Assessment of Non-Sessile Invertebrates Associated with Mats of the Red Alga Phyllophora crispa at Giglio Island, Mediterranean Sea
Abstract
1. Introduction
- What are the abundances and diversity of non-sessile invertebrates associated with P. crispa?
- Which are the most abundant families in P. crispa mats?
- Are there spatial differences in associations with P. crispa?
2. Materials and Methods
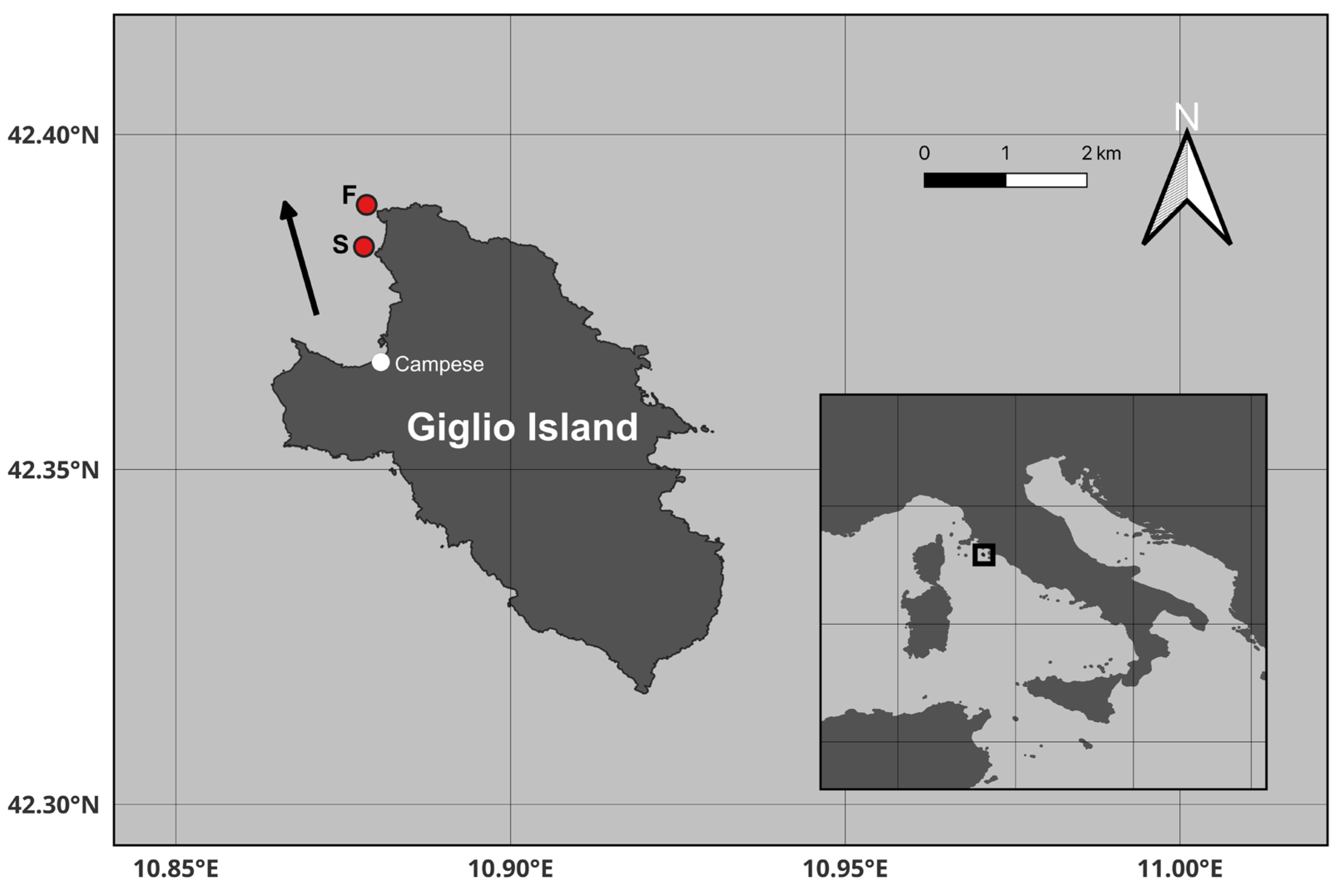
2.1. Study Area and Sampling Activities
2.2. Species Identification
2.3. Diversity Descriptors
3. Results
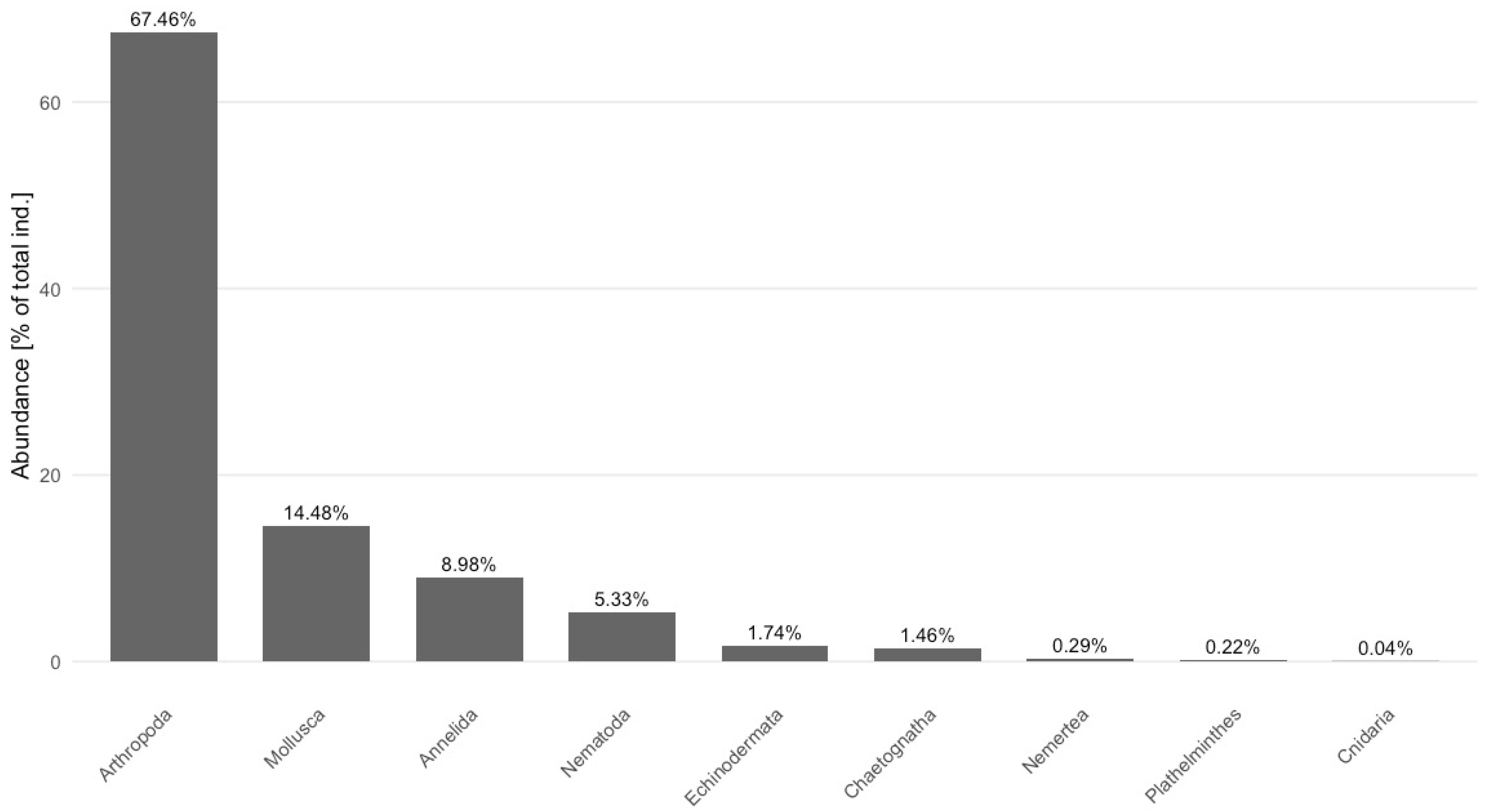
3.1. Analyses of Non-Sessile Invertebrate Assemblages
3.2. Diversity Descriptors
4. Discussion
4.1. Composition of Non-Sessile Invertebrate Assemblages in P. crispa Mats
4.2. Abundance and Diversity of Non-Sessile Invertebrates in P. crispa Mats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Author(s) | Year | Title |
|---|---|---|
| Riedl, R. | 2011 | Fauna und Flora des Mittelmeers |
| Stresemann, E. | 1992 | Wirbellose |
| Hayward, P.J. & Ryland, J.S. | 1999 | Handbook of the Marine Fauna of North-West Europe |
| Larink, O. & Westheide, W. | 2006 | Coastal Plankton. Photo guide for European Seas |
| Sabelli, B. | 1982 | Conchiglie. Caratteristiche e ambienti di vita dei Molluschi |
| Doneddu, M. & Trainito, E. | 2005 | Conchiglie del Mediterraneo |
| Debelius, H. & Kuiter, R.H. | 2007 | Nacktschnecken der Weltmeere |
| Suarez-Morales, E. et al. | 2020 | Class Copepoda |
