Abstract
Zoological collections are essential for preserving regional biodiversity but often lack taxonomic updates, risking data loss. This work supposes the continuation of a review of the African marine crabs housed at the Crustacean collection of the Museo Nacional de Ciencias Naturales (MNCN) in Madrid. Part I, Heterotremata, revealed that only 21.9% of the specimens were correctly identified. In this new study focused on Thoracotremata species, 197 specimens (59 records) were reviewed, and this new study reveals similar results to Part I: 22.9% of the reviewed specimens were previously correctly identified (less than 29% when considering records), meaning that around 77.2% of the African Thoracotremata specimens housed in the MNCN were either misidentified or not identified at all. This highlights the importance of the taxonomic reviews made by specialists of specimens housed in both historical and non-historical scientific collections. This time we have used DNA barcodes (16S and COI) to confirm identifications or to add new molecular data to species without previous DNA sequences known. DNA sequences (16S and/or COI) were obtained for seven species. It has been a challenge to obtain sequences from specimens preserved 60 to 200 years ago.
1. Introduction
Natural history museums (NHMs) and other institutional collections are key institutions for knowledge, conservation, and education [1]. They house scientific collections which help in understanding Earth’s evolution and bio- and geodiversity [2]. Zoological collections play a vital role in preserving biodiversity within specific regions and taxonomic groups. However, these collections are increasingly at risk of deterioration and often lack essential taxonomic updates, as pointed out in recent research on historical specimens [2,3]. Many specimens lose their preservatives and dry out, thus becoming at risk of dehydration, decay, or fungal growth. Specimens deliberately preserved in a dry state are prone to breaking easily and may also be attacked by fungi or other insects. In some collections, the lids used for the containers are made of metal, and over the years these lids often corrode. The resulting rust may fall into the preservative, potentially affecting the specimens stored within.
The present study constitutes the second part of a taxonomic revision of all African brachyuran specimens housed in the Arthropod collection of the Museo Nacional de Ciencias Naturales (MNCN) in Madrid, one of the oldest natural history museums in Europe. In part I, Heterotremata [3], significant limitations were identified in the taxonomic accuracy of the specimens. This previous study documented a total of 82 species of Heterotremata, revealing that only 21.9% of the specimens had been correctly identified, pointing out a considerable loss of biodiversity information and limiting the collection’s potential for discovering new species. Many of the specimens were identified at the genus level, and in other cases only at the family level, which were not always correct. This may be since the collectors of these specimens were largely naturalist entomologists rather than brachyuran specialists. Subsequently, the museum curators focused on the preservation, labelling, and cataloguing of the specimens, but not on their taxonomic revision.
Building on these initial findings, the present study seeks to continue and expand this taxonomic review, in this case, with the taxonomic revision of the specimens belonging to the Thoracotremata subsection (Crustacea, Brachyura) and using DNA barcoding for confirming identifications or adding molecular data for species without previous DNA sequences known. As with the first part of this research, these findings will be valuable for future studies, furthering the knowledge and preservation of biodiversity not only in African waters but also in other regions of the world.
The MNCN is one of the emblematic centres of the Consejo Superior de Investigaciones Científicas (CSIC) with a heritage of over 250 years and housed many important faunal collections. See Part I of this study for a detailed description of the Arthropod collection housed in the MNCN [3].
The Arthropod collection of the MNCN contains 1302 decapod specimens from African marine waters, among these, the Thoracotremata are represented by specimens from seven countries, including specimens from the Canary Islands (Spain). As was pointed out in the first part of this study [3], many important collectors collaborated in the collections or donations of the specimens housed in the MNCN such as Félix Édouard Guérin-Méneville, Manuel Martínez de la Escalera, Emilio Rolán, or Émile Deyrolle.
