Metabolites Fingerprinting Variations and Chemotaxonomy of Related South African Hypoxis Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Collection and Comminution
2.2. Plant Extraction
2.3. RP-UPLC-QTOF-MS Analysis
2.4. GC-MS Analysis
2.5. HPTLC Analysis
2.6. Chemometric Data Analysis
3. Results
3.1. Chemical Fingerprinting of Wild Hypoxis by HPTLC
3.2. Chemical Fingerprinting of Wild Hypoxis by RP-UPLC-QTOF-MS Using a Targeted Approach
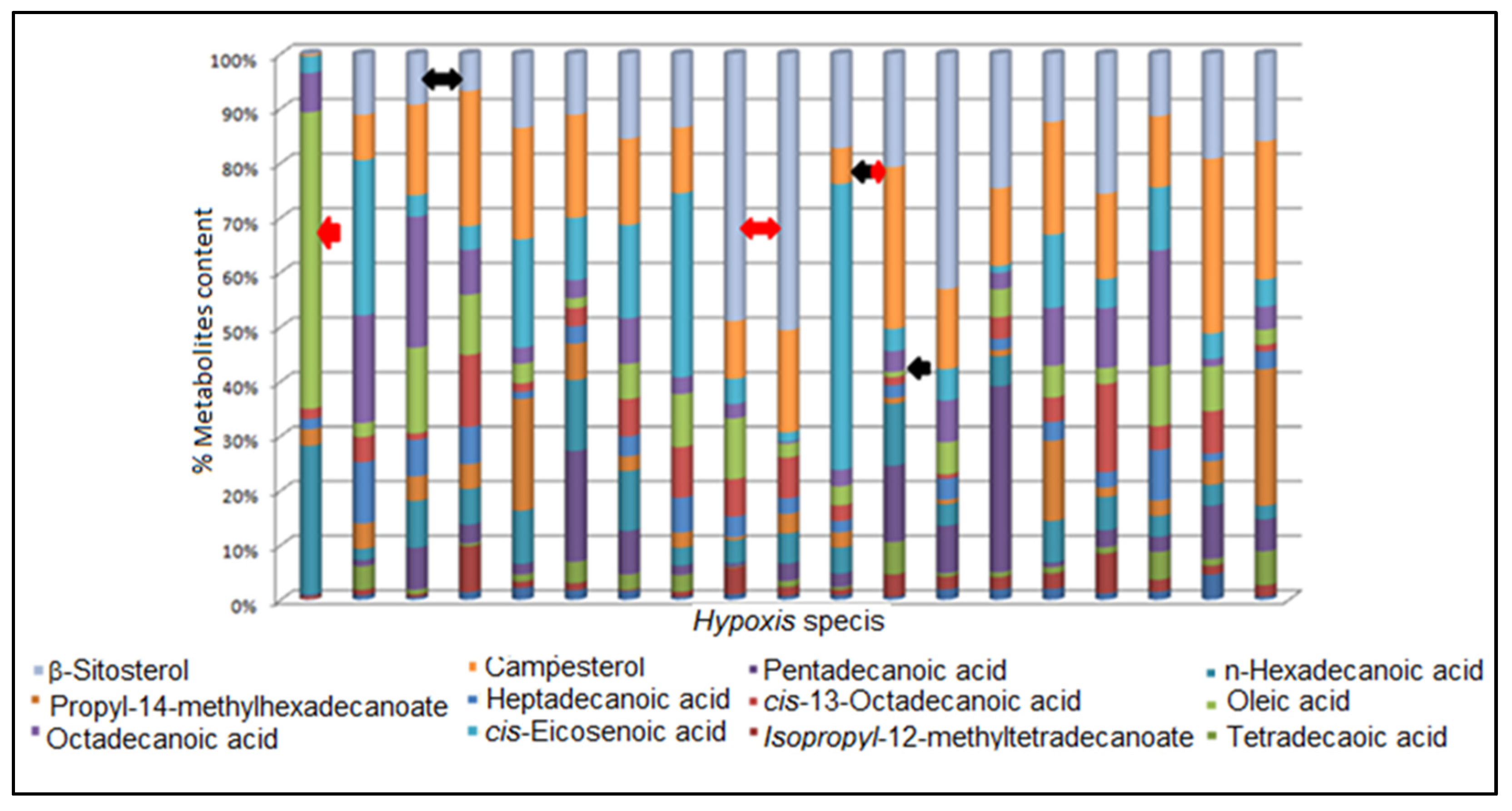
3.3. Chemical Fingerprinting of Wild Hypoxis by GC-MS Using a Targeted Approach
| Peaks | Retention Time (min) | Mass to Charge Ratio [M]+ (m/z) | Mass Fragmentation Pattern | Compound |
|---|---|---|---|---|
| A | 11.29 | 224 | 224, 111, 97, 69, 57 | 2-hexadecanol |
| B | 13.33 | 248 | 248, 242, 225, 185, 143, 129, 102 | isopropyl-propyl 12-methyltetradecanoate |
| C | 14.68 | 288 | 199, 185, 171, 166, 143, 129, 115 | tetradecanoic acid |
| D | 15.32 | 242 | 242, 213, 199, 157, 143, 129, 97 | pentadecanoic acid |
| E | 16.50 | 256 | 239, 227, 213, 199, 185, 171, 157 | n-hexadecanoic (palmitic) acid |
| F | 17.11 | 312 | 283, 270, 253, 241, 227, 205, 199 | propyl-14-methylhexadecanoate |
| G | 17.34 | 270 | 241, 227, 213, 171, 143, 129, 115 | heptadecanoic acid |
| H | 17.71 | 282 | 264, 222, 180, 137, 125, 111, 97 | cis-13-octadecanoic oleic acid |
| I | 18.16 | 264 | 246, 235, 222, 207, 193, 180, 165 | oleic acid |
| J | 18.33 | 284 | 284, 255, 241, 227, 199, 185, 171 | octadecanoic acid |
| K | 18.72 | 310 | 259, 241, 121, 199, 173, 157, 147 | cis-13-eicosenoic acid |
| L | 20.21 | 268 | 341, 313, 272, 241, 199, 147, 129 | hexanedioc acid-bis(2-ethylhexyl) ester |
| M | 21.20 | 341 | 380, 351, 323, 295, 267, 239, 211 | heptacosane |
| N | 22.64 | 365 | 341, 322, 281, 264, 250, 207, 183 | oleic acid-3-(octadecyloxy) propyl ester |
| O | 24.05 | 490 | 462, 418, 392, 348, 320, 292, 267 | 17-pentatriacontene |
| P | 24.79 | 380 | 337, 309, 281, 253, 225, 197, 169 | heptacosane |
| Q | 25.45 | 400 | 367, 253, 213, 185, 159, 145, 121 | ethylisoallocholate |
| R | 25.65 | 484 | 424, 365, 330, 304, 287, 244, 227 | 7,8-epoxylanostan-11-ol |
| S | 26.65 | 450 | 421, 393, 365, 337, 309, 281, 253 | dotriacontane |
| T | 26.95 | 400 | 382, 367, 340, 315, 289, 273, 255 | campesterol |
| U | 27.77 | 364 | 337, 295, 281, 251, 225, 197, 183 | octadecane-3-ethyl-5-(2-ethylbutyl) |
| V | 28.02 | 414 | 396, 354, 329, 303, 255, 231, 213 | β-sitosterol |
| W | 28.49 | 490 | 462, 418, 391, 348, 320, 292, 267 | 17-pentatriacontene |
| X | 29.54 | 410 | 395, 368, 296, 241, 229, 187, 174 | stigmasta-3,5-dien-7-one |