| Rodriguez-Lazaro, J. & Ruiz-Munoz, F. | 2012 | A General Introduction to Ostracods: Morphology, Distribution, Fossil Record and Applications |
| Debelius, H. | 2000 | Krebsführer |
| Bellan-Santini et al. | 1980 | The Amphipoda of the Mediterranean |
| Holdich, D.M. & Jones, J.A. | 1983 | Tanaids |
| Smaldon, G. | 1993 | Coastal Shrimps and Prawns |
| Ballesteros, E. & Llobet, T. | 2015 | Illustrierter Naturführer Mittelmeer |
| Wirtz, P. & Debelius, H. | 2003 | Niedere Tiere Mittelmeer und Atlantik |
| Geiß, Günter | 1990 | Weichtiere, Krebse, Stachelhäuter des Mittelmeeres |
| Green, J. & Macquitty | 1987 | Halacarid Mites |
References
- Turley, C. The changing Mediterranean Sea—A sensitive ecosystem? Prog. Oceanogr. 1999, 44, 387–400. [Google Scholar] [CrossRef]
- Wilson, E.O.; Peter, F.M. The Current State of Biological Diversity. Environ. Sci. 1988, 521, 3–18. [Google Scholar]
- Defant, A. Physical Oceanography; Pergamon Press: London, UK, 1961; Volume 2. [Google Scholar]
- Bianchi, C.N.; Morri, C. Marine biodiversity of the Mediterranean Sea: Situation, problems and Prospects for Future research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; Volume 1, p. 55. [Google Scholar]
- Ponti, M.; Turicchia, E.; Ferro, F.; Cerrano, C.; Abbiati, M. The understorey of gorgonian forests in mesophotic temperate reefs. PLoS ONE 2018, 28, 1153–1166. [Google Scholar] [CrossRef]
- Ballesteros, E. Mediterranean Coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar] [CrossRef]
