Abstract
Acmella radicans (Jacquin) R.K.Jansen is an annual herb native to Central America. In China, it is becoming increasingly invasive and often co-occurs with the native congener A. paniculata (Wall. ex DC.) R.K.Jansen in some habitats. In order to understand the invasion mechanism of A. radicans, we investigated the growth parameters of both the invasive A. radicans and the native congener, A. paniculata, under different light conditions (5%, 25%, 50%, 75%, and 100% of light availability) using potted plants in a glasshouse. Light level, plant species, and their interaction were significant, with plant species generally having a greater effect than light level. Acmella radicans and A. paniculata showed great phenotypic plasticity to various light intensities and had a similar trend with increased shade. The plasticity indices of all parameters of A. radicans, except for branch length and inflorescence number, were greater than those of A. paniculata under the same light intensity. The physiological parameters for A. radicans under both favorable (high light intensity) and unfavorable (low light intensity) conditions showed less inhibition than those of A. paniculata. All these responses indicated that A. radicans had greater phenotypic plasticity and higher adaptability to low light, which may contribute to its invasion success.
1. Introduction
Biological invasions have become increasingly common with increasing globalization of the world economy and have emerged as a major global concern [1,2,3]. Invasive alien species can cause significant social, economic, and environmental problems by reducing productivity and affecting ecosystem structure and function [4,5,6]. The invasion of an exotic species is mainly dependent on its own invasiveness and the invasibility of particular environments [7,8,9]. The invasion process of an exotic species often involves a series of complex processes, such as transport, introduction, establishment, and spread of the species [10]. Thus, the probability of an alien species becoming a harmful invasive species tends to be low [11]. Consequently, determining the factors and biological characteristics that facilitate the invasion of invasive species has become one of the priorities of research worldwide.
Invasive alien plants typically possess biological and ecological characteristics that give them inherent advantages over native plant species [12,13]. They also often have a higher tolerance to various abiotic environmental factors than native congeners [14,15]. Among the conditions affecting growth, the amount of available light is one of the most critical abiotic factors [16,17]. Compared to native species, invasive alien plants tend to be better competitors for light and/or exhibit comparatively higher shade tolerances through responding at various scales ranging from the cellular level to the whole plant [15,18,19]. Hence, research on how invasive and native congeners occupying similar niches respond under different light conditions is an important avenue for exploring plant invasion mechanisms [20,21].
The annual herb Acmella radicans (Jacquin) R.K.Jansen (Asteraceae) originates in Central America and has successfully invaded many countries and regions worldwide [22,23,24,25]. In China, A. radicans is found widely in southern regions of Yunnan Province and is now considered as a serious invasive species, infesting a diverse range of agricultural and non-agricultural habitats with high rates of disturbance [6,26]. The invasion of A. radicans has caused severe negative impacts to crop production, biodiversity, structure, function, and soil nutrients of invaded ecosystems, through strong competitiveness, a large long-term soil seedbank, a broad seed germination niche, and a high ability to release allelochemicals [6,26,27,28].
To better understand mechanisms of invasiveness, it is useful to compare the ecophysiology of invasive plants with that of native plants, and, in particular, it is useful to do pairwise comparisons between plant species in the same genus [29]. Acmella paniculata (Wall. ex DC.) R.K.Jansen, is an indigenous and traditional Chinese medicinal plant, and is a native congener of A. radicans [30]. This species is generally found in freshwater habitats, paddy, and sugar cane fields, as well as waste wetlands [31]. In Yunnan Province, A. radicans generally has a wider distribution and causes greater negative impacts than A. paniculata when they co-occur in similar environments [6]. In an earlier study, A. radicans was found to grow in a wide range of environmental conditions, including light and moisture [32]. However, this study was limited in that it focused solely on A. radicans without comparing these physiological traits with other plant species.
The overall objectives of the current study are to determine effects of light intensity on the growth, biomass, morphology, physiology, and plant nutrients of A. radicans and its native congener A. paniculata to assess ecophysiological differences between the two species. This comparison is to highlight what traits of A. radicans comprise the mechanism by which it behaves as an invasive species. Our hypothesis is that the invasive plant A. radicans would exhibit greater adaptability to varying light intensity, enabling it to grow more rapidly under a greater variety of light intensities than its congener A. paniculata. Greater knowledge of the invasive mechanism employed by A. radicans should provide helpful insights into its management.
2. Methods
2.1. Study Species
Mature seeds of A. radicans and A. paniculata were collected separately from numerous plants of the same populations within cultivated lands in Bangbing Township (23°33′ N, 99°77′ E), Shuangjiang County of Yunnan Province in December 2023. Seeds were taken to the laboratory at the Agricultural Environment and Resource Research Institute, Yunnan Academy of Agricultural Sciences, China, in paper bags, dried at room temperature for three weeks and then stored in the refrigerator at −4 °C.