A Brief Historical Review of the Provenance of the Studied Specimens
The largest number of specimens (56, belonging to 13 species) came from Equatorial Guinea; roughly half of it originates from the Río Muni expedition in 1901, led by Manuel Martínez de la Escalera. In 1902, the Muni commission requested from the Spanish Ministry of State the necessary funding to publish a volume of memoirs containing the results of the expedition to the Spanish Guinea (now Equatorial Guinea) [4]. A second group of specimens came from a scientific expedition organised by the MNCN to Equatorial Guinea, the Annobón island, and Fernando Poo (present-day Bioko). In 1932, the MNCN organised an entomological expedition, entrusted to Drs. Juan Gil Collado and Federico Bonet Marco, which aimed to study the entomological fauna of these largely unexplored regions. The expedition included a one-month stay in Annobón and a period of approximately six to eight weeks in the southern highlands of Fernando Poo. This initiative reflected the scientific interest in documenting the biodiversity of these areas, whose fauna was presumed to possess characteristics distinct from those known in other regions of the Gulf of Guinea [5]. Although those sent on these expeditions were usually entomologists and their main objective was to collect specimens for the entomology collection, they also gathered other faunal specimens, both marine and terrestrial. Decapod specimen collection was typically carried out by hand on beaches and in other coastal waters.
After the Spanish Civil War (1936–1939), the Project for Scientific Expeditions to the Spanish Territories in the Gulf of Guinea, the Spanish Sahara (now Western Sahara), and Sidi Ifni [6], sought to resume research activities in the colonies and the Moroccan Protectorate, focusing primarily on ornithological studies and rare animal species, as well as geographic–geological surveys, mining studies, and assessments of fishery resources in the Sahara.
Other expeditions developed in the entomological framework in Equatorial Guinea, were carried out by Salvador Vicente Peris, the Director of the Instituto Español de Entomología until 1984 (now part of the MNCN). These campaigns included trips to the Annobón island (1959) and the Gulf of Guinea, visiting Fernando Poo, the Annobón island, and, once again, Muni River in 1961.
Although there are documents attesting to the existence of many other expeditions aimed at collecting items or specimens for the MNCN, the delivery of materials, mainly ethnographic but not only so, was documented as being carried out by the Naval Officer Luis Sorela himself, to be incorporated into the Museum’s collections [7].
Other records are from the Indian Ocean, from Mauritius and were acquired through a purchase to Félix Édouard Guérin-Méneville (1799–1874), a renowned entomologist of the time, and other specimens were obtained throughout an exchange with Pere Antiga i Suñer (1854–1904). More details can be found in Muñoz et al. [3]. The more recent collections were made by Francisco de las Barras de Aragón (1869–1955) from Djibouti during the Red Sea Expedition, as part of the Extremo Oriente university expedition in 1935 [8].
Duperrey et al. [9] documented the voyage of the corvette La Coquille around the world, which passed through Mauritian waters between 11 September and 16 November in 1824, and the specimens were catalogued in Guérin-Méneville [10]. The author explained that he was entrusted by the renowned naturalist Latreille with continuing the publication on articulated animals from the voyage around the world undertaken by the corvette La Coquille. He acknowledged that this was a challenging task and that he chose to approach it with great care and dedication, with the aim of honouring Latreille’s legacy. The author also emphasised that he chose to devote considerable time to researching and comparing species rather than publishing hastily, as he believed that a well-developed and rigorous work brought more value to science than rushed publications. He also noted that he sacrificed personal time and resources to complete this work, driven by his love for science and his desire to make an honourable contribution to the naval department and to the natural sciences. He criticised the tendency of some to publish superficial works, with names and descriptions lacking sufficient scientific backing, which created confusion and hindered the work of other entomologists. He pointed out, for example, the difficulty in correctly identifying species and genera due to the lack of clear references and the proliferation of names without official publications. In summary, the author defended the importance of patience, in-depth research, and accuracy in science, in contrast to the speed and superficiality that sometimes prevailed in scientific publishing.
Some specimens from Eritrea reviewed in this study list “Raffray? Deyrolle” as the collector. It appears that Deyrolle was an animal trader who operated a shop in Paris starting in 1831 and became a prominent figure in the trade of natural history specimens and illustrations. These specimens may have been collected during the Hemprich and Ehrenberg expedition (1824–1825). The German naturalists Friedrich Wilhelm Hemprich and Christian Gottfried Ehrenberg were part of a Prussian archaeological mission to Egypt (under General von Minutoli) in 1820. In November 1824, they sailed along the Red Sea coast, stopping at key ports including Massawa (April–June 1825), before proceeding to the highlands of Abyssinia. Their journey is documented in Ehrenberg [11]. Many of the specimens from this expedition are housed at the Museum of Berlin.
Following this investigation, we consider that the specimens from Eritrea may have been collected in 1825, and those from Mauritius in 1824.