- Marba, N.; Diaz-Almela, E.; Duarte, C.M. Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biol. Conserv. 2014, 176, 183–190. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Mayot, N.; Pergent, G. The outstanding traits of the functioning of the Posidonia oceanica seagrass ecosystem. Biol. Mar. Medit 2006, 13, 109–113. [Google Scholar]
- Mazzella, L.; Buia, M.C.; Gambi, M.C.; Lorenti, M.; Russo, G.F.; Scipione, M.B.; Zupo, V. Plant-animal trophic relationships in the Posidonia oceanica ecosystem of the Mediterranean Sea: A review. In Plant-Animal Interactions in the Marine Benthos; Clarendon Press: Oxford, UK, 1992; pp. 165–187. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Elsevier Ebooks 2018, 79, 61–136. [Google Scholar] [CrossRef]
- Meadows, P.S.; Meadows, A.; Murray, J.M.H. Biological modifiers of marine benthic seascapes: Their role as ecosystem engineers. Geomorphology 2012, 157–158, 31–48. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Litsi-Mizan, V.; Efthymidias, P.T.; Gerakaris, V.; Serrano, O.; Tsapakis, M.; Apostolaki, E.T. Decline of seagrass (Posidonia oceanica) production over two decades in the face of warming of the Eastern Mediterranean Sea. New Phytol. Found. 2023, 239, 2126–2137. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Bernardeau-Esteller, J.; García-Muñoz, R.; Ramos, A.; Ontoria, Y.; Romero, J.; Pérez, M.; Ruiz, J.M.; Procaccini, G. Carbon economy of Mediterranean seagrasses in response to thermal stress. Mar. Pollut. Bull. 2018, 135, 617–629. [Google Scholar] [CrossRef]
- CEAM. Available online: https://www.ceam.es/ceamet/SST/SST-climatology.html (accessed on 24 September 2025).
