9, 167. [Google Scholar] [CrossRef]
- Huang, P.C.; Yuan, P.G.; Grunseich, J.M.; Taylor, J.; Tiénébo, E.O.; Pierson, E.A.; Bernal, J.S.; Kenerley, C.M.; Kolomiets, M.V. Trichoderma virens and Pseudomonas chlororaphis Differentially Regulate Maize Resistance to Anthracnose Leaf Blight and Insect Herbivores When Grown in Sterile versus Non-Sterile Soils. Plants 2024, 13, 1240. [Google Scholar] [CrossRef]
- Singh, S.P.; Keswani, C.; Singh, S.P.; Sansinenea, E.; Hoat, T.X. Trichoderma spp. Mediated Induction of Systemic Defense Response in Brinjal against Sclerotinia sclerotiorum. Curr. Res. Microb. Sci. 2021, 2, 100051. [Google Scholar] [CrossRef]
- Galletti, S.; Paris, R.; Cianchetta, S. Selected Isolates of Trichoderma gamsii Induce Different Pathways of Systemic Resistance in Maize upon Fusarium verticillioides Challenge. Microbiol. Res. 2020, 233, 126406. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone Production Profiles in Trichoderma Species and Their Relationship to Wheat Plant Responses to Water Stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Hariharan, G.; Rifnas, L.M.; Prasannath, K. Role of Trichoderma spp. in Biocontrol of Plant Diseases. In Microbial Biocontrol: Food Security and Post Harvest Management; Springer International Publishing: Cham, Switzerland, 2022; pp. 39–78. [Google Scholar]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum Strains from Argentina Produce Indole-3 Acetic Acid and Phosphorus Solubilization, Promote Growth and Control Wilt Disease on Tomato (Solanum lycopersicum L.). J. King Saud. Univ. Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-Induced Plant Immunity Likely Involves Both Hormonal- and Camalexindependent Mechanisms in Arabidopsis thaliana and Confers Resistance against Necrotrophic Fungus Botrytis cinerea. Plant Signal Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.J.; Liu, K.J.; Liang, B.B.; Gong, H.X.; Chen, L.; Dong, H.Y. Application of Difenoconazole and Trichoderma Broth Combination for Synergistic Control of Corn Leaf Blight and Stalk Rot in Straw-Returned Fields in Liaoning Province, China. Appl. Sci. 2025, 15, 7834. [Google Scholar] [CrossRef]
- Rana, A.; Kumar, N.; Roy, R.; Kumari, A.; Kumar, R.; Gautam, A.; Das, S. Field Efficacy of Trichoderma viride, Pseudomonas fluorescens, and Consortia of Microbial Agents against Tomato Late Blight, Phytophthora infestans (Mont.) de Bary in Indo-Gangetic Plain of Bihar. Crop Prot. 2025, 190, 107128. [Google Scholar] [CrossRef]
- Romlumduan, K.; Kaewkrajay, C.; Dethoup, T. Synergistic Effects of Wettable Powder of Trichoderma and Fungicides in Controlling Phytophthora and Corynespora Leaf Fall Diseases in Rubber. Eur. J. Plant Pathol. 2025, 71, 1–11. [Google Scholar] [CrossRef]
- Haque, Z.; Nawaz, S.; Haidar, L.; Ansari, M.S.A. Development of Novel Trichoderma Bioformulations against Fusarium Wilt of Chickpea. Sci. Rep. 2025, 15, 9564. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.H.A.; Mostafa, N.A.; Yousef, S.A.M.; El-Blasy, S.A.S.; Abd El Badeea, O. Enhancing Trichoderma Efficacy in Managing Wheat Stem Rust Disease and Boosting Production through the Application of Certain Chemical Inducers. BMC Plant Biol. 2025, 25, 562. [Google Scholar] [CrossRef]
- Yu, N.; Gao, Y.J.; Chang, F.; Liu, W.T.; Guo, C.H.; Cai, H.S. Screening of Antagonistic Trichoderma Strains to Enhance Soybean Growth. J. Fungi 2025, 11, 159. [Google Scholar] [CrossRef]
- Tang, W.X.; Lu, Y.; Zhang, R.; Wang, X.; Wang, H.Y.; Wang, M.; Chen, X.S.; Shen, X.; Yin, C.M.; Mao, Z.Q. Mixed Application of Raw Amino Acid Powder and Trichoderma harzianum Fertilizer for the Prevention and Management of Apple Replant Disease. J. Integr. Agric. 2025, 24, 1126–1139. [Google Scholar] [CrossRef]
- Raval, P.; Gami, S.; Pandya, A.; Pandya, S.; Nagar, P.; Kumar, T. Effect of Trichoderma and Humicil Application on Oroxylum indicum Grown in Root Trainers: A Case Study. J. Sustain. For. 2025, 44, 350–360. [Google Scholar] [CrossRef]
- dos Santos, D.K.Q.; dos Santos, F.M.; Roso, A.S.; Camargo, D.P.; Viera, L.D.; Morandini, G.B.; Santos, J.R.P.; da Silva, J.C.P. Friends or Foes: Co-Application of Trichoderma- and Bacillus-Based Products with Chemicals to Manage Soybean Foliar Diseases. Biocontrol 2025, 70, 257–270. [Google Scholar] [CrossRef]
- de Lima, E.M.; Martins, A.P.; da Costa, D.P.; de Barros, J.A.; da Franca, R.F.; Lima, J.R.D.; Duda, G.P.; da Silva, M.M.; Araujo, A.S.F.; de Medeiros, E.V. Winery Residues Transformed into Biochar and Co-Applied with Trichoderma Increase Grape Productivity and Soil Quality. Sustainability 2025, 17, 4150. [Google Scholar] [CrossRef]
- Fagundes-Nacarath, I.R.; Cavalcante, G.P.; Brito, R.A.S.; Costa, P.M.A.; Debona, D.; Maffia, L.A.; Rodrigues, F.A. Antagonistic Potential of Different Species of Trichoderma Against Sclerotinia sclerotiorum. J. Phytopathol. 2025, 173, e70012. [Google Scholar] [CrossRef]
- Kumar, N.; Sow, S.; Rana, L.; Ranjan, S.; Singh, A.K. Trash Amended with Trichoderma Effects on Cane Yield, Soil Carbon Dynamics, and Enzymatic Activities under Plant-Ratoon System of Sugarcane in Calcareous Soil. Sugar Tech 2025, 27, 134–147. [Google Scholar] [CrossRef]
- Seekham, N.; Kaewsalong, N.; Jantasorn, A.; Dethoup, T. Field Biocontrol Efficacy of Trichoderma spp. in Fresh and Dry Formulations against Rice Blast and Brown Spot Diseases and Yield Effect. Eur. J. Plant Pathol. 2024, 170, 1–13. [Google Scholar] [CrossRef]
- Zarafshar, M.; Besnard, O.; Thomas, A.; Perrot, B.; Vincent, G.; Bazot, S. Unlocking the Promising Potential: Trichoderma TrB (CNCM Strain I-5327) in Golf Course Management. Pedobiologia 2024, 105, 150972. [Google Scholar] [CrossRef]
- Saini, S.; Raj, K.; Kumar, R.; Saini, A.K. Potency of Formulated Biocontrol Agents from Lab to Field: A Sustainable Approach for Onion Purple Blotch Suppression. J. Plant Pathol. 2024. [Google Scholar] [CrossRef]
- Xia, X.R.; Wang, Q.Z.; Guo, K.; Yuan, G.Q.; Deng, T.; Zhao, Z.Y.; Guo, Q.C.; Wu, K.; Chen, B.; Pan, Y.H. Combined Application of Resistance Inducer and Trichoderma Control Two Tobacco Soil-Borne Diseases by Regulating the Field Soil Microbial Composition. J. Phytopathol. 2024, 172, e13333. [Google Scholar] [CrossRef]
- Djaenuddin, N.; Yusnawan, E.; Nugraha, Y.; Muis, A.; Nasruddin, A.; Kuswinanti, T. Biological Control of Downy Mildew Peronosclerospora spp. L. by Application of Endophytic Agents in Corn. Biocontrol Sci. Technol. 2024, 34, 942–968. [Google Scholar] [CrossRef]
- Banan, A.; Homayoonzadeh, M.; Torabi, E.; Ghasemi, V.; Shahbazi, S.; Talebi, K. Effects of Fungicides Propiconazole and Trichoderma spp. on the Mortality and the Physiological Status of Larvae and Adult Worker Honey Bees (Apis mellifera L.). J. Apic. Res. 2024, 63, 932–945. [Google Scholar] [CrossRef]
- Dutta, R.; Kumar, S.; Jayalakshmi, K.; Radhakrishna, A.; Bhagat, K.; Gowda, D.C.M.; Karuppaiah, V.; Bhandari, H.R.; Bomble, R.; Gurav, V.; et al. Potential of Trichoderma Strains to Positively Modulate Plant Growth Processes and Bulb Yield in Rabi Onion. Front. Sustain. Food Syst. 2024, 8, 1427303. [Google Scholar] [CrossRef]
- Gomez-Garay, A.; Calderon, S.A.; Mariscal, M.L.T.; López, B.P. Effective Control of Neofusicoccum Parvum in Grapevines: Combining Trichoderma spp. with Chemical Fungicides. Agronomy 2024, 14, 2766. [Google Scholar] [CrossRef]
- de Medeiros, E.V.; da Costa, D.P.; Silva, E.L.D.; de Franca, A.F.; Lima, J.R.D.; Hammecker, C.; Mendes, L.W.; Pereira, A.P.D.; Araujo, A.S.F. Biochar and Trichoderma as an Eco-Friendly and Low-Cost Alternative to Improve Soil Chemical and Biological Properties. Waste Biomass Valorization 2024, 15, 1439–1450. [Google Scholar] [CrossRef]