| Y | 29.91 | 484 | 424, 365, 330, 304, 287, 244, 227 | 8-epoxylanostan-11-ol |
| Z | 31.73 | 490 | 460, 432, 390, 362, 334, 306, 285 | pentatricontanol |
| AA | 32.95 | 428 | 413, 400, 328, 285, 269, 227, 213 | citrost-7-en-3-ol |
3.4. Investigation of Phytochemical Variation by RP-UPLC-QTOF-MS Using a Targeted Approach
3.5. Qualitative Variation in the Methanol and Dichloromethane Extracts of the Hypoxis Samples
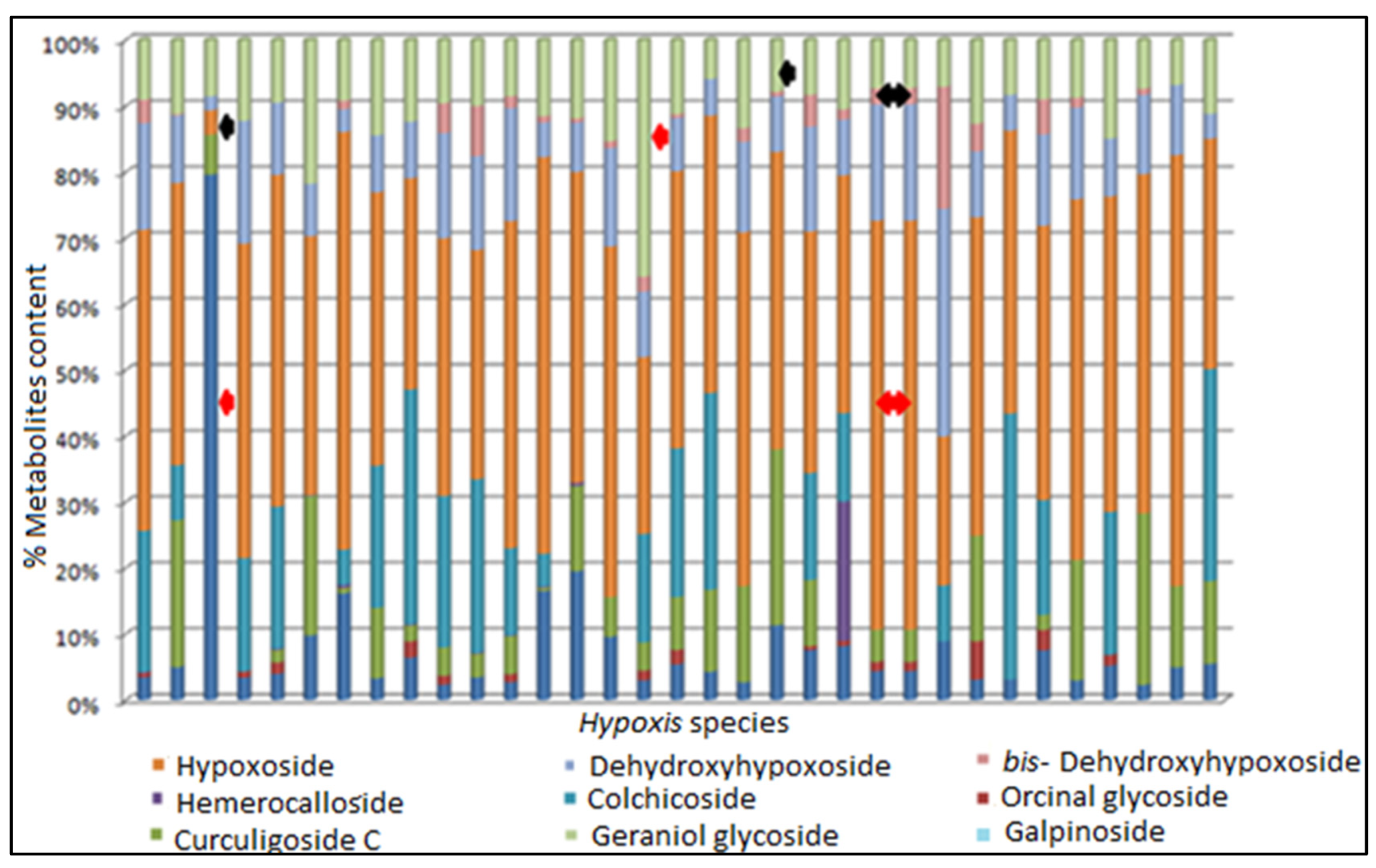
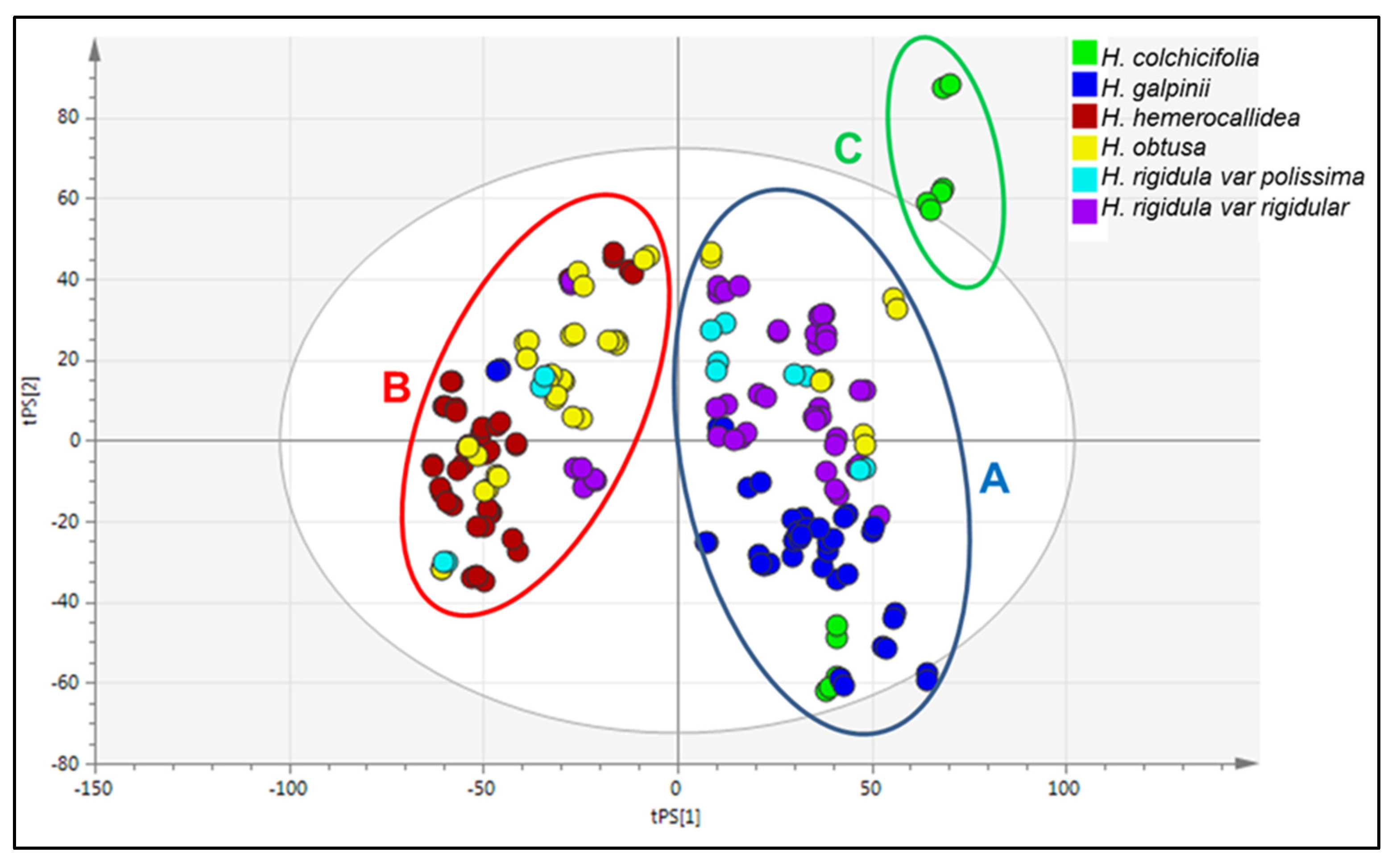
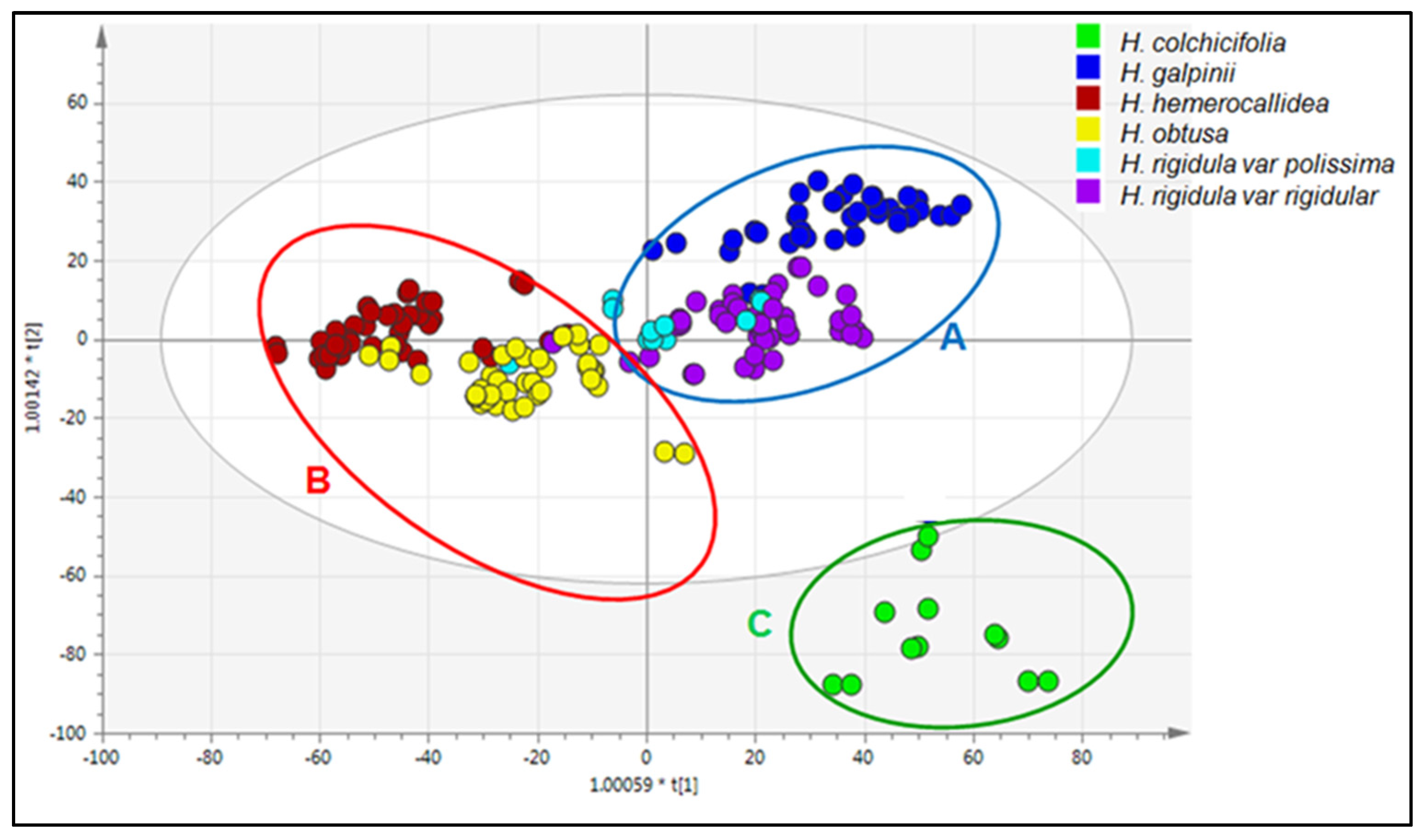
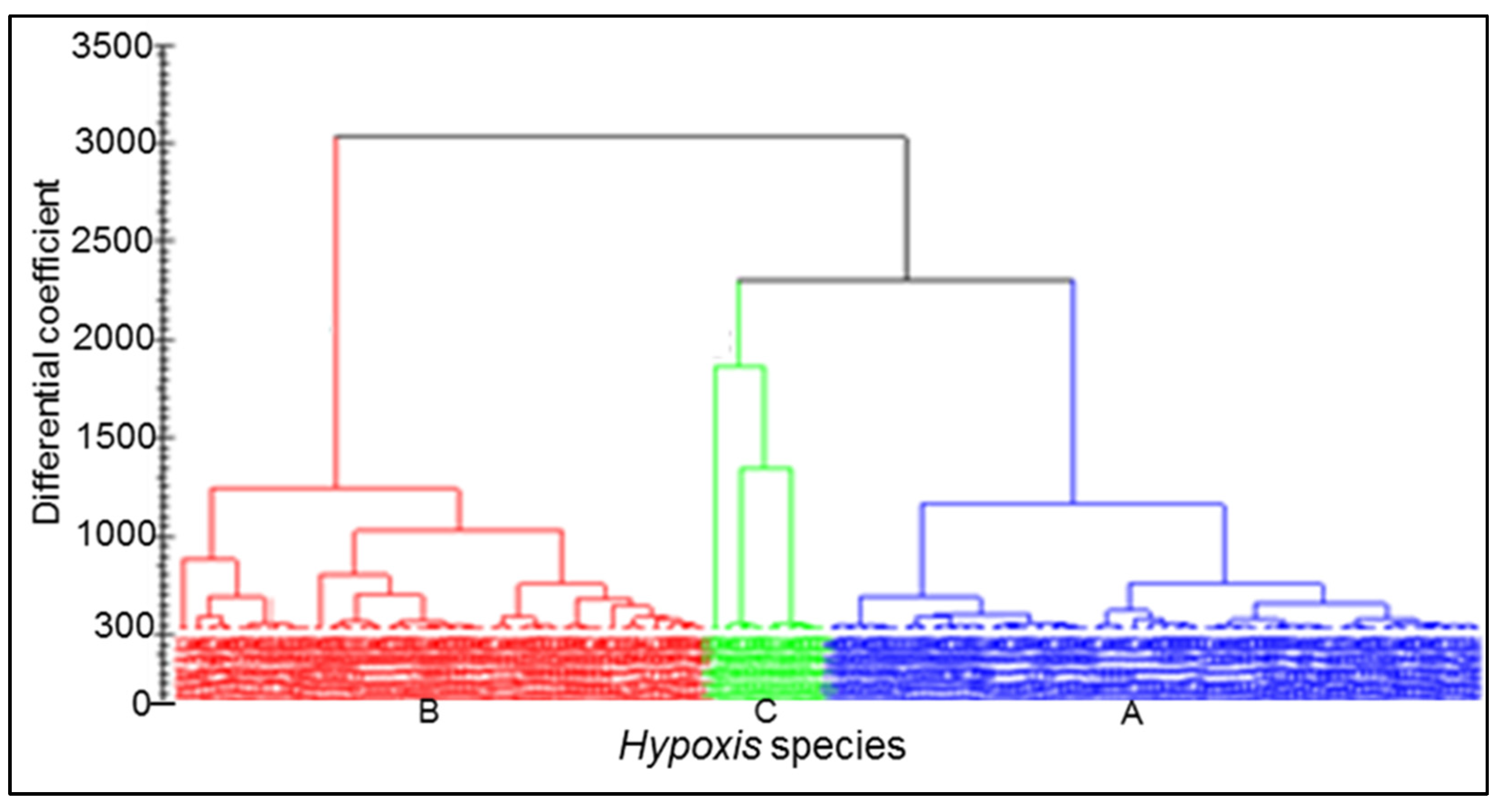
3.6. Chemotype Identification and Variations as Determined by RP-UPLC-QTOF-MS and Metabolomic Analysis
4. Discussions
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCA | Principal Component Analysis |
| OPLS-DA | Orthogonal to Partial Least Square-Discriminant Analysis |
| LC | Liquid Chromatography |
| GC | Gass Chromatography |
| NMR | Nuclear Magnetic Resonance |
| MVD | Multivariate Data Analysis |
| CHCl3 | Chloroform |
| MeOH | Methanol |
| RP-UPLC-QTOF-PDA/MS | Reverse Phase Ultra Performance |
| QTOF | Quadrupole Time of Flight |
| PDA | Photodiode Array Detector |
| MS | Mass Spectrometry |
| HPTLC | High Performance Thin Layer Chromatography |
| GC-MS. | Gas-Chromatography -Mass Spectrometry |
| MSD | Mass Selective Detector |
| EtOAc | Ethyl Acetate |
| H2SO4 | Sulphuric Acid |
| UV | Ultra-Violet |
| AUC | Area Under the Curve |
References
- Hu, L.F.; Li, S.P.; Cao, H.; Liu, J.J.; Gao, J.L.; Yang, F.Q.; Wang, Y.T. GC-MS fingerprint of Pogostemon cablin in China. J. Pharm. Biomed. Anal. 2006, 42, 200–206. [Google Scholar] [PubMed]
- Jayanthy, A.; Prakash, K.U.; Remashree, A.B. Seasonal and geographical variations in cellular characters and chemical contents in Desmodium gangeticum (L.) DC: An Ayurvedic medicinal plant. Int. J. Herb. Med. 2013, 1, 34–37. [Google Scholar]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479–3485. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Pinto, M.E.S.; Holloway, D.E.; Bramley, P.M. Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2000, 24, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, Y.; Jefthas, E.; Goliath, J.; Smith, M.; Langenhoven, P.; Acquaye, D.; Juliani, R.; Letchamo, W.; Renaud, E.; Zimba, N.; et al. Quality assurance and quality control for African natural plant products. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Joshi, D.D. Herbal Drugs and Fingerprints: Evidence Based Herbal Drugs; Springer Science & Business Media: Delhi, India, 2012. [Google Scholar]