- Ontaria, Y.; Cuesta-Gracia, A.; Ruiz, J.M.; Romero, J.; Perez, M. The negative effects of short-term extreme thermal events on the seagrass Posidonia oceanica are exacerbated by ammonium additions. PLoS ONE 2014, 32, e0222798. [Google Scholar] [CrossRef]
- Marba, N.; Duarte, C.M. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Change Biol. 2010, 16, 2366–2375. [Google Scholar] [CrossRef]
- Meybeck, M. The global change of continental Aquatic Systems: Dominant impacts of human activities. Water Sci. Technol. 2004, 49, 73–83. [Google Scholar] [CrossRef]
- Piazzi, L.; Gennaro, P.; Balata, D. Threats to macroalgal coralligenous assemblages in the Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 2623–2629. [Google Scholar] [CrossRef]
- Pergent, G.; Bazairi, H.; Bianchi, C.N.; Boudouresque, C.F.; Buia, M.C.; Calvo, S.; Clabaut, P.; Harmelin-Vivien, M.; Mateo, M.Á.; Montefalcone, M.; et al. Climate Change and Mediterranean Seagrass Meadows: A Synopsis for Environmental Managers. Mediterr. Mar. Sci. 2014, 15, 462. [Google Scholar] [CrossRef]
- Sunday, J.M.; Fabricius, K.; Kroeker, K.J.; Anderson, K.M.; Brown, N.E.M.; Barry, J.; Connell, S.D.; Dupont, S.; Gaylord, B.; Hall-Spencer, J.M.; et al. Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat. Clim. Change 2016, 7, 81–85. [Google Scholar] [CrossRef]
- Rocha, J.; Yletyinen, J.; Biggs, R.; Blenckner, T.; Peterson, G.D. Marine Regime shifts: Drivers and impacts on ecosystems services. Philos. Trans. R. Soc. B 2015, 370, 20130273. [Google Scholar] [CrossRef]
- Ainsworth, C.H.; Mumby, P.J. Coral–algal phase shifts alter fish communities and reduce fisheries production. Glob. Change Biol. 2015, 21, 165–172. [Google Scholar] [CrossRef]
- Chase, J.M.; Blowes, S.A.; Knight, T.M.; Gerstner, K.; May, F. Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 2020, 584, 238–243. [Google Scholar] [CrossRef]
- El-Khaled, Y.C.; Daraghmeh, N.; Tilstra, A.; Roth, F.; Huettel, M.; Roßbach, F.; Casoli, E.; Koester, A.; Beck, M.; Meyer, R.L.; et al. Fleshy red algae mats act as temporary reservoirs for sessile invertebrate biodiversity. Commun. Biol. 2022, 5, 579. [Google Scholar] [CrossRef]
- Bonifazi, A.; Ventura, D.; Gravina, M.F.; Lasinio, G.J.; Belluscio, A.; Ardizzone, G. Unusual algal turfs associated with the Rhodophyta phyllophora crispa: Benthic assemblages along a depth gradient in the Central Mediterranean Sea. Estuar. Coast. Shelf Sci. 2017, 185, 77–93. [Google Scholar] [CrossRef]
- Salomidi, M.; Katsanevakis, S.; Borja, A.; Braeckman, U.; Damalas, D.; Galparsoro, I.; Mifsud, R.; Mirto, S.; Pascual, M.; Pipitone, C.; et al. Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: A stepping stone towards ecosystem-based marine spatial management. Mediterr. Mar. Sci. 2012, 13, 49–88. [Google Scholar] [CrossRef]
- Schmidt, N.; El-Khaled, Y.C.; Roßbach, F.; Wild, C. Fleshy red algae mats influence their environment in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 721626. [Google Scholar] [CrossRef]
- El-Khaled, Y.C.; Tilstra, A.; Mezger, S.D.; Wild, C. Red and brown algae mats overgrow classical marine biodiversity hotspots in the Mediterranean Sea. Bull. Mar. Sci. 2023, 99, 51–56. [Google Scholar] [CrossRef]
- Navone, A.; Bianchi, C.N.; Orru, P.; Ulzega, A. Saggio di cartografia geomorfologica e bionomica nel parco marino di Tavolara-Capo Coda Cavallo. Oebalia 1992, 17, 469–478. [Google Scholar]
