Drivers of Alpine Mire Vegetation at Their Range Limit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Study of Environmental Variables and Vegetation
2.2.1. Sampling Plots
2.2.2. Vegetation Relevés
2.2.3. Water Table Depth
2.2.4. Water Chemistry
2.3. Statistical Analyses
2.3.1. Numerical Classification
2.3.2. Ordination Analyses
3. Results
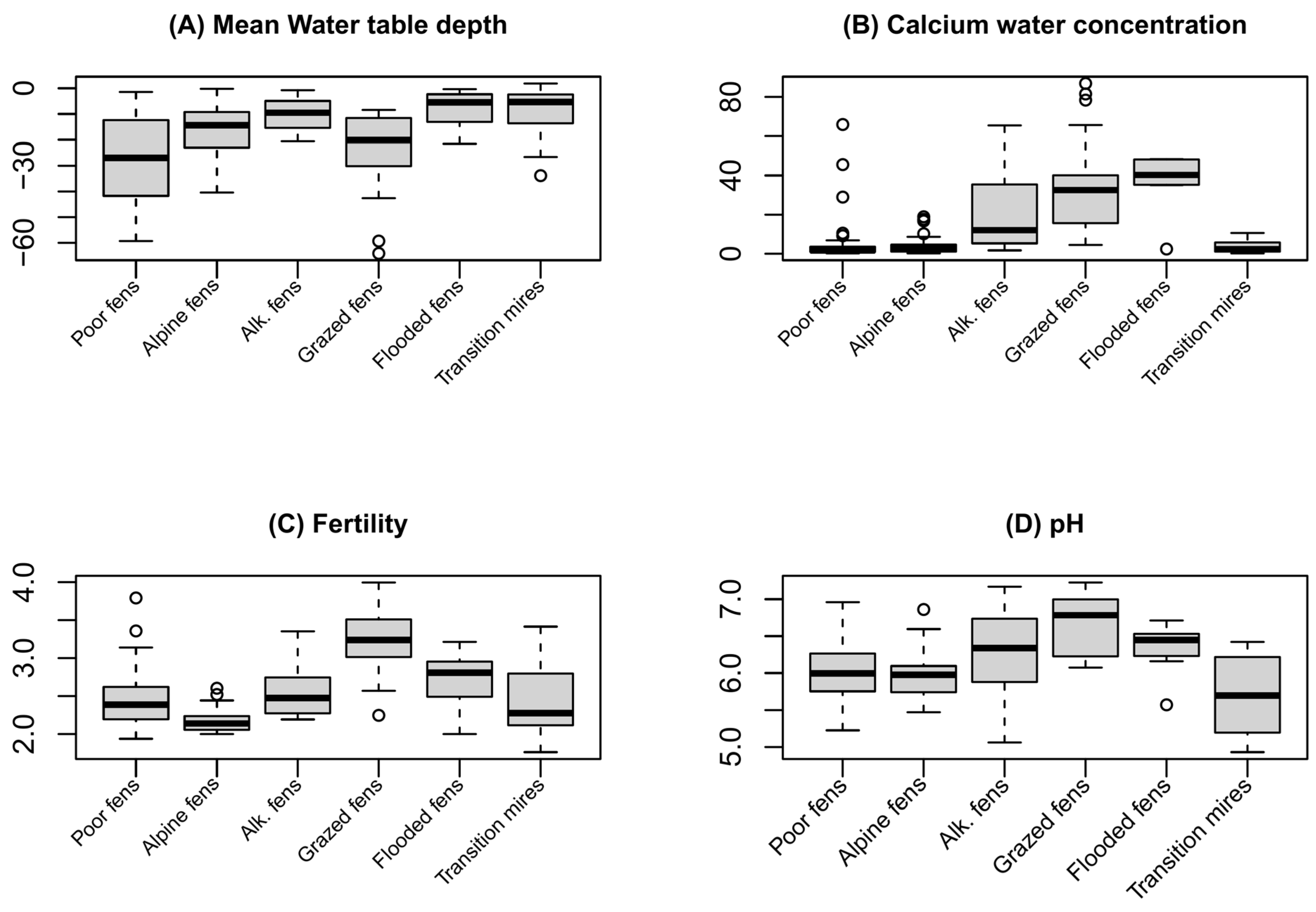
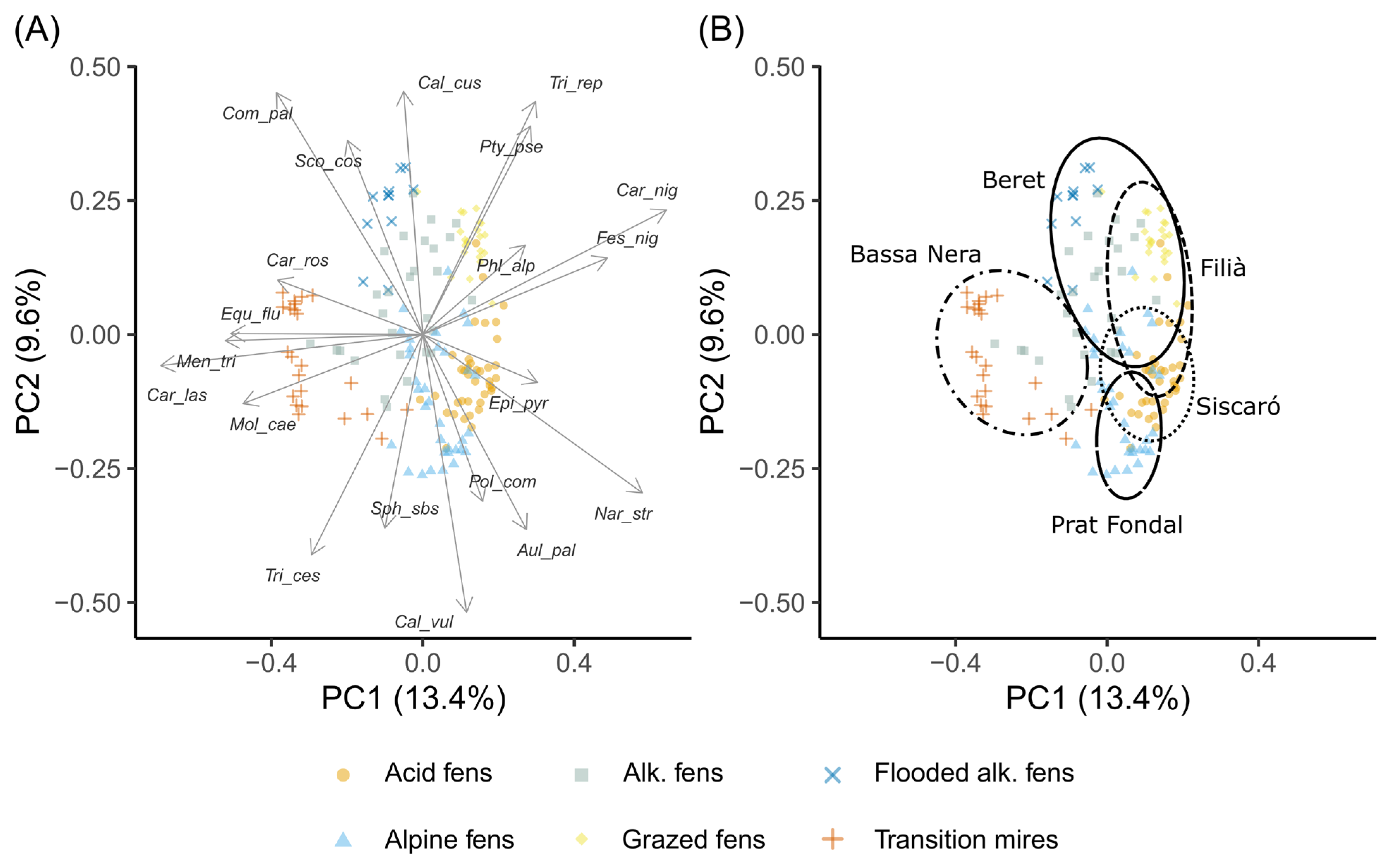
3.1. Plant Vegetation Types
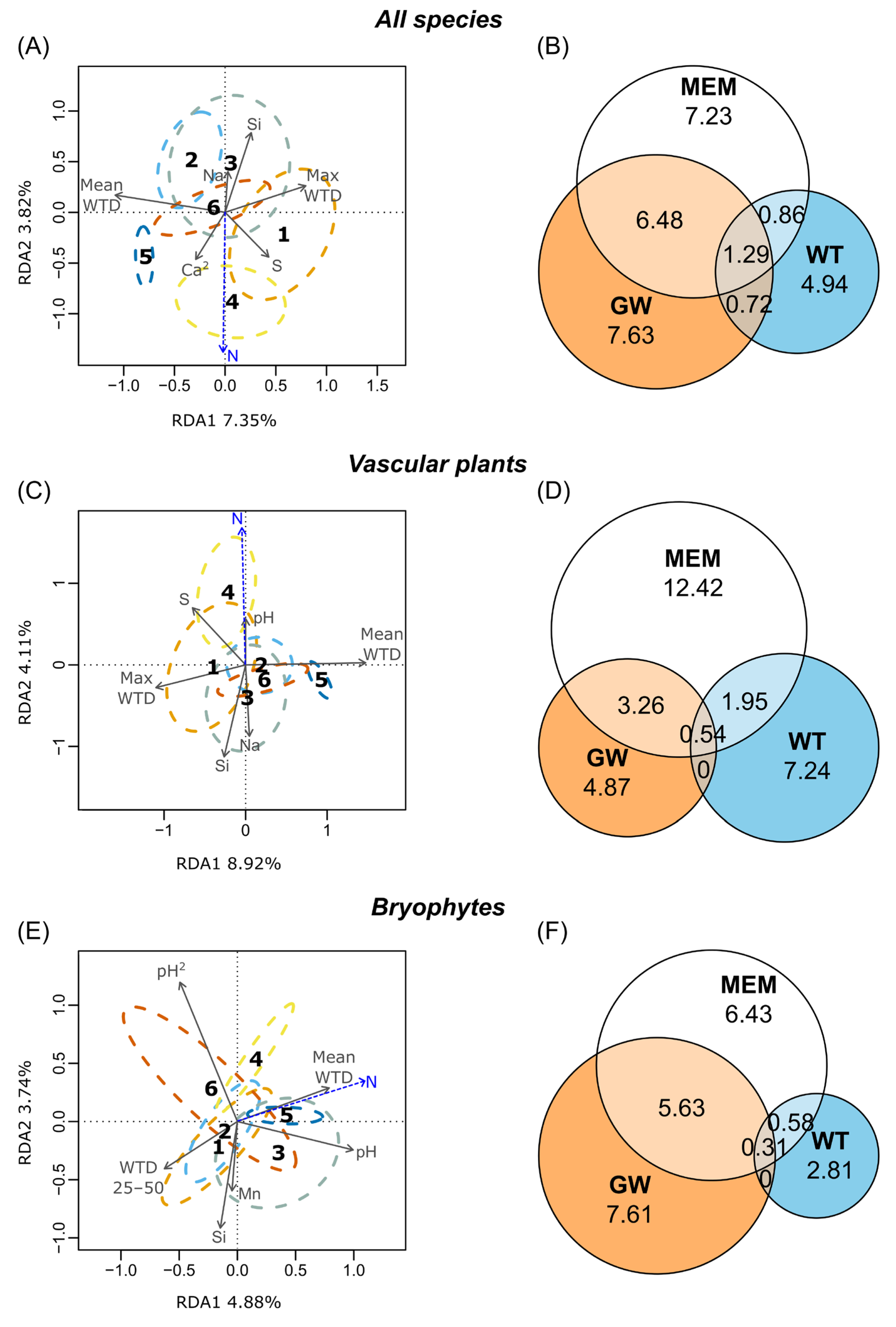
3.2. tb-PCA and RDA Ordinations
4. Discussion
4.1. Vegetation Types
4.2. Environmental Drivers of the Plant Communities
4.3. Comparison of Ecological Drivers of Vascular Plants and Bryophytes
4.4. The Role of Spatial Structure on Vegetation Patterns
4.5. Future Trajectories of Pyrenean Mires
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | Group | Phi Coefficient | Taxon | Group | Phi Coefficient |
|---|---|---|---|---|---|
| Festuca nigrescens | 1. Poor fens | 0.67 | Anthoxanthum odoratum | 3. Alk. fens | 0.52 |
| Carex echinata | 1. Poor fens | 0.67 | Eriophorum latifolium | 3. Alk. fens | 0.52 |
| Viola palustris | 1. Poor fens | 0.64 | Pinguicula grandiflora | 3. Alk. fens | 0.46 |
| Nardus stricta | 1. Poor fens | 0.63 | Pedicularis pyrenaica | 3. Alk. fens | 0.46 |
| Pedicularis pyrenaica | 1. Poor fens | 0.62 | Juncus pyrenaeus | 3. Alk. fens | 0.45 |
| Aulacomnium palustre | 1. Poor fens | 0.6 | Alchemilla vulgaris | 3. Alk. fens | 0.44 |
| Luzula sudetica | 1. Poor fens | 0.59 | Caltha palustris | 3. Alk. fens | 0.43 |
| Trifolium alpinum | 1. Poor fens | 0.56 | Thalictrum alpinum | 3. Alk. fens | 0.42 |
| Potentilla erecta | 1. Poor fens | 0.55 | Tomentypnum nitens | 3. Alk. fens | 0.41 |
| Trifolium spadiceum | 1. Poor fens | 0.51 | Riccardia chamaedryfolia | 3. Alk. fens | 0.38 |
| Trifolium pratense | 1. Poor fens | 0.51 | Trifolium repens | 4. Grazed fens | 0.8 |
| Dactylorhiza maculata | 1. Poor fens | 0.5 | Ptytochosmum pseudotriquetrum | 4. Grazed fens | 0.75 |
| Dicranum bonjeanii | 1. Poor fens | 0.47 | Ranunculus acris | 4. Grazed fens | 0.73 |
| Gentiana pyrenaica | 1. Poor fens | 0.47 | Scorzoneroides carpetana subsp. duboisii | 4. Grazed fens | 0.64 |
| Euphrasia stricta | 1. Poor fens | 0.46 | Agrostis capillaris | 4. Grazed fens | 0.61 |
| Scapania irrigua | 1. Poor fens | 0.46 | Palustriella falcata | 4. Grazed fens | 0.59 |
| Sphagnum russowii | 1. Poor fens | 0.43 | Phleum alpinum | 4. Grazed fens | 0.56 |
| Juncus filiformis | 1. Poor fens | 0.42 | Poa annua | 4. Grazed fens | 0.56 |
| Rhinanthus minor | 1. Poor fens | 0.4 | Plantago media | 4. Grazed fens | 0.55 |
| Agrostis canina | 2. Alpine fens | 0.87 | Veronica serpyllifolia | 4. Grazed fens | 0.53 |
| Festuca airoides | 2. Alpine fens | 0.75 | Carex nigra | 4. Grazed fens | 0.49 |
| Pinguicula vulgaris | 2. Alpine fens | 0.71 | Gentiana verna | 4. Grazed fens | 0.48 |
| Ranunculus pyrenaeus | 2. Alpine fens | 0.69 | Philonotis calcarea | 4. Grazed fens | 0.43 |
| Eriophorum angustifolium | 2. Alpine fens | 0.67 | Galium uliginosum | 4. Grazed fens | 0.41 |
| Primula integrifolia | 2. Alpine fens | 0.63 | Cerastium fontanum | 4. Grazed fens | 0.41 |
| Polytrichum commune | 2. Alpine fens | 0.61 | Comarum palustre | 5. Flooded fens | 0.84 |
| Sphagnum subsecundum | 2. Alpine fens | 0.57 | Calliergonella cuspidata | 5. Flooded fens | 0.78 |
| Straminergon stramineum | 2. Alpine fens | 0.51 | Eleocharis quinqueflora | 5. Flooded fens | 0.67 |
| Sarmentypnum exannulatum | 2. Alpine fens | 0.49 | Scorpidium cossonii | 5. Flooded fens | 0.59 |
| Euphrasia minima | 2. Alpine fens | 0.48 | Juncus articulatus | 5. Flooded fens | 0.55 |
| Cetraria islandica | 2. Alpine fens | 0.44 | Epilobium palustre | 5. Flooded fens | 0.46 |
| Salix lapponum | 2. Alpine fens | 0.4 | Carex lasiocarpa | 6. Transition mires | 0.95 |
| Carex davalliana | 3. Alk. fens | 0.9 | Equisetum fluviatile | 6. Transition mires | 0.87 |
| Primula farinosa | 3. Alk. fens | 0.85 | Menyanthes trifoliata | 6. Transition mires | 0.81 |
| Briza media | 3. Alk. fens | 0.79 | Molinia caerulea | 6. Transition mires | 0.65 |
| Bartsia alpina | 3. Alk. fens | 0.75 | Sphagnum papillosum | 6. Transition mires | 0.64 |
| Campylium stellatum | 3. Alk. fens | 0.7 | Sphagnum divinum | 6. Transition mires | 0.63 |