- Zhu, L.X.; Wang, Y.F.; Wang, G.D.; Xiao, M.J.; Luo, H.L.; Zhang, F.L.; Li, L.L. Manure and Trichoderma harzianum Increase Cotton Yield via Regulating Soil Bacterial Community and Physicochemical Properties. Scienceasia 2024, 50, 1. [Google Scholar] [CrossRef]
- Rigobelo, E.C.; de Carvalho, L.A.L.; Santos, C.H.B.; Frezarin, E.T.; Pinheiro, D.G.; Nicodemo, D.; Babalola, O.O.; Desoignies, N. Growth Promotion and Modulation of the Soybean Microbiome INTACTA RR PRO with the Application of the Fungi Trichoderma harzianum and Purpureocillum lilacinum. Sci. Rep. 2024, 14, 21004. [Google Scholar] [CrossRef]
- Swe, W.L.L.; Tóth, F.; Petrikovszki, R.; Ladányi, M.; Fail, J. Harnessing the Impact of Beneficial Microorganisms to Control Meloidogyne incognita in Tomato Cultivation across Diverse Environments. Egypt. J. Biol. Pest. Control 2024, 34, 64. [Google Scholar] [CrossRef]
- Sofia, J.; Sofia, R.; Vila-Maior, J. Evaluating the Effect of Trichoderma Atroviride (i-1237) on Grapevine Phomopsis Cane and Leaf Spot: A Promising and Reproducible Trial. Cienc. E Tec. Vitivinic. 2024, 39, 64–73. [Google Scholar] [CrossRef]
- Bandara, A.Y.; Kang, S.G. Trichoderma Application Methods Differentially Affect the Tomato Growth, Rhizomicrobiome, and Rhizosphere Soil Suppressiveness against Fusarium oxysporum. Front. Microbiol. 2024, 15, 1366690. [Google Scholar] [CrossRef] [PubMed]
- Szczech, M.; Kowalska, B.; Wurm, F.R.; Ptaszek, M.; Jarecka-Boncela, A.; Trzcinski, P.; Lovschall, K.B.; Velasquez, S.T.R.; Maciorowski, R. The Effects of Tomato Intercropping with Medicinal Aromatic Plants Combined with Trichoderma Applications in Organic Cultivation. Agronomy 2024, 14, 2572. [Google Scholar] [CrossRef]
- Iqbal, S.; Ashfaq, M.; Rao, M.J.; Khan, K.S.; Malik, A.H.; Mehmood, M.A.; Fawaz, M.S.; Abbas, A.; Shakeel, M.T.; Naqvi, S.A.H.; et al. Trichoderma viride: An Eco-Friendly Biocontrol Solution Against Soil-Borne Pathogens in Vegetables Under Different Soil Conditions. Horticulturae 2024, 10, 1277. [Google Scholar] [CrossRef]
- dela Cruz, T.E.E.; Behr, J.H.; Geistlinger, J.; Grosch, R.; Witzel, K. Monitoring of an Applied Beneficial k Strain in Root-Associated Soil of Field-Grown Maize by MALDI-TOF MS. Microorganisms 2023, 11, 1655. [Google Scholar] [CrossRef]
- Senger, M.; Urrea-Valencia, S.; Nazari, M.T.; Vey, R.T.; Piccin, J.S.; Martin, T.N. Evaluation of Trichoderma asperelloides-Based Inoculant as Growth Promoter of Soybean (Glycine max (L.) Merr.): A Field-Scale Study in Brazil. J. Crop Sci. Biotechnol. 2023, 26, 255–263. [Google Scholar] [CrossRef]
- Ayyandurai, M.; Akila, R.; Manonmani, K.; Mini, M.L.; Vellaikumar, S.; Brindhadevi, S.; Theradimani, M. Combined Application of Trichoderma longibrachiatum T(SP)-20 and Trichoderma asperellum T(AR)-10 in the Management of Stem Rot of Groundnut. Legume Res. 2023, 46, 215–221. [Google Scholar] [CrossRef]
- Limdolthamand, S.; Songkumarn, P.; Suwannarat, S.; Jantasorn, A.; Dethoup, T. Biocontrol Efficacy of Endophytic Trichoderma spp. in Fresh and Dry Powder Formulations in Controlling Northern Corn Leaf Blight in Sweet Corn. Biol. Control 2023, 181, 105217. [Google Scholar] [CrossRef]
- Nujthet, Y.; Jantasorn, A.; Dethoup, T. Biological Efficacy of Marine-Derived Trichoderma in Controlling Chili Anthracnose and Black Spot Disease in Chinese Kale. Eur. J. Plant Pathol. 2023, 166, 369–383. [Google Scholar] [CrossRef]
- Petcu, V.; Bubueanu, C.; Casarica, A.; Savoiu, G.; Stoica, R.; Bazdoaca, C.; Lazar, D.A.; Iordan, H.L.; Horhocea, D. Efficacy of Trichoderma harzianum and Bacillus subtilis as seed and vegetation application combined with integrated agroecology measures on maize. Rom. Agric. Res. 2023, 40, 439–448. [Google Scholar] [CrossRef]
- Saharan, R.; Patil, J.A.; Yadav, S.; Kumar, A.; Goyal, V. The Nematicidal Potential of Novel Fungus, Trichoderma asperellum FbMi6 against Meloidogyne incognita. Sci. Rep. 2023, 13, 6603. [Google Scholar] [CrossRef]
- da Silva, J.O.N.; Pessoa, L.G.M.; da Silva, E.M.; da Silva, L.R.; Freire, M.; de Souza, E.S.; Ferreira-Silva, S.L.; de França, J.G.E.; da Silva, T.G.F.; Alencar, E.L.D. Effects of Silicon Alone and Combined with Organic Matter and Trichoderma harzianum on Sorghum Yield, Ions Accumulation and Soil Properties under Saline Irrigation. Agriculture 2023, 13, 2146. [Google Scholar] [CrossRef]
- Gonçalves, G.C.; Ferbonink, G.F.; Hemkemeier, C.; Caione, G.; Yamashita, O.M.; Luiz, S.A.R.; de Carvalho, M.A.C. Vegetative and Productive Characteristics of Soybean under Doses of Boron and Inoculation of Trichoderma atroviride. Chil. J. Agric. Res. 2023, 83, 159–167. [Google Scholar] [CrossRef]
- Kirillova, N.I.; Degtyareva, I.A.; Prishchepenko, E.A. Prospects of a Modified Biofungicide Based on Trichoderma viride for Soil Health Improvement. Izv. Vuzov-Prikl. Khimiya I Biotekhnologiya 2023, 13, 107–114. [Google Scholar]
- Nagy, V.D.; Zhumakayev, A.; Vörös, M.; Bordé, A.; Szarvas, A.; Szucs, A.; Kocsubé, S.; Jakab, P.; Monostori, T.; Skrbic, B.D.; et al. Development of a Multicomponent Microbiological Soil Inoculant and Its Performance in Sweet Potato Cultivation. Microorganisms 2023, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Forlano, P.; Mang, S.M.; Caccavo, V.; Fanti, P.; Camele, I.; Battaglia, D.; Trotta, V. Effects of Below-Ground Microbial Biostimulant Trichoderma harzianum on Diseases, Insect Community, and Plant Performance in Cucurbita pepo L. under Open Field Conditions. Microorganisms 2022, 10, 2242. [Google Scholar] [CrossRef] [PubMed]
- Abbey, J.A.; Percival, D.; Anku, K.; Schilder, A.; Asiedu, S.K. Managing Botrytis Blossom Blight of Wild Blueberry through Field Sanitation, Lime Sulfur and Trichoderma Application. Can. J. Plant Pathol. 2022, 44, 361–371. [Google Scholar] [CrossRef]
- Lyu, R.T.; Huang, C.H. Supplementation of Manure Compost with Trichoderma asperellum Improves the Nutrient Uptake and Yield of Edible Amaranth under Field Conditions. Sustainability 2022, 14, 5389. [Google Scholar] [CrossRef]
- Vij, S.; Sharma, N.; Sharma, M.; Mohanta, T.K.; Kaushik, P. Application of Trichoderma viride and Pseudomonas fluorescens to Cabbage (Brassica oleracea L.) Improves Both Its Seedling Quality and Field Performance. Sustainability 2022, 14, 7583. [Google Scholar] [CrossRef]
- Tandon, A.; Anshu, A.; Kumar, S.; Yadav, U.; Mishra, S.K.; Srivastava, S.; Chauhan, P.S.; Srivastava, P.K.; Bahadur, L.; Shirke, P.A.; et al. Trichoderma-Primed Rice Straw Alters Structural and Functional Properties of Sodic Soil. Land. Degrad. Dev. 2022, 33, 698–709. [Google Scholar] [CrossRef]
- Hang, X.N.; Meng, L.X.; Ou, Y.N.; Shao, C.; Xiong, W.; Zhang, N.; Liu, H.J.; Li, R.; Shen, Q.R.; Kowalchuk, G.A. Trichoderma-Amended Biofertilizer Stimulates Soil Resident Aspergillus Population for Joint Plant Growth Promotion. NPJ Biofilms Microbiomes 2022, 8, 57. [Google Scholar] [CrossRef]
- Expósito, A.; García, S.; Giné, A.; Escudero, N.; Herranz, S.; Pocurull, M.; Lacunza, A.; Sorribas, F.J. Effect of Molasses Application Alone or Combined with Trichoderma asperellum T-34 on Meloidogyne spp. Management and Soil Microbial Activity in Organic Production Systems. Agronomy 2022, 12, 1508. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, Y.X.; Zhang, Z.P.; Li, J.Q.; Zhu, S.S.; Yang, M.; Luo, L.X. Application of Plant Survival-Promoting and Pathogen-Suppressing Trichoderma Species for Crop Biofertilization and Biocontrol of Root Rot in Panax notoginseng. J. Plant Pathol. 2022, 104, 1361–1369. [Google Scholar] [CrossRef]
- Brecchia, M.; Buscaroli, E.; Mazzon, M.; Blasioli, S.; Braschi, I. Effect of the Growing Season, Trichoderma, and Clinoptilolite Application on Potentially Toxic Elements Uptake by Cucumis melo L. Hortscience 2022, 57, 1548. [Google Scholar] [CrossRef]