- Guiry, M. Macroalgae of Rhodophycota, Phaeophycota, Chlorophycota, and two genera of Xanthophycota. In European Register of Marine Species: A Check-List of the Marine Species in Europe and a Bibliography of Guides to their Identification; Costello, M.J., Emblow, C., White, R., Eds.; Collection Patrimoines Naturels: Paris, France, 2001; pp. 20–38. [Google Scholar]
- Zaitsev, Y. An Introduction to the Black Sea Ecology; Smil Edition and Publishing Agency Ltd.: Odessa, Ukraine, 2008. [Google Scholar]
- Berov, D.; Todorova, V.; Dimitrov, L.; Rinde, E.; Karamfilov, V. Distribution and abundance of phytobenthic communities: Implications for connectivity and ecosystem functioning in a Black Sea Marine Protected Area. Estuar. Coast. Shelf Sci. 2018, 200, 234–247. [Google Scholar] [CrossRef]
- Bunker, F.; Brodie, J.A.; Maggs, C.A.; Bunker, A.R. Seaweeds of Britain and Ireland; Wild Nature Press: Oxford, UK, 2017. [Google Scholar]
- Rossbach, F.; Casoli, E.; Beck, M.; Wild, C. Mediterranean red macro algae mats as habitat for high abundances of Serpulid polychaetes. Diversity 2021, 13, 265. [Google Scholar] [CrossRef]
- Casoli, E.; Bonifazi, A.; Ardizzone, G.; Gravina, M.F.; Russo, G.F.; Sandulli, R.; Donnarumm, L. Comparative analysis of mollusc assemblages from different hard bottom habitats in the Central Tyrrhenian Sea. Diversity 2019, 11, 74. [Google Scholar] [CrossRef]
- Hamlet, C.L.; Strickland, W.C.; Battista, N.; Miller, L.A. Multiscale flow between the branches and polyps of gorgonians. J. Exp. Biol. 2023, 226, jeb244520. [Google Scholar] [CrossRef]
- Rossbach, F.; Casoli, E.; Plewka, J.; Schmidt, N.; Wild, C. New insights into a Mediterranean sea benthic habitat: High diversity of epiphytic bryozoan assemblages on Phyllophora crispa (Rhodophyta) mats. Diversity 2022, 14, 346. [Google Scholar] [CrossRef]
- Rossbach, F.; Merk, B.; Wild, C. High diversity and abundance of foraminifera associated with Mediterranean benthic red algae mats. Diversity 2022, 14, 21. [Google Scholar] [CrossRef]
- Mabrouk, L.; Ben Brahim, M.; Hamza, A.; Bradai, M.N. Diversity and temporal fluctuations of epiphytes and sessile invertebrates on the rhizomes Posidonia oceanica in a seagrass meadow off Tunisia. Mar. Ecol. 2014, 35, 212–220. [Google Scholar] [CrossRef]
- Verdura, J.; Linares, C.; Ballesteros, E.; Coma, R.; Uriz, M.J.; Bensoussan, N.; Cebrian, E. Biodiversity loss in a Mediterranean ecosystem due to an extreme warming event unveils the role of an engineering gorgonian species. Sci. Rep. 2019, 9, 5911. [Google Scholar] [CrossRef]
- Donato, G.; Rossana, S.; Basso, D.; Bazzicalupo, P.; Bertolino, M.; Bracchi, V.A.; Cipriani, M.; D’Alpa, F.; Guido, A.; Negri, M.P.; et al. Biodiversity associated with a coralligenous build-up off Sicily (Ionian Sea). Reg. Stud. Mar. Sci. 2024, 80, 103868. [Google Scholar] [CrossRef]
- Ballesteros, E.; Garrabou, J.; Hereu, B.; Zabala, M.; Cebrian, E.; Sala, E. Deep-water stands of Cystoseira zosteroides C. Agardh (Fucales, Ochrophyta) in the Northwestern Mediterranean: Insights into assemblage structure and population dynamics. Estuar. Coast. Shelf Sci. 2009, 82, 477–484. [Google Scholar] [CrossRef]