| Tofieldia calyculata | 3. Alk. fens | 0.68 | Sphagnum subnitens | 6. Transition mires | 0.55 |
| Valeriana dioica | 3. Alk. fens | 0.63 | Drosera anglica | 6. Transition mires | 0.53 |
| Carex panicea | 3. Alk. fens | 0.63 | Drosera rotundifolia | 6. Transition mires | 0.48 |
| Succisa pratensis | 3. Alk. fens | 0.61 | Melampyrum pratense | 6. Transition mires | 0.42 |
| Selaginella selaginoides | 3. Alk. fens | 0.58 | Utricularia minor | 6. Transition mires | 0.42 |
| Prunella vulgaris | 3. Alk. fens | 0.56 | Sphagnum angustifolium | 6. Transition mires | 0.42 |
| Sphagnum warnstorfii | 3. Alk. fens | 0.53 | Vaccinium myrtillus | 6. Transition mires | 0.41 |
| Species | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Agrostis canina | 0.095 | −0.469 | 0.633 | −0.387 |
| Aulacomnium palustre | 0.453 | −0.653 | 0.256 | 0.250 |
| Calliergonella cuspidata | −0.084 | 0.814 | 0.484 | −0.107 |
| Calluna vulgaris | 0.191 | −0.930 | −0.163 | −0.311 |
| Campylium stellatum | −0.226 | 0.123 | −0.402 | −0.627 |
| Carex davalliana | −0.171 | 0.344 | −0.733 | −0.929 |
| Carex flava agg. | 0.157 | 0.232 | 0.185 | −0.817 |
| Carex lasiocarpa | −1.144 | −0.104 | −0.173 | 0.654 |
| Carex nigra | 1.064 | 0.417 | 0.718 | −0.582 |
| Carex panicea | −0.201 | 0.339 | −0.488 | −0.795 |
| Carex rostrata | −0.635 | 0.182 | 0.358 | 0.421 |
| Comarum palustre | −0.639 | 0.810 | 0.775 | 0.419 |
| Eleocharis quinqueflora | −0.020 | 0.489 | 0.208 | −0.690 |
| Epikeros pyrenaeum | 0.501 | −0.160 | −0.155 | −0.069 |
| Equisetum fluviatile | −0.839 | 0.003 | −0.068 | 0.523 |
| Eriophorum angustifolium | −0.076 | −0.475 | 0.404 | −0.572 |
| Festuca airoides | 0.138 | −0.536 | 0.638 | −0.423 |
| Festuca nigrescens | 0.806 | 0.256 | −0.691 | 0.669 |
| Menyanthes trifoliata | −0.863 | −0.021 | 0.109 | 0.673 |
| Molinia caerulea | −0.785 | −0.231 | −0.599 | 0.034 |
| Nardus stricta | 0.959 | −0.530 | −0.363 | 0.881 |
| Palustriella falcata | 0.203 | 0.503 | 0.049 | −0.183 |
| Polytrichum commune | 0.262 | −0.559 | 0.520 | −0.116 |
| Potentilla erecta | 0.365 | −0.341 | −1.099 | 0.185 |
| Ptychostomum pseudotriquetrum | 0.471 | 0.698 | −0.098 | 0.095 |
| Scorpidium cossonii | −0.329 | 0.650 | −0.098 | −0.671 |
| Sphagnum subsecundum | −0.166 | −0.648 | 0.580 | −0.108 |
| Sphagnum warnstorfii | 0.042 | −0.129 | −0.539 | −0.275 |
| Straminergon stramineum | −0.045 | −0.503 | 0.318 | −0.047 |
| Succisa pratensis | 0.193 | −0.282 | −0.903 | 0.061 |
| Trichophorum cespitosum | −0.486 | −0.738 | −0.791 | −0.620 |
| Trifolium repens | 0.493 | 0.780 | −0.128 | 0.442 |
| Variable | Ca | Al | Mg | P | S | Fe | Na | Si | Zn | Mn | pH | EC | WTD Above0 | WTD 0_10 | WTD 10_25 | WTD 25_50 | WTD 50_75 | Mean WTD | Min WTD | Max WTD | WT_Fluctuation | Slope | Lon |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | 1.00 | −0.09 | 0.75 | −0.09 | 0.37 | −0.03 | 0.25 | −0.36 | −0.22 | 0.10 | 0.69 | 0.90 | −0.12 | 0.19 | 0.00 | −0.16 | −0.08 | 0.15 | −0.10 | −0.05 | −0.09 | 0.33 | −0.45 |
| Al | −0.09 | 1.00 | −0.09 | 0.19 | 0.20 | 0.21 | 0.33 | 0.02 | 0.11 | 0.02 | −0.04 | 0.11 | −0.07 | −0.27 | −0.03 | 0.29 | 0.24 | −0.38 | 0.48 | 0.15 | 0.47 | 0.07 | 0.01 |
| Mg | 0.75 | −0.09 | 1.00 | −0.09 | 0.63 | −0.07 | 0.05 | −0.25 | −0.20 | 0.18 | 0.65 | 0.68 | −0.08 | 0.14 | −0.08 | −0.04 | 0.03 | 0.07 | −0.05 | 0.01 | −0.06 | 0.21 | −0.39 |
| P | −0.09 | 0.19 | −0.09 | 1.00 | 0.01 | 0.11 | 0.13 | 0.07 | 0.07 | −0.04 | −0.20 | 0.01 | −0.04 | −0.21 | 0.03 | 0.30 | 0.02 | −0.19 | 0.20 | 0.19 | 0.16 | −0.02 | 0.03 |
| S | 0.37 | 0.20 | 0.63 | 0.01 | 1.00 | −0.12 | 0.13 | −0.20 | −0.02 | 0.03 | 0.35 | 0.41 | 0.01 | −0.02 | −0.18 | 0.18 | 0.14 | −0.15 | 0.18 | 0.09 | 0.16 | 0.22 | −0.17 |
| Fe | −0.03 | 0.21 | −0.07 | 0.11 | −0.12 | 1.00 | 0.17 | 0.31 | −0.02 | −0.04 | −0.17 | 0.07 | 0.02 | −0.14 | 0.06 | 0.08 | 0.12 | −0.12 | 0.19 | −0.05 | 0.22 | −0.01 | 0.11 |
| Na | 0.25 | 0.33 | 0.05 | 0.13 | 0.13 | 0.17 | 1.00 | 0.01 | 0.14 | 0.00 | 0.17 | 0.46 | −0.08 | −0.10 | −0.04 | 0.12 | 0.14 | −0.17 | 0.29 | 0.13 | 0.27 | −0.12 | −0.07 |
| Si | −0.36 | 0.02 | −0.25 | 0.07 | −0.20 | 0.31 | 0.01 | 1.00 | 0.32 | −0.09 | −0.29 | −0.25 | −0.03 | −0.34 | 0.03 | 0.27 | 0.40 | −0.37 | 0.29 | 0.15 | 0.27 | −0.21 | 0.59 |
| Zn | −0.22 | 0.11 | −0.20 | 0.07 | −0.02 | −0.02 | 0.14 | 0.32 | 1.00 | −0.05 | −0.11 | −0.14 | −0.05 | −0.24 | 0.11 | 0.18 | 0.03 | −0.19 | 0.21 | 0.00 | 0.23 | −0.23 | 0.52 |
