Abstract
Eutrophication of water bodies significantly accelerates water quality degradation, leading to the decline of aquatic organisms. To evaluate the synergistic restoration effects of submerged macrophyte Hydrilla verticillata and filter-feeding bivalve Anodonta woodiana on hypereutrophic water, a 40-day mesocosm simulation experiment in hypereutrophic aquatic ecosystems was conducted by setting up four treatments: control group (CK), A. woodiana group (Aw), H. verticillata group (Hv), and combined H. verticillata + A. woodiana group (HA). The results indicated that the combined application of H. verticillata and A. woodiana significantly reduced total phosphorus (TP), chlorophyll a (Chl a) concentration, and turbidity in the water, with removal rates reaching 58.3%, 60.6%, and 85.4%, respectively. The introduction of A. woodiana substantially altered the algal community composition. At the end of the experiment, the average proportion of cyanobacteria in the CK and Hv groups was 55.6%, whereas in the Aw and HA groups it decreased to 36.0%. Both total phosphorus and water-soluble phosphorus contents in H. verticillata tissues were significantly lower in HA compared to Hv, indicating that the combined treatment could reduce the risk of internal phosphorus release after H. verticillata senescence. These findings collectively demonstrate that the combination of H. verticillata and A. woodiana represents an efficient and environmentally friendly ecological restoration technology of eutrophic waters.
1. Introduction
Eutrophication has become a global environmental issue, leading to algal blooms and a decline in biodiversity, which pose serious threats to drinking water safety and the health of freshwater ecosystems [1]. Current restoration techniques for eutrophic water bodies mainly include physical, chemical, and biological methods. However, physical methods are often costly and cause significant disturbance to water bodies, while chemical methods may introduce secondary pollution, disrupt ecological balance, and have limited long-term effectiveness [2,3]. In contrast, ecological restoration techniques based on biological synergies have gained widespread attention in recent years due to their high cost-effectiveness, environmental friendliness, and strong sustainability [4].
Submerged macrophytes are important primary producers in aquatic ecosystems. They can improve water quality through photosynthesis, compete with algae for nutrients, and release allelochemicals to inhibit algae growth, thereby exerting a significant influence on the structure and function of lake ecosystems [5]. Filter-feeding animals are also key factors in forming and maintaining clear water conditions in lakes. They can efficiently ingest phytoplankton, organic detritus, and other suspended particulates, significantly reducing water turbidity and increasing transparency. Additionally, their biodeposition and excretion processes contribute to and regulate the nutrient cycling of the aquatic ecosystem, indirectly promoting the growth of submerged macrophytes [6]. Numerous studies have demonstrated that submerged macrophytes, filter feeders, and other functionally relevant aquatic organisms can effectively suppress cyanobacteria and reduce nutrient concentrations in water bodies [7,8,9]. However, most of these studies are limited to examining the effect of either aquatic plants or animals in isolation on algae or nutrient removal. Although some scholars have developed ecological restoration systems combining submerged macrophytes and filter feeders and confirmed their mutual facilitation and effectiveness in treating slightly polluted water bodies [10,11,12,13], there remains a lack of research on such combined restoration strategies under hypereutrophic conditions. In such conditions, cyanobacterial blooms serve as stress factors for both submerged macrophytes and filter feeders, as cyanobacteria compete with plants for nutrients and release cyanotoxins that pose threats to aquatic organisms [14,15]. Currently, research on the effectiveness and stability of combined restoration approaches involving submerged macrophytes and filter feeders in hypereutrophic water bodies is still limited.
In this study, submerged macrophyte Hydrilla verticillata and the filter-feeding bivalve Anodonta woodiana were selected as target organisms for controlled experiments. The objective was to elucidate the synergistic mechanisms and effectiveness of the H. verticillata–A. woodiana system in restoring hypereutrophic water bodies under cyanobacterial stress. This research aims to provide essential theoretical support and practical references for the ecological management of eutrophic water bodies and cyanobacterial blooms.
2. Materials and Methods
2.1. Experimental Site
The experiment was conducted at the Poyang Lake Model Experimental Research Base (29°13′1.97″ N, 115°50′16.09″ E) in Gongqingcheng City, Jiangxi Province, located on the western side of Poyang Lake, the largest freshwater lake in China. It was carried out in a plant cultivation greenhouse measuring 180 m in length, 20 m in width, and 10 m in height, with a plexiglass roof and shading nets.
2.2. Materials
The experimental setup consisted of 100 L polyethylene barrels with dimensions of 40 cm (bottom diameter) × 55 cm (top diameter) × 58 cm (height) (Figure 1). Both water and sediments used in the experiment were collected from the Nanhu Lake inlet on the eastern side of the Poyang Lake Model Experimental Research Base. This inlet has a summer water depth ranging from approximately 1 to 5 m and currently functions as the effluent discharge zone of the Gongqingcheng City Wastewater Treatment Plant. Due to its long-term use for waterfowl farming, the water body has become severely eutrophic. The eutrophic water from the inlet was pumped and filtered through a plankton net, after which the filtered water was mixed and added to the experimental containers. Sediments were collected using a sediment grab sampler, homogenized, and cleared of debris before being transferred into the barrels for later use.
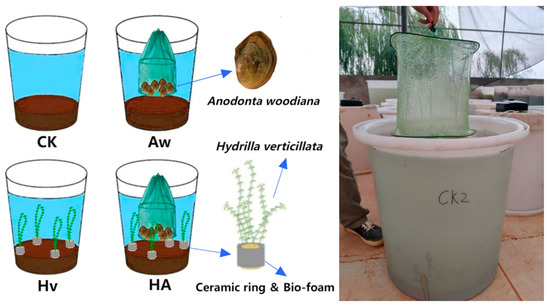
Figure 1.
Schematic diagram of the experimental device and experimental design. CK: control group; Aw: A. woodiana group; Hv: H. verticillata group; HA: A. woodiana–H. verticillata group.
This study selected the submerged macrophyte Hydrilla verticillata and the benthic bivalve Anodonta woodiana as research subjects, both of which are widely distributed in lake ecosystems. H. verticillata is an erect-type submerged plant with a relatively balanced development of roots, stems, and leaves, characterized by small, numerous leaves, high growth rates, strong water purification capacity, and high tolerance to adverse environmental conditions. It has been proven to be a dominant species for aquatic ecosystem restoration [16]. The H. verticillata used in the experiment was collected from the plant cultivation greenhouse at the Poyang Lake Model Experimental Research Base. Healthy, unbranched apical shoots, approximately 15 cm in length, were selected from H. verticillata, washed thoroughly, and secured with ceramic rings and biochemical cotton, with three shoots placed per planting ring.