- Singh, Y. Systematics of Hypoxis (Hypoxidaceae) in South Africa. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2009. [Google Scholar]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Oksaman-Caldentey, K.-M.; Inze, D. Plant cell factories in the post genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef]
- Villas-Boas, S.G.; Moxley, J.F.; Akesson, M.; Stephanopoulos, G.; Nielsen, J. High-throughput metabolic state analysis: The missing link in integrated functional genomics of yeasts. Biochem. J. 2005, 388, 669–677. [Google Scholar] [CrossRef]
- De Vos, R.C.H.; Moco, S.; Lomen, A.; Keurrentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef]
- Griffin, J.L.; VIdal-Puig, A. Current challenges in metabolomics for diabetes research: A vital functional genomic tool or just a ploy for gaining funding? Physiol. Genom. 2008, 34, 1–5. [Google Scholar] [CrossRef][Green Version]
- Weiss, E.A. Essential Oil Crops; CAB International: New York, NY, USA, 1997. [Google Scholar][Green Version]
- Li, Y.; Hu, Z.; He, L. An approach to the development of binary chromatographic fingerprints of the total alkaloids from Caulophyllum robustum by high performance liquid chromatography/diode array detector and gas chromatography/mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 1667–1672. [Google Scholar][Green Version]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Lomen, A.; Van der Weg, G.; Van Engelen, M.C.; Bor, G.; Hoogenboom, L.A.P.; Nielen, M.W.F. An untargeted metabolomic approach to contaminant analysis: Pinpointing potential unknown compounds. Anal. Chim. Acta 2007, 584, 43–49. [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettaaneh-wold, N.; Trygg, J.; Wikström, C.; Wold, S. Multi- and Megavariate Data Analysis. Part 1: Basic Principles and Applications, 2nd ed.; Umetrics AB: Umea, Sweden, 2006. [Google Scholar]
- Van Der Kooy, F.; Verpoorte, R.; Meyer, M.J.J. Metabolomic quality control of claimed anti-malarial Artemisia afra herbal remedy and A. afra and A. annua plant extracts. S. Afr. J. Bot. 2008, 74, 186–189. [Google Scholar] [CrossRef]
- Naes, T.; Isaksson, T.; Fearn, T.; Davies, T. A User Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2007. [Google Scholar]
- Zimudzi, C. African potato (Hypoxis spp.): Diversity and comparison of the phytochemical profiles and cytotoxicity evaluation of four Zimbabwean species. J. Appl. Pharm. Sci. 2014, 4, 79–83. [Google Scholar] [CrossRef]
- Boukes, J.G.; Van de venter, M.; Oosthuizen, V. Quantitative and qualitative analysis of sterols/sterolins and hypoxoside contents of three Hypoxis (African potato) spp. Afr. J. Biotechnol. 2008, 7, 1624–1629. [Google Scholar] [CrossRef]