| Mn | 0.10 | 0.02 | 0.18 | −0.04 | 0.03 | −0.04 | 0.00 | −0.09 | −0.05 | 1.00 | 0.14 | 0.09 | −0.03 | −0.08 | 0.07 | 0.04 | 0.04 | −0.07 | 0.08 | −0.06 | 0.11 | 0.03 | −0.10 |
| pH | 0.69 | −0.04 | 0.65 | −0.20 | 0.35 | −0.17 | 0.17 | −0.29 | −0.11 | 0.14 | 1.00 | 0.66 | −0.02 | 0.08 | −0.03 | −0.09 | −0.01 | 0.03 | −0.01 | −0.09 | 0.01 | 0.30 | −0.33 |
| EC | 0.90 | 0.11 | 0.68 | 0.01 | 0.41 | 0.07 | 0.46 | −0.25 | −0.14 | 0.09 | 0.66 | 1.00 | −0.10 | 0.11 | −0.04 | −0.07 | 0.03 | 0.03 | 0.07 | −0.04 | 0.09 | 0.25 | −0.41 |
| WTD_above0 | −0.12 | −0.07 | −0.08 | −0.04 | 0.01 | 0.02 | −0.08 | −0.03 | −0.05 | −0.03 | −0.02 | −0.10 | 1.00 | 0.05 | −0.21 | −0.14 | −0.11 | 0.24 | −0.21 | −0.34 | −0.12 | −0.09 | 0.02 |
| WTD_0_10 | 0.19 | −0.27 | 0.14 | −0.21 | −0.02 | −0.14 | −0.10 | −0.34 | −0.24 | −0.08 | 0.08 | 0.11 | 0.05 | 1.00 | −0.57 | −0.63 | −0.42 | 0.77 | −0.70 | −0.57 | −0.58 | −0.05 | −0.32 |
| WTD_10_25 | 0.00 | −0.03 | −0.08 | 0.03 | −0.18 | 0.06 | −0.04 | 0.03 | 0.11 | 0.07 | −0.03 | −0.04 | −0.21 | −0.57 | 1.00 | −0.10 | −0.17 | −0.07 | 0.08 | 0.21 | 0.02 | 0.17 | −0.02 |
| WTD_25_50 | −0.16 | 0.29 | −0.04 | 0.30 | 0.18 | 0.08 | 0.12 | 0.27 | 0.18 | 0.04 | −0.09 | −0.07 | −0.14 | −0.63 | −0.10 | 1.00 | 0.34 | −0.68 | 0.60 | 0.65 | 0.44 | −0.07 | 0.32 |
| WTD_50_75 | −0.08 | 0.24 | 0.03 | 0.02 | 0.14 | 0.12 | 0.14 | 0.40 | 0.03 | 0.04 | −0.01 | 0.03 | −0.11 | −0.42 | −0.17 | 0.34 | 1.00 | −0.76 | 0.73 | 0.27 | 0.69 | −0.04 | 0.23 |
| Mean_WTD | 0.15 | −0.38 | 0.07 | −0.19 | −0.15 | −0.12 | −0.17 | −0.37 | −0.19 | −0.07 | 0.03 | 0.03 | 0.24 | 0.77 | −0.07 | −0.68 | −0.76 | 1.00 | −0.90 | −0.54 | −0.79 | 0.00 | −0.33 |
| Min_WTD | −0.10 | 0.48 | −0.05 | 0.20 | 0.18 | 0.19 | 0.29 | 0.29 | 0.21 | 0.08 | −0.01 | 0.07 | −0.21 | −0.70 | 0.08 | 0.60 | 0.73 | −0.90 | 1.00 | 0.35 | 0.96 | −0.04 | 0.29 |
| Max_WTD | −0.05 | 0.15 | 0.01 | 0.19 | 0.09 | −0.05 | 0.13 | 0.15 | 0.00 | −0.06 | −0.09 | −0.04 | −0.34 | −0.57 | 0.21 | 0.65 | 0.27 | −0.54 | 0.35 | 1.00 | 0.08 | 0.04 | 0.05 |
| WT_fluctuation | −0.09 | 0.47 | −0.06 | 0.16 | 0.16 | 0.22 | 0.27 | 0.27 | 0.23 | 0.11 | 0.01 | 0.09 | −0.12 | −0.58 | 0.02 | 0.44 | 0.69 | −0.79 | 0.96 | 0.08 | 1.00 | −0.05 | 0.29 |
| Slope | 0.33 | 0.07 | 0.21 | −0.02 | 0.22 | −0.01 | −0.12 | −0.21 | −0.23 | 0.03 | 0.30 | 0.25 | −0.09 | −0.05 | 0.17 | −0.07 | −0.04 | 0.00 | −0.04 | 0.04 | −0.05 | 1.00 | −0.19 |
| Lon | −0.45 | 0.01 | −0.39 | 0.03 | −0.17 | 0.11 | −0.07 | 0.59 | 0.52 | −0.10 | −0.33 | −0.41 | 0.02 | −0.32 | −0.02 | 0.32 | 0.23 | −0.33 | 0.29 | 0.05 | 0.29 | −0.19 | 1.00 |
| Forward Selection Steps | Variable | Adjusted R2 | Pseudo-F Ratio | p-Value |
|---|---|---|---|---|
| Combined dataset | ||||
| 1 | Mean_WTD | 0.041 | 8.292 | 0.001 |
| 2 | Si | 0.059 | 4.377 | 0.001 |
| 3 | Ca | 0.073 | 3.531 | 0.001 |
| 4 | Mean_WTD2 | 0.083 | 2.948 | 0.001 |
| 5 | pH | 0.091 | 2.506 | 0.001 |
| 6 | pH2 | 0.100 | 2.579 | 0.001 |
| 7 | S | 0.107 | 2.411 | 0.001 |
| 8 | Na | 0.114 | 2.295 | 0.002 |
| 9 | Fe | 0.120 | 2.064 | 0.001 |
| 10 | Max_WTD | 0.125 | 1.938 | 0.004 |
| 11 | Ca2 | 0.129 | 1.769 | 0.010 |
| 12 | Mn | 0.133 | 1.764 | 0.008 |
| Vascular plants | ||||
| 1 | Mean_WTD | 0.055 | 11.281 | 0.001 |
| 2 | Si | 0.075 | 4.961 | 0.001 |
| 3 | Ca | 0.087 | 3.307 | 0.001 |
| 4 | S | 0.099 | 3.353 | 0.001 |
| 5 | Mean_WTD2 | 0.110 | 3.089 | 0.001 |
| 6 | pH | 0.118 | 2.603 | 0.002 |
| 7 | Na | 0.124 | 2.264 | 0.005 |
| 8 | Max_WTD | 0.129 | 2.062 | 0.008 |
| Bryophytes | ||||
| 1 | pH | 0.027 | 5.619 | 0.001 |
| 2 | pH2 | 0.046 | 4.282 | 0.001 |
| 3 | Mean_WTD | 0.064 | 4.114 | 0.001 |
| 4 | Si | 0.077 | 3.300 | 0.001 |
| 5 | Fe | 0.086 | 2.738 | 0.001 |
| 6 | WTD_25_50 | 0.094 | 2.439 | 0.004 |
| 7 | Mn | 0.102 | 2.287 | 0.003 |
References
- Molina, J.A. Aquatic and Wetland Vegetation of the Iberian Peninsula. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Plant and Vegetation; Springer International Publishing: Cham, Switzerland, 2017; Volume 13, pp. 355–396. ISBN 978-3-319-54866-1. [Google Scholar]
- Ninot, J.M.; Carreras, J.; Carrillo, E.; Vigo, J. Syntaxonomic Conspectus of the Vegetation of Catalonia and Andorra. I: Hygrophilous herbaceous communities. Acta Bot. Barc. 2000, 46, 191–237. [Google Scholar]