- Zhang, W.T.; Xiong, Y.W.; Li, Y.P.; Qiu, Y.C.; Huang, G.H. Effects of Organic Amendment Incorporation on Maize (Zea mays L.) Growth, Yield and Water-Fertilizer Productivity under Arid Conditions. Agric. Water Manag. 2022, 269, 107663. [Google Scholar] [CrossRef]
- Conte, E.D.; Dal Magro, T.; Dal Bem, L.C.; Dalmina, J.C.; Matte, J.A.; Schenkel, V.O.; Schwambach, J. Use of Trichoderma spp. in No-Tillage System: Effect on Soil and Soybean Crop. Biol. Control 2022, 171, 104941. [Google Scholar] [CrossRef]
- Pradhan, P.C.; Mukhopadhyay, A.; Kumar, R.; Kundu, A.; Patanjali, N.; Dutta, A.; Kamil, D.; Bag, T.K.; Aggarwal, R.; Bharadwaj, C.; et al. Performance Appraisal of Trichoderma viride Based Novel Tablet and Powder Formulations for Management of Fusarium Wilt Disease in Chickpea. Front. Plant Sci. 2022, 13, 990392. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, S.; Metruccio, E.G.; Moretti, S.; Nocentini, M.; Carella, G.; Pacetti, A.; Battiston, E.; Osti, F.; Mugnai, L. Activity of Trichoderma asperellum Strain ICC 012 and Trichoderma gamsii Strain ICC 080 Toward Diseases of Esca Complex and Associated Pathogens. Front. Microbiol. 2022, 12, 813410. [Google Scholar] [CrossRef]
- Gallegos-Morales, G.; Espinoza-Ahumada, C.A.; Figueroa-Reyes, J.; Méndez-Aguilar, R.; Rodríguez-Guerra, R.; Salas-Gómez, A.L.; Peña-Ramos, F.M. Trichoderma Species Compatibility for Production and Wilt Chili Biocontrol. Ecosistemas Y Recur. Agropecu. 2022, 9, e3066. [Google Scholar] [CrossRef]
- Funahashi, F.; Myrold, D.D.; Parke, J.L. The Effects of Soil Solarization and Application of a Trichoderma Biocontrol Agent on Soil Fungal and Prokaryotic Communities. Soil Sci. Soc. Am. J. 2022, 86, 369–383. [Google Scholar] [CrossRef]
- Acosta-González, U.; Silva-Rojas, H.V.; Fuentes-Aragón, D.; Hernández-Castrejón, J.; Romero-Bautista, A.; Rebollar-Alviter, A. Comparative Performance of Fungicides and Biocontrol Products in the Management of Fusarium Wilt of Blackberry. Plant Dis. 2022, 106, 1419–1427. [Google Scholar] [CrossRef]
- Degani, O.; Gordani, A. New Antifungal Compound, 6-Pentyl-α-Pyrone, against the Maize Late Wilt Pathogen, Magnaporthiopsis maydis. Agronomy 2022, 12, 2339. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Raja, M.; Singh, B.; Katoch, S.; Kumar, S.; Sharma, P. Potential Native Trichoderma Strains against Fusarium verticillioides Causing Post Flowering Stalk Rot in Winter Maize. Crop Prot. 2022, 152, 105838. [Google Scholar] [CrossRef]
- Al-Abbas, H.H.A.; Salih, Y.A. Evaluation of the Efficiency of the Bioagent Trichoderma longibrachiatum Against Root Rot Disease of Cucumber Plant Caused by the Fungus Fusarium solani. Int. J. Agric. And. Stat. Sci. 2022, 18, 1395–1401. [Google Scholar]
- Mao, T.T.; Jiang, X.L. Changes in Microbial Community and Enzyme Activity in Soil under Continuous Pepper Cropping in Response to Trichoderma hamatum MHT1134 Application. Sci. Rep. 2021, 11, 21585. [Google Scholar] [CrossRef]
- Moragrega, C.; Carmona, A.; Llorente, I. Biocontrol of Stemphylium vesicarium and Pleospora allii on Pear by Bacillus subtilis and Trichoderma spp.: Preventative and Curative Effects on Inoculum Production. Agronomy 2021, 11, 1455. [Google Scholar] [CrossRef]
- Mutlu, A.; Kucuk, C.; Cevheri, C. Effect of Trichoderma ssp. Isolates Applications On Grain Yield And Some Yield Components In Organic Barley Cultivation (Hordeum vulgare L.). Fresenius Environ. Bull. 2021, 30, 723–730. [Google Scholar]
- Jurys, A.; Feiziene, D. The Effect of Specific Soil Microorganisms on Soil Quality Parameters and Organic Matter Content for Cereal Production. Plants 2021, 10, 2000. [Google Scholar] [CrossRef] [PubMed]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; Álvarez-García, S.; Gutiérrez, S.; Casquero, P.A. The Influence of Temperature on the Growth, Sporulation, Colonization, and Survival of Trichoderma spp. in Grapevine Pruning Wounds. Agronomy 2021, 11, 1771. [Google Scholar] [CrossRef]
- Syam, N.; Hidrawati; Sabahannur, S.; Nurdin, A. Effects of Trichoderma and Foliar Fertilizer on the Vegetative Growth of Black Pepper (Piper nigrum L.) Seedlings. Int. J. Agron. 2021, 2021, 9953239. [Google Scholar] [CrossRef]
- Pedrero-Mendez, A.; Insuasti, H.C.; Neagu, T.; Illescas, M.; Rubio, M.B.; Monte, E.; Hermosa, R. Why Is the Correct Selection of Trichoderma Strains Important? The Case of Wheat Endophytic Strains of T. harzianum and T. simmonsii. J. Fungi 2021, 7, 1087. [Google Scholar] [CrossRef]
- Harbawi, M.A.; AlBadrani, W.A.; Ghazal, R.A. The Effect of Fertilisation Using Trichoderma harzianum and Cow Manure on Releasing CO2 in Two Soils with Different Textures in North of Iraq. Asian J. Water Environ. Pollut. 2021, 18, 117–122. [Google Scholar] [CrossRef]
- Chen, D.W.; Hou, Q.Z.; Jia, L.Y.; Sun, K. Combined Use of Two Trichoderma Strains to Promote Growth of Pakchoi (Brassica chinensis L.). Agronomy 2021, 11, 726. [Google Scholar] [CrossRef]
- Win, T.T.; Bo, B.; Malec, P.; Khan, S.; Fu, P.C. Newly Isolated Strain of Trichoderma asperellum from Disease Suppressive Soil Is a Potential Bio-Control Agent to Suppress Fusarium Soil Borne Fungal Phytopathogens. J. Plant Pathol. 2021, 103, 549–561. [Google Scholar] [CrossRef]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.S.; Digilio, M.C. Temperature Differentially Influences the Capacity of Trichoderma Species to Induce Plant Defense Responses in Tomato Against Insect Pests. Front. Plant Sci. 2021, 12, 678830. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.D.; Vieira, V.D.; da Silva, R.C.; Pinheiro, D.G.; Soares, M.A. Introduction of Trichoderma spp. Biocontrol Strains against Sclerotinia sclerotiorum (Lib.) de Bary Change Soil Microbial Community Composition in Common Bean (Phaseolus vulgaris L.) Cultivation. Biol. Control 2021, 163, 104755. [Google Scholar] [CrossRef]
- Manzar, N.; Singh, Y.; Kashyap, A.S.; Sahu, P.K.; Rajawat, M.V.S.; Bhowmik, A.; Sharma, P.K.; Saxena, A.K. Biocontrol Potential of Native Trichoderma spp. against Anthracnose of Great Millet (Sorghum bicolour L.) from Tarai and Hill Regions of India. Biol. Control 2021, 152, 104474. [Google Scholar] [CrossRef]
- Atalla, S.M.M.; Abdel-Kader, M.M.; El-Gamal, N.G.; El-Mougy, N.S. Using Maize Wastes, Fermented by Co-Cultures of Trichoderma harzianum and Pseudomonas fluorescens, as Grain Dressing against m Maize Diseases under Field Conditions. Egypt. J. Biol. Pest. Control 2020, 30, 37. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.C.; Li, J.S.; Zou, Z.R.; Cao, K. Bio-Organic Fertilizer with Reduced Rates of Chemical Fertilization Improves Soil Fertility and Enhances Tomato Yield and Quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef]
- Herrera, W.; Valbuena, O.; Pavone-Maniscalco, D. Formulation of Trichoderma asperellum TV190 for Biological Control of Rhizoctonia solani on Corn Seedlings. Egypt. J. Biol. Pest. Control 2020, 30, 44. [Google Scholar] [CrossRef]
- Zhang, F.L.; Dou, K.; Liu, C.; Chen, F.J.; Wu, W.W.; Yang, T.W.; Li, L.L.; Liu, T.X.; Yu, L.J. The Application Potential of Trichoderma T-Soybean Containing 1-Aminocyclopropane-1-Carboxylate for Maize Production. Physiol. Mol. Plant Pathol. 2020, 110, 101475. [Google Scholar] [CrossRef]
- Stummer, B.E.; Zhang, Q.; Zhang, X.; Warren, R.A.; Harvey, P.R. Quantification of Trichoderma afroharzianum, Trichoderma harzianum and Trichoderma gamsii Inoculants in Soil, the Wheat Rhizosphere and in Planta Suppression of the Crown Rot Pathogen Fusarium pseudograminearum. J. Appl. Microbiol. 2020, 129, 971–990. [Google Scholar] [CrossRef]
- Moradtalab, N.; Ahmed, A.; Geistlinger, J.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Synergisms of Microbial Consortia, N Forms, and Micronutrients Alleviate Oxidative Damage and Stimulate Hormonal Cold Stress Adaptations in Maize. Front. Plant Sci. 2020, 11, 396. [Google Scholar] [CrossRef]