It is important to highlight that many expeditions included the presence of curators, illustrators or draftsmen, photographers, as well as specialists in taxonomic groups, as the second scientific-fishing campaign to the coasts of the Spanish Sahara Project [6].
The use of DNA from historical museum specimens is becoming increasingly relevant, especially for taxa with limited fresh material available. Although many collections were not originally intended for molecular studies, museums are gradually opening up to the controlled use of such specimens, recognising their value for advancing taxonomic and evolutionary research [12,13,14,15,16]. In the case of African marine crabs, where fresh material is often scarce or unavailable, museum collections represent an essential resource for expanding our understanding of species diversity and evolutionary relationships.
2. Materials and Methods
The specimens considered in this work are those catalogued in any family of the Subsection Thoracotremata from African marine waters, housed in the Arthropod collection of the MNCN. Materials and methods were explained in detail in Part I of this study focused on Heterotremata [3].
General publications on specific areas, more or less extensive, have been used to confirm the identification of the species, such as [17,18,19,20,21,22,23]; or more specific to the Atlantic Ocean [20,22,24,25,26,27]. As well as others specific to groups mentioned in the species sections. In addition, a general book for genera identification is Poore & Ahyong [28]. Sasaki [29] and Decanet [30] have been the reference for checking distribution range of the species.
For checking, the current valid scientific names of species have been used [30,31].
Since many of these specimens are historical, donated, or collected by entomologists during their travels, the records lack some of the data that would be desirable for any collection. Consequently, some collection dates are missing, collectors’ names are absent, and the locations are often vague and lack details. On some dates, the symbol “?” is used when it is possible but not entirely certain.
All specimens and labels from each record (59 jars) have been carefully reviewed, identified, measured, and photographed whenever possible. Additionally, tissue samples have been taken from many of them for molecular studies. In the Material Examined Section for each species, the term “leg.” (legit, legerunt) includes not only the actual collectors but also those who donated, sold, or exchanged specimens with the MNCN. The collection date is approximate in many cases and may refer to the date of purchase, donation, or exchange. Therefore, surely many of the specimens were collected years before the date listed in this work.
Measurements, given in millimetres (mm), refer to carapace at its maximum width including teeth or spines if present (cw) and carapace length (cl) as length of the dorsal midline from the middle of the frontal region to the posterior margin. In all the measured specimens, the measurement will be given as follows: (cw × cl). In some cases, the identification presented limitations, such as in deteriorated specimens or remains, or in dry specimens where the pleon could not be studied to avoid damaging the specimens, as they are glued to cardboard and displayed in boxes. The term “unsexed” is used to indicate immature specimens or those whose sex cannot be determined. Darwin Core (DwC) terms have been used to cite records and specimens in the Material Examined Section.
Total genomic DNA of a sample of the specimens studied herein was extracted from muscle tissue from one pereiopod, eye, or female pleopod, following a modified Qiagen DNeasy Blood and Tissue Kit protocol by Ruane & Austin [32]. Target mitochondrial DNA from the 16S rRNA and COI genes was amplified with polymerase chain reaction (PCR) using the following cycling conditions: 15 min at 95 °C, and 40 cycles of 30 s at 95 °C, 30 s at 44–54 °C (depending on primer combination), and 30 s at 72 °C, and a final 5 min at 72 °C. Primers used, combinations, and size of sequences obtained are listed in Table 1. PCR products were sent to Stab Vida company to be purified and then bidirectionally sequenced. Sequences were edited using the software Chromas, version 2.6.4. A BLAST (Basic Local Alignment Search Tool) on the NCBI (National Center for Biotechnology Information) web facility on the GenBank sequences database [33] was performed with the obtained final DNA sequences to obtain the best matches for species identification.
Identifications were considered to belong to the same species when comparative sequences showed similarity values greater than 99%, with differences in 1–4 mutations in 16S and >97% (1–15 mutations) in COI, in this last case according to Meyer & Paulay [34] and depending on sequence size. Lower similarity values are highlighted as questionable species relationships that need further study. All sequences obtained for both genes were deposited in GenBank (accession codes: PX418377-PX418384 (16S), PX418257-PX418259 (COI)).

Table 1.
List of sequenced genes including primers used for each gene, pair combinations, length of the sequences obtained (bp), and references.
Table 1.