- Pérez-Haase, A.; Ninot, J.M. Hydrological Heterogeneity Rather than Water Chemistry Explains the High Plant Diversity and Uniqueness of a Pyrenean Mixed Mire. Folia Geobot. 2017, 52, 143–160. [Google Scholar] [CrossRef]
- Økland, R.H. A Phytoecological Study of the Mire Northern Kisselbergmosen, SE Norway. II. Identification of Gradients by Detrended (Canonical) Correspondence Analysis. Nord. J. Bot. 1990, 10, 79–108. [Google Scholar] [CrossRef]
- Wheeler, B.D.; Proctor, M.C.F. Ecological Gradients, Subdivisions and Terminology of North-west European Mires. J. Ecol. 2000, 88, 187–203. [Google Scholar] [CrossRef]
- Bragazza, L.; Rydin, H.; Gerdol, R. Multiple Gradients in Mire Vegetation: A Comparison of a Swedish and an Italian Bog. Plant Ecol. 2005, 177, 223–236. [Google Scholar] [CrossRef]
- Harbert, B.L.; Cooper, D.J. Environmental Drivers of Subalpine and Alpine Fen Vegetation in the Southern Rocky Mountains, Colorado, USA. Plant Ecol. 2017, 218, 885–898. [Google Scholar] [CrossRef]
- Benavides, J.C.; Vitt, D.H.; Cooper, D.J. The High-Elevation Peatlands of the Northern Andes, Colombia. Plants 2023, 12, 955. [Google Scholar] [CrossRef]
- Sjörs, H. On the Relation between Vegetation and Electrolytes in North Swedish Mire Waters. Oikos 1950, 2, 241. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Pastor, J.; Janssens, J.A.; Chapin, C.; Malterer, T.J. Multiple Limiting Gradients in Peatlands: A Call for a New Paradigm. Wetlands 1996, 16, 45–65. [Google Scholar] [CrossRef]
- Sjörs, H.; Gunnarsson, U. Calcium and pH in North and Central Swedish Mire Waters. J. Ecol. 2002, 90, 650–657. [Google Scholar] [CrossRef]
- Laitinen, J.; Nyberg, J.; Kaakinen, E.; Küttim, M.; Muurinen, L.; Ulvinen, T.; Virtanen, R.; Tahvanainen, T. Site Types, Species Composition, Species Richness and Ecological Gradients of Rich Fens in the Oulanka Region, North-Boreal Finland. Phytocoenologia 2024, 52, 1–27. [Google Scholar] [CrossRef]
- Bragazza, L.; Gerdol, R. Are Nutrient Availability and Acidity-alkalinity Gradients Related in Sphagnum-dominated Peatlands? J. Veg. Sci. 2002, 13, 473–482. [Google Scholar] [CrossRef]
- Hájek, M.; Hekera, P.; Hájková, P. Spring Fen Vegetation and Water Chemistry in the Western Carpathian Flysch Zone. Folia Geobot. 2002, 37, 205–224. [Google Scholar] [CrossRef]
- Sekulová, L.; Hájek, M.; Hájková, P.; Mikulášková, E.; Rozbrojová, Z. Alpine Wetlands in the West Carpathians: Vegetation Survey and Vegetation–Environment Relationships. Preslia 2011, 83, 1–24. [Google Scholar]
- Peterka, T.; Hájek, M.; Jiroušek, M.; Jiménez-Alfaro, B.; Aunina, L.; Bergamini, A.; Dítě, D.; Felbaba-Klushyna, L.; Graf, U.; Hájková, P.; et al. Formalized Classification of European Fen Vegetation at the Alliance Level. Appl. Veg. Sci. 2017, 20, 124–142. [Google Scholar] [CrossRef]
- Jiroušek, M.; Peterka, T.; Chytrý, M.; Jiménez-Alfaro, B.; Kuznetsov, O.L.; Pérez-Haase, A.; Aunina, L.; Biurrun, I.; Dítě, D.; Goncharova, N.; et al. Classification of European Bog Vegetation of the Oxycocco-Sphagnetea Class. Appl. Veg. Sci. 2022, 25, e12646. [Google Scholar] [CrossRef]
- Tanneberger, F.; Moen, A.; Barthelmes, A.; Lewis, E.; Miles, L.; Sirin, A.; Tegetmeyer, C.; Joosten, H. Mires in Europe—Regional Diversity, Condition and Protection. Diversity 2021, 13, 381. [Google Scholar] [CrossRef]
- Sekulová, L.; Hájek, M.; Syrovátka, V. Vegetation–Environment Relationships in Alpine Mires of the West Carpathians and the Alps. J. Veg. Sci. 2013, 24, 1118–1128. [Google Scholar] [CrossRef]
- Hrivnák, R.; Hájek, M.; Blanár, D.; Kochjarová, J.; Hájková, P. Mire Vegetation of the Muránska Planina Mts—Formalised Classification, Ecology, Main Environmental Gradient and Influence of Geographical Position. Biologia 2008, 63, 368–377. [Google Scholar] [CrossRef]
- Damman, A.W.H.; French, T.W. The Ecology of Peat Bogs of the Glaciated Northeastern United States: A Community Profile; (Biological) Report; Fish and Wildlife Service, U.S. Department of the Interior: Washington, DC, USA, 1987; Volume 85 (7.16). [Google Scholar]
- Malmer, N. Vegetational Gradients in Relation to Environmental Conditions in Northwestern European Mires. Can. J. Bot. 1986, 64, 375–383. [Google Scholar] [CrossRef]
- Vitt, D.H.; Chee, W.-L. The Relationships of Vegetation to Surface Water Chemistry and Peat Chemistry in Fens of Alberta, Canada. Vegetatio 1990, 89, 87–106. [Google Scholar] [CrossRef]