- Cleary, D.; Polónia, A.; Renema, W.; Hoeksema, B.; Rachello-Dolmen, P.; Moolenbeek, R.; Budiyanto, A.; Yahmantoro; Tuti, Y.; Giyanto; et al. Variation in the composition of corals, fishes, sponges, echinoderms, ascidians, molluscs, foraminifera and macroalgae across a pronounced in-to-offshore environmental gradient in the Jakarta Bay–Thousand Islands coral reef complex. Mar. Pollut. Bull. 2016, 110, 701–717. [Google Scholar] [CrossRef]
- Milne, R.; Griffiths, C. Invertebrate biodiversity associated with algal turfs ona coral-dominated reef. Mar. Biodivers. 2014, 44, 181–188. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Fosså, J.H. Species diversity and spatial distribution of invertebrates on deep–water Lophelia reefs in Norway. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004; Volume 1868, pp. 1849–1868. [Google Scholar]
- Henry, L.A.; Davies, A.J.; Roberts, J.M. Beta diversity of cold-water coral reef communities off western Scotland. Coral Reefs 2010, 29, 427–436. [Google Scholar] [CrossRef]
- Farnsworth, E.J.; Ellison, A.M. Scale-dependent spatial and temporal variability in biogeography of mangrove root epibiont communities. Ecol. Monogr. 1996, 66, 45–66. [Google Scholar] [CrossRef]
- Graham, M.H. Effects of local deforestation on the diversity and structure of southern California giant kelp forest food webs. Ecosystems 2004, 7, 341–357. [Google Scholar] [CrossRef]
- Gutt, J.; Sirenko, B.I.; Arntz, W.E.; Smirnov, I.S.; Broyer, C.D.E. Biodiversity of the Weddell Sea: Macrozoobenthic species (demersal fish included) sampled during the expedition ANT Xllll3 (EASIZ I) with RV ‘Polarstern’. Berichte Polarforsch. Meeresforsch. 2000, 372, 118. [Google Scholar]
- McNeil, M.; Firn, J.; Nothdurft, L.D.; Pearse, A.R.; Webster, J.M.; Pitcher, C.R. Inter-reef Halimeda algal habitats within the Great Barrier Reef support a distinct biotic community and high biodiversity. Nat. Ecol. Evol. 2021, 5, 647–655. [Google Scholar] [CrossRef]
- Gozler, A.M.; Kopuz, U.; Agirbas, E. Seasonal changes of invertebrate fauna associated with Cystoseira barbata facies of Southeastern Black Sea coast. Afr. J. Biotechnol. 2010, 9, 8852–8859. [Google Scholar]
- Kocatas, A.; Katağan, T.; Sezgin, M.; Kirkim, F.; Koçak, C. Crustacean diversity among the Cystoseira facies of the Aegean coast of Turkey. Turk. Zooloji Derg. Turk. J. Zool. 2004, 28, 309–316. Available online: https://journals.tubitak.gov.tr/zoology/vol28/iss4/5 (accessed on 24 September 2025).
- Turner, J.T. The Importance of Small Planktonic Copepods and Their Roles in Pelagic Marine Food Webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Arroyo, N.L.; Aarnio, K.; Ólafsson, E. Interactions between two closely related phytal harpacticoid copepods, asymmetric positive and negative effects. J. Exp. Mar. Biol. Ecol. 2006, 341, 219–227. [Google Scholar] [CrossRef]
- Karaytuğ, S.; Koçak, C. Faunistic assessment of the marine Harpacticoida (Crustacea: Copepoda) fauna of Turkey with remarks on harpacticoid diversity in the eastern Mediterranean Sea. Mar. Biodiv. 2018, 48, 273–280. [Google Scholar] [CrossRef]
- Hooper, G.J.; Davenport, J. Epifaunal composition and fractal dimensions of intertidal marine macroalgae in relation to emersion. J. Mar. Biol. Assoc. United Kingd. 2006, 86, 1297–1304. [Google Scholar] [CrossRef]