2.2. Experiment Design and Data Collection
The study was conducted from June to December in 2024 in the greenhouse of Yunnan Academy of Agricultural Sciences, Songming County (25°35′ N, 103°11′ E), Yunnan Province, China. On 25 June 2024, fully developed A. radicans and A. paniculata were sown separately in seedbeds in the greenhouse. After six weeks, two seedlings of similar size of each species were transplanted separately into each pot (20 cm diameter and 25 cm deep), filled with soil collected from bare ground in the glasshouse, such that each pot had two similar-sized seedlings of the same species. The soil had a pH of 6.80 ± 0.11, an organic matter content of 27.58 ± 0.39 g·kg−1, available N 86.76 ± 1.35 mg·kg−1, available P 32.01 ± 1.12 mg·kg−1, and available K 190.06 ± 8.16 mg·kg−1. A total of 200 pots of each species were set up. The seedlings were maintained under optimal growing conditions.
After 25 days, seedlings were of suitable size (about 7 cm high, with five fully expanded leaves) and the experiments commenced, using a completely randomized design with different light levels. Within the glasshouse, different light levels were obtained within 5 m × 4 m × 1.5 m black nylon shade sheds with different shade ratings: non-irradiance (NI) treatment received 5%, the low-irradiance (LI) treatment received 25%, the medium-irradiance (MI) treatment received 50%, the high-irradiance treatment (HI) received 75%, and the full-irradiance treatments (FI) received 100%. Twenty shade sheds were constructed for each, and each shed was separated by 2.5 m distance. Each shed contained eight pots randomly selected of one or the other species. There were 5 light treatments × 4 replicate sheds × 8 pots × 2 species = 320 total pots, with the full irradiance acting as the control treatment. During the experiment, sufficient water was provided to each plant equally, and the pots were weeded by hand. No synthetic fertilizers were applied to any pot.
On 15 November 2024, the net photosynthetic rate (Pn) of leaves of individual plants of each of A. radicans and A. paniculata from each pot was measured with a Portable Photosynthesis System (LI-COR Biosciences LI6400XT, Lincoln, NE, USA). On 28 December 2024, all plants were harvested and the plant height, branch length (total branch length not including the main stem), basal stem diameter, petiole length, leaf area (all leaves) (Li-3000A; LI-COR Biosciences, Lincoln, NE, USA), inflorescence number, seed number, and biomass (dried weight) of each species were recorded. The chlorophyll content of fresh leaves and plant nutrients from whole plants were analyzed.
2.3. Statistical Analysis
The normality and homogeneity of variances were tested, and log transformations of the data were performed to correct deviation before analysis, if necessary. A two-way ANOVA using General Linear Models (GLMs) (IBM SPSS 26.0, Armonk, NY, USA) was used to test the effect of plant species, light intensity and their interactions on the morphological and physiological characteristics, and plant nutrient composition of A. radicans and A. paniculata. The inhibitory rate for plant morphological, physiological, and plant nutrient characteristics was calculated using the formula (C − T)/C × 100%, where C is the mean value of FI, and T is the mean value of each shade treatment; IR N 0 shows inhibition, IR b 0 shows promotion, and the magnitude of IR values reflects the intensity of the shade effect. The F, P, and R2 statistics were calculated, considering plant species and light levels, with their interaction as factors (5% significance level) (ns, *, and ** indicate p > 0.05, p ≤ 0.05, and p ≤ 0.01, respectively). Tukey’s honestly significant difference (HSD), post hoc multiple comparisons, and homogeneity of variance tests were explored to detect significant differences among light level treatments.
3. Results
3.1. Effects of Species and Light on Plant Growth Parameters of Acmella radicans and Acmella paniculata
Plant species, light level, and their interaction had significant effects on all plant parameters tested for A. radicans and A. paniculata (Table 1). Plant species had a greater effect than light intensity on all morphological and physiological characteristics tested except for inflorescence number, seed number, Pn, and K content of the two species (Table 1).

Table 1.
Two-way ANOVA results of effects of light intensity, species, and their interaction on plant growth.
3.2. Effect of Light on Plant Growth of Acmella radicans and Acmella paniculata
Plant height, total branch length, and basal stem diameter of A. radicans and A. paniculata were significantly affected by light level (Figure 1A–C). Plant height and basal stem diameter of A. radicans were greater than those of A. paniculata, but its total branch length was less than A. paniculata. Plant height and total branch length of both species increased significantly with decreasing light intensity (Figure 1A,B), while basal stem diameter was clearly reduced (Figure 1C). Inhibition of most characteristics of A. radicans was less than that of A. paniculata under same light conditions (Table 2).