- Kabdwal, B.C.; Sharma, R.; Tewari, R.; Tewari, A.K.; Singh, R.P.; Dandona, J.K. Field Efficacy of Different Combinations of Trichoderma harzianum, Pseudomonas fluorescens, and Arbuscular Mycorrhiza Fungus against the Major Diseases of Tomato in Uttarakhand (India). Egypt. J. Biol. Pest. Control 2019, 29, 1. [Google Scholar] [CrossRef]
- Vázquez-Martínez, J.A.; González-Cárdenas, J.C.; Chiquito-Contreras, R.; Sangabriel-Conde, W.; Alvarado-Castillo, G. Evaluation of the Biofertilizer Potential of Five Species of Trichoderma in the Production of Native Ear Corn and Hybrid under Field Conditions. Itea-Inf. Tec. Econ. Agrar. 2019, 115, 213–218. [Google Scholar]
- Joshi, D.; Singh, P.; Holkar, S.K.; Kumar, S. Trichoderma-Mediated Suppression of Red Rot of Sugarcane Under Field Conditions in Subtropical India. Sugar Tech 2019, 21, 496–504. [Google Scholar] [CrossRef]
- Rosmana, A.; Taufik, M.; Asman, A.; Jayanti, N.J.; Hakkar, A.A. Dynamic of Vascular Streak Dieback Disease Incidence on Susceptible Cacao Treated with Composted Plant Residues and Trichoderma asperellum in Field. Agronomy 2019, 9, 650. [Google Scholar] [CrossRef]
- Khan, M.R.; Haque, Z.; Rasoo, F.; Salati, K.; Khan, U.; Mohiddin, F.A.; Zuhaib, M. Management of Root-Rot Disease Complex of Mungbean Caused by Macrophomina phaseolina and Rhizoctonia solani through Soil Application of Trichoderma spp. Crop Prot. 2019, 119, 24–29. [Google Scholar] [CrossRef]
- He, A.L.; Liu, J.; Wang, X.H.; Zhang, Q.G.; Song, W.; Chen, J. Soil Application of Trichoderma asperellum GDFS1009 Granules Promotes Growth and Resistance to Fusarium graminearum in Maize. J. Integr. Agric. 2019, 18, 599–606. [Google Scholar] [CrossRef]
- Daza, F.F.F.; Roman, G.R.; Rodriguez, M.V.; Vargas, I.A.G.; Heano, H.C.; Cereda, M.P.; Mulet, R.A.C. Spores of Beauveria bassiana and Trichoderma lignorum as a Bioinsecticide for the Control of Atta cephalotes. Biol. Res. 2019, 52, 51. [Google Scholar] [CrossRef]
- Wang, S.Q.; Ma, J.; Wang, M.; Wang, X.H.; Li, Y.Q.; Chen, J. Combined Application of Trichoderma harzianum SH2303 and Difenoconazole-Propiconazolein Controlling Southern Corn Leaf Blight Disease Caused by Cochliobolus heterostrophus in Maize. J. Integr. Agric. 2019, 18, 2063–2071. [Google Scholar] [CrossRef]
- Rosa, R.; Franczuk, J.; Zaniewicz-Bajkowska, A.; Hajko, L. Effects of Trichoderma harzianum And Boron On Spring Broccoli. Appl. Ecol. Environ. Res. 2019, 17, 4397–4407. [Google Scholar] [CrossRef]
- Konappa, N.; Krishnamurthy, S.; Siddaiah, C.N.; Ramachandrappa, N.S.; Chowdappa, S. Evaluation of Biological Efficacy of Trichoderma asperellum against Tomato Bacterial Wilt Caused by Ralstonia solanacearum. Egypt. J. Biol. Pest. Control 2018, 28, 63. [Google Scholar] [CrossRef]
- Szczech, M.; Nawrocka, J.; Felczynski, K.; Malolepsza, U.; Sobolewski, J.; Kowalska, B.; Maciorowski, R.; Jas, K.; Kancelista, A. Trichoderma atroviride TRS25 Isolate Reduces Downy Mildew and Induces Systemic Defence Responses in Cucumber in Field Conditions. Sci. Hortic. 2017, 224, 17–26. [Google Scholar] [CrossRef]
- Oskiera, M.; Szczech, M.; Stepowska, A.; Smolinska, U.; Bartoszewski, G. Monitoring of Trichoderma Species in Agricultural Soil in Response to Application of Biopreparations. Biol. Control 2017, 113, 65–72. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Li, Y.Q.; Yu, C.J.; Wang, Q.Q.; Wang, M.; Sun, J.A.; Gao, J.X.; Chen, J. Effect of Trichoderma harzianum on Maize Rhizosphere Microbiome and Biocontrol of Fusarium Stalk Rot. Sci. Rep. 2017, 7, 1771. [Google Scholar] [CrossRef]
- Duc, N.H.; Mayer, Z.; Pék, Z.; Helyes, L.; Posta, K. Combined Inoculation of Arbuscular Mycorrhizal Fungi, Pseudomonas fluorescens And Trichoderma spp. For Enhancing Defense Enzymes And Yield of Three Pepper Cultivars. Appl. Ecol. Environ. Res. 2017, 15, 1815–1829. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and Its Secondary Metabolites Improve Yield and Quality of Grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Tobacco Mosaic Virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium Head Blight: Interactions between Trichoderma and Mycotoxigenic Fusarium. Microbiol. 2012, 158, 98–106. [Google Scholar] [CrossRef]
- Figueira, E.P.P.; Kuhn, O.J.; Martinazzo-Portz, T.; Stangarlin, J.R.; Pereira, M.D.P.; Lampugnani, C. Histochemical Changes Induced by Trichoderma spp. And Potassium Phosphite in Common Bean (Phaseolus vulgaris) in Response to the Attack by Colletotrichum lindemuthianum. Semin. Cienc. Agrar. 2020, 41, 811–828. [Google Scholar] [CrossRef]
- Ayvar-Serna, S.; Nájera, J.F.D.; Valdez-Hernández, E.F.; Delgado-Núñez, E.J.; Mena-Bahena, A. Antagonismo de Cepas Nativas y Foráneas de Trichoderma spp., Contra Colletotrichum gloeosporioides Causante de Antracnosis En Maracuyá. Investig. Y Estud.-UNA 2024, 15, 110–116. [Google Scholar] [CrossRef]
- Gutiérrez-Chávez, A.; Robles-Hernández, L.; Guerrero, B.I.; González-Franco, A.C.; Medina-Pérez, G.; Acevedo-Barrera, A.A.; Hernández-Huerta, J. Potential of Trichoderma asperellum as a Growth Promoter in Hydroponic Lettuce Cultivated in a Floating-Root System. Plants 2025, 14, 382. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Kong, S. Effects of Trichoderma asperellum and Its Siderophores on Endogenous Auxin in Arabidopsis thaliana under Iron-Deficiency Stress. Int. Microbiol. 2020, 23, 501–509. [Google Scholar] [CrossRef]
- Ali, I.; Khan, A.; Ali, A.; Ullah, Z.; Dai, D.Q.; Khan, N.; Khan, A.; Al-Tawaha, A.R.; Sher, H. Iron and Zinc Micronutrients and Soil Inoculation of Trichoderma harzianum Enhance Wheat Grain Quality and Yield. Front. Plant Sci. 2022, 13, 960948. [Google Scholar] [CrossRef]
- Sánchez-Bermúdez, M.; del Pozo, J.C.; Pernas, M. Effects of Combined Abiotic Stresses Related to Climate Change on Root Growth in Crops. Front. Plant Sci. 2022, 13, 918537. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for Plant Growth Promotion Activated by Trichoderma in Natural and Managed Terrestrial Ecosystems. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive Secondary Metabolites from Trichoderma spp. Against Phytopathogenic Fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to Plant Health and Productivity From Enhancing Plant Microbial Symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; García-García, A.L.; Herrera, A.J.; Valdés, F.; Luis, J.C.; Borges, A.A. A Beginner’s Guide to Osmoprotection by Biostimulants. Plants 2021, 10, 363. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Saha, K.C.; Uddin, M.K.; Shaha, P.K.; Hossain Chowdhury, M.A.; Hassan, L.; Saha, B.K. Application of Trichoderma harzianum Enhances Salt Tolerance and Yield of Indian Mustard through Increasing Antioxidant Enzyme Activity. Heliyon 2025, 11, e41114. [Google Scholar] [CrossRef]
- Eftekhari, F.; Sarcheshmehpour, M.; Lohrasbi-Nejad, A.; Boroomand, N. Effects of Mycorrhizal and Trichoderma Treatment on Enhancing Maize Tolerance to Salinity and Drought Stress, through Metabolic and Enzymatic Evaluation. BMC Plant Biol. 2025, 25, 687. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shamim, M.; Azad, C. Compatibility Assessment of Indigenous Trichoderma Strains with Fungicide Molecules Through In Vitro Analysis. Plant Arch. 2024, 24, 619–625. [Google Scholar]