- Sathekge, N.R. Comparison of Secondary Metabolites Content and Anti-Microbial Activity of Four Hypoxis Species Used in Traditional Medicine. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2010. [Google Scholar]
- Germishuizen, G.; Meyer, N.L. (Eds.) Plants of Southern Africa: An Annotated Checklist; Strelitzia 14; National Botanical Institute: Pretoria, South Africa, 2003. [Google Scholar]
- BoukeS, G.J.; Van de Venter, M. Cytotoxicity and mechanism(s) of action of Hypoxis spp. (African potato) against HeLa, HT-29 and MC-7 cancer cell lines. J. Med. Plant Res. 2011, 5, 2766–2774. [Google Scholar]
- Van Wyk, B.-E.; Van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa; Briza Publications: Pretoria, South Africa, 1997. [Google Scholar]
- Singh, Y. Hypoxis (Hypoxidaceae) in Africa: List of species and infraspecific names. Bothalia 2006, 36, 13–23. [Google Scholar] [CrossRef][Green Version]
- Da-costa-Rocha, I.; Edwards, S.; Lawrence, M.J.; Cable, C.; Heinrich, M. Quality and safety of herbal products: Part 1-new legislation and production. Pharm. J. 2012, 288, 658. [Google Scholar][Green Version]
- Bassey, K.; Viljoen, A.; Combrinck, S.; Choi, Y.H. New phytochemicals from the corms of medicinally important South African Hypoxis species. Phytochem. Lett. 2014, 10, lxix–lxxv. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. PLS regression on wavelet compressed NIR spectra. Chemom. Intell. Lab. Syst. 2001, 42, 209–220. [Google Scholar] [CrossRef]
- Sandasi, M.; Vermaak, I.; Chen, W.; Viljoen, A.M. Hyperspectral imaging and chemometric modelling of Echinacea—A Novel Approach in the quality control of herbal medicines. Molecules 2014, 19, 13104–13121. [Google Scholar] [CrossRef] [PubMed]
- Laporta, O.; Perez-Fons, L.; Mallavia, R.; Caturla, N. Isolation, characterization and antioxidant capacity assessment of the bioactive compounds derived from Hypoxis rooperi corm extract (African potato). Food Chem. 2007, 101, 1425–1437. [Google Scholar] [CrossRef]
- Fu, D.X.; Lei, G.Q.; Cheng, X.W.; Chen, J.K.; Zhou, T.S. Cureuligoside C, a New Phenolic Glucoside from Rhizomes of Curculigo orchioides. Acta Bot. Sin. Engl. Ed. 2004, 46, 621–624. [Google Scholar]
- Marini-Bettolo, G.B.; Nicoletti, P.M.; Galeffi, C.; Messana, I. Hypoxoside a new glycoside with uncommon structure from Hypoxis obtusa Busch. Tetrahedron 1982, 38, 1683–1687. [Google Scholar]
- Bredenkamp, M.W.; Drewes, S.E.; Wentler, G.L. A geraniol glycoside from Hypoxis acuminata. Phytochemistry 1989, 28, 263–265. [Google Scholar] [CrossRef]