- Bragazza, L.; Gerdol, R. Hydrology, Groundwater Chemistry and Peat Chemistry in Relation to Habitat Conditions in a Mire on the South—Eastern Alps of Italy. Plant Ecol. 1999, 144, 243–256. [Google Scholar] [CrossRef]
- Økland, R.H.; Økland, T.; Rydgren, K. A Scandinavian Perspective on Ecological Gradients in North-west European Mires: Reply to Wheeler and Proctor. J. Ecol. 2001, 89, 481–486. [Google Scholar] [CrossRef]
- Hájek, M.; Horsák, M.; Hájková, P.; Dítě, D. Habitat Diversity of Central European Fens in Relation to Environmental Gradients and an Effort to Standardise Fen Terminology in Ecological Studies. Perspect. Plant Ecol. Evol. Syst. 2006, 8, 97–114. [Google Scholar] [CrossRef]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-960299-5. [Google Scholar]
- Hájková, P.; Hájek, M.; Apostolova, I.; Zelený, D.; Dítě, D. Shifts in the Ecological Behaviour of Plant Species between Two Distant Regions: Evidence from the Base Richness Gradient in Mires. J. Biogeogr. 2008, 35, 282–294. [Google Scholar] [CrossRef]
- Hájek, M.; Těšitel, J.; Tahvanainen, T.; Peterka, T.; Jiménez-Alfaro, B.; Jansen, F.; Pérez-Haase, A.; Garbolino, E.; Carbognani, M.; Kolari, T.H.M.; et al. Rising Temperature Modulates pH Niches of Fen Species. Glob. Change Biol. 2022, 28, 1023–1037. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Lamentowicz, Ł.; Van Der Knaap, W.O.; Gąbka, M.; Mitchell, E.A.D. Contrasting Species—Environment Relationships in Communities of Testate Amoebae, Bryophytes and Vascular Plants Along the Fen–Bog Gradient. Microb. Ecol. 2010, 59, 499–510. [Google Scholar] [CrossRef]
- Horsák, M.; Hájek, M. Composition and Species Richness of Molluscan Communities in Relation to Vegetation and Water Chemistry in the Western Carpathian Spring Fens: The Poor–Rich Gradient. J. Molluscan Stud. 2003, 69, 349–357. [Google Scholar] [CrossRef]
- Hájková, P.; Hájek, M. Bryophyte and Vascular Plant Responses to Base-Richness and Water Level Gradients in Western CarpathianSphagnum-Rich Mires. Folia Geobot. 2004, 39, 335–351. [Google Scholar] [CrossRef]
- Hettenbergerová, E.; Hájek, M.; Zelený, D.; Jiroušková, J. Changes in Species Richness and Species Composition of Vascular Plants and Bryophytes along a Moisture Gradient. Preslia 2013, 85, 369–388. [Google Scholar]
- Hájek, M.; Poulíčková, A.; Vašutová, M.; Syrovátka, V.; Jiroušek, M.; Štěpánková, J.; Opravilová, V.; Hájková, P. Small Ones and Big Ones: Cross-Taxon Congruence Reflects Organism Body Size in Ombrotrophic Bogs. Hydrobiologia 2014, 726, 95–107. [Google Scholar] [CrossRef]
- Essl, F.; Dullinger, S.; Moser, D.; Rabitsch, W.; Kleinbauer, I. Vulnerability of Mires under Climate Change: Implications for Nature Conservation and Climate Change Adaptation. Biodivers. Conserv. 2012, 21, 655–669. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Zhao, L.; Fu, S.; Cui, Y.; Fulati, G.; Wang, X.; Zhou, J. Dynamic Monitoring and Restorability Evaluation of Alpine Wetland in the Eastern Edge of Qinghai–Tibet Plateau. Glob. Ecol. Conserv. 2024, 51, e02948. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; García-Calvo, L.; García, P.; Acebes, J.L. Anticipating Extinctions of Glacial Relict Populations in Mountain Refugia. Biol. Conserv. 2016, 201, 243–251. [Google Scholar] [CrossRef]
- Colomer, J.; Pérez-Haase, A.; Carrillo, E.; Ventura, M.; Ninot, J.M. Fine-scale Vegetation Mosaics in Pyrenean Mires Are Driven by Complex Hydrological Regimes and Threatened by Extreme Weather Events. Ecohydrology 2019, 12, e2070. [Google Scholar] [CrossRef]
- Sperle, T.; Bruelheide, H. Climate Change Aggravates Bog Species Extinctions in the Black Forest (Germany). Divers. Distrib. 2021, 27, 282–295. [Google Scholar] [CrossRef]
- Spitale, D. A Warning Call from Mires of the Southern Alps (Italy): Impacts Which Are Changing the Bryophyte Composition. J. Nat. Conserv. 2021, 61, 125994. [Google Scholar] [CrossRef]
- Gorham, E. The Development of Peat Lands. Q. Rev. Biol. 1957, 32, 145–166. [Google Scholar] [CrossRef]
- Peterka, T.; Syrovátka, V.; Dítě, D.; Hájková, P.; Hrubanová, M.; Jiroušek, M.; Plesková, Z.; Singh, P.; Šímová, A.; Šmerdová, E.; et al. Is Variable Plot Size a Serious Constraint in Broad-scale Vegetation Studies? A Case Study on Fens. J. Veg. Sci. 2020, 31, 594–605. [Google Scholar] [CrossRef]