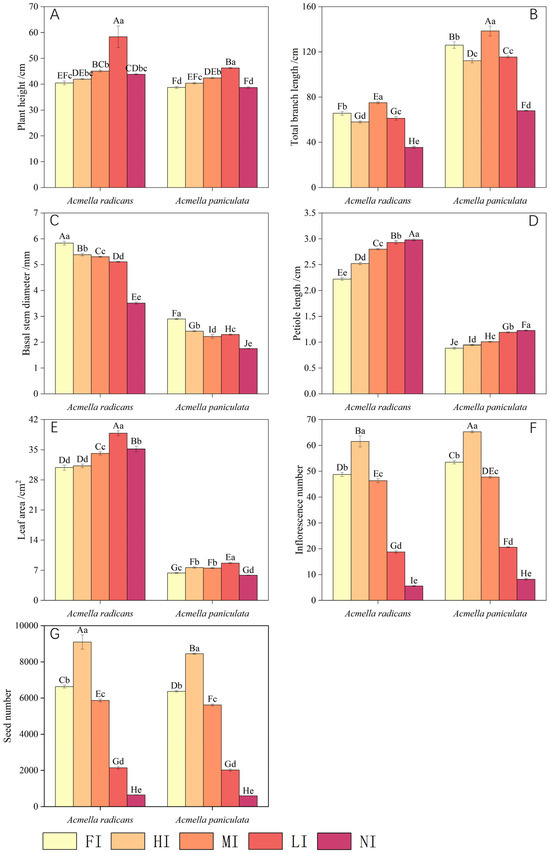
Figure 1.
Plant growth and reproductive characteristics of Acmella radicans and Acmella paniculata under different light levels [FI = full irradiance (100%), HI = high irradiance (75%), MI = moderate irradiance (50%), LI = low irradiance (25%), NI = non irradiance (5%)] for (A) plant height, (B) total branch length, (C) basal stem diameter, (D) petiole length, (E) leaf area, (F) inflorescence number, and (G) seed number. Different small and capital letters above bars indicate a significant difference at 0.05 level for one species and between two species under different treatments, respectively.

Table 2.
Inhibitory rate (%) of shade rate on plant growth and physiological characteristics of Acmella radicans and Acmella paniculata.
The petiole length and leaf area were much larger in A. radicans than in A. paniculata (Figure 1D,E). Petiole length and leaf area of both species increased significantly with a decrease in light intensity, usually with less inhibition for A. radicans than for A. paniculata (Table 1). Acmella radicans produced a greater seed number while A. paniculata produced a greater inflorescence number. Both inflorescence and seed number of A. radicans and A. paniculata were greatest at high irradiance and then were markedly reduced with decreasing light intensity (Figure 1F,G), generally with less inhibition for A. radicans than for A. paniculata (Table 2).
The leaf, stem, inflorescence, root, aboveground, and total biomass of A. radicans were obviously greater than those of A. paniculata under the same light conditions (Figure 2). With a decline in light intensity, the inflorescence and root biomass of both species were significantly reduced (Figure 2C,D), whereas the leaf, stem, and total biomass were obviously increased (Figure 2A,B,E,F), usually with less inhibition for A. radicans than for A. paniculata (Table 2).
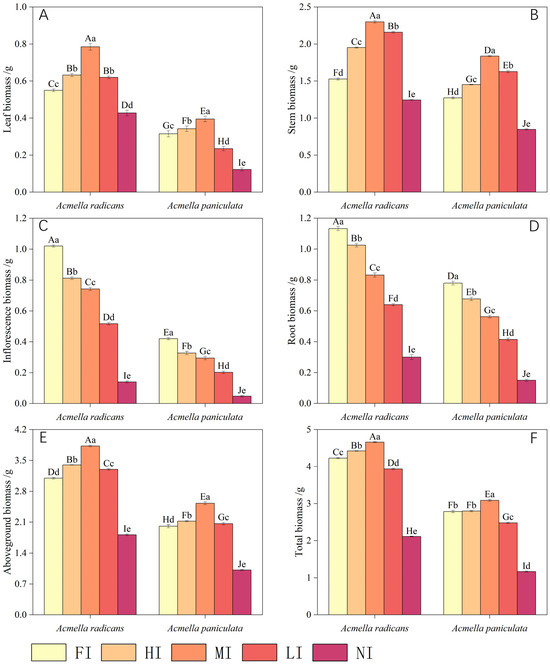
Figure 2.