- Moghadasian, M.H. Pharmacological properties of plant sterols in vivo and in vitro observations. Life Sci. 2000, 67, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Grata, E.; Boccard, J.; Guillarme, D.; Glauser, G.; Carrupt, P.A.; Farme, E.E.; Wolfender, J.L.; Rudas, S. UPLC-TOF-MS for plant metabolomics: A sequential approach for wound marker analysis of Arabidopsis thaliana. J. Chromatogr. B 2008, 871, 261–270. [Google Scholar] [CrossRef] [PubMed]
- AbidI, S.L. Chromatographic analysis of plant sterols in foods and vegetables oils. J. Chromatogr. A 2001, 935, 173–201. [Google Scholar] [CrossRef]
- Nair, V.D.P.; Kanfer, I. Sterols and sterolins in Hypoxis hemerocallidea (African Potato). S. Afr. J. Sci. 2008, 104, 322–324. [Google Scholar]
- Jackson, J.E. A User’s Guide to Principal Components; John Wiley: New York, NY, USA, 1991. [Google Scholar]
- Matseke, B.; Poka, M.; Demana, P.; Bassey, K. Unravelling the Potentials of Managing Metabolic Diabetes and Related Oxidative Stresses with Extracts from Five South African Hypoxis Species. Stresses 2025, 5, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, J.; Guo, S.; Wang, Z.; Wang, S.; Yu, X.; He, X.; Zhang, A.; Qiu, S. Integrated and proteomics revealed mangiferin alleviating oxidative stress and mitochondrial dysfunction of HT22 cells via regulating the abnormal metabolic pathways. Food Biosci. 2025, 66, 1061380. [Google Scholar] [CrossRef]
- Altarjami, L.R. Anticancer and antioxidant activities of polyphenolic pomegranate peel extracts obtained by a novel hybrid ultrasound-microwave method: In vitro and in vivo studies in albino mice with HeLa, colon, and HepG2 cancerous cell lines. Pak. Vet. J. 2025, 45, 723–734. [Google Scholar] [CrossRef]
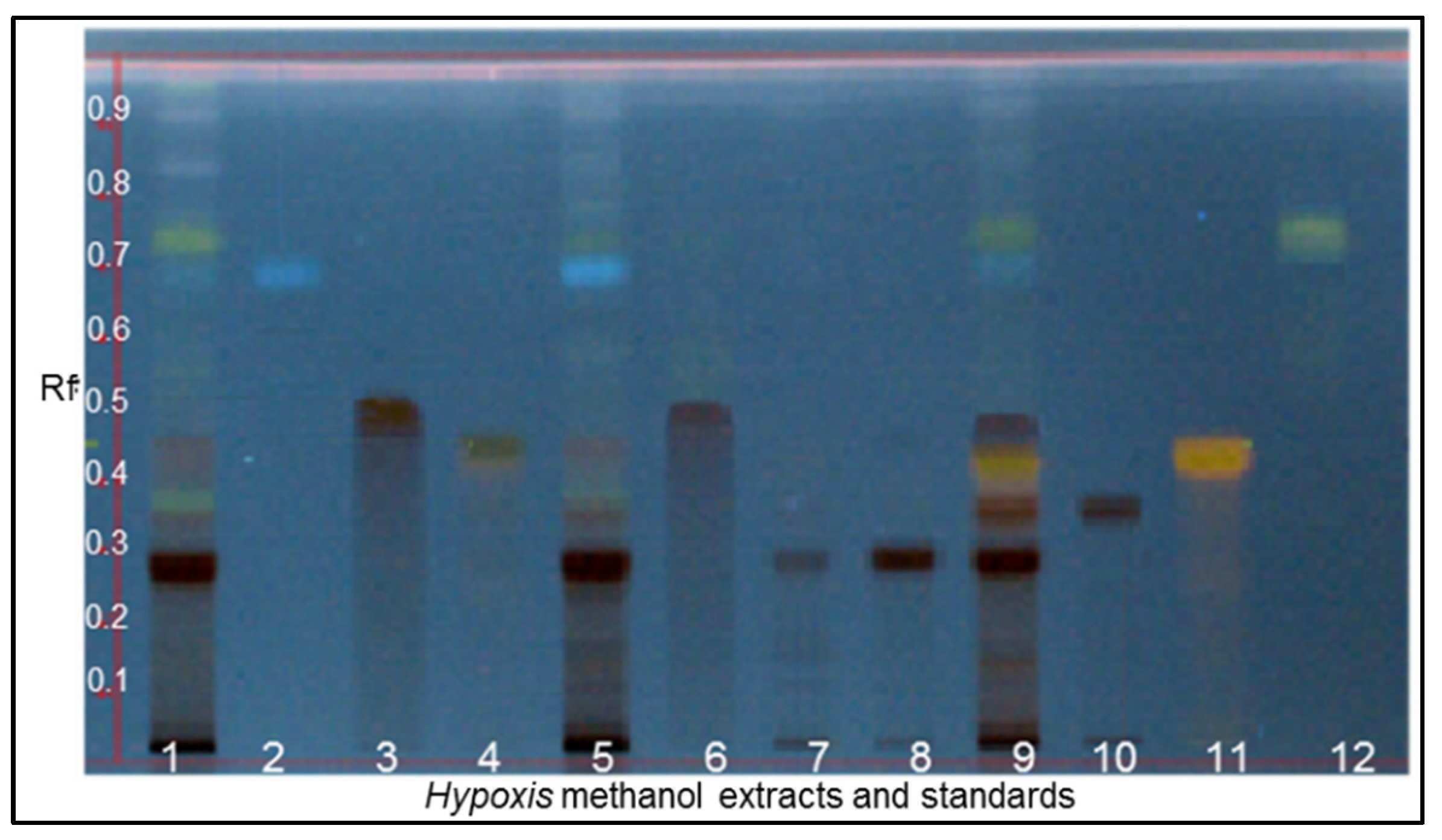
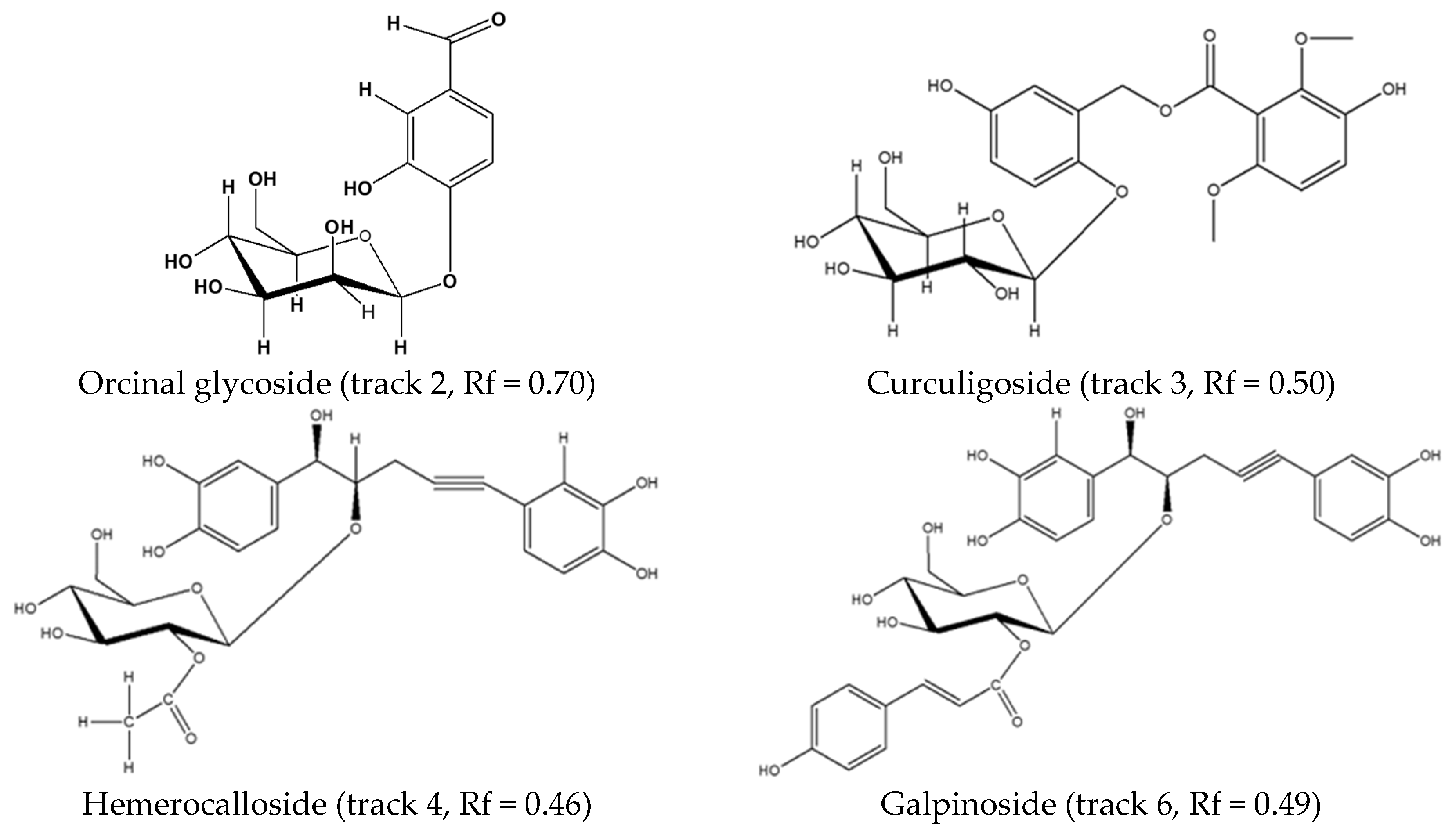
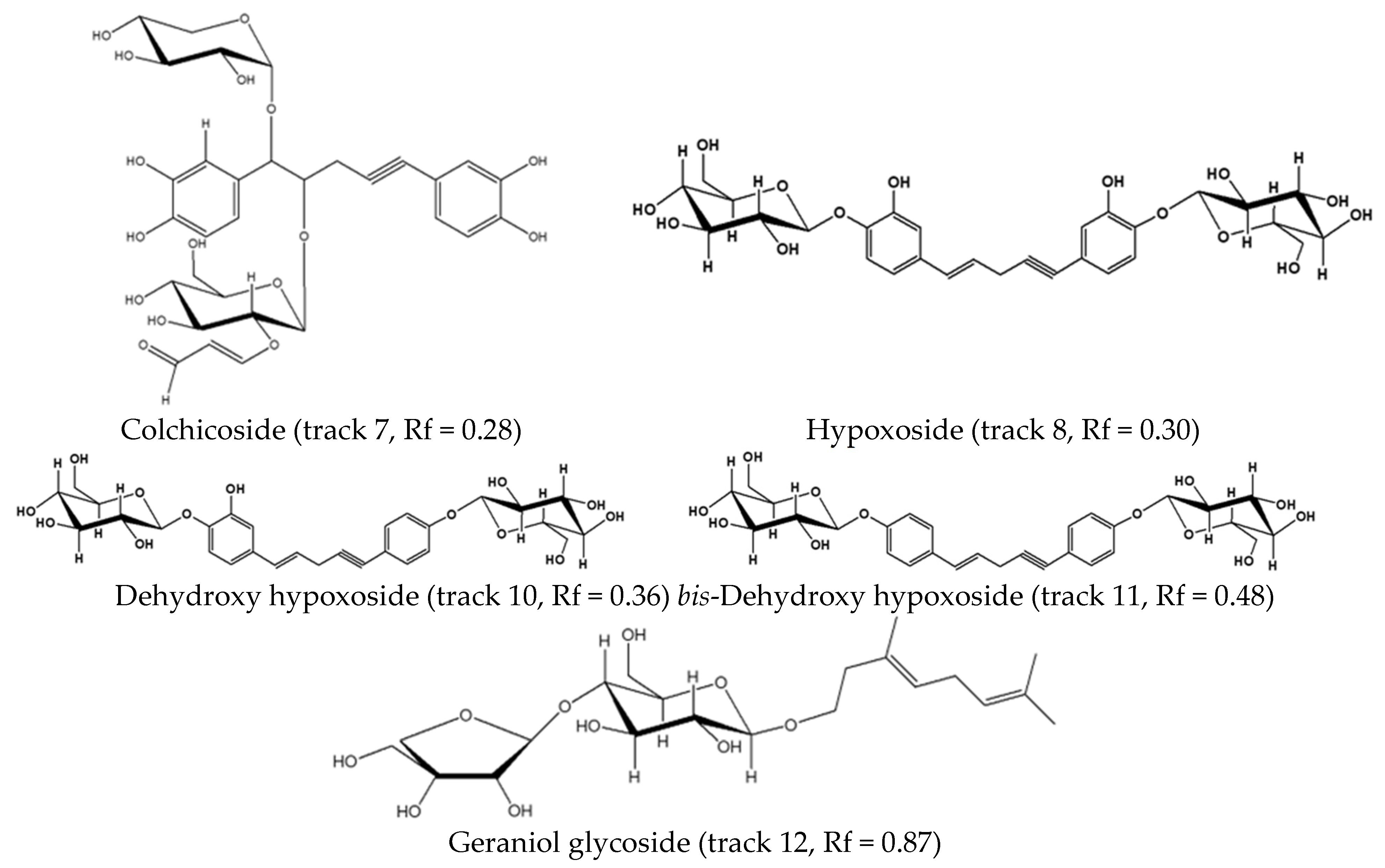
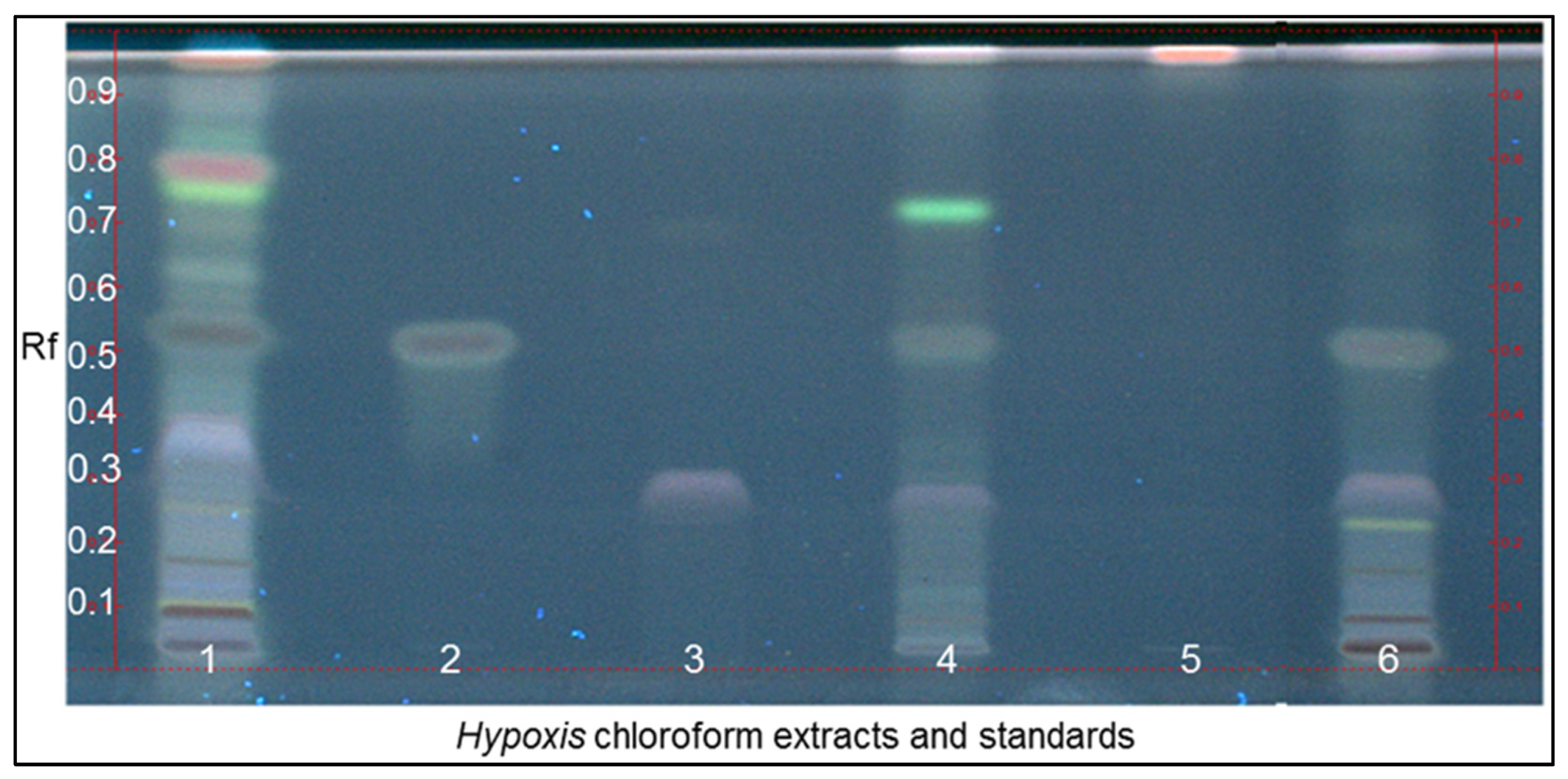
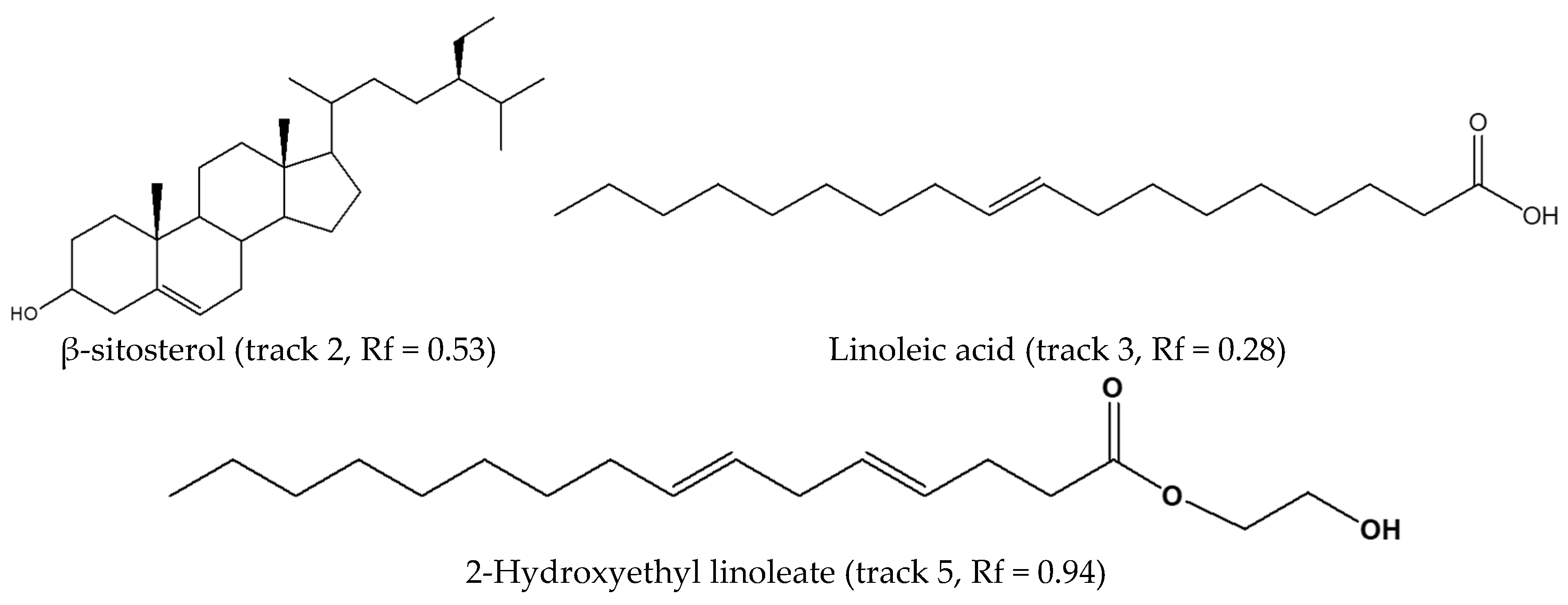
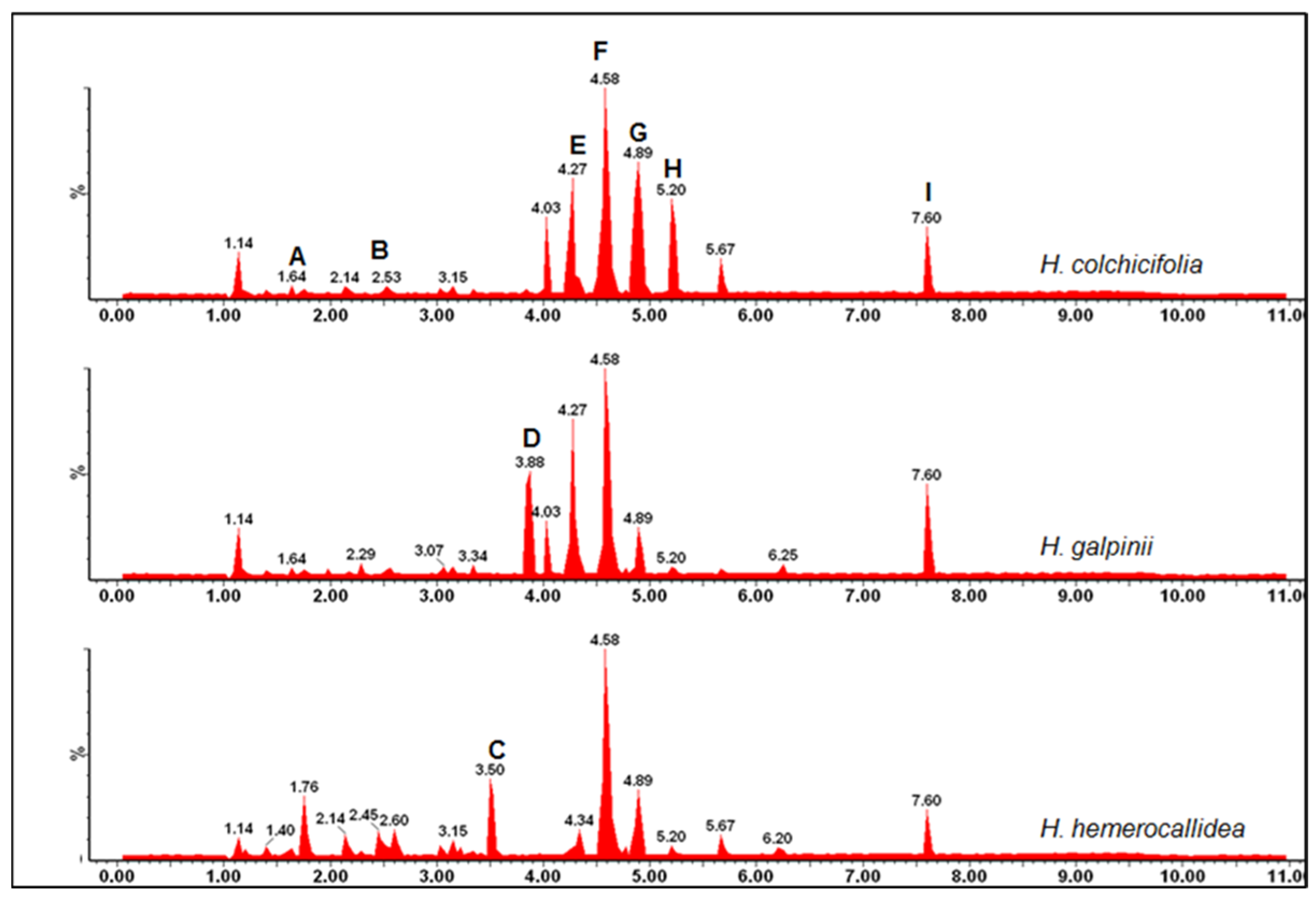
| Hypoxis Species | Province | Town | Locality | Voucher Specimen No. | No. of Specimen | Altitude (mASL) |
|---|---|---|---|---|---|---|
| H. argentea | Northern Cape | Kimberley | L3 | HA 007-HA 009 | 3 | 1555 |
| H. colchicifolia | Eastern Cape | Port Edward | L22 | HC 070-HC 072 | 3 | 415 |
| H. colchicifolia | KwaZulu-Natal | Midmar | L24 | HC 076-HC 078 | 3 | 1036 |
| H. galpinii | Mpumalanga | Wakkerstroom | L8 | HG 024-HG 026 | 3 | 1791 |
| H. galpinii | KwaZulu-Natal | Groenvlei | L9 | HG 027-HG 029 | 3 | 1914 |