- Bacilio, M.; Moreno, M.; Lopez-Aguilar, D.R.; Bashan, Y. Scaling from the Growth Chamber to the Greenhouse to the Field: Demonstration of Diminishing Effects of Mitigation of Salinity in Peppers Inoculated with Plant Growth-Promoting Bacterium and Humic Acids. Appl. Soil Ecol. 2017, 119, 327–338. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Płaza, A.; Wolna-Maruwka, A.; Budka, A.; Głuchowska, K.; Rudziński, R.; Kaczmarek, T. Consortia of Plant Growth-Promoting Rhizobacteria and Selected Catch Crops for Increasing Microbial Activity in Soil under Spring Barley Grown as an Organic Farming System. Appl. Sci. 2023, 13, 5120. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, W.; Cooke, R.; Oladeji, O.; Tian, G.; Bhattarai, R. Sorption Materials for Phosphorus Reduction in Drained Agricultural Fields: Gaps between the Results from Laboratory Evaluation and Field Application. Ecol. Eng. 2024, 207, 107351. [Google Scholar] [CrossRef]
- Athul, P.P.; Patra, R.K.; Sethi, D.; Panda, N.; Mukhi, S.K.; Padhan, K.; Sahoo, S.K.; Sahoo, T.R.; Mangaraj, S.; Pradhan, S.R.; et al. Efficient Native Strains of Rhizobia Improved Nodulation and Productivity of French Bean (Phaseolus vulgaris L.) under Rainfed Condition. Front. Plant Sci. 2022, 13, 1048696. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant Growth-Promoting Rhizobacterial Biofertilizers for Crop Production: The Past, Present, and Future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V.; Gautam, S.S.; Agarwal, A.; Punetha, A.; Upadhayay, V.K.; Kukreti, B.; Bundela, V.; Jugran, A.K.; Goel, R. Microbial Bioformulation: A Microbial Assisted Biostimulating Fertilization Technique for Sustainable Agriculture. Front. Plant Sci. 2023, 14, 1270039. [Google Scholar] [CrossRef]
- Fertiberia Trichoderma Inteligente. Biofertilizantes Que Revolucionan La Producción y La Salud Del Suelo. 2024. Available online: https://www.fertiberia.com/Trichoderma-inteligente-biofertilizantes-que-revolucionan-la-produccion-y-la-salud-del-suelo/ (accessed on 14 September 2025).
- Mahato, S.; Bhuju, S.; Shrestha, J. Effect of Trichoderma viride As Biofertilizer on Growth and Yield of Wheat. Malays. J. Sustain. Agric. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Nicot, P.C.; Bardin, M.; Alabouvette, C.C.; Köhl, J.; Ruocco, M. Potential of Biological Control Based on Published Research. 1. Protection against Plant Pathogens of Selected Crops. In Classical and Augmentative Biological Control Against Diseases and Pests: Critical Status Analysis and Review of Factors Influencing Their Success; Nicot, P.C., Ed.; IOBC-WPRS: Zurich, Switzerland, 2011; pp. 1–11. [Google Scholar]
- Harman, G.E. Trichoderma—Not Just for Biocontrol Anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The Genomics of Opportunistic Success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Gomes, E.N.; Elsherbiny, E.A.; Aleem, B.; Bennett, J.W. Beyond Classical Biocontrol: New Perspectives on Trichoderma. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Domingues, M.V.P.F.; de Moura, K.E.; Salomão, D.; Elias, L.M.; Patricio, F.R.A. Effect of Temperature on Mycelial Growth of Trichoderma, Sclerotinia minor and S. sclerotiorum, as Well as on Mycoparasitism. Summa Phytopathol. 2016, 42, 222–227. [Google Scholar] [CrossRef]
- Rivera-Méndez, W. Trichoderma Interactions in Vegetable Rhizosphere Under Tropical Weather Conditions. In Trichoderma; Springer: Singapore, 2020; pp. 293–314. [Google Scholar]
- Missbach, K.; Flatschacher, D.; Bueschl, C.; Samson, J.M.; Leibetseder, S.; Marchetti-Deschmann, M.; Zeilinger, S.; Schuhmacher, R. Light-Induced Changes in Secondary Metabolite Production of Trichoderma atroviride. J. Fungi 2023, 9, 785. [Google Scholar] [CrossRef]
- Zehra, A.; Dubey, M.K.; Meena, M.; Upadhyay, R.S. Effect of Different Environmental Conditions on Growth and Sporulation of Some Trichoderma Species. J. Environ. Biol. 2017, 38, 197–203. [Google Scholar] [CrossRef]
- Bazghaleh, N.; Prashar, P.; Woo, S.; Vandenberg, A. Effects of Lentil Genotype on the Colonization of Beneficial Trichoderma Species and Biocontrol of Aphanomyces Root Rot. Microorganisms 2020, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.G.; Vitaglione, P.; Troise, A.D.; Pigna, M.; Ruocco, M. Influence of Three Different Soil Types on the Interaction of Two Strains of Trichoderma harzianum with Brassica rapa subsp. sylvestris Cv. Esculenta, under Soil Mineral Fertilization. Geoderma 2019, 350, 11–18. [Google Scholar]
- Schmidt, J.; Dotson, B.R.; Schmiderer, L.; van Tour, A.; Kumar, B.; Marttila, S.; Fredlund, K.M.; Widell, S.; Rasmusson, A.G. Substrate and Plant Genotype Strongly Influence the Growth and Gene Expression Response to Trichoderma afroharzianum T22 in Sugar Beet. Plants 2020, 9, 1005. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Bissett, J.; Druzhinina, I.; Kullnig-Gradinger, C.; Szakacs, G. Genetic and Metabolic Diversity of Trichoderma: A Case Study on South-East Asian Isolates. Fungal Genet. Biol. 2003, 38, 310–319. [Google Scholar] [CrossRef]
- Cripps-Guazzone, N.; Ridgway, H.J.; Condron, L.M.; McLean, K.L.; Stewart, A.; Jones, E.E. Isolate and Plant Host Specificity of Rhizosphere Competence in Trichoderma Species. Fungal Biol. 2025, 129, 101554. [Google Scholar] [CrossRef]
- Pedrero-Méndez, A.; Cesarini, M.; Mendoza-Salido, D.; Petrucci, A.; Sarrocco, S.; Monte, E.; Hermosa, R. Trichoderma Strain-Dependent Direct and Indirect Biocontrol of Fusarium Head Blight Caused by Fusarium graminearum in Wheat. Microbiol. Res. 2025, 296, 128153. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma Secondary Metabolites That Affect Plant Metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar] [CrossRef]
- Wang, C.; Zhuang, W.Y. Carbon Metabolic Profiling of Trichoderma Strains Provides Insight into Potential Ecological Niches. Mycologia 2020, 112, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Moreno, K.; Ruocco, M.; Monti, M.M.; de la Vega, O.M.; Heil, M. Context-Dependent Effects of Trichoderma Seed Inoculation on Anthracnose Disease and Seed Yield of Bean (Phaseolus vulgaris): Ambient Conditions Override Cultivar-Specific Differences. Plants 2021, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The Beneficial Effect of Trichoderma spp. on Tomato Is Modulated by the Plant Genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Mengiste, T.D.; Myers, J.R.; Egel, D.S.; Hoagland, L.A. Tomato Domestication Attenuated Responsiveness to a Beneficial Soil Microbe for Plant Growth Promotion and Induction of Systemic Resistance to Foliar Pathogens. Front. Microbiol. 2020, 11, 604566. [Google Scholar] [CrossRef]
- Bridžiuvienė, D.; Raudonienė, V.; Švedienė, J.; Paškevičius, A.; Baužienė, I.; Vaitonis, G.; Šlepetienė, A.; Šlepetys, J.; Kačergius, A. Impact of Soil Chemical Properties on the Growth Promotion Ability of Trichoderma ghanense, T. tomentosum and Their Complex on Rye in Different Land-Use Systems. J. Fungi 2022, 8, 85. [Google Scholar] [CrossRef]
- Delory, B.M.; Schempp, H.; Spachmann, S.M.; Störzer, L.; van Dam, N.M.; Temperton, V.M.; Weinhold, A. Soil Chemical Legacies Trigger Species-Specific and Context-Dependent Root Responses in Later Arriving Plants. Plant Cell Environ. 2021, 44, 1215–1230. [Google Scholar] [CrossRef]
- Segarra, G.; Casanova, E.; Bellido, D.; Odena, M.A.; Oliveira, E.; Trillas, I. Proteome, Salicylic Acid, and Jasmonic Acid Changes in Cucumber Plants Inoculated with Trichoderma asperellum Strain T34. Proteomics 2007, 7, 3943–3952. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Madden, L.V.; Dorrance, A.E. Systemic Resistance Induced by Trichoderma spp.: Interactions between the Host, the Pathogen, the Biocontrol Agent, and Soil Organic Matter Quality. Phytopathology 2006, 96, 186–189. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, S.; Peng, H.; Zhang, L.; Li, S.; Berendsen, R.L.; Yang, T.; Dong, C.; Wei, Z.; Xu, Y.; et al. Synergic Interactions between Trichoderma and the Soil Microbiomes Improve Plant Iron Availability and Growth. NPJ Biofilms Microbiomes 2025, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Okoth, S.A.; Odhiambo, J. Influence of Soil Chemical And Physical Properties On Ocurrence of Trichoderma spp. in Embu, Kenya. Trop. Subtrop. Agroecosyst. 2009, 11, 403–413. [Google Scholar]