Plant biomass of Acmella radicans and Acmella paniculata under different light levels [FI = full irradiance (100%), HI = high irradiance (75%), MI = moderate irradiance (50%), LI = low irradiance (25%), NI = non irradiance (5%)] for (A) leaf biomass, (B) stem biomass, (C) inflorescence biomass, (D) root biomass, (E) aboveground biomass, and (F) total biomass. Different small and capital letters above bars indicate a significant difference at 0.05 level for one species and between two species under different treatments, respectively.
3.3. Effect of Light on Physiological Parameters of Acmella radicans and Acmella paniculata
Pn, chlorophyll a, and chlorophyll b content of A. radicans were much higher than for A. paniculata (Figure 3A–C). The Pn of both species was usually greater at higher light intensities but was less at the lowest light intensity (Figure 3A). The chlorophyll levels of A. radicans and A. paniculata at full irradiance were reduced by more 50% compared to other light intensities. Pn, chlorophyll a, and chlorophyll b content of both A. radicans and A. paniculata were initially noticeably increased with decreasing light intensity, and then markedly reduced, with less inhibition observed for A. radicans than for A. paniculata (Table 2).
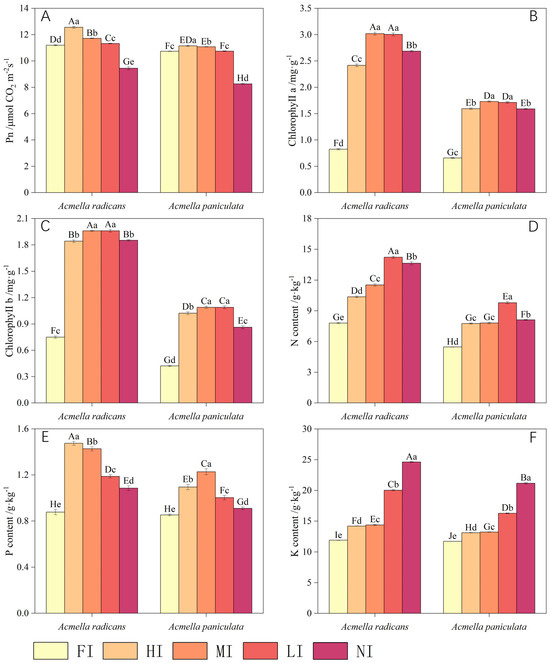
Figure 3.
Photosynthetic and plant nutrient properties of Acmella radicans and Acmella paniculata under different light levels [FI = full irradiance (100%), HI = high irradiance (75%), MI = moderate irradiance (50%), LI = low irradiance (25%), NI = non irradiance (5%)] for (A) Pn, (B) chlorophyll a, (C) chlorophyll b, (D) N content, (E) P content, and (F) K content. Different small and capital letters above bars indicate a significant difference at 0.05 level for one species and between two species under different treatments, respectively.
3.4. Effect of Light on Plant Nutrients of Acmella radicans and Acmella paniculata
Plant N, P, and K content for both A. radicans and A. paniculata were significantly increased, with decreasing light intensity (Figure 3D–F), with less inhibition for A. radicans than for A. paniculata (Table 2). Plant N, P, and K content of A. radicans were higher than those of A. paniculata under the same light conditions (Figure 3D–F).
4. Discussion
Plant growth and reproduction for both A. radicans and A. paniculata responded significantly to a change in light intensity. However, A. radicans appeared to exhibit more efficient resource use, as is common with many invasive plants. Acmella radicans generally exhibited competitive advantages over A. paniculata in terms of having greater plant height, leaf area, biomass, seed number, Pn, chlorophyll content, and plant nutrient content, even at the lowest light level (5% solar irradiance). In addition, from high light intensities to low light intensities, most inhibition rates of plant parameters of A. radicans were less than those of A. paniculata under the same light intensities, indicating that the morphological and physiological characteristics of the invasive species were least affected by low light intensities.
Previous studies showed that A. radicans possessed a large leaf area, a high plant height, a large long-term soil seedbank, a broad seed germination niche, a strong allelopathic effect, and a high adaptability for light and soil water conditions [6,26,27,28,32]. However, there is a lack of comparative studies with its native congeners to determine if these characteristics help facilitate its invasion over native species.
Invasive alien plants are generally more tolerant to environmental stresses than native species. Higher morphological characteristics (e.g., plant height and biomass of plants) tend to increase competitive ability of invasive plants compared to sympatric species [33,34]. However, their population growth and spread may be limited by extreme environmental conditions such as low light levels [14,18,19]. Light is one of the most limiting environmental factors in terms of its ability to restrict population growth and spread of invasive plants [16,17].