| H. galpinii | KwaZulu-Natal | Wagendrift | L17 | HG 054-HG 057 | 4 | 1497 |
| H. galpinii | KwaZulu-Natal | Ukahlamba | L18 | HG 058-HG 060 | 3 | 1662 |
| H. galpinii | KwaZulu-Natal | Impendle | L26 | HG 082-HG 084 | 3 | 1599 |
| H. galpinii | KwaZulu-Natal | Ukahlamba | L29 | HG 091-HG 093 | 3 | 1714 |
| H. hemerocallidea | Northern Cape | Boshof | L2 | HH 004-HH 006 | 3 | 1555 |
| H. hemerocallidea | KwaZulu-Natal | Mount Frere | L14 | HH 042-HH 044 | 3 | 1100 |
| H. hemerocallidea | KwaZulu-Natal | Kokstad | L20 | HH 064-HH 066 | 3 | 1340 |
| H. hemerocallidea | Eastern Cape | Port Edward | L23 | HH 073-HH 075 | 3 | 415 |
| H. hemerocallidea | Gauteng | Walter Sisulu Garden | L31 | HH 097-HH099 | 3 | 1550 |
| H. hemerocallidea | Gauteng | Tshwane Market | L32 | HH 100-HH 102 | 3 | 1280 |
| H. hemerocallidea | Gauteng | Marabastad Market | L33 | HH 103-HH 105 | 3 | 1280 |
| H. multiceps | Mpumalanga | Breyten | L7 | HM 021-HM 023 | 3 | 1784 |
| H. obtusa | Mpumalanga | Breyten | L6 | HO 018-HO 020 | 3 | 1785 |
| H. obtusa | KwaZulu-Natal | Chelmsford | L11 | HO 033-HO 035 | 3 | 1284 |
| H. obtusa | Gauteng | Randfontein | L13 | HO 039-HO 041 | 3 | 1709 |
| H. obtusa | KwaZulu-Natal | Mount Frere | L15 | HO 045-HO 050 | 6 | 1102 |
| H. obtusa | KwaZulu-Natal | Kokstad | L19 | HO 061-HO 063 | 3 | 1451 |
| H. obtusa | KwaZulu-Natal | Ukahlamba | L28 | HO 088-HO 090 | 3 | 1637 |
| H. rigidula var. polissisima | KwaZulu-Natal | Dundee | L12 | HRP 036-HRP 037 | 3 | 1284 |
| H. rigidula var. polissisima | KwaZulu-Natal | Wagendrift | L16 | HRP 051-HRP 053 | 3 | 1345 |
| H. rigidula var. rigidula | Kwazulu-Natal | Boshof | L1 | HRP 001-HRP003 | 3 | 1556 |
| H. rigidula var. rigidula | Northern Cape | Boshof | L4 | HRR 010-HRR 012 | 3 | 1562 |
| H. rigidula var. rigidula | Mpumalanga | Breyten | L5 | HRR 013-HRR 017 | 5 | 1780 |
| H. rigidula var. rigidula | KwaZulu-Natal | Groenvlei | L10 | HRR030-RHH 032 | 3 | 1915 |
| H. rigidula var. rigidula | Eastern Cape | Port Edward | L21 | HRR 67-HRR 069 | 3 | 418 |
| H. rigidula var. rigidula | KwaZulu-Natal | Impendle | L25 | HRR 79-HRR 081 | 3 | 1598 |
| H. rigidula var. rigidula | KwaZulu-Natal | Ukahlamba | L27 | HRR 085-HRR 087 | 3 | 1455 |
| H. rigidula var. rigidula | Free State | Sterkfontein | L30 | HRR 94-HRR 096 | 3 | 1694 |
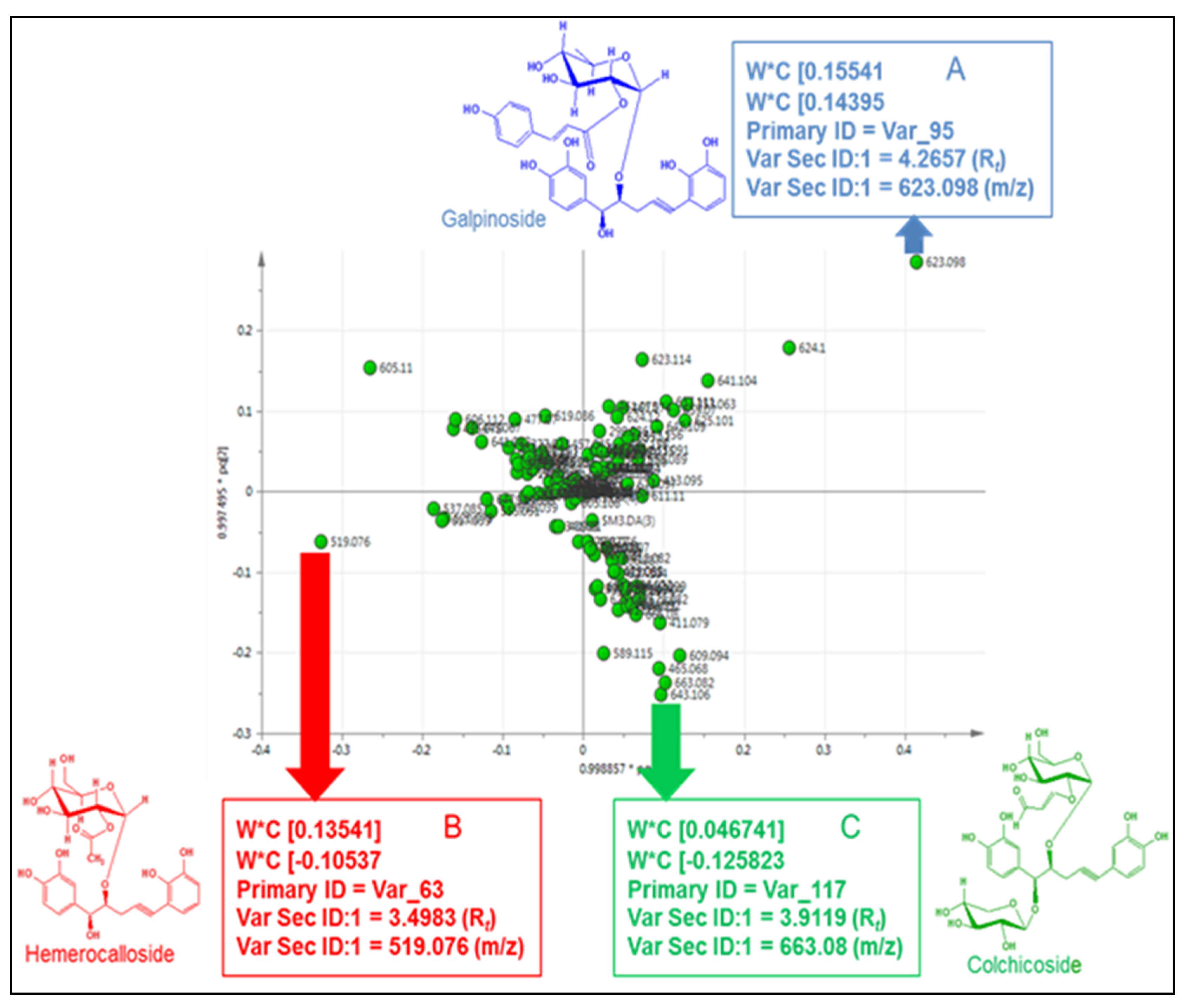
| Peaks | Retention Time (min) | Mass to Charge Ratio [M − 1]− (m/z) | UV (λmax) (nm) | Hypoxis Species Detected | Mass Fragmentation Pattern [M − H]− | Fragment Identification | Compound |
|---|---|---|---|---|---|---|---|