List of sequenced genes including primers used for each gene, pair combinations, length of the sequences obtained (bp), and references.
| Genes | Primers (Forward and Reverse) | Pair | (bp) | References |
|---|---|---|---|---|
| 16S | L12c (5′-TGA CYG TGC AAA GGT AGS ATA A-3′) | br | 438 | [3] |
| MiDecaFb (5′- RGA CGA TAA GAC CCT RTA AA-3′) | 1472 | 338 | [3] | |
| br (5′-CCG GTC TGA ACT CAG ATC ACG T-3′) | L12c | [35] | ||
| 1472 (5′-AGA TAG AAA CCA ACC TGG-3′) | MiDecaFb | [36] | ||
| COI | mlCOIintF-XT (5′-GGW ACW RGW TGR ACW NTN TAY CCY CC-3′) | H6 | 345 | [37] |
| HCO2198-JJ | 345 | |||
| H6 (5′-TAD ACT TCD GGR TGD CCA AAR AAY CA-3′) | mlCOIintF-XT | [38] | ||
| HCO2198-JJ (5′-AWA CTT CVG GRT GVC CAA ARA ATC A -3′) | mlCOIintF-XT | [39] |
3. Results
3.1. Taxonomic Synopsis
| Superfamily Grapsoidea MacLeay, 1838 |
| Family Gecarcinidae MacLeay, 1838 |
| Genus Cardisoma Latreille in Latreille, Le Peletier, Serville & Guérin, 1828 |
| Cardisoma armatum (Herklots, 1851) |
| Figure 1 |

Figure 1.
Cardisoma armatum from Ghana, MNCN 20.04/03582. Scale bars: 1 cm.
Material examined. GHANA • Two ♂♂ (65.1 × 52.1, 62.1 × 51.4); March 1993; MNCN 20.04/03582.
Distribution. West Africa from the Cape Verde Islands and Senegal to Angola, including the islands in the Gulf of Guinea, and São Tomé Island. An observation has been reported in Singapore, but it is from an aquarium escape [40].
Remarks. The specimens were previously identified as “No identificado” (unidentified) and assigned to Grapsidae. We considered including this species, although C. armatum is classified as a terrestrial species because it spends most of its life on land. However, its primary habitat is mangrove areas, and it relies on saltwater for reproduction and hydration; in fact, their larvae develop in the open sea [41].
For identification and distribution, see also [17,42].
| Family Grapsidae MacLeay, 1838 |
| Genus Geograpsus Stimpson, 1858 |
| Geograpsus grayi (H. Milne Edwards, 1853) |
| Figure 2 |
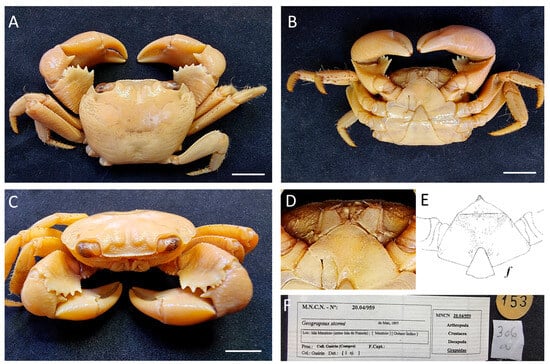
Figure 2.
(A–C) Geograpsus grayi, from Mauritius, MNCN 20.04/00959; (D) Pubescent second sternite; (E) drawing of the second sternite from Banerjee [18]; (F) old and posterior labels. Scale bars: 1 cm.
Material examined. MAURITIUS • One ♂ (33.6 × 27.6); 1824?; “La Coquille” corvette; Guérin-Méneville leg.; MNCN 20.04/00959.
Distribution. IWP: Red Sea, Tanzania, Madagascar, Mayotte Islands, Seychelles, Réunion, Mauritius to Indonesia, Taiwan, Japan, Australia, Solomon, New Caledonia, Loyalty, Fiji, Kermadec, Wallis & Futuna, Niue, Cook, French Polynesia, and Henderson.
Remarks. Throughout the old labels, it is possible to see the original identification as G. lividus.
For identification and distribution, see also [18,19,21,43,44,45].
This species is considered terrestrial, but it maintains a close association with marine environments. It is commonly found in the supratidal zone at night, where it seeks to conserve the humidity of its branchial cavity.
| Geograpsus lividus (H. Milne Edwards, 1837) |
| Figure 3 |

Figure 3.
Geograpsus lividus from Equatorial Guinea, MNCN 20.04/01049. Scale bars: 1 cm.