Many invasive plants have greater plant height, greater basal stem diameter, and higher biomass than sympatric species to capture more light and soil nutrient resources in shaded environments [29,35,36]. For example, Lantana camara L. (Verbenaceae) exhibits acclimation to low light levels via increased plant height and leaf biomass [37]. Phytolacca americana L. (Phytolaccaceae) had the greatest plant height and total biomass, and the lowest reduction in biomass of each plant part under low light conditions when compared with those of its native congener, P. acinosa Roxb. [29]. This study found that plant height, basal stem diameter, and biomass of A. radicans were all greater than that of A. paniculata, enabling it to utilize limited light and soil nutrient resources more effectively under extreme conditions. In addition, from high light intensities to low light intensities, most inhibition rates of plant height, basal stem diameter, and biomass of A. radicans were less than those of A. paniculata under the same light conditions, indicating that the morphological characteristics of the invasive species were least less affected by low light.
In addition to greater morphological traits, many successful invasive plants are more likely to have a greater leaf area, higher photosynthetic rates, and lower tissue construction costs than native species, leading superior growth rates, biomass accumulation, and overall production [38,39,40,41,42]. As an example, the invasive tree fern Sphaeropteris cooperi (Hook. ex F.Muell.) R.M.Tryon (Cyatheaceae) had approximately five times larger total leaf surface area per plant, higher maximum rates of photosynthesis, and higher chlorophyll content compared to native tree ferns, Cibotium chamissoi Kaulf. (Cibotiaceae), C. menziesii Hook. & Arn. and C. glaucum (Sm.) Hook. & Arn. in the sun and shade [41]. A previous study showed that A. radicans exhibited high growth even at 25% solar irradiance [32], and this study confirmed that both A. radicans and A. paniculata can survive, grow, and reproduce even at 5% solar irradiance. The bigger leaf area, higher Pn, and chlorophyll content of A. radicans compared to that of A. paniculata, demonstrated that the invasive species A. radicans has the potential to outcompete the native species A. paniculata through higher growth rates and more biomass accumulation. Moreover, from high light intensity to low light intensity, most inhibition rates of leaf area, Pn, and chlorophyll content of A. radicans were less than those of A. paniculata under the same light conditions, indicating that the physiological traits of the invasive species were least affected by low-light intensities.
These physiological traits tend to translate into higher reproductive ability than native species [43]. In fact, reproductive ability is positively correlated with the invasiveness of alien plants and high light levels could increase the seed production of invasive plants [40,43,44,45]. For example, high light level increased the production of racemes of both P. americana and its congener P. acinosa, but the invasive P. americana had more racemes than P. acinosa [42]. Similarly, the current study found that A. radicans generally had a greater reproductive ability than that of A. paniculata, and both species can also successfully reproduce even at 5% solar irradiance. However, A. radicans generally had a greater reproductive ability than that of A. paniculata. From high light intensity to low light intensity, inhibition rates of inflorescence number and seed number of A. radicans were less than those of A. paniculata under the same light conditions, showing that the reproductive traits of the invasive species were least affected by low light intensities. Additional data collections are needed to quantify these hypothetical differences in seed biology (e.g., seed length, size, and germination) between A. radicans and A. paniculata to explore its invasion mechanism under different environmental conditions.
Many invasive alien plants employ invasion mechanisms involving alterations of soil conditions with the resultant modifications in nutrient levels and availability which may actually lead to further invasion [33,34,46,47]. Invasive plant species tend to exhibit better functional traits and higher adaptability under high soil nutrient environments compared to surrounding native species [15,48,49,50]. Higher N, P, and K content in plants can be related to higher soil nutrient absorption which can promote increased growth rates, biomass accumulation, and overall production of exotic species [34]. Invasive plant species tend to have higher N and P concentrations in photosynthetic tissues by comparison to their native competitors [48], and rapidly growing invasive species may strategically promote nutrient cycling via resource acquisition [51]. A previous study indicated that intermediate irradiance levels tended to maximize nutrient absorption by A. radicans [32]. In this current study, plant N, P, and K content of A. radicans and A. paniculata increased significantly with increasing shade level, but A. radicans showed less inhibition than A. paniculata. This further demonstrated that A. radicans had higher soil nutrient or fertilizer nutrient absorption ability than A. paniculata. From high light intensity to shade, the inhibition of N, P, and K content of A. radicans was less than that of A. paniculata under the same light conditions.