Anodonta woodiana, belonging to the phylum Mollusca, class Bivalvia, family Unionidae, and genus Cristaria. It is widely distributed in freshwater environments such as lakes, rivers, and ponds. This species feeds on plankton and organic detritus and possesses a strong filtering capacity, with proven effectiveness in removing phytoplankton and suspended particulate matter, and in regulating algal community structure [17]. The A. woodiana used in the experiment was collected from the Freshwater Fisheries Research Center of the Chinese Academy of Fishery Sciences. Individuals of similar size, weight, and healthy growth status were selected. The average shell length, width, and height are 76.2 mm, 55.7 mm, and 24.9 mm, respectively.
2.3. Experimental Design
The experimental setup consisted of 12 polyethylene barrels, each filled with approximately 12 kg of homogenized sediment to form a layer about 4.5 cm thick. Subsequently, 80 L of treated algae-laden eutrophic water was slowly added until the water level reached approximately 56 cm. After a stabilization period of three days, mid-depth water samples were collected to determine the initial water quality parameters. The measured concentrations of total phosphorus (TP), total nitrogen (TN), ammonia nitrogen (NH3-N), orthophosphate phosphorus (PO4-P), and permanganate index (CODMn) in the water were 0.293, 7.378, 0.110, 0.007, and 12.882 mg/L, respectively. The TN, TP, and organic matter content of the sediment were 2.96 mg/g, 1.79 mg/g, and 9.0%, respectively.
The experiment included four treatment groups: the control group (CK), the A. woodiana group (Aw), the H. verticillata group (Hv), and the combined H. verticillata + A. woodiana group (HA) (Figure 1). Each treatment was conducted in triplicate. CK received no treatment. On day 4 of the experiment, Aw received six A. woodiana individuals, Hv received four H. verticillata planting rings, and HA received both six A. woodiana individuals and four H. verticillata planting rings simultaneously. Each ring contained H. verticillata with a total fresh weight of approximately 8.5 g and a planting density of 49.30 g/m2. A. woodiana were placed at the bottom of cylindrical mesh bags (30 cm in diameter and 30 cm in height), which were secured at the center of each barrel. The A. woodiana were positioned approximately 10 cm above the sediment surface and 40 cm below the water surface. The total biomass of A. woodiana per group was approximately 236 g, and the stocking density was 60 individuals per cubic meter. To prevent hypoxic death of A. woodiana, all groups were aerated using oxygen pumps (SB-948, Songbao Electrical Appliances Co., Ltd., Zhongshan, China) at an oxygenation rate of 1000 mg O2/min.
On days 0, 5, 8, 13, 19, 26, 33, and 40, key physicochemical parameters of the overlying water in each treatment group were measured in situ, including electrical conductivity (EC), dissolved oxygen (DO), oxidation-reduction potential (ORP), water temperature (WT), pH, turbidity, and Chl a. Water samples of 200 mL were collected from each mesocosm to determine TP, TN, NH3-N, PO4-P, and CODMn. After sampling, ultrapure water was added to restore the original water level. At the end of the experiment, Hydrilla verticillata was harvested, thoroughly rinsed with ultrapure water, measured for fresh weight, then dried, pulverized, and sieved through a 100-mesh sieve for later use. The wet weight, shell length, and shell width of Anodonta woodiana were measured at both the beginning and end of the experiment. During measurement, the shells were cleaned and dried with paper towels. Wet weight was measured using an electronic balance, and shell length and width were measured using a vernier caliper.
2.4. Parameter Measurement
During the experimental period, the concentrations of TN, NH3-N, NO3−-N, TP, PO4-P, and CODMn were measured following standard methods [18]. Meanwhile, the physicochemical parameters such as Water Temperature, EC, DO, turbidity, and pH were measured using a portable multi-parameter water quality analyzer (YSI Pro Plus, Xylem Corporation, Rye Brook, NY, USA).
Chl a was measured using a rapid fluorescence analyzer for qualitative and quantitative assessment of algae (PHYTO-PAM-II ELD Compact Version, Heinz Walz GmbH, Nuremberg, Germany). The PHYTO-PAM-II chlorophyll fluorescence is equipped with five measurement wavelengths (440, 480, 540, 590, and 625 nm) and can simultaneously excite four types of phytoplankton pigment groups (cyanobacteria, green algae, diatoms/dinoflagellates, and phycoerythrin-containing organisms). Given that the concentration of green algae and phycoerythrin-containing organisms in each treatment group was negligible, this study only reported the Chl a concentrations of cyanobacteria and diatoms/dinoflagellates.
The method for determining total phosphorus (TP) in submerged macrophytes involves initial digestion using concentrated H2SO4-H2O2 on a temperature-controlled electric heating plate, followed by measurement of the phosphorus content in the digested solution [19]. Phosphorus within the submerged macrophyte is sequentially extracted using different reagents to obtain water-soluble phosphorus (H2O-P), non-reactive organic phosphorus (NaOH-P), and calcium-bound phosphorus (HCl-P). The main extraction steps are as follows: (1) Add 100 mg of pretreated dried plant sample into a 100 mL centrifuge tube, then add 50 mL of ultrapure water (with a resistivity of 18.25 MΩ cm). Place it on a shaker at a speed of 180 rpm for 30 min of extraction, after which measure the phosphorus content in the extract to obtain H2O-P. (2) Discard the extract from the previous step, add 50 mL of 1 mol/L sodium hydroxide solution to the residue, place it on a shaker at a speed of 180 rpm for 16 h of extraction, and then measure the phosphorus content in the extract to obtain NaOH-P. (3) Discard the extract from the previous step, add 50 mL of 1 mol/L hydrochloric acid solution to the residue, place it on a shaker at a speed of 180 rpm for 30 min of extraction, and then measure the phosphorus content in the extract to obtain HCl-P [20,21,22]. The phosphorus content in both the digested and extracted solutions is measured using the Ammonium molybdate spectrophotometric method [23,24].