- Mascart, T.; Lepoint, G.; De Troch, M. Meiofauna and harpacticoid copepods in different habitats of a Mediterranean seagrass meadow. J. Mar. Biol. Assoc. United Kingd. 2013, 93, 1557–1566. [Google Scholar] [CrossRef]
- Mascart, T.; Lepoint, G.; Deschoemaeker, S.; Binard, M.; Remy, F.; De Troch, M. Seasonal variability of meiofauna, especially harpacticoid copepods, in Posidonia oceanica macrophytodetritus accumulations. J. Sea Res. 2015, 95, 149–160. [Google Scholar] [CrossRef]
- Suárez-Morales, E.; Gutiérrez-Aguirre, M.; Gómez, S.; Perbiche-Neves, G.; Previattelli, D.; Dos Santos-Silva, E.N.; Da Rocha, C.E.F.; Mercado-Salas, N.F.; Marques, T.M.; Cruz-Quintana, Y.; et al. Class Copepoda. In Thorp and Covich’s Freshwater Invertebrates; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Bell, S.S.; Walters, K.; Hall, M.O. Habitat utilization by harpacticoid copepods: A morphometric approach. Mar. Ecol.—Prog. Ser. 1987, 35, 59–64. [Google Scholar] [CrossRef]
- Coull, B.C. Role of meiofauna in estuarine soft—Bottom habitats. Aust. J. Ecol. 1999, 24, 327–343. [Google Scholar] [CrossRef]
- Hermosillo-Núñez, B.B. Contribution of echinoderms to keystone species complexes and macroscopic properties in kelp forest ecosystems (northern Chile). Hydrobiologia 2020, 847, 739–756. [Google Scholar] [CrossRef]
- Birdsey, E.M.; Johnston, E.L.; Poore, A.G.B. Diversity and cover of a sessile animal assemblage does not predict its associated mobile fauna. Mar. Biol. 2012, 159, 551–560. [Google Scholar] [CrossRef]
- Gilinski, E. The Role of Fish Predation and Spatial Heterogeneity in Determining Benthic Community Structure. Ecology 1984, 65, 455–468. [Google Scholar] [CrossRef]
- Dean, R.L.; Connell, J.H. Marine invertebrates in an algal succession. III. Mechanisms linking habitat complexity with diversity. J. Exp. Mar. Biol. Ecol. 1987, 109, 249–273. [Google Scholar] [CrossRef]
- Gibbons, E.G.; Quijon, P.A. Macroalgal features and their influence on associated biodiversity: Implications for conservation and restoration. Front. Mar. Sci. 2023, 10, 1304000. [Google Scholar] [CrossRef]
- Chemello, R.; Milazzo, M. Effect of algal architecture on associated fauna: Some evidence from phytal molluscs. Mar. Biol. 2002, 140, 981–990. [Google Scholar] [CrossRef]
- Carvalho, L.R.S.; Barros, F. Physical habitat structure in marine ecosystems: The meaning of complexity and heterogeneity. Hydrobiologia 2017, 797, 1–9. [Google Scholar] [CrossRef]
- Tokeshi, M.; Arakaki, S. Habitat complexity in aquatic systems: Fractals and beyond. Hydrobiologia 2012, 685, 27. [Google Scholar] [CrossRef]


| Habitat | Location | Richness | Taxa | Evenness | H′ | D | Reference |
|---|---|---|---|---|---|---|---|
| Phyllophora crispa | Giglio Island, Secca II | 92 x,y | 9 A,AN,C,c,E,M,N,NE,P,p | 0.8 | 3.0 | 0.9 | Present study |
| Phyllophora crispa | Giglio Island, Fenaio | 92 x,y | 9 A,AN,C,c,E,M,N,NE,P,p | 0.9 | 3.2 | 0.9 | Present study |
| Phyllophora crispa | Giglio Island, Secca II | 25 | 1 CO | 0.9 | 2.6 | 0.9 | Present study |
| Phyllophora crispa | Giglio Island, Fenaio | 22 | 1 CO | 0.9 | 2.4 | 0.9 | Present study |
| Phyllophora crispa | Giglio Island, Secca II | 17 | 1 OS | 0.8 | 1.9 | 0.8 | Present study |
| Phyllophora crispa | Giglio Island, Fenaio | 18 | 1 OS | 0.8 | 2.0 | 0.8 | Present study |
| Phyllophora crispa | NW Mediterranean | 223 | 9 a,b,c,e,f,m,p,r,s | 0.7 | 2.2 | 0.3 | [28] |