5. Conclusions
Our results indicated that the invasive plant A. radicans had a greater phenotypic plasticity and higher ability to adapt different light conditions compared to the indigenous species A. paniculata. Except for total branch length and inflorescence number, plant height, leaf area, seed production, biomass, Pn, chlorophyll content, and plant nutrient content of A. radicans were clearly higher than those of A. paniculata under the same light conditions, showing that the invasive species has higher phenotypic plasticity than that of A. paniculata. The morphological and physiological traits of A. radicans and A. paniculata were initially increased with increasing shade before markedly declining at the lowest light levels, with A. radicans generally less inhibited than A. paniculata, indicating that A. radicans is better adapted for shade than A. paniculata. Thus, the higher phenotypic plasticity and environmental adaptability of A. radicans adapted different light conditions by comparison to A. paniculata help make it more invasive. Although these general characteristics have been recognized in A. radicans in previous work, this is the first study to compare them with those of a non-invasive plant in the same genus. In order to improve the better understanding of A. radicans and explore more efficient control methods for A. radicans, additional comparing details on ecological impacts, biochemical traits, and molecular mechanisms of A. radicans and A. paniculata should be further examined.
Author Contributions
X.W.: data curation; investigation; writing—original draft. F.Z. (Fengping Zheng): investigation; supervision. Z.W.: data curation; investigation. Q.L.: investigation. K.Y.: investigation. G.X.: investigation. Y.Y.: investigation. D.R.C.: writing—review and editing. S.Y.: investigation. B.Y.: investigation. G.J.: investigation. S.S.: conceptualization; data curation; funding acquisition; investigation; supervision; writing—review and editing. F.Z. (Fudou Zhang): conceptualization; funding acquisition; project administration. M.D.D.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Yunnan Fundamental Research Project (202501AS070027), National Key Research and Development Program of China (2024YFC2607600), Yunnan Provincial Agricultural Basic Research Joint Special Project (202401BD070001-019), National Natural Science Foundation of China (31960569), Key Research and Development Program of Yunnan Province (202103AF140007, 202203AE140008), and Ten Thousand Talent Program (Young Top-Notch Talent) of Yunnan Province (YNWR-QNBJ-2018-201).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We wish to thank Zewen Fan from the School of Agriculture, Yunnan University, for his great experimental support.
Conflicts of Interest
The authors declare no conflicts of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Change Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, E.J.; Cuthbert, R.N.; Haubrock, P.J.; Taylor, N.G.; Kourantidou, M.; Nguyen, D.; Bang, A.; Turbelin, A.J.; Moodley, D.; Briski, E.; et al. Unevenly distributed biological invasion costs among origin and recipient regions. Nat. Sustain. 2023, 6, 1113–1124. [Google Scholar] [CrossRef]
- Peller, T.; Altermatt, F. Invasive species drive cross-ecosystem effects worldwide. Nat. Ecol. Evol. 2024, 8, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zheng, F.; Zhang, W.; Xu, G.; Li, D.; Yang, S.; Jin, G.; Clements, D.R.; Nikkel, E.; Chen, A.; et al. Potential distribution and ecological impacts of Acmella radicans (Jacquin) R.K. Jansen (a new Yunnan invasive species record) in China. BMC Plant Biol. 2024, 24, 494. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; Eren, Ö.; Hierro, J.L. Community assembly theory as a framework for biological invasions. Trends Ecol. Evol. 2018, 33, 313–325. [Google Scholar] [CrossRef]
- Zheng, Y.; Burns, J.H.; Liao, Z.; Li, Y.; Yang, J.; Chen, Y.; Zhang, J.; Zheng, Y. Species composition, functional and phylogenetic distances correlate with success of invasive Chromolaena odorata in an experimental test. Ecol. Lett. 2018, 21, 1211–1220. [Google Scholar] [CrossRef]