2.5. Data Calculation and Analysis
2.5.1. Trophic Level Index Method
The trophic level index (TLI) method is widely used for assessing eutrophication in lakes and reservoirs in China. Where TLI is the sum of indices of all nutrient parameters, TLIj is the TLI of the jth parameter, m is the number of participating evaluation parameters, and Wj is the weight of the jth parameter in TLI, Wj were determined using the correlation method within the Chinese lake eutrophication assessment framework. This methodology calculates the weights based on the correlation between each nutrient parameter (e.g., TP, TN, Chl a) and the benchmark parameter, Chl a. The specific correlation coefficients and resultant weights were derived from extensive historical data on Chinese lakes. In this study, the Wj values for Chl a, TP, TN, SD, and CODMn were determined to be 0.27, 0.18, 0.19, 0.18, and 0.18, respectively. Jin et al. [25] established significant correlations among TP, TN, Chl a, and other water quality parameters. Although multiple evaluation parameters were considered, their analysis demonstrated that the concentrations of Chl a, TP, TN, CODMn, and Secchi disk depth (SD) constitute the five most critical indicators for assessing eutrophication in Chinese lakes within the TLI framework. The TLI of Chl a, TP, TN, SD, and CODMn is represented by the Specification for lake eutrophication survey [26].
where the unit of Chl a is μg/L, and that of TP, TN, and CODMn is mg/L.
TLI (Chl a) = 10 × (2.5 + 1.086 × lnChl a)
TLI (TP) = 10 × (9.436 + 1.624 × lnTP)
TLI (TN) = 10 × (5.453 + 1.694 × lnTN)
TLI (SD) = 10 × (5.118 + 1.94 × lnSD)
TLI (CODMn) = 10 × (0.109 + 2.661 × lnCODMn)
The TLI of the Lake was divided into five levels: oligotrophic (TLI < 30), mesotrophic (30 ≤ TLI ≤ 50), slightly eutrophic (50 < TLI ≤ 60), moderately Eutrophic (60 < TLI ≤ 70), and hypereutrophic (TLI > 70).
The individual Trophic Level Index (TLI) formulas (Equations (1)–(5)) were developed based on large-scale survey data from Chinese lakes, establishing empirical relationships between the concentration of each parameter (Chl a, TP, TN, SD, CODMn) and trophic status. The constants in these formulas (e.g., 2.5 and 1.086 in TLI(Chl a)) are regression coefficients obtained through data fitting, while the coefficient 10 serves as a scaling factor to amplify the index to a practical range of 0–100 for enhanced interpretability and application. This methodology ensures that the TLI calculation is specifically adapted to the unique conditions and characteristics of Chinese lakes.
2.5.2. The Calculation of Relative Growth Rate (RGR)
The relative growth rate (RGR) of Hydrilla verticillata and Anodonta woodiana was calculated using Equation (7):
where m1 and m0 represent the mass (g) at the end and beginning of the experiment, respectively, expressed as fresh weight for plants and wet weight for bivalves, and t is the experimental duration (days).
RGR = ln(m1/m0)/t
2.5.3. Statistical Analysis
Origin 2021 (OriginLab Corporation, Northampton, MA, USA) was used for plotting. The data were statistically analyzed in SPSS Statistics 22.0 (IBM Corporation, Armonk, NY, USA). One-way analysis of variance (ANOVA) and Tukey’s test were used to analyze the differences in N and P content in the aquatic environment among different treatments. The significance level for experimental data was set at p < 0.05 (the same letters indicate no significant difference, while other letters indicate a significant difference).
3. Results
3.1. Effects of Different Treatments on the Physicochemical Indicators of the Water
During the experimental period, the water temperature averaged 23.5 ± 4.3 °C. The average pH in all treatment groups decreased from 9.61 at the beginning of the experiment to 8.76 at the end, with the pH in the CK being significantly higher than in the other treatment groups (p < 0.05) (Figure 2a). The CODMn concentrations in all treatment groups showed a trend of initial decline followed by an increase. At the end of the experiment, CODMn concentrations in groups containing Anodonta woodiana (Aw and HA) were significantly lower than those in groups without A. woodiana (CK and Hv) (p < 0.05). Compared with CK, CODMn concentrations in the Aw and HA groups decreased by 27.5% and 17.5%, respectively (Figure 2b).
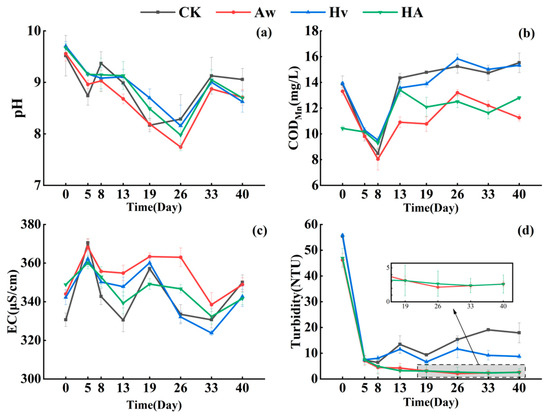
Figure 2.
Changes in pH (a), CODMn (b), EC (c), and Turbidity (d) (means ± standard deviation) of the overlying water with time. Error bars indicate the standard deviations. CK: control group; Aw: A. woodiana group; Hv: H. verticillata group; HA: A. woodiana–H. verticillata group; CODMn: permanganate index; EC: electrical conductivity.
The mean initial electrical conductivity (EC) across all treatment groups was 342.6 μS/cm. Throughout the experimental period, the EC values in all groups exhibited fluctuating trends, reaching their peak and lowest levels on days 5 and 33, respectively. By the end of the experiment, the mean EC had increased to 347.4 μS/cm, with no significant differences observed among the treatment groups (p > 0.05) (Figure 2c). From day 0 to day 5, turbidity in all treatment groups decreased sharply. Thereafter, turbidity in the Aw and HA groups declined slowly, whereas that in the CK and Hv groups increased slightly. At the end of the experiment, the turbidity levels in the Aw and HA groups were 2.65 and 2.62 NTU, respectively, which were significantly lower than those in the Hv and CK groups (p < 0.05). Compared with CK, turbidity in the Aw and HA groups decreased by 85.2% and 85.4%, respectively (Figure 2c).
3.2. Effects of Different Treatments on Nutrient Salts in the Overlying Water
The TN concentration in all treatment groups exhibited a declining trend throughout the experiment. At the end of the experiment, the TN concentrations in the Aw and HA groups were 1.125 and 1.511 mg/L, respectively, significantly lower than those in the CK and Hv groups (p < 0.05). However, during days 0–33, the TN concentrations in the Aw and HA groups remained consistently higher than those in the CK and Hv groups (Figure 3a). The TP concentrations in all treatment groups decreased sharply from day 0 to day 8. Subsequently, TP concentrations in the CK and Hv groups fluctuated, whereas those in the Aw and HA groups continued to decrease. At the end of the experiment, the TP concentrations in the Aw and HA groups were 0.054 and 0.048 mg/L, respectively, significantly lower than those in the CK and Hv groups. Compared with CK, the TP concentrations in the Aw and HA groups were reduced by 53.0% and 58.3%, respectively (Figure 3b).