| A | 1.64 | 299 | 279 | H. colchicifolia | 299, 163, 137 | [M − H]−, [ M − C7H5O3]−, [M − C6H11O5]− | orcinal glycoside |
| B | 2.48 | 517 | 210 | H. colchicifolia | 517, 481, 330, 197, 162 | [M − Cl2]−, [M − H]−, [M − C8H9O3]−, [M − C13H17O7]−, [M − C16H15O7]− | curculigoside C [32] |
| C | 3.50 | 519 | 255 | H. hemerocallidea and H. obtusa | 519, 461, 315, 299, 221, 205 163 | [M − H]−, [M − C2H3O2]−, [M − C8H13O6]−, [M − C8H13O7]−, [M − C17H15O5] −, [M − C17H15O6]−, [M − C17H15O6 − C2H3O − 2H]− | hemerocalloside |
| D | 3.88 | 663 | 278 | Predominantly H. colchicifolia | 663, 627, 465, 315, 217 | [M − H]−, [M − C2H2O2 − 5H]−, [M − C9H13O6 − H2O]−, [M − C9H13O6 − C8H15O2]−, [M − C22H23O10]−. | colchicoside |
| E | 4.27 | 623 | 256, (299) | H. galpinii and H. rigidula var. rigidula | 623, 461, 325, 299, 163, 160 | [M − H]−, (M − C9H7O3]−, [M − C17H15O5]−, [C15H17O8]−, [M − C23H25O10]−, [M − C17H15O8 − C9H7O3]− | galpinoside |
| F | 4.58 | 605 | 257 | All | 605, 443, 281 | [M − H]−, [M − C6H11O5]−, [M − 2C6H11O5]−, | hypoxoside [33] |
| G | 4.89 | 589 | 258 | Most | 589, 427, 256, 163 | [M − H]−, [M − C6H11O5] −, [M− 2C6H11O5]−, [M − C23H23O8 | dehydroxyhypoxoside [31] |
| H | 5.20 | 609 | 259 | Most | 609, 411, 248, 163 | [M − Cl]−, [M − C6H11O5]−, [M − 2C6H11O5]−, [M − C23H23O7]− | bis-dehydroxyhypoxoside [31] |
| I | 7.60 | 447 | 200 | All | 483, 447, 295, 163, 155 | [M − Cl2], [M − 1]−, [M − C10H19O], [M − C10H19O− C11H19O9–C5H9O4]−, [M − C5H9O4]− | geraniol glycoside [34] |
| Hypoxis Species | Secondary Metabolites Identified in the MeOH Extract of Hypoxis Samples | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| H. hemerocallidea | √ | X | X | √ | X | √ | √ |
| H. obtusa | √ | X | X | √ | √ | √ | √ |
| H. rigidula var. rigidula | X | X | √ | √ | √ | X | √ |
| H. rigidula var. polissisima | X | X | √ | √ | √ | √ | √ |
| H. galpinii | X | √ | X | √ | √ | X | √ |
| H. colchicifolia | X | √ | √ | √ | √ | √ | √ |
| H. multiceps | X | X | X | √ | √ | X | √ |
| H. argentea | X | X | √ | √ | √ | √ | √ |
| Hypoxis Species | Province | Town | Locality Id | Voucher Specimen No | Chemotype |
|---|---|---|---|---|---|
| H. hemerocallidea | Gauteng | Westcliff | L23 | HH 073 HH 074 HH 075 | B B B |
| Gauteng | Walter Sisulu | L31 | HH 097 HH 098 HH 099 | B B B | |
| Gauteng | Tshwane market | L32 | HH 100 HH 101 HH 102 | B B B | |
| Gauteng | Marabastad Market | L33 | HH 103 HH 104 HH 105 | B B B | |
| Eastern Cape | Mount Frere | L14 | HH 042 HH 043 HH 044 | B B B | |
| KwaZulu-Natal | Kokstad | L20 | HH 064 HH 065 HH 066 | B B B | |
| Northern Cape | Boshof | L2 | HH 004 HH 005 HH 006 | B B B | |