- Guo, K.; Pyšek, P.; van Kleunen, M.; Kinlock, N.L.; Lučanová, M.; Leitch, I.J.; Pierce, S.; Dawson, W.; Essl, F.; Kreft, H.; et al. Plant invasion and naturalization are influenced by genome size, ecology and economic use globally. Nat. Commun. 2024, 15, 1330. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yan, J.; Li, W.; Yin, L.; Li, P.; Yu, H.; Xing, L.; Cai, M.; Wang, H.; Zhao, M.; et al. Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nat. Commun. 2020, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ning, Y.; Li, J.; Shi, Z.; Zhang, Q.; Li, L.; Kang, B.; Du, Z.; Luo, J.; He, M.; et al. Invasion stage and competition intensity co-drive reproductive strategies of native and invasive saltmarsh plants: Evidence from field data. Sci. Total Environ. 2024, 954, 176383. [Google Scholar] [CrossRef] [PubMed]
- Dawson, W.; Burslem, D.F.R.P.; Hulme, P.E. Factors explaining alien plant invasion success in a tropical ecosystem differ at each stage of invasion. J. Ecol. 2009, 97, 657–665. [Google Scholar] [CrossRef]
- Funk, J.L. The physiology of invasive plants in low-resource environments. Conserv. Physiol. 2013, 1, cot026. [Google Scholar] [CrossRef]
- Parendes, L.A.; Jones, J.A. Role of light availability and dispersal in exotic plant invasion along roads and streams in the H. J. Andrews Experimental Forest, Oregon. Conserv. Biol. 2000, 14, 64–75. [Google Scholar] [CrossRef]
- Yu, H.; He, W. Congeneric invasive versus native plants utilize similar inorganic nitrogen forms but have disparate use efficiencies. J. Plant Ecol. 2021, 14, 180–190. [Google Scholar] [CrossRef]
- Quan, G.; Mao, D.; Zhang, J.; Xie, J.; Xu, H.; An, M. Response of invasive Chromolaena odorata and two coexisting weeds to contrasting irradiance and nitrogen. Photosynthetica 2015, 53, 419–429. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Wang, Y.; Li, Q. Long period exposure to serious cadmium pollution benefits an invasive plant (Alternanthera philoxeroides) competing with its native congener (Alternanthera sessilis). Sci. Total Environ. 2021, 786, 147456. [Google Scholar] [CrossRef]
- Kempel, A.; Nater, P.; Fischer, M.; van Kleunen, M. Plant-microbe-herbivore interactions in invasive and non-invasive alien plant species. Funct. Ecol. 2013, 27, 498–508. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.H.; Guo, K.; Guo, W.Y.; Wang, Y.J. Native plant species are more resistant than invasive aliens to escalating environmental change factors. Glob. Change Biol. 2025, 31, e70282. [Google Scholar] [CrossRef]
- Bagga, J.; Deshmukh, U.B. Acmella radicans (Jacquin) R.K. Jansen (Asteraceae)—A new distributional plant record for Jharkhand State (India). J. New Biol. Rep. 2018, 7, 24–27. [Google Scholar]
- Rahman, M.M.; Khan, S.A.; Hossain, G.M.; Jakaria, M.; Rahim, M.A. Acmella radicans (Jacq.) R.K. Jansen (Asteraceae)—A new angiosperm record. Jahangirnagar Univ. J. Biol. Sci. 2016, 5, 87–93. [Google Scholar] [CrossRef]
- Maity, D.; Sardar, A.; Dash, S.S. Acmella radicans (Asteraceae), and American weed new to Eastern India. Nelumbo 2017, 59, 54–57. [Google Scholar] [CrossRef]
- Panyadee, P.; Inta, A. Taxonomy and ethnobotany of Acmella (Asteraceae) in Thailand. Biodiversitas 2022, 23, 2177–2186. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Y.; Wu, X.; Zheng, F.; Xu, G.; Yang, S.; Jin, G.; Clements, D.R.; Shen, S.; Zhang, F. Allelopathic potential and chemical composition of essential oil from the invasive plant Acmella radicans. Agronomy 2024, 14, 342. [Google Scholar] [CrossRef]
- Wu, X.; Yang, K.; Zheng, F.; Xu, G.; Fan, Z.; Clements, D.R.; Yang, Y.; Yang, S.; Jin, G.; Zhang, F.; et al. Effects of Acmella radicans invasion on soil seed bank community characteristics in different habitats. Plants 2024, 13, 2644. [Google Scholar] [CrossRef]
- Wu, X.; Yang, K.; Zheng, F.; Yang, Y.; Xu, G.; Clements, D.R.; Yang, S.; Yao, B.; Jin, G.; Shen, S.; et al. Impact of environmental factors on seed germination and seedling emergence of the invasive plant Acmella radicans. Plant Ecol. 2025, 226, 995–1004. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, C.; Gong, W.; Xiong, Y.; Zhou, Y.; Guo, W.; Li, B.; Wang, Y. Trade-off between shade tolerance and chemical resistance of invasive Phytolacca americana under different light levels compared with its native and exotic non-invasive congeners. Environ. Exp. Bot. 2022, 196, 104809. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Li, C.; Yue, L. GC-MS analysis of volatile oil components from different parts of Acmella paniculata. China Food Addit. 2022, 12, 233–240. [Google Scholar] [CrossRef]