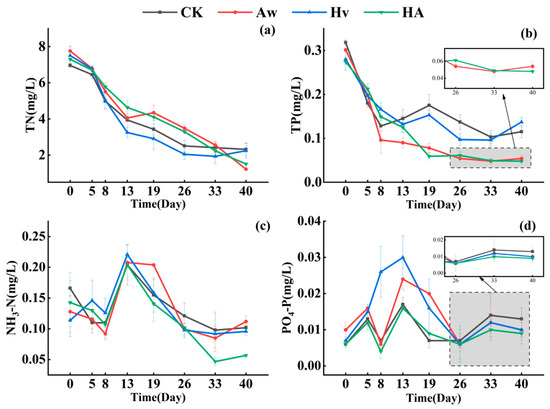
Figure 3.
Changes in TN (a), TP (b), NH3-N (c), and PO4-P (d) concentrations (means ± standard deviations) of the overlying water with time. Error bars indicate the standard deviations. CK: control group; Aw: A. woodiana group; Hv: H. verticillata group; HA: A. woodiana–H. verticillata group; TP: total phosphorus; TN: total nitrogen; NH3-N: ammonia nitrogen; PO4-P: orthophosphate Phosphorus.
The NH3-N concentrations in all treatment groups increased briefly between days 8 and 13, followed by a rapid decline. At the end of the experiment, the NH3-N concentration in HA was 0.057 mg/L, significantly lower than that in the other treatment groups (p < 0.05), representing a 44.1% reduction compared with CK (Figure 3c). The PO4-P concentrations in the Aw and Hv groups substantially fluctuated during the mid-experimental period. At the end of the experiment, there were no significant differences in PO4-P concentrations among the treatment groups, and the values remained statistically unchanged compared to the initial levels (p > 0.05) (Figure 3c).
3.3. The Restoration Effect on Eutrophic Water
At the beginning of the experiment, all treatment groups exhibited hypereutrophic conditions. From day 0 to day 8, the TLI values in all groups showed a decreasing trend. Subsequently, the TLI values in the CK and Hv groups gradually stabilized at a moderately eutrophic level (60 < TLI(∑) ≤ 70), while those in the Aw and HA groups continued to decline. At the end of the experiment, the TLI values in the Aw and HA groups decreased to 58.02 and 57.31, respectively, representing reductions of 14.6% and 15.7% compared to CK. These values were significantly lower than those in the CK and Hv groups (p < 0.05) and met the criteria for slightly eutrophic (50 < TLI(∑) ≤ 60) (Figure 4).
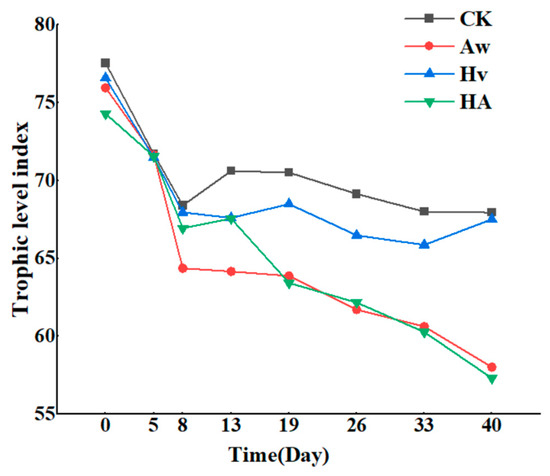
Figure 4.
Change in the TLI of the overlying water with time. CK: control group; Aw: A. woodiana group; Hv: H. verticillata group; HA: A. woodiana–H. verticillata group.
3.4. Change in the Composition of Algae in Water
During the initial stage, the contribution of cyanobacterial Chl a (154.75–162.65 µg/L) to the algal communities of all treatment groups was significantly greater than the contributions from diatoms/dinoflagellates (21.0–23.13 µg/L), with cyanobacteria accounting for 86.3–88.2% of the total, thereby achieving absolute dominance. At the end of the experiment, although the algal community composition in the CK and Hv groups changed significantly, the contribution of cyanobacteria (CK: 27.8 μg/L; Hv: 32.7 μg/L) remained slightly higher than that of diatoms/dinoflagellates (CK: 26.8 μg/L; Hv: 22.1 μg/L), with cyanobacteria accounting for an average of 55.6% of the total. In contrast, the cyanobacterial contribution in the water of the Aw and HA groups (Aw: 7.7 μg/L; HA: 8.9 μg/L) was significantly lower than that of diatoms/dinoflagellates (Aw: 13.8 μg/L; HA: 15.6 μg/L), with cyanobacteria accounting for only 36.0% of the total, a proportion significantly lower than the biomass of diatoms/dinoflagellates in these groups. This result indicates that, in the treatment groups Aw and HA introduced with Anodonta woodiana, a fundamental shift in algal community structure was successfully achieved, transitioning from initial cyanobacteria-dominated assemblages to diatom- and dinoflagellate-dominated assemblages (Figure 5).
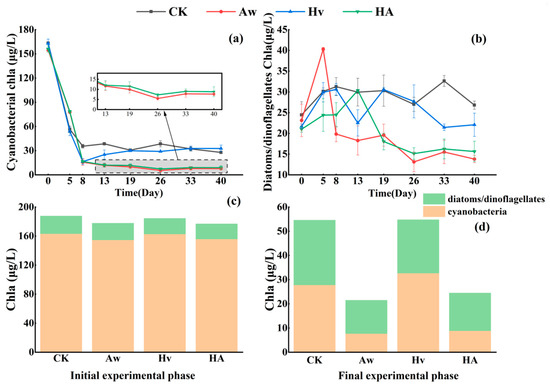
Figure 5.
Changes in Chl a concentrations (means ± standard deviations) of cyanobacteria (a) and diatoms/dinoflagellates (b) in water over time. Temporal changes in contributions of cyanobacteria (c) and diatoms/dinoflagellates (d) to total Chl a concentrations in water during the experiment. CK: control group; Aw: A. woodiana group; Hv: H. verticillata group; HA: A. woodiana–H. verticillata group.
3.5. Effects of Different Treatments on the Survival Rate and Growth Performance of Anodonta Woodiana and Hydrilla verticillata
At the end of the experiment, the survival rates of Anodonta woodiana and Hydrilla verticillata in all treatment groups were 100%. In the Aw and HA groups, the wet weight, shell length, and shell width of A. woodiana showed slight increases compared to the initial values, with growth ranges of 0.1% to 0.2%. The relative growth rates of H. verticillata in the Hv and HA groups were 0.043 and 0.045, respectively, with no significant difference between the two treatment groups (p > 0.05).