- van der Maarel, E. Transformation of Cover-Abundance Values in Phytosociology and Its Effects on Community Similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Sáez, L.; Aymerich, P. An Annotated Checklist of the Vascular Plants of Catalonia (Northeastern Iberian Peninsula); Kit-Book Serveis Editorials; SCP: Barcelona, Spain, 2021. [Google Scholar]
- Sáez, L.; Ruiz, E.; Brugués, M. Bryophyte Flora of Catalonia (Northeastern Iberian Peninsula). Checkl. Red List. Bol. Soc. Esp. Briol. 2019, 51, 1–126. [Google Scholar]
- Wildi, O. Why Mean Indicator Values Are Not Biased. J. Veg. Sci. 2016, 27, 40–49. [Google Scholar] [CrossRef]
- Julve, P. Baseflor. Index Botanique, Écologique et Chronologique de La Flore de France; Institut Catholique de Lille: Lille, France, 2016. [Google Scholar]
- Hill, M.O.; Preston, C.D.; Bosanquet, S.D.S.; Roy, D.B. Attributes of British and Irish Mosses, Liverworts, and Hornworts; NFRC Centre for Ecology and Hydrology and Countryside Council for Wales; Saxon Print Group: Norwich, UK, 2007. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7975-9. [Google Scholar]
- Roberts, D.W. Vegetation Classification by Two New Iterative Reallocation Optimization Algorithms. Plant Ecol. 2015, 216, 741–758. [Google Scholar] [CrossRef]
- Aho, K.; Roberts, D.W.; Weaver, T. Using Geometric and Non-geometric Internal Evaluators to Compare Eight Vegetation Classification Methods. J. Veg. Sci. 2008, 19, 549–562. [Google Scholar] [CrossRef]
- Tichý, L.; Chytrý, M.; Šmarda, P. Evaluating the Stability of the Classification of Community Data. Ecography 2011, 34, 807–813. [Google Scholar] [CrossRef]
- Tichý, L.; Chytrý, M.; Hájek, M.; Talbot, S.S.; Botta-Dukát, Z. OptimClass: Using Species-to-Cluster Fidelity to Determine the Optimal Partition in Classification of Ecological Communities. J. Veg. Sci. 2010, 21, 287–299. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Zelený, D.; Schaffers, A.P. Too Good to Be True: Pitfalls of Using Mean E Llenberg Indicator Values in Vegetation Analyses. J. Veg. Sci. 2012, 23, 419–431. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.scirp.org/reference/referencespapers?referenceid=3456808 (accessed on 10 January 2025).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.3-7. 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 30 July 2025).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions, R Package Version 2.1.6. 2023. Available online: https://CRAN.R-project.org/package=cluster (accessed on 30 July 2025).
- Cáceres, M.D.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology, R Package Version 2.7-1. 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 July 2025).
- Hothorn, T.; Hornik, K.; Wiel, M.A.V.D.; Zeileis, A. Implementing a Class of Permutation Tests: The Coin Package. J. Stat. Soft. 2008, 28, 1–23. [Google Scholar] [CrossRef]
- Hájek, M.; Dítě, D.; Horsáková, V.; Mikulášková, E.; Peterka, T.; Navrátilová, J.; Jiménez-Alfaro, B.; Hájková, P.; Tichý, L.; Horsák, M. Towards the Pan-European Bioindication System: Assessing and Testing Updated Hydrological Indicator Values for Vascular Plants and Bryophytes in Mires. Ecol. Indic. 2020, 116, 106527. [Google Scholar] [CrossRef]
- Hájek, M.; Hájková, P.; Goia, I.; Dítě, D.; Plášek, V. Variability and Classification of Carpathian Calcium-Rich Fens: Breaking the State Borders. Preslia 2021, 93, 203–235. [Google Scholar] [CrossRef]
- Deane, D.C.; Fordham, D.A.; Stevens, A.K.; Bradshaw, C.J.A. Dispersal-driven Homogenization of Wetland Vegetation Revealed from Local Contributions to Β-diversity. J. Veg. Sci. 2017, 28, 893–902. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual Synthesis in Community Ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef]
- Pärtel, M.; Zobel, M.; Zobel, K.; Van Der Maarel, E.; Partel, M. The Species Pool and Its Relation to Species Richness: Evidence from Estonian Plant Communities. Oikos 1996, 75, 111. [Google Scholar] [CrossRef]
- Wheeler, B.D. Water and Plants in Freshwater Wetlands. In Eco-Hydrolgy; Routledge: London, UK, 1999; pp. 127–180. [Google Scholar]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Epstein, E. The Anomaly of Silicon in Plant Biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Is Plant Ecology More Siliceous than We Realise? Trends Plant Sci. 2011, 16, 61–68. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Gocke, M.; Liang, W.; Sommer, M.; Kuzyakov, Y. Silicon Uptake by Wheat: Effects of Si Pools and pH. Z. Pflanzenernähr. Bodenk. 2013, 176, 551–560. [Google Scholar] [CrossRef]