- Dutta, P.; Deb, L.; Pandey, A.K. Trichoderma- from Lab Bench to Field Application: Looking Back over 50 Years. Front. Agron. 2022, 4, 932839. [Google Scholar] [CrossRef]
- Mulder, D. Compendium of Soil Fungi. Geoderma 1982, 28, 63–64. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Vi Nguyen, D.; Rostás, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental Growth Conditions of Trichoderma spp. Affects Indole Acetic Acid Derivatives, Volatile Organic Compounds, and Plant Growth Promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial Formulates of Trichoderma Induce Systemic Plant Resistance to Meloidogyne incognita in Tomato and the Effect Is Additive to That of the Mi-1.2 Resistance Gene. Front. Microbiol. 2020, 10, 3042. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 Induces Systemic Resistance in Tomato Infected by Cucumber Mosaic Virus. Front. Plant Sci. 2016, 7, 1520. [Google Scholar] [CrossRef] [PubMed]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the Efficacy of Biological Control against Plant Diseases Likely to Be More Durable than That of Chemical Pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Guo, Y.; Ghirardo, A.; Weber, B.; Schnitzler, J.P.; Philipp Benz, J.; Rosenkranz, M. Trichoderma Species Differ in Their Volatile Profiles and in Antagonism toward Ectomycorrhiza Laccaria bicolor. Front. Microbiol. 2019, 10, 891. [Google Scholar] [CrossRef]
- Dutta, P.; Kakati, N.; Das, A.; Kaushik, H.; Boruah, S.; Bhowmick, P.; Kaman, P.; Bhuyan, R.P.; Hazarika, G.N. Trichoderma pseudokoningii Showed Compatibility with Certain Commonly Used Inorganic Pesticides, Fertilizers and Sticker Cum Spreaders. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 140–146. [Google Scholar] [CrossRef][Green Version]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; González-López, Ó.; Gutiérrez, S.; Casquero, P.A. Influence of Fungicide Application and Vine Age on Trichoderma Diversity as Source of Biological Control Agents. Agronomy 2021, 11, 446. [Google Scholar] [CrossRef]
- Mendarte-Alquisira, C.; Alarcón, A.; Ferrera-Cerrato, R. Growth, Tolerance, and Enzyme Activities of Trichoderma Strains in Culture Media Added with a Pyrethroids-Based Insecticide. Rev. Argent. Microbiol. 2024, 56, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Szpyrka, E.; Podbielska, M.; Zwolak, A.; Piechowicz, B.; Siebielec, G.; Słowik-Borowiec, M. Influence of a Commercial Biological Fungicide Containing Trichoderma harzianum rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil. Molecules 2020, 25, 1391. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Vargas, A.M.; Wilson-Krugg, J.; Rojas-Villacorta, W.; De La Cruz-Noriega, M.; Otiniano, N.M.; Rojas-Flores, S.; Mendoza-Villanueva, K. In Vitro Compatibility of Three Native Isolates of Trichoderma with the Insecticide Chlorpyrifos. Appl. Sci. 2023, 13, 811. [Google Scholar] [CrossRef]
- Ramanagouda, G.; Naik, M.K. Compatibility Studies of Indigenous Trichoderma Isolates with Pesticides. Indian. Phytopathol. 2021, 74, 241–248. [Google Scholar] [CrossRef]
- Manandhar, S.; Timila, R.D.; Karkee, A.; Gupt, S.K.; Baidya, S. Compatibility Study of Trichoderma Isolates with Chemical Fungicides. J. Agric. Environ. 2020, 21, 9–18. [Google Scholar] [CrossRef]
- Khandelwal, M.; Datta, S.; Mehta, J.; Naruka, R.; Makhijani, K.; Sharma, G.; Kumar, R.; Chandra, S. Isolation, Characterization & Biomass Production of Trichoderma viride Using Various Agro Products—A Biocontrol Agent. Adv. Appl. Sci. Res. 2012, 3, 3950–3955. [Google Scholar]
- Mendoza-Mendoza, A.; Clouston, A.; Li, J.H.; Nieto-Jacobo, M.F.; Cummings, N.; Steyaert, J.; Hill, R. Isolation and Mass Production of Trichoderma. Methods Mol. Biol. 2016, 1477, 13–20. [Google Scholar]
- Kumar, V.; Koul, B.; Taak, P.; Yadav, D.; Song, M. Journey of Trichoderma from Pilot Scale to Mass Production: A Review. Agriculture 2023, 13, 2022. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Golo, P.S.; de Souza Ribeiro-Silva, C.; Muniz, E.R.; de Oliveira Franco, A.; Kobori, N.N.; Fernandes, É.K.K. Advances in Submerged Liquid Fermentation and Formulation of Entomopathogenic Fungi. Appl. Microbiol. Biotechnol. 2024, 108, 451. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil Microbial Inoculants for Sustainable Agriculture: Limitations and Opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Tensi, A.F.; Ang, F.; van der Fels-Klerx, H.J. Microbial Applications and Agricultural Sustainability: A Simulation Analysis of Dutch Potato Farms. Agric. Syst. 2024, 214, 103797. [Google Scholar] [CrossRef]
- Ghoghari, N.; Bharwad, K.; Champaneria, A.; Rajkumar, S. Microbial Consortia for Augmentation of Plant Growth-Revisiting the Promising Approach towards Sustainable Agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering: Sustainable Agriculture: Microorganisms as Biostimulants; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ma, G.; Zhang, T.; Li, R.; Li, M.; Lian, H. Effects of Trichoderma pseudokoningii Agents on Growth, Antioxidant System and Control Effect against Fusarium Wilt of Cucumber Seedlings. Agric. Res. Arid Areas 2022, 40, 17606. [Google Scholar]
- Cumagun, C.J.R. Advances in Formulation of Trichoderma for Biocontrol. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 527–531. [Google Scholar]
- Sanjeev, K.; Manibhushan, T.; Archana, R. Trichoderma: Mass Production, Formulation, Quality Control, Delivery and Its Scope in Commercialization in India for the Management of Plant Diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar]
- Martínez-Medina, A.; Appels, F.V.W.; van Wees, S.C.M. Impact of Salicylic Acid- and Jasmonic Acid-Regulated Defences on Root Colonization by Trichoderma harzianum T-78. Plant Signal Behav. 2017, 12, e1345404. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Bilesky-José, N.; de Lima, R.; Fraceto, L.F. Encapsulation of Trichoderma harzianum Preserves Enzymatic Activity and Enhances the Potential for Biological Control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef]
- Mamabolo, E.; Mashala, M.J.; Mugari, E.; Mogale, T.E.; Mathebula, N.; Mabitsela, K.; Ayisi, K.K. Application of Precision Agriculture Technologies for Crop Protection and Soil Health. Smart Agric. Technol. 2025, 12, 101270. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial Consortia: Promising Probiotics as Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Kredics, L.; Chen, L.; Kedves, O.; Büchner, R.; Hatvani, L.; Allaga, H.; Nagy, V.D.; Khaled, J.M.; Alharbi, N.S.; Vágvölgyi, C. Molecular Tools for Monitoring Trichoderma in Agricultural Environments. Front. Microbiol. 2018, 9, 1599. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational Research on Trichoderma: From ’omics to the Field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Chi, S.; Xue, X.; Zhang, R.; Zhang, L.; Yu, J. Taugt17b1 Overexpression in Trichoderma atroviride Enhances Its Ability to Colonize Roots and Induce Systemic Defense of Plants. Pathogens 2023, 12, 264. [Google Scholar] [CrossRef]
- Nitzko, S. Consumer Evaluation of Food from Pesticide-Free Agriculture in Relation to Conventional and Organic Products. Farming Syst. 2024, 2, 100112. [Google Scholar] [CrossRef]
- Koppert. Trianum®-P. 2025. Available online: https://www.koppert.com/trianum-p/ (accessed on 9 October 2025).
- Plant Health Care. PHC® RootShield Plus®. 2025. Available online: https://phcmexico.com.mx (accessed on 9 October 2025).
- US EPA Trichoderma Harzianum Rifai Strain T-39 (119200) Technical Document. Available online: https://share.google/fYJa7JWXU22SMFUXn (accessed on 9 October 2025).