3.6. Effects of Different Treatments on the P Speciation of Submerged Plants
The TP and H2O-P contents in Hydrilla verticillata from Hv were significantly higher than those in HA (p < 0.05), while no significant differences were observed in NaOH-P and HCl-P contents between the two groups (p > 0.05) (Figure 6).
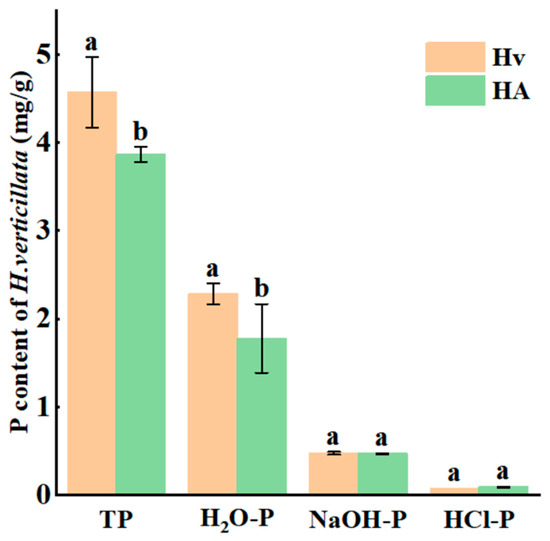
Figure 6.
P content in different forms within the shoots of H. verticillata (means ± standard deviations). Different lowercase letters above the bars in the same section indicate significant differences among experimental groups (p < 0.05). Error bars indicate the standard deviations. Hv: H. verticillata group; HA: A. woodiana–H. verticillata group.
4. Discussion
In this study, two inexpensive and readily available aquatic organisms, Hydrilla verticillata and Anodonta woodiana, were used for the ecological restoration of severely eutrophic water, achieving favorable outcomes. Compared with the CK and Hv groups, the two treatment groups with A. woodiana exhibited significantly lower concentrations of TP, Chl a, CODMn, and turbidity. The composition of algal communities in water also underwent significant changes, shifting from a cyanobacteria-dominated type to a diatoms/dinoflagellates-dominated type, with a notable improvement in the degree of eutrophication. In addition, compared with the CK, Hv, and Aw groups, HA showed a significant reduction in NH3-N concentration. At the end of the experiment, both aquatic organisms exhibited good growth status, with the relative growth rate of H. verticillata and the wet weight growth rate, shell length growth rate, and shell width growth rate of A. woodiana showing a slight upward trend, suggesting a mutually promotive relationship. Furthermore, compared with Hv, the TP contents and its component H2O-P in the shoots of H. verticillata were significantly reduced in HA.
Hydrilla verticillata is a narrow-leaved submerged macrophyte, and previous studies have shown that narrow-leaved submerged macrophytes have a stronger inhibitory effect on algal growth than broad-leaved species such as Potamogeton crispus, and exhibit a more pronounced suppression of odor compound synthesis [27]. Some studies have reported that, under conditions of high turbidity, the microbial communities attached to the leaves of submerged macrophytes can secrete extracellular polymeric substances (EPS)—mainly composed of proteins, polysaccharides, and humic acid-like substances—which can efficiently flocculate suspended particles in the water, thereby enhancing water transparency [28,29]. However, in practical applications, the purification efficacy of submerged macrophytes is considerably constrained by several factors: the low tolerance threshold of algae to phosphorus concentrations, combined with the release of cyanotoxins and nutrient competition from cyanobacteria. Consequently, relying solely on submerged macrophytes for regulation during algal blooms often fails to achieve significant suppression of algal biomass [30]. Anodonta woodiana can feed on phytoplankton and organic detritus in the water through filter feeding, forming a functional complementarity with submerged macrophytes. In this study, the introduction of A. woodiana resulted in a significant reduction in Chl a, suspended solids concentration, and turbidity in the water within a short period, with increased transparency. This effect effectively improved the photosynthetic conditions for H. verticillata, enhancing nutrient uptake by the plants, thereby driving a continuous decrease in TP concentration in the treatments with A. woodiana, which remained significantly lower than in treatments without A. woodiana.
In contrast to Anodonta woodiana, the growth and metabolism of Hydrilla verticillata directly depend on the uptake of dissolved reactive phosphorus (e.g., PO4-P) from the water. Therefore, although the TP removal efficiency of H. verticillata may be compromised by cyanobacteria suppression, its continuous absorption of dissolved reactive phosphorus in Hv resulted in lower PO4-P concentrations compared to CK, which exhibited almost no effective removal. In HA, A. woodiana reduced the algal consumption of PO4-P through filter-feeding, while its excretion likely facilitated the conversion of particulate organic phosphorus into dissolved forms [31]. This process consequently enhanced the bioavailable phosphorus supply for H. verticillata, thereby achieving highly efficient removal of this key nutrient. In addition, we found that the TP and H2O-P contents in H. verticillata from HA were significantly lower than those in Hv. This difference was primarily due to the filter-feeding and flocculation effects of the A. woodiana in HA, which reduced the concentration of bioavailable phosphorus in the water [32], thereby diminishing the phosphorus acquisition capacity of H. verticillata. H2O-P is the most active phosphorus fraction in submerged macrophytes; thus, the reduction in H2O-P content in H. verticillata also indicates a lower risk of phosphorus release after plant senescence. Therefore, under hypereutrophic conditions, while A. woodiana plays a dominant role in the bivalve-macrophyte synergistic system, H. verticillata establishes a sustainable pathway for phosphorus removal through its unique ecological function of modifying nutrient speciation. This complements the physical filtration provided by the bivalves, and its contribution to phosphorus speciation transformation and long-term sequestration is indispensable. Furthermore, subsequent studies could focus on optimizing the planting density of H. verticillata to enhance its ecological restoration efficacy.