| Posidonia oceanica | NW Mediterranean | 179 | 7 a,b,c,f,m,p,s | 0.8 | 2.1 | 0.3 | [28] |
| Phyllophora crispa | NW Mediterranean | 17 | 1 ST | 0.6 | 1.1 | / | [38] |
| Posidonia oceanica leaves | NW Mediterranean | 10 | 1 ST | 0.4 | 0.2 | / | [38] |
| Posidonia oceanica shoots | NW Mediterranean | 10 | 1 ST | 0.4 | 0.2 | / | [38] |
| Phyllophora crispa | NW Mediterranean | 33 | 1 b | 0.2 | 2.2 | / | [41] |
| Posidonia oceanica leaves | NW Mediterranean | 29 | 1 b | 0.2 | 1.3 | / | [41] |
| Posidonia oceanica shoots | NW Mediterranean | 29 | 1 b | 0.2 | 2 | / | [41] |
| Phyllophora crispa | NW Mediterranean | 81 | 1 f | 0.8 | 2.5 | / | [42] |
| Posidonia oceanica leaves | NW Mediterranean | 58 | 1 f | 0.9 | 1.3 | / | [42] |
| Posidonia oceanica shoots | NW Mediterranean | 58 | 1 f | 0.7 | 1.2 | / | [42] |
| Phyllophora crispa | NW Mediterranean | 26 | 1 M | 0.9 | 3.4 | / | [39] |
| Posidonia oceanica | NW Mediterranean | 33 | 5 a,b,f,p,s | 0.9 | 2.0 | 0.3 | [43] |
| Coralligenous reefs | NW Mediterranean | 55 | 6 a,b,c,f,p,s | 0.8 | 2.1 | 0.3 | [44] |
| Coralligenous reefs | Mediterranean | 786 t | 7 a,b,c,f,m,p,s | 0.9 | 2.6 | 0.2 | [9] |
| Coralligenous reefs canopy | E Mediterranean | 209 | 5 f,M,OS,ST | 0.3 | 1.8 | / | [45] |
| Coralligenous reefs frame | E Mediterranean | 209 | 5 f,M,OS,ST | 0.5 | 1.4 | / | [45] |
| Coralligenous reefs canopy | E Mediterranean | 136 | 1 b | 0.29 | 5.95 | / | [45] |
| Coralligenous reefs frame | E Mediterranean | 136 | 1 b | 0.51 | 13.5 | / | [45] |
| Cystoseira zosteroides | NW Mediterranean | 78 | 6 a,b,c,f,p,s | 0.8 | 2.0 | 0.3 | [46] |
| Coral reef | SW Indian Ocean | 457 | 5 a,c,f,m,s | 0.9 | 2.0 | 0.3 | [47] |
| Coral reef turf algae | W Indian Ocean | 48 u | 2 p,m | 1.0 | 1.0 | 0.5 | [48] |
| Coldwater coral reef | N Atlantic Ocean | 213 | 7 a,b,c,f,m,p,s | 1.0 | 2.7 | 0.2 | [49] |
| Coldwater coral reef | N Atlantic Ocean | 77 | 4 a,b,c,s | 0.8 | 1.6 | 0.4 | [50] |
| Mangrove forest | Caribbean Sea | 54 | 6 a,b,c,m,p,s | 0.8 | 1.9 | 0.3 | [51] |
| Kelp forest | NE Pacific Ocean | 79 v | 6 a,b,c,m,p,s | 1.0 | 2.4 | 0.2 | [52] |
| Antarctic hard bottom | Weddell Sea | 608 x | 6 a,b,c,f,m,s | 0.9 | 2.2 | 0.3 | [53] |
| Halimeda bioherm | Coral Sea | 474 x | 5 a,b,c,m,s | 0.7 | 1.6 | 0.4 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Töpfel, A.; Steinhoff, M.; Wild, C. Assessment of Non-Sessile Invertebrates Associated with Mats of the Red Alga Phyllophora crispa at Giglio Island, Mediterranean Sea. Diversity 2025, 17, 728. https://doi.org/10.3390/d17100728
Töpfel A, Steinhoff M, Wild C. Assessment of Non-Sessile Invertebrates Associated with Mats of the Red Alga Phyllophora crispa at Giglio Island, Mediterranean Sea. Diversity. 2025; 17(10):728. https://doi.org/10.3390/d17100728
Chicago/Turabian StyleTöpfel, Alexander, Melissa Steinhoff, and Christian Wild. 2025. "Assessment of Non-Sessile Invertebrates Associated with Mats of the Red Alga Phyllophora crispa at Giglio Island, Mediterranean Sea" Diversity 17, no. 10: 728. https://doi.org/10.3390/d17100728
APA StyleTöpfel, A., Steinhoff, M., & Wild, C. (2025). Assessment of Non-Sessile Invertebrates Associated with Mats of the Red Alga Phyllophora crispa at Giglio Island, Mediterranean Sea. Diversity, 17(10), 728. https://doi.org/10.3390/d17100728