- Fertiberia. VELLTRIX®. 2025. Available online: https://www.fertiberia.com/productos/agricultura/velltrix/ (accessed on 9 October 2025).
- Biocontrol Technologies. T34 Biocontrol®. 2025. Available online: https://biocontroltechnologies.com/t34-biocontrol/ (accessed on 9 October 2025).
- Bi-PA. Vintec®. 2025. Available online: https://www.bi-pa.com/vintec/ (accessed on 9 October 2025).
- Gowan. Tenet® WP. 2025. Available online: https://mx.gowanco.com/products/tenet (accessed on 9 October 2025).
- Certis-Biologicals. SoilGard®. 2025. Available online: https://www.certisbio.com/products/biofungicides/soilgard-12g (accessed on 9 October 2025).
- Agrosavia. TRICOTEC®. 2025. Available online: https://www.agrosavia.co/en/product-and-services/technological-offer/agricultural-line/vegetables-and-aromatic-plants/bioproducts/331-tricotec-tomato-and-lettuce (accessed on 9 October 2025).
- Plant Health Products. Eco-T® (T-Gro). 2025. Available online: https://plant-health.co.za/biocontrol-products/eco-t-biofungicide-biostimulant-t-gro/ (accessed on 9 October 2025).
- Yuan, G. Collaborations in 2024 Biological Industry: How Enterprises Lead an Industry Revolution through Multi-Dimensional Industry Chain Collaboration. Available online: https://news.agropages.com/News/NewsDetail---53169.htm (accessed on 9 October 2025).
- Jeon, W.; Kim, T.; Choi, S.; Yang, K. Bridging the Lab-to-Field Gap in Plant Disease Diagnosis through Unsupervised Domain Adaptation Enhanced by Background Recomposition. SSRN 2025. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E. The Molecular Basis of Shoot Responses of Maize Seedlings to Trichoderma harzianum T22 Inoculation of the Root: A Proteomic Approach. Plant Physiol. 2008, 147, 2147–2163. [Google Scholar] [CrossRef]
- Harman, G.E.; Petzoldt, R.; Comis, A.; Chen, J. Interactions between Trichoderma harzianum Strain T22 and Maize Inbred Line Mo17 and Effects of These Interactions on Diseases Caused by Pythiuin ultimum and Colletotrichum graminicola. Phytopathology 2004, 94, 147–153. [Google Scholar] [CrossRef]
- Khan, M.Y.; Nadeem, S.M.; Sohaib, M.; Waqas, M.R.; Alotaibi, F.; Ali, L.; Zahir, Z.A.; Al-Barakah, F.N.I. Potential of Plant Growth Promoting Bacterial Consortium for Improving the Growth and Yield of Wheat under Saline Conditions. Front. Microbiol. 2022, 13, 958522. [Google Scholar] [CrossRef]
- Lin, L.; Li, L.; Tao, M.; Wu, Q.; Zhou, L.; Wang, B.; Wang, L.; Shao, X.; Zhong, C.; Qian, G. Assembly of an Active Microbial Consortium by Engineering Compatible Combinations Containing Foreign and Native Biocontrol Bacteria of Kiwifruit. Comput. Struct. Biotechnol. J. 2023, 21, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Sohaib, M.; Zahir, Z.A.; Khan, M.Y.; Ans, M.; Asghar, H.N.; Yasin, S.; Al-Barakah, F.N.I. Comparative Evaluation of Different Carrier-Based Multi-Strain Bacterial Formulations to Mitigate the Salt Stress in Wheat. Saudi J. Biol. Sci. 2020, 27, 777–787. [Google Scholar] [CrossRef]
- Mawarda, P.C.; Le Roux, X.; Dirk van Elsas, J.; Salles, J.F. Deliberate Introduction of Invisible Invaders: A Critical Appraisal of the Impact of Microbial Inoculants on Soil Microbial Communities. Soil. Biol. Biochem. 2020, 148, 107874. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, X.; Kuramae, E.E. Insight into Farming Native Microbiome by Bioinoculant in Soil-Plant System. Microbiol. Res. 2024, 285, 127776. [Google Scholar] [CrossRef]
- Farda, B.; Pasquarelli, F.; Djebaili, R.; Spera, D.M.; Del Gallo, M.; Pellegrini, M. Co-Culturing a Multistrain Gram-Negative Inoculant Useful in Sustainable Agriculture. Front. Ind. Microbiol. 2024, 2, 1380037. [Google Scholar] [CrossRef]
- Martignoni, M.M.; Kolodny, O. Microbiome Transfer from Native to Invasive Species May Increase Invasion Risk and Shorten Invasion Lag. Proc. R. Soc. B Biol. Sci. 2023, 291, 20241318. [Google Scholar] [CrossRef]
- Fernandez-Gnecco, G.; Gégu, L.; Covacevich, F.; Consolo, V.F.; Bouffaud, M.L.; Buscot, F.; Smalla, K.; Babin, D. Alone as Effective as Together: AMF and Trichoderma Inoculation Boost Maize Performance but Differentially Shape Soil and Rhizosphere Microbiota. J. Sustain. Agric. Environ. 2024, 3, e12091. [Google Scholar] [CrossRef]
- De Vita, P.; Avio, L.; Sbrana, C.; Laidò, G.; Marone, D.; Mastrangelo, A.M.; Cattivelli, L.; Giovannetti, M. Genetic Markers Associated to Arbuscular Mycorrhizal Colonization in Durum Wheat. Sci. Rep. 2018, 8, 10612. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Nakata, N.; Suzuki, T.; Shirasu, K. Markers to Differentiate Species of Anthracnose Fungi Identify Colletotrichum fructicola as the Predominant Virulent Species in Strawberry Plants in Chiba Prefecture of Japan. J. Gen. Plant Pathol. 2017, 83, 14–22. [Google Scholar] [CrossRef]

| Trichoderma Species | Crop/Plant | Target Pathogen/Disease | Reference |
|---|---|---|---|
| Trichoderma spp. | Corn | Leaf blight and Stalk Rot | [50] |
| T. viride | Tomato | Phytophthora infestans, Late blight | [51] |
| Trichoderma sp. (formulation) T. harzianum | Rubber tree | Phytophthora and Corynespora | [52] |
| Trichoderma bioformulations: T. harzianum | Chickpea | Fusarium oxysporum f. sp. ciceris | [53] |
| T. harzianum | Wheat | Stem rust (Puccinia graminis f. sp. tritici) | [54] |
| Trichoderma strains | Soybean | Fusarium oxysporum | [55] |
| T. harzianum | Apple | Apple replant disease (ARD) | [56] |
| Trichoderma formulation | Oroxylum indicum | NA | [57] |
| T. asperellum | Soybean | Several diseases | [58] |
| T. aureoviride and T. hamatum | Grapevines | NA | [59] |
| Several Trichoderma isolates | Soybean and common bean | Sclerotinia sclerotiorum | [60] |
| Trichoderma spp. | Sugarcane | NA | [61] |
| Trichoderma strains: T. harzianum | Rice | Brown spot, Rice blast | [62] |
| Trichoderma TrB | Grass (Golf courses) | Fusarium sp. | [63] |
| Different Trichoderma formulations | Onion | Purple blotch disease (Alternaria porri) | [64] |
| Trichoderma sp. | Tobbacco | Tobacco black shank (TBS) and tobacco root black rot (TRBR) | [65] |
| T. asperellum | Corn | Downy mildew (Peronosclerospora spp. L.) | [66] |
| Trichoderma spp. | NA | Honey bee | [67] |
| Trichoderma strains | Onion | NA | [68] |
| Trichoderma spp. | Grapevines | Neofusicoccum parvum | [69] |
| Trichoderma spp. | Tomatoes | NA | [70] |
| T. harzianum | Cotton | NA | [71] |
| T. harzianum | Soybean | NA | [72] |
| T. virens | Maize | Anthracnose, Leaf blight, and insect herbivores | [42] |
| T. asperellum | Tomato | Meloidogyne incognita | [73] |
| T. atroviride | Grapevine | Phomopsis viticola | [74] |
| T. virens | Tomato | Fusarium oxysporum | [75] |
| T. atroviride and commercial Trianum | Tomato, thyme (Thymus vulgaris), and basil (Ocimum basilicum L.) | Late blight | [76] |
| T. viride | Chilli and Tomato | Chilli pathogens (Pythium aphanidermatum, Phytophthora capsici, and Fusarium oxysporum) and tomato (Pythium aphanidermatum, Phytophthora infestans, and F. oxysporum) | [77] |
| T. harzianum | Maize | NA | [78] |
| T. asperelloides | Soybean | NA | [79] |
| T. longibrachiatum, T. asperellum | Groundnut | Stem rot | [80] |
| Trichoderma spp. | Sweet Corn | Leaf blight | [81] |
| T. asperellum and T. harzianum | Chilli and Chinese Kale | Anthracnose | [82] |
| T. harzianum | Maize | NA | [83] |
| T. asperellum | Not specified | Meloidogyne incognita | [84] |
| T. harzianum | Sorghum | NA | [85] |
| T. atroviride | Soybean | NA | [86] |
| T. viride | winter wheat (Sultan variety) | Aspergillus, Mucor, Fusarium | [87] |
| T. ghanense | Seet potato | NA | [88] |
| T. harzianum T22 | Cucurbita pepo L | plant viruses, powdery mildew, and the arthropod community | [89] |
| T. harzianum T22 | Blueberry | Botrytis Blossom | [90] |
| T. asperellum | Amaranth | NA | [91] |