- Patel, S.; Gamit, S.; Qureshimatva, U.; Solanki, H. Distribution patterns of Acmella paniculata (Wall. Ex DC.) R. K. Jansen in Gujarat, India. Int. J. Res. Advent. Technol. 2019, 7, 186–191. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, F.; Xu, G.; Yang, K.; Clements, D.R.; Yang, Y.; Yang, S.; Jin, G.; Zhang, F.; Shen, S. Plant growth and physiological responses of the invasive plant Acmella radicans to contrasting light and soil water conditions. Discov. Life 2024, 54, 11. [Google Scholar] [CrossRef]
- Oduor, A.M.D. Evolutionary responses of native plant species to invasive plants: A review. New Phytol. 2013, 200, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Čuda, J.; Skálová, H.; Janovský, Z.; Pyšek, P. Competition among native and invasive Impatiens species: The roles of environmental factors, population density and life stage. AoB Plants 2015, 7, plv033. [Google Scholar] [CrossRef]
- Wyka, T.; Robakowski, P.; Zytkowiak, R. Acclimation of leaves to contrasting irradiance in juvenile trees differing in shade tolerance. Tree Physiol. 2007, 27, 1293–1306. [Google Scholar] [CrossRef]
- Tecco, P.A.; Díaz, S.; Cabido, M.; Urcelay, C. Functional traits of alien plants across contrasting climatic and land-use regimes: Do aliens join the locals or try harder than them? J. Ecol. 2010, 98, 17–27. [Google Scholar] [CrossRef]
- Carrión-Tacuri, J.; Rubio-Casal, A.E.; de Cires, A.; Figueroa, M.E.; Castillo, J.M. Lantana camara L.: A weed with great light-acclimation capacity. Photosynthetica 2011, 49, 321–329. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. S. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Sang, W. Biomass allocation, morphology and photosynthesis of invasive and noninvasive exotic species grown at four irradiance levels. Acta Oecol. 2007, 31, 40–47. [Google Scholar] [CrossRef]
- Durand, L.Z.; Goldstein, G. Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 2001, 126, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, L.; Chen, C.; Zhou, Y.; Xiao, F.; Wang, Y.; Li, Q. Effect of plant VOCs and light intensity on growth and reproduction performance of an invasive and a native Phytolacca species in China. Ecol. Evol. 2022, 12, e8522. [Google Scholar] [CrossRef] [PubMed]
- Assad, R.; Rashid, I.; Reshi, Z.A.; Sofi, I.A. Invasiveness traits help Amaranths to invade Kashmir Himalaya, India. Trop. Ecol. 2021, 62, 209–217. [Google Scholar] [CrossRef]
- Callaway, J.C.; Josselyn, M.N. The introduction and spread of smooth cordgrass (Spartina alterniflora) in South San Francisco Bay. Estuaries 1992, 15, 218–226. [Google Scholar] [CrossRef]
- Walck, J.L.; Baskin, J.M.; Baskin, C.C. Why is Solidago shortii narrowly endemic and S. altissmima geographically wide spread? A comprehensive comparative study of biological trait. J. Biogeogr. 2001, 28, 1221–1237. [Google Scholar] [CrossRef]
- Callaway, R.M.; Newingham, B.; Zabinski, C.A.; Mahall, B.E. Compensatory growth and competitive ability of an invasive weed are enhanced by soil fungi and native neighbours. Ecol. Lett. 2001, 4, 429–433. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Huang, F.; Wang, R.; Lu, B.; Shen, Y.; Fan, Z.; Lin, P. Nutrient addition increases the capacity for division of labor and the benefits of clonal integration in an invasive plant. Sci. Total Environ. 2018, 643, 1232–1238. [Google Scholar] [CrossRef]
- Sardans, J.; Bartrons, M.; Margalef, O.; Gargallo-Garriga, A.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sigurdsson, B.D.; Chen, H.Y.H.; Peñuelas, J. Plant invasion is associated with higher plant–soil nutrient concentrations in nutrient-poor environments. Glob. Change Biol. 2017, 23, 1282–1291. [Google Scholar] [CrossRef]
- Xun, Z.; Bai, L.; Qu, B.; Xu, Y.; Li, G.; Zhan, Z.; Shi, J. Effect of nitrogen treatments on growth of the invasive plant Xanthium strumarium, the native plant Xanthium sibiricum, and their reciprocal crosses. Acta Pratacult. Sin. 2017, 26, 51–61. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Y.; Ma, J.; Wang, H.; Wang, K.; Wang, T.; Jiang, S.; Jiao, J.; Sun, Y.; Jiang, X.; et al. Nitrogen deposition effects on invasive and native plant competition: Implications for future invasions. Ecotox. Environ. Safe. 2023, 259, 115029. [Google Scholar] [CrossRef]
- Fridley, J.D.; Craddock, A. Contrasting growth phenology of native and invasive forest shrubs mediated by genome size. New Phytol. 2015, 207, 659–668. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).