Filter-feeding bivalves can substantially reduce phytoplankton biomass through their filtration activity. Typically, larger phytoplankton species (e.g., cyanobacteria) are more susceptible to removal by filtration, while some smaller algae may temporarily “escape” this physical selection. Consequently, this size-selective feeding behavior induces shifts in the algal community structure. The excretion of filter-feeding bivalves directly releases nutrients (e.g., NH3-N and reactive phosphate) back into the water column. Undigested materials, such as algal debris, form dense biodeposits (fecal and pseudofecal pellets) that rapidly settle to the sediment. This biodeposition process facilitates the growth of benthic algae [31,33,34]. Hydrilla verticillata plays a critical role in driving the succession of dominant phytoplankton from cyanobacteria to diatoms/dinoflagellates. Although cyanobacteria remained dominant in both the CK and Hv groups at the end of the experiment, the biomass of diatoms/dinoflagellates in Hv was higher than in CK. The presence of H. verticillata, whether alone (Hv) or combined with Anodonta woodiana (HA), creates a complex three-dimensional structure that provides favorable attachment surfaces for benthic diatoms and enhances habitat conditions for planktonic dinoflagellates. Submerged macrophytes such as H. verticillata release specific allelochemicals that selectively inhibit cyanobacterial growth while exerting minimal or even favorable effects on diatoms and dinoflagellates [5,27]. In HA, bivalve filtration reduced turbidity while the leaves of H. verticillata provided suitable attachment substrates for light-favoring diatoms. Together, these mechanisms established a “High-Light, Low-Disturbance” positive feedback loop. Diatoms are important primary producers in lakes, and their sensitive response to water quality, particularly to total phosphorus, has been well documented [35,36]. Oxygen production by diatoms, photosynthetic autotrophs, makes substantial contributions to ecosystem services that rely on this process [37]. Due to their high density and rapid sinking rates, diatom cells reduce the concentration of suspended solids and improve water transparency [38]. Therefore, the H. verticillata–A. woodiana system promotes the shifts from a turbid-water stable state to a clear-water stable state in shallow aquatic ecosystems.
It is noteworthy that the NH3-N concentration in HA was significantly lower than in the other treatment groups, and this can be mainly attributed to three factors. First, Hydrilla verticillata is an efficient absorber of NH3-N in aquatic ecosystems [39]. Beyond direct plant uptake, it primarily converts NH3-N into NO3−-N and N2 through rhizosphere nitrification and denitrification processes [8]. Second, although Anodonta woodiana excreta contain high concentrations of NH3-N, the nitrification process converts the toxic form of NH3-N into nitrate, which is toxic only at high concentrations. This process, in synergy with the photosynthesis of H. verticillata, synergistically enhanced nitrogen transformation efficiency [40]. Finally, the shift in dominant phytoplankton species to diatoms/dinoflagellates not only increased the photosynthetic rate of submerged macrophytes, thereby indirectly enhancing nitrification, but also, compared with cyanobacteria, diatoms possess higher nitrogen assimilation efficiency [41].
Although the TN concentrations in the Aw and HA groups decreased, they became significantly lower than those in the CK and Hv groups only at the experiment’s final stage, during days 33 to 40. The delayed response suggests that Hydrilla verticillata requires a physiological adaptation period in eutrophic environments, and the functional activation of nitrifying/denitrifying bacterial communities also takes time to accumulate. Additionally, through filter feeding on plankton, Anodonta woodiana introduces nitrogen-containing excretions directly into the water, and the decomposition of undigested planktonic debris releases dissolved organic nitrogen [32]. This process was confirmed between days 8 and 13, when NH3-N concentrations in all treatment groups showed a brief increase, with the increase in the Aw and HA groups being significantly greater than in CK. Therefore, the nitrogen transformation efficiency of the H. verticillata–A. woodiana synergistic system only became apparent in the later stage of the experiment. This phased characteristic also aligns with the dynamic changes in water Chl a concentrations—in the first 8 days, the decrease in Chl a in the Aw and HA groups was significantly greater than in the CK and Hv groups (p < 0.05), indicating that A. woodiana filter feeding accelerated the reduction in plankton biomass, but the accompanying nitrogen release temporarily masked the purification effect.
In eutrophic waters, the respiration of high-density algal populations and the decomposition of senescent algal cells lead to oxygen depletion [42]. While promoting photosynthetic oxygen production by submerged macrophytes, the activity of Anodonta woodiana indirectly enhances dissolved oxygen levels through significantly reducing algal biomass and filtering organic suspended particulates from the water column [17]. The decomposition of these particulates would otherwise consume substantial oxygen, thereby reducing the biochemical oxygen demand (BOD) of the water body. However, as this experiment was conducted under laboratory conditions with relatively high initial temperatures, uniform aeration was applied across all treatment groups to ensure the survival of A. woodiana and prevent hypoxia-induced mortality. The potential impacts of aeration were neutralized in inter-group comparisons. Nevertheless, compared to the equally aerated CK and Hv groups, the Aw and HA groups still demonstrated significant improvements in water quality parameters (p < 0.05). These differences should be attributed to the presence of bivalves and their synergistic interactions with submerged macrophytes.
In this study, both Hydrilla verticillata and Anodonta woodiana exhibited positive growth performance. At the end of the experiment, the growth indices of A. woodiana in all groups showed no significant increase compared with the initial value, which may be attributed to the substantial reduction in cyanobacterial abundance at the beginning of the experiment, resulting in reduced food availability for the A. woodiana. Although the relative growth rates of H. verticillata in the Hv and HA groups did not differ significantly, HA exhibited slightly higher values, suggesting that the excretion and filter-feeding activities of A. woodiana may have had a slight promotive effect on the growth of H. verticillata. The CODMn concentration in the water increased after day 8, primarily because A. woodiana excreta introduced organic matter into the water. The pH of the water decreased during the early stage of the experiment, likely due to the consumption of CO2 by plant photosynthesis and the production of organic acids from the microbial decomposition of planktonic debris and feces. With the replacement of cyanobacteria by diatoms/dinoflagellates, the sedimentation of diatoms drove the carbonate system in the water toward alkalinity by exporting organic carbon and silicic acid, resulting in a pH increase [43], although it remained within a reasonable range. The trophic level index (TLI) of all treatment groups showed a sharp decline during the initial 0–8 days, primarily attributable to natural purification processes including sediment adsorption of dissolved phosphorus and gravitational settling of suspended algae and organic debris, which temporarily removed nutrients from the water column. As the experiment progressed, these initial natural remediation processes weakened. Concurrently, settled algae and organic matter were decomposed by heterotrophic microorganisms at the sediment-water interface, releasing nutrients back into the water column. This led to a rebound in TLI values in the CK and Hv groups, whereas the Aw and HA groups maintained a continuous decline in TLI, benefiting from the restorative effects of A. woodiana and H. verticillata.
5. Conclusions
This study investigated the effectiveness of combining Hydrilla verticillata and Anodonta woodiana in the restoration of severely eutrophic water bodies. The main conclusions are as follows: (1) The sole introduction of A. woodiana (Aw group) significantly reduced TP, Chl a concentration, and turbidity, effectively mitigating eutrophication. The water quality improvement effect of planting H. verticillata alone (Hv group) was inferior to that of Aw. However, the synergistic interaction of H. verticillata and A. woodiana (HA group) exhibited the optimal purification performance, with greater reductions in TP, NH3-N, Chl a, and turbidity than individual treatments. (2) The filter-feeding activity of A. woodiana effectively regulated the algal community structure in eutrophic waters, shifting the dominant phytoplankton from cyanobacteria to diatom/dinoflagellate. This shift reflects a notable improvement in the ecological state of the aquatic ecosystem. (3) The combined application of A. woodiana and H. verticillata significantly reduced the TP and H2O-P content associated with H. verticillata. Such a reduction alleviated the risk of phosphorus release during the senescence and decomposition of the submerged macrophytes.