- Schoelynck, J.; Müller, F.; Vandevenne, F.; Bal, K.; Barão, L.; Smis, A.; Opdekamp, W.; Meire, P.; Struyf, E. Silicon–Vegetation Interaction in Multiple Ecosystems: A Review. J. Veg. Sci. 2014, 25, 301–313. [Google Scholar] [CrossRef]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon Increases the Phosphorus Availability of Arctic Soils. Sci. Rep. 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Schoelynck, J.; Struyf, E.; Meire, P. Silicon Affects Nutrient Content and Ratios of Wetland Plants. Silicon 2016, 8, 479–485. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon Pools and Fluxes in Soils and Landscapes—A Review. Z. Pflanzenernähr. Bodenk. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Struyf, E.; Van Damme, S.; Gribsholt, B.; Bal, K.; Beauchard, O.; Middelburg, J.J.; Meire, P. Phragmites Australis and Silica Cycling in Tidal Wetlands. Aquat. Bot. 2007, 87, 134–140. [Google Scholar] [CrossRef]
- Schaller, J.; Wu, B.; Amelung, W.; Hu, Z.; Stein, M.; Lehndorff, E.; Obst, M. Silicon as a Potential Limiting Factor for Phosphorus Availability in Paddy Soils. Sci. Rep. 2022, 12, 16329. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Dudel, E.G. Silicon Availability Changes Structural Carbon Ratio and Phenol Content of Grasses. Environ. Exp. Bot. 2012, 77, 283–287. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Zhao, F.; Xu, C. Ecological Stoichiometry of N:P:Si in China’s Grasslands. Plant Soil 2014, 380, 165–179. [Google Scholar] [CrossRef]
- Garbuzov, M.; Reidinger, S.; Hartley, S.E. Interactive Effects of Plant-Available Soil Silicon and Herbivory on Competition between Two Grass Species. Ann. Bot. 2011, 108, 1355–1363. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. Global Silicate Weathering and CO2 Consumption Rates Deduced from the Chemistry of Large Rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Miserere, L.; Montacchini, F.; Buffa, G. Ecology of Some Mire and Bog Plant Communities in the Western Italian Alps. J. Limnol. 2003, 62, 88. [Google Scholar] [CrossRef]
- Ribichich, A.M. From Null Community to Non-randomly Structured Actual Plant Assemblages: Parsimony Analysis of Species Co-occurrences. Ecography 2005, 28, 88–98. [Google Scholar] [CrossRef]
- Batriu, E.; Pino, J.; Rovira, P.; Ninot, J.M. Environmental Control of Plant Species Abundance in a Microtidal Mediterranean Saltmarsh: Environmental Control of Marsh Plant Species Abundance. Appl. Veg. Sci. 2011, 14, 358–366. [Google Scholar] [CrossRef]
- Fukami, T. Historical Contingency in Community Assembly: Integrating Niches, Species Pools, and Priority Effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef]
- García-Girón, J.; Lindholm, M.; Heino, J.; Toivonen, H.; Alahuhta, J. Historical Contingency via Priority Effects Counteracts Environmental Change on Metacommunity Dynamics across Decades. Limnol. Oceanogr. 2022, 67, S38–S53. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M. Pattern and Process in Macroecology; Blackwell Science: Oxford, UK; Malden, MA, USA, 2000; ISBN 978-0-470-99959-2. [Google Scholar]
- Götzenberger, L.; De Bello, F.; Bråthen, K.A.; Davison, J.; Dubuis, A.; Guisan, A.; Lepš, J.; Lindborg, R.; Moora, M.; Pärtel, M.; et al. Ecological Assembly Rules in Plant Communities—Approaches, Patterns and Prospects. Biol. Rev. 2012, 87, 111–127. [Google Scholar] [CrossRef]
- Miller, N.G.; McDaniel, S.F. Bryophyte Dispersal Inferred from Colonization of an Introduced Substratum on Whiteface Mountain, New York. Am. J. Bot. 2004, 91, 1173–1182. [Google Scholar] [CrossRef]
- Sundberg, S. Spore Rain in Relation to Regional Sources and Beyond. Ecography 2013, 36, 364–373. [Google Scholar] [CrossRef]
- Terrádez, J.; Arauzo, I. Climate Change in the Pyrenees: Impacts, Vulnerabilities and Adaptation. Bases of Knowledge for the Future Climate Change Adaptation Strategy in the Pyrenees; Pyrenean Observatory of Climate Change: Huesca, Spain, 2018; ISBN 978-84-09-06268-3. Available online: https://www.opcc-ctp.org/sites/default/files/editor/opcc-informe-en-paginas.pdf (accessed on 4 September 2025).