Material examined. EQUATORIAL GUINEA—Annobón Island • Two ♀♀ (23 × 17.3, 18.8 × 15.1); 1959; S. V. Peris & J. Álvarez leg.; MNCN 20.04/01049.
Distribution. East coast of USA, Antilles, northern South America, and Brazil; West Africa, from the Cape Verde Islands and Senegal to Angola and São Tomé. There are some records from the Indian Ocean, but they are inaccurate https://www.marinespecies.org/aphia.php?p=taxdetails&id=241196#distributions (accessed on 10 July 2025).
Although there are records of this species in the Pacific Ocean in DecaNet [30], Schubart [46] clarified that the eastern Pacific representatives are G. occidentalis Stimpson, 1860, and not G. lividus.
Remarks. The specimens were accompanied by different labels and two different identifications, but the most current one is as Geograpsus stormi (species present in the East Africa). Geograpsus lividus has a wide distribution, with records in West Atlantic, but molecular data suggest that it could actually be two different species [46].

Figure 4.
Geograpsus stormi from Mauritius, MNCN 20.04/00954. Scale bars: 1 cm.
Material examined. MAURITIUS • One ♂ (49.5 × 39); 1824?; “La Coquille” Corvette; Guérin-Méneville leg.; MNCN 20.04/00954.
Distribution. IWP: From the eastern coast of Africa from Somalia to South Africa, across the Indian Ocean and Southeast Asia, through Japan and the Pacific Islands, reaching as far east as French Polynesia and possibly Hawaii. An isolated record also exists from the eastern Pacific (Gulf of California), a record that would need to be reviewed.
Remarks. In contrast to the description provided in the key, where the lateral margins of the carapace are described as parallel or slightly diverging posteriorly and the antero-lateral margin as perfectly straight, our specimen shows slightly more convex lateral margins, giving the carapace a subtly more rounded appearance in dorsal view.
For identification and distribution, see also [18,19,21,29,44].
DNA barcodes. Only the 16S sequence (PX418378) was obtained for this specimen preserved about 200 years ago. The sequence (437 bp) has a 99.77% similarity (only differing in one mutation) with the FR871290 sequence obtained by Schubart [46] from the specimen SMF38441 from Taiwan, and KM510098 obtained by Ip et al. [47] from the specimen R452-3 from Taiwan as well.
| Genus Goniopsis De Haan, 1833 |
| Goniopsis pelii (Herklots, 1851) |
| Figure 5 |
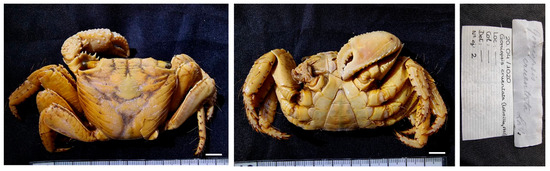
Figure 5.
Goniopsis pelii from Equatorial Guinea, MNCN 20.04/01020. Scale bars: 1 cm.
Material examined. EQUATORIAL GUINEA—Cabo San Juan • One ♂ (46.4 × 38); 1901; M. Martínez de la Escalera leg.; MNCN 20.04/01020.
Distribution. West Africa, from Senegal to Angola, and São Tomé.
Remarks. The specimen was previously identified as Goniopsis cruentata (Latreille, 1803). Goniopsis cruentata has been the species cited in West Africa for a long time [24,48,49]; however, Manning & Holthuis [26] clarified that the species distributed in the African Atlantic is G. pelli and not G. cruentata, providing a list of differences between the two species. This had also been supported by molecular data [46].
For identification, see [26,50].
DNA barcodes. Only a sequence of 375 bp of 16S (PX418377) was obtained for this specimen preserved 66 years ago. The sequence has a 99.09% similarity (only differing in three mutations) with the sequence FR871291 obtained by Schubart [46] from the specimen ULLZ5492 from Ghana, and 100% with the sequence KU313175 obtained by Buranelli & Mantelatto (unpublished) from the same specimen. The explanation of these differences is that sequence KU313175 is shorter than FR871291 and the mutations are in the final part of the sequence. These are the only two 16S sequences of this species in GenBank.
| Genus Grapsus Lamarck, 1801 |
| Grapsus adscensionis (Osbeck, 1765) |
| Figure 6 |

Figure 6.