| H. obtusa | Gauteng | Randfontein | L13 | HO 039 HO 040 HO 041 | B B B |
| KwaZulu-Natal | Chelmsford | L11 | HO 033 HO 034 HO 035 | B B B | |
| Eastern Cape | Mount Frere | L15 | HO 045 HO 046 HO 047 HO 048 HO 049 HO 050 | B B B B B B | |
| KwaZulu-Natal | Kokstad | L19 | HO 061 HO 062 HO 063 | B B B | |
| KwaZulu-Natal | Ukahlamba | L28 | HO 088 HO 089 HO 090 | B B B | |
| Mpumalanga | Breyten | L6 | HO 018 HO 019 HO 020 | B B B | |
| H. rigidula var. rigidula | Eastern Cape | Port Edward | L21 | HRR 067 HRR 068 HRR 069 | A A A |
| Free State | Sterkfontein | L30 | HRR 094 HRR 095 HRR 096 | A A A | |
| KwaZulu-Natal | Groenvlei | L10 | HRR 030 HRR 031 HRR 032 | A A A | |
| KwaZulu-Natal | Impendle | L25 | HRR 079 HRR 080 HRR 081 | B B B | |
| KwaZulu-Natal | Ukahlamba | L27 | HRR 85 HRR 86 HRR 87 | B B B | |
| Mpumalanga | Breyten | L5 | HRR 013 HRR 014 HRR 015 HRR016 HRR 017 | A A A A A | |
| Northern Cape | Boshof | L4 | HRR 010 HRR011 HRR 012 | A A A | |
| H. rigidula var. polissisima | KwaZulu-Natal | Boshof | L1 | HRP 001 HRP 002 HRP 003 | A A A |
| KwaZulu-Natal | Dundee | L12 | HRP 036 HRP 037 HRP 038 | A A A | |
| KwaZulu-Natal | Wagendrift | L16 | HRP 051 HRP 052 HRP 053 | B A B | |
| H. galpinii | KwaZulu-Natal | Groenvlei | L9 | HG 027 HG 028 HG 029 | A A A |
| KwaZulu-Natal | Wagendrift | L17 | HG 054 HG 055 HG 056 HG 057 | A A A A | |
| KwaZulu-Natal | Ukahlamba | L18 | HG 058 HG 059 HG 060 | A A A | |
| KwaZulu-Natal | Impendle | L26 | HG 082 HG 083 HG 084 | A A A | |
| KwaZulu-Natal | Ukahlamba | L29 | HG 091 HG 092 HG 093 | A A A | |
| Mpumalanga | Wakkerstroom | L8 | HG 024 HG 025 HG 026 | A A A | |
| H. colchicifolia | Eastern Cape | Port Edward | L22 | HC 070 HC 071 HC 072 | C C C |
| Eastern Cape | Port Elizabeth | L24 | HC 076 HC 077 HC 078 | C C C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassey, K. Metabolites Fingerprinting Variations and Chemotaxonomy of Related South African Hypoxis Species. Diversity 2025, 17, 729. https://doi.org/10.3390/d17100729
Bassey K. Metabolites Fingerprinting Variations and Chemotaxonomy of Related South African Hypoxis Species. Diversity. 2025; 17(10):729. https://doi.org/10.3390/d17100729
Chicago/Turabian StyleBassey, Kokoette. 2025. "Metabolites Fingerprinting Variations and Chemotaxonomy of Related South African Hypoxis Species" Diversity 17, no. 10: 729. https://doi.org/10.3390/d17100729
APA StyleBassey, K. (2025). Metabolites Fingerprinting Variations and Chemotaxonomy of Related South African Hypoxis Species. Diversity, 17(10), 729. https://doi.org/10.3390/d17100729