- Gonzalez-Saldias, F.; Pérez-Haase, A.; Pladevall-Izard, E.; Gomà, J. Environmental and Overgrazing Effects on Diatom Communities in High Mountain Mires. Sci. Total Environ. 2025, 969, 178983. [Google Scholar] [CrossRef]
- Breeuwer, A.; Robroek, B.J.M.; Limpens, J.; Heijmans, M.M.P.D.; Schouten, M.G.C.; Berendse, F. Decreased Summer Water Table Depth Affects Peatland Vegetation. Basic Appl. Ecol. 2009, 10, 330–339. [Google Scholar] [CrossRef]
- Ballesteros, M.; Řehounková, K.; Šebelíková, L.; Müllerová, A.; Vítovcová, K.; Prach, K. Participation of Grassland Species in Various Successional Series in a Temperate European Region and Implications for Habitat Management. Glob. Ecol. Conserv. 2024, 49, e02761. [Google Scholar] [CrossRef]
- Lin, M.; Bergamini, A.; Pichon, N.A.; Allan, E.; Boch, S. Nitrogen Enrichment and Vascular Plant Richness Loss Reduce Bryophyte Richness. Sci. Rep. 2025, 15, 4049. [Google Scholar] [CrossRef]
- Stapanian, M.A.; Schumacher, W.; Gara, B.; Adams, J.V.; Viau, N. Moss and Vascular Plant Indices in Ohio Wetlands Have Similar Environmental Predictors. Ecol. Indic. 2016, 62, 138–146. [Google Scholar] [CrossRef]

| Beret | Bassa Nera | Filià | Siscaró | Prat Fondal | |
|---|---|---|---|---|---|
| Geographical coordinates (long., lat.) | 0.95358, 42.71462 | 0.92421, 42.63818 | 0.95196, 42.45417 | 1.70454, 42.59528 | 1.79272, 42.47692 |
| Number of plots | 32 | 35 | 29 | 30 | 22 |
| Mire size (ha) | 4.8 | 1.9 | 3.9 | 4.6 | 3.1 |
| Elevation (m a.s.l.) | 1857–1875 | 1889–1893 | 2050–2122 | 2142–2149 | 2304–2305 |
| Mean annual precipitation (mm) | 1004 | 1063 | 1435 | 1221 | 1056 |
| Mean annual temperature (°C) | 4.8 | 4.6 | 4.2 | 4.9 | 3.2 |
| Mean July temperature (°C) | 11.6 | 12.9 | 12.2 | 12.8 | 10.6 |
| Bedrock | Schists and limestones | Granites | Lutites and limestones | Gneiss | Granites |
| Main hydrological types | |||||
| Topogenous | ✓ | ✓ | ✓ | ✓ | ✓ |
| Soligenous | ✓ | ✓ | ✓ | ✓ | ✓ |
| Limnogenous | ✓ | ||||
| Ombrogenous (Sphagnum hummocks) | ✓ | ✓ | ✓ | ✓ |
| Explanatory Variables | Adjusted R2 | Pseudo-F Ratio |
|---|---|---|
| Combined dataset | ||
| Mean_WTD | 0.041 | 8293 *** |
| Si | 0.018 | 4.37 *** |
| Ca | 0.014 | 3.531 *** |
| Mean_WTD2 | 0.010 | 2.948 *** |
| pH | 0.008 | 2.506 *** |
| Vascular plants | ||
| Mean_WTD | 0.054 | 11.28 *** |
| Si | 0.021 | 4.96 *** |
| Ca | 0.012 | 3.31 *** |
| S | 0.012 | 3.35 *** |
| Mean_WTD2 | 0.011 | 3.09 *** |
| Bryophytes | ||
| pH | 0.027 | 5.62 *** |
| pH2 | 0.019 | 4.28 *** |
| Mean_WTD | 0.018 | 4.11 *** |
| Si | 0.013 | 3.30 *** |
| Fe | 0.010 | 2.74 *** |
| Type | Adjusted R2 (%) | |
|---|---|---|
| Combined dataset | Conditioned | 17.6 |
| Constrained | 18.8 | |
| Unconstrained | 63.6 | |
| Conditioned (without controlling for covariates) | 22.0 | |
| Intersection (Constrained and conditioned parts) | 8.6 | |
| Vascular plants | Conditioned | 19.8 |
| Constrained | 16.4 | |
| Unconstrained | 63.7 | |
| Conditioned (without controlling for covariates) | 20.2 | |
| Intersection (Constrained and conditioned parts) | 7.3 | |
| Bryophytes | Conditioned | 14.7 |
| Constrained | 13.6 | |
| Unconstrained | 71.6 | |
| Conditioned (without controlling for covariates) | 16.7 | |
| Intersection (Constrained and conditioned parts) | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Haase, A.; Ninot, J.M. Drivers of Alpine Mire Vegetation at Their Range Limit. Diversity 2025, 17, 702. https://doi.org/10.3390/d17100702
Pérez-Haase A, Ninot JM. Drivers of Alpine Mire Vegetation at Their Range Limit. Diversity. 2025; 17(10):702. https://doi.org/10.3390/d17100702
Chicago/Turabian StylePérez-Haase, Aaron, and Josep M. Ninot. 2025. "Drivers of Alpine Mire Vegetation at Their Range Limit" Diversity 17, no. 10: 702. https://doi.org/10.3390/d17100702
APA StylePérez-Haase, A., & Ninot, J. M. (2025). Drivers of Alpine Mire Vegetation at Their Range Limit. Diversity, 17(10), 702. https://doi.org/10.3390/d17100702