| T. viride | Cabbage (Brassica oleracea L.) | NA | [92] |
| T. koningiopsis NBRI-PR5 (PR5) and T. asperellum NBRI-K14 (K14) consortium | Rice | NA | [93] |
| T. harzianum | Cucumber | Aspergillus tamarii and A. niger | [94] |
| T. asperellum | Tomato | Meloidogyne spp. | [95] |
| Trichoderma species | Panax notoginseng | Root rot | [96] |
| Trichoderma spp. | Cucumis melo L. | NA | [97] |
| T. harzianum | Maize | NA | [98] |
| Trichoderma spp. | Soybean | NA | [99] |
| T. viride | Chickpea | Fusarium wilt | [100] |
| T. asperellum, T. gamsii | Grapevines | Esca complex disease | [101] |
| Trichoderma species | Pepper | Fusarium oxysporum and Rhizoctonia solani | [102] |
| T. asperellum | Soil | Phytophthora ramorum | [103] |
| Trichoderma strains | Blackberry | Fusarium wilt | [104] |
| Trichoderma 6-PP compound | Maize | Magnaporthiopsis maydis | [105] |
| Trichoderma strains | Maize | Fusarium verticillioides | [106] |
| T. longibrachiatum | Cucumber | Fusarium solani | [107] |
| T. hamatum | Pepper | Microbial communities | [108] |
| Trichoderma spp. | Pear | Stemphylium vesicarium and Pleospora allii | [109] |
| Trichoderma spp. | Barley | NA | [110] |
| T. reesei bio-product | winter wheat (Triticum aestivum L.) cv. Ada and spring barley (Hordeum vulgare L.) cv. Luokė. | NA | [111] |
| Not specified | Grapevines | Grapevine pruning wounds | [112] |
| T. harzianum | Black pepper | NA | [113] |
| Trichoderma strains: T. harzianum, T. simmonsii, T. afroharzianum | Wheat | NA | [114] |
| T. harzianum | Soil | NA | [115] |
| T. atroviride LX-7 and T. citrinoviride HT-1 | Pakchoi (Brassica chinensis L.) | NA | [116] |
| T. asperellum | Banana | Fusarium species | [117] |
| T. afroharzianum T22, T. atroviride P1 | Tomato | Macrosiphum euphorbiae, and Spodoptera littoralis | [118] |
| Trichoderma spp. | Common bean (Phaseolus vulgaris L.) | Sclerotinia sclerotiorum | [119] |
| Trichoderma spp. | Great millet (Sorghum bicolour L.) | Anthracnose | [120] |
| T. harzianum | Maize | Root rot, damping-off (Rhizoctonia solani, Fusarium spp.), stalk rot (Erwinia carotovora), gray leaf spot (Cercospora zeae-maydis), late wilt (Cephalosporium maydis), and ear rot (Fusarium verticillioides, F. graminearum) | [121] |
| T. harzianum | Tomato | NA | [122] |
| T. asperellum | Corn | Rhizoctonia solani | [123] |
| Trichoderma T-soybean | Maize | Maize stalk rot | [124] |
| T. afroharzianum, T. harzianum, T. gamsii | Wheat | Crown rot pathogen Fusarium pseudograminearum | [125] |
| T. harzianum | Maize | Cold stress | [126] |
| T. harzianum | Tomato | Wilt and root rot diseases | [127] |
| Trichoderma sp., T. reesei Simmons, T. virens Miller, T. harzianum Rifai, | Corn | NA | [128] |
| T. harzianum, T. virens, T. asperellum, and T. longibrachiatum | Sugarcane | Red Rot | [129] |
| T. asperellum | Cacao | Vascular-streak dieback (VSD) disease (Ceratobasidium theobromae) | [130] |
| Trichoderma spp. | Mungbean | Macrophomina phaseolina and Rhizoctonia solani | [131] |
| T. asperellum | Maize | Fusarium graminearum | [132] |
| T. lignorum | Not specified | Atta cephalotes | [133] |
| T. harzianum | Corn, Maize | Leaf blight (Cochliobolus heterostrophus) | [134] |
| T. harzianum | Broccoli | NA | [135] |
| T. asperellum | Tomato | Wilt caused by Ralstonia solanacearum | [136] |
| T. atroviride | Cucumber | Downy mildew | [137] |
| T. atroviride/T. harzianum | Soil-Lettuce | NA | [138] |
| T. harzianum | Maize | Fusarium graminearum | [139] |
| Trichoderma spp. | Pepper | Not specified | [140] |
| T. harzianum M10 and T. atroviride P1 | Grapes | NA | [141] |
| Product Name | Trichoderma Strain | Country or Supplier (Origin) | Principal Crops of Application | Target Diseases |
|---|---|---|---|---|
| Trianum/Trianum-P (Trianum-WG, T-22) | Trichoderma harzianum strain T-22 * | Koppert (The Netherlands/Koppert) Global Product. | Ornamentals, horticulture, protected fruits and crops (greenhouse). | Soil phytopathogens: Pythium, Rhizoctonia, Fusarium, Sclerotinia, Thielaviopsis; Root and vigor enhancer [225]. |
| RootShield/RootShield PLUS | Trichoderma harzianum strain T-22 * (in combination with T. virens G-41 in RootShield PLUS) | Plant Health Care PHC (EE. UU.)—distributed in North America and Europe. | Horticulture, ornamentals, transplants, pot crops, greenhouses. | Soil phytopathogens: Pythium, Rhizoctonia, Fusarium, Thielaviopsis, etc. [226]. |
| Trichodex | Trichoderma harzianum strain T-39 | Israel/historically commercialized by Makhteshim-Agan | Strawberries, greenhouse horticulture, grape vine, field horticulture. | Botrytis (grey mildew), other soil and foliar pathogens (integrated use against Botrytis, Sclerotinia, etc [227]. |
| VELLTRIX (TRICHODEX line) | Trichoderma asperellum (commercial strain from the group TRICHODEX) | Fertiberia/TRICHODEX (Spain/Europe) | Widely used in field and horticultural crops to improve root development; used in cereals, vegetables, and fruit trees, depending on the formulation. | Biofertilizer/bioprotection: improves rooting, tolerance to water stress, and support against soil diseases (broad action, growth promotion) [228]. |
| T34 Biocontrol (Trademark/T34) | Trichoderma asperellum strain T34 | Biocontrol Technologies, Manufacturer in Spain (EPA-registered product/US regulatory documentation also available). | Greenhouse use: ornamental crops (e.g., carnations) and trials on other protected crops. | Aimed at controlling Fusarium oxysporum (stimulation of plant defenses and control of Fusarium wilt) [229]. |
| Vintec | T. atroviride SC1 | Bi-PA (Belgium). | Protection of grapevine, greenhouse tomato, and other fruiting crops. | Phaeomoniella chlamydospora, Phaeoacremonium aleophilum, Botrytis cinerea, Coryneum, Monilinia and Taphrina [230]. |
| TENET WP/Remedier/BLINDAR | T. asperellum ICC 012 and T. gamsii ICC 080 | Gowan LLC (multinational) | Control of soil diseases of soy and wheat, eggplant, zucchini, strawberry, lettuce, melon, cucumber, pepper, tomato, and watermelon. | Phytophthora spp., Pythium spp. [231]. |
| SOILGARD 12 G | Trichoderma virens/Gliocladium virens GL-21 | Certis Biologicals (USA) | Nightshades, cucurbits, ornamentals, lettuce, spinach | Overturning/Pythium spp., Fusarium oxysporum, Rhizoctonia spp., and root rots [232]. |
| Tricotec® | Trichoderma koningiopsis Th003 | Agrosavia (Colombia and Latin America) | Tomato, lettuce, rice, berries, roses, and ornamentals, potato | Fusarium oxysporum, Rhizoctonia solani, Sclerotinia spp., Botrytis cinerea [233]. |
| Eco-T® | T. harzianum strain Eco-T | Plant Health Products (Africa) | Horticultural and field crops | Fusarium, Phytophthora, Pythium, Rhizoctonia, Sclerotinia, Verticillium [234]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Moreno, K.; Olguín-Martínez, A.I.; Montoya-Martínez, A.C.; de los Santos-Villalobos, S. Trichoderma in Sustainable Agriculture and the Challenges Related to Its Effectiveness. Diversity 2025, 17, 734. https://doi.org/10.3390/d17100734
Gutiérrez-Moreno K, Olguín-Martínez AI, Montoya-Martínez AC, de los Santos-Villalobos S. Trichoderma in Sustainable Agriculture and the Challenges Related to Its Effectiveness. Diversity. 2025; 17(10):734. https://doi.org/10.3390/d17100734
Chicago/Turabian StyleGutiérrez-Moreno, Karina, Ana I. Olguín-Martínez, Amelia C. Montoya-Martínez, and Sergio de los Santos-Villalobos. 2025. "Trichoderma in Sustainable Agriculture and the Challenges Related to Its Effectiveness" Diversity 17, no. 10: 734. https://doi.org/10.3390/d17100734
APA StyleGutiérrez-Moreno, K., Olguín-Martínez, A. I., Montoya-Martínez, A. C., & de los Santos-Villalobos, S. (2025). Trichoderma in Sustainable Agriculture and the Challenges Related to Its Effectiveness. Diversity, 17(10), 734. https://doi.org/10.3390/d17100734