Author Contributions
Conceptualization, X.L. and W.L.; methodology, W.L.; validation, X.L., S.Q., W.S., B.H. and W.L.; formal analysis, X.L. and T.D.; investigation, W.L.; resources, W.L. and J.Z.; data curation, W.L. and B.H.; writing—original draft preparation, X.L. and W.S.; writing—review and editing, W.L., T.D., M.G., S.Q. and J.Z.; supervision, W.L., T.D., M.G. and J.Z.; project administration, W.L. and J.Z.; funding acquisition, W.L. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 52260026) and the Science and Technology Project of the Jiangxi Provincial Department of Water Resources (Grant No. 202325ZDKT09).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Gerasimova, T.N.; Sadchikov, A.P. Eutrophication of water bodies and control of this process with the use of zooplankton and planktophage fish. J. Gen. Chem. 2024, 94, 3596–3601. [Google Scholar] [CrossRef]
- Olukowi, O.M.; Xie, Y.; Zhou, Z.; Adebayo, I.O.; Zhang, Y. Performance improvement and mechanism of composite PAC/PDMDAAC coagulant via enhanced coagulation coupled with rapid sand filtration in the treatment of micro-polluted surface water. J. Environ. Chem. Eng. 2022, 10, 108450. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, P.; Zhao, S.; Kang, S.; Wang, P.; Zhou, M.; Lyu, J. Control and remediation methods for eutrophic lakes in the past 30 years. Water Sci. Technol. 2020, 81, 1099–1113. [Google Scholar] [CrossRef]
- Pan, M.; Dong, J.; Zhang, Z.; Zhang, L.; Guo, Y.; Yang, J.; Huang, L.; Wang, C.; Shan, K.; Wang, H.; et al. Changes in the ecosystem structure and function of a cyanobacteria bloom-dominated, shallow lake after ten-year eutrophication management. J. Oceanol. Limnol. 2024, 42, 1726–1740. [Google Scholar] [CrossRef]
- Maredová, N.; Altman, J.; Kaštovský, J. The effects of macrophytes on the growth of bloom-forming cyanobacteria: Systematic review and experiment. Sci. Total Environ. 2021, 792, 148413. [Google Scholar] [CrossRef]
- Mao, Z.; Cao, Y.; Gu, X.; Cai, Y.; Chen, H.; Zeng, Q.; Jeppesen, E. Effects of nutrient reduction and habitat heterogeneity on benthic macroinvertebrate assemblages in a large shallow eutrophic lake. Sci. Total Environ. 2023, 867, 161538. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Hei, P.; Zhang, J.; Yang, J.; Luo, T.; Zhou, G.; Liu, C.; Wang, R.; Chen, F. Internal phosphorus cycling in macrophyte-dominated eutrophic lakes and its implications. J. Environ. Manag. 2022, 306, 114424. [Google Scholar] [CrossRef]
- Trentman, M.T.; Atkinson, C.L.; Brant, J.D. Native freshwater mussel effects on nitrogen cycling: Impacts of nutrient limitation and biomass dependency. Freshw. Sci. 2018, 37, 276–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, R.; Gu, X.; Li, K.; Chen, H.; He, H.; Mao, Z.; Johnson, R.K. Simultaneous increases of filter-feeding fish and bivalves are key for controlling cyanobacterial blooms in a shallow eutrophic lake. Water Res. 2023, 245, 120579. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Song, D.; Wang, H.; Yang, J.; Liu, H.; Huo, T. The combined effects of filter-feeding bivalves (Cristaria plicata) and submerged macrophytes (Hydrilla verticillata) on phytoplankton assemblages in nutrient-enriched freshwater mesocosms. Front. Plant Sci. 2023, 14, 1069593. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, F.; Sun, J.; Hu, Y.; Huang, T.; Zhang, Y.; Wu, Z. Effects of three biological control approaches and their combination on the restoration of eutrophicated waterbodies. Limnology 2017, 18, 301–313. [Google Scholar] [CrossRef]
- He, H.; Liu, X.; Liu, X.; Yu, J.; Li, K.; Guan, B.; Jeppesen, E.; Liu, Z. Effects of cyanobacterial blooms on submerged macrophytes alleviated by the native Chinese bivalve Hyriopsis cumingii: A mesocosm experiment study. Ecol. Eng. 2014, 71, 363–367. [Google Scholar] [CrossRef]
- Gu, J.; Li, K.; Jeppesen, E.; Han, Y.; Jin, H.; He, H.; Ning, X. Using Freshwater Bivalves (Corbicula fluminea) to Alleviate Harmful Effects of Small-Sized Crucian Carp (Carassius carassius) on Growth of Submerged Macrophytes during Lake Restoration by Biomanipulation. Water 2020, 12, 3161. [Google Scholar] [CrossRef]
- Cheng, C.; Steinman, A.D.; Xue, Q.; Wan, X.; Xie, L. The disruption of calcium and hydrogen ion homeostasis of submerged macrophyte Vallisneria natans (Lour.) Hara caused by microcystin-LR. Aquat. Toxicol. 2023, 254, 106377. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Siedlecka-Kroplewska, K.; Woźniak, M.; Mazur-Marzec, H. Presence of ß-N-methylamino-L-alanine in cyanobacteria and aquatic organisms from waters of Northern Poland; BMAA toxicity studies. Toxicon 2021, 194, 90–97. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yao, W.; Shangguan, L.; Zhang, X.; Jin, B.; Cong, X.; Qian, P.; Xu, Y. Improving the efficacy of different life-form macrophytes in phytoremediation of artificial eutrophic water by combined planting. Environ. Sci. Pollut. Res. 2023, 30, 67621–67633. [Google Scholar] [CrossRef]
- Jin, Z.; Jin, H.; Gao, B.; Tong, C.; Jeppesen, E.; Rudstam, L.G.; Dumont, H.J.; de los Ángeles González Sagrario, M.; Razlutskij, V.; Liu, Z.; et al. Effects of filter-feeding bivalves in benthic and pelagic habitats on plankton community and water quality in shallow systems: Implications for lake rehabilitation. Aquat. Ecol. 2025, 59, 53–66. [Google Scholar] [CrossRef]
- Huang, X.; Chen, W.; Cai, Q. Survey, Observation and Analysis of Lake Ecology, 1st ed.; Standards Press of China: Beijing, China, 1999; pp. 72–81. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis. Part 3: Chemical Methods, 1st ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 894–895. [Google Scholar]
- Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F.; Ferguson, J.D. Laboratory Procedures for Characterizing Manure Phosphorus. J. Environ. Qual. 2000, 29, 508–514. [Google Scholar] [CrossRef]
- Siong, K.; Asaeda, T. Does calcite encrustation in Chara provide a phosphorus nutrient sink? J. Environ. Qual. 2006, 35, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Pardo, P.; Rauret, G.; López-sánchez, J.F. Shortened screening method for phosphorus fractionation in sediments. Anal. Chim. Acta 2004, 508, 201–206. [Google Scholar] [CrossRef]
- Luo, R.; Li, W.; Zhong, J.; Dai, T.; Liu, J.; Zhang, X.; Chen, Y.; Gao, G. Combining Multiple Remediation Techniques Is Effective for the Remediation of Eutrophic Flowing Water. Water 2024, 16, 858. [Google Scholar] [CrossRef]
- Rydin, E. Potentially mobile phosphorus in Lake Erken sediment. Water Res. 2000, 34, 2037–2042. [Google Scholar] [CrossRef]
- Jin, X. Lake Environment in China, 1st ed.; Ocean Press: Beijing, China, 1995; pp. 88–91. [Google Scholar]
- Wang, M.; Liu, X.; Zhang, J. Evaluate method and classification standard on lake eutrophication. Environ. Monit. China 2002, 18, 47–49. [Google Scholar] [CrossRef]
- Yang, C.; Shen, X.; Shi, X.; Cui, Z.; Nan, J.; Lu, H.; Li, J.; Huang, Q. Impact of submerged macrophytes on growth and 2-MIB release risk of Pseudanabaena sp.: From field monitoringa to cultural experiments. J. Hazard. Mater. 2023, 442, 130052. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Lv, X.; Guo, S.; Ma, Y.; Han, B.; Hu, X. Interactions between suspended sediments and submerged macrophytes-epiphytic biofilms under water flow in shallow lakes. Water Res. 2022, 222, 118911. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, B.; An, Q.; Qiu Guo, Z.; Huang, C. The characteristics and flocculation mechanisms of SMP and B-EPS from a bioflocculant-producing bacterium Pseudomonas sp. XD-3 and the application for sludge dewatering. Chem. Eng. J. 2024, 479, 147584. [Google Scholar] [CrossRef]
- Li, W.; Dai, T.; Liu, J.; Zhong, J.; Wu, K.; Gao, G.; Chen, Y.; Fan, H. Ferric- and calcium-loaded red soil assist colonization of submerged macrophyte for the in-situ remediation of eutrophic shallow lake: From mesocosm experiment to field enclosure application. Sci. Total Environ. 2024, 924, 171730. [Google Scholar] [CrossRef]
- Cyr, H.; Collier, K.J.; Clearwater, S.J.; Hicks, B.J.; Stewart, S.D. Feeding and nutrient excretion of the New Zealand freshwater mussel Echyridella menziesii (Hyriidae, Unionida): Implications for nearshore nutrient budgets in lakes and reservoirs. Aquat. Sci. 2017, 79, 557–571. [Google Scholar] [CrossRef]
- Hoellein, T.J.; Zarnoch, C.B.; Bruesewitz, D.A.; DeMartini, J. Contributions of freshwater mussels (Unionidae) to nutrient cycling in an urban river: Filtration, recycling, storage, and removal. Biogeochemistry 2017, 13, 307–324. [Google Scholar] [CrossRef]
- Lucas, L.V.; Cloern, J.E.; Thompson, J.K.; Stacey, M.T.; Koseff, J.R. Bivalve Grazing Can Shape Phytoplankton Communities. Front. Mar. Sci. 2016, 3, 14. [Google Scholar] [CrossRef]
- Qiao, L.; Chang, Z.; Li, J.; Li, T. Selective feeding of three bivalve species on the phytoplankton community in a marine pond revealed by high-throughput sequencing. Sci. Rep. 2022, 12, 6163. [Google Scholar] [CrossRef]
- Hall, R.I.; Smol, J.P. A weighted—Averaging regression and calibration model for inferring total phosphorus concentration from diatoms in British Columbia (Canada) lakes. Freshw. Biol. 1992, 27, 417–434. [Google Scholar] [CrossRef]
- Yang, X.; Anderson, N.J.; Dong, X.; Shen, J. Surface sediment diatom assemblages and epilimnetic total phosphorus in large, shallow lakes of the Yangtze floodplain: Their relationships and implications for assessing long-term eutrophication. Freshwater Biol. 2008, 53, 1273–1290. [Google Scholar] [CrossRef]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.; Selmeczy, G.B.; Lengyel, E.; Tapolczai, K. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia 2023, 850, 2707–2733. [Google Scholar] [CrossRef]
- Gaiser, E.E. Using Diatoms to Guide Successful Ecological Restoration. In Diatom Ecology, 1st ed.; Maidana, N.I., Licursi, M., Morales, E., Eds.; Wiley-Scrivener: Hoboken, NJ, USA, 2024; pp. 1–39. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Xu, J.; Li, L. Nitrogen removal performance and microbial community characteristics of a submerged macrophyte pond–constructed wetland composite system. J. Water Process. Eng. 2025, 72, 107492. [Google Scholar] [CrossRef]
- Ismall, N.S.; Dodd, H.; Sassoubre, L.M.; Horne, A.J.; Boehm, A.B.; Luthy, R.G. Improvement of Urban Lake Water Quality by Removal of Escherichia coli through the Action of the Bivalve Anodonta californiensis. Environ. Sci. Technol. 2015, 49, 1664–1672. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Interactions between NH+4 and NO−3 uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperatures. Mar. Biol. 1999, 133, 541–551. [Google Scholar] [CrossRef]
- Chen, J.; Gao, X.; Xu, X.; Zhu, C.; She, X.; Kong, D.; Xue, K.; Li, Y. Algal blooms in Lake Taihu: Earlier onset and extended duration. Harmful Algae 2025, 148, 102917. [Google Scholar] [CrossRef]
- Gately, J.A.; Kim, S.M.; Jin, B.; Brzezinski, M.A.; Iglesias-Rodriguez, M.D. Coccolithophores and diatoms resilient to ocean alkalinity enhancement: A glimpse of hope? Sci. Adv. 2023, 9, 6066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).