Grapsus adscensionis. (A,B) Specimens from Equatorial Guinea, MNCN 20.04/01059; (C–E) specimen from Cape Vert, MNCN 20.04/00995; (F–H) specimen from Equatorial Guinea, MNCN 20.04/01074. Scale bars: 1 cm.
Material examined. CAPE VERT—San Vicente Island • Two ♂♂ (18.8 × 15.7, 14.4 × 12.5), 1 ♀ (18.2 × 15.1), one unsexed (13.4 × 11.8); 22 August 1862; Martínez & Sáez leg.; MNCN 20.04/00995.
EQUATORIAL GUINEA—Annobón Island • Eight specimens (not sized, not sexed); 1959; S. V. Peris & J. Álvarez leg.; MNCN 20.04/01059. • One ♂ (51.2 × 45.1); 1959; S. V. Peris & J. Álvarez leg.; MNCN 20.04/01074.
Distribution. East Atlantic, from Azores to Angola, Madeira, Canary Islands, Cape Verde Islands, islands of the Gulf of Guinea, Saint Helen Island, Ascension Island, and St. Paul’s Rocks.
Remarks. Specimens MNCN 20.04/00995 were previously identified as G. tenuicrustatus and MNCN 20.04/01059 and MNCN 20.04/01074 as G. grapsus.
For a long time, all Atlantic populations of Grapsus were generally assigned to G. grapsus, until Manning & Chace [27] resurrected the oldest available scientific name for the species, Cancer adscensionis Osbeck, 1765, for the other inhabiting species in West Africa, G. adscensionis. Although DecaNet [30] reports the distribution of this species as extending into the Mediterranean Sea, this claim should be treated with caution due to the lack of published records.
For identification and distribution, see also [18,26,27].
| Grapsus tenuicrustatus (Herbst, 1783) |
| Figure 7 |
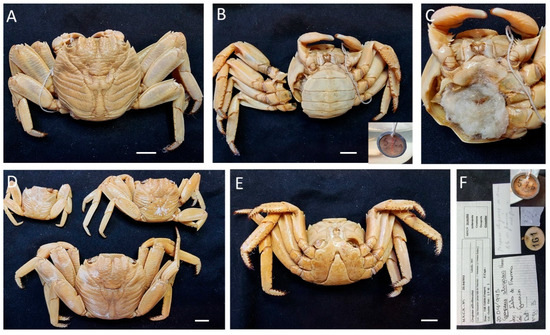
Figure 7.
Grapsus tenuicrustatus. (A–C) specimen MNCN 20.04/00989; (D–F) specimens MNCN 20.04/00993. All from Mauritius. Scale bars: 1 cm.
Material examined. MAURITIUS • One ♂ (56.6 × 53.2); May 1885; Antiga leg.; MNCN 20.04/00989 • Three ♂♂ (50.3 × 46.2, 34 × 32.4, 24.8 × 24); 1824?; “La Coquille” corvette; Guérin-Méneville leg.; MNCN 20.04/00993.
Distribution. Widely distributed across the Indo-Pacific, from East Africa to Japan, Hawaii, and the South Pacific. It is found in the Red Sea, Madagascar, the Seychelles, Taiwan, Japan, Australia, and many islands in the Pacific and Indian Oceans, including the Philippines, Indonesia, Sri Lanka, and the Maldives.
Remarks. The female MNCN 20.04/00989 is stuffed with cotton, which means it was displayed dry at some point of its storage.
For identification and distribution, see also [19,21,29,30,51].
| Genus Metopograpsus H. Milne Edwards, 1853 |
| Metopograpsus messor (Forskål, 1775) |
| Figure 8 |
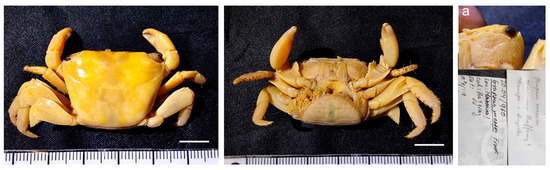
Figure 8.
Metopograpsus messor from Eritrea, MNCN 20.04/00960. a: orbital region. Scale bars: 1 cm.
Material examined. ERITREA—Massawa • One ♀ ov. (26.3 × 19.7); 1825?; Hemprich & Ehrenberg Expedition (1824–1825)?; Raffray? Deyrolle leg.; MNCN 20.04/00960.
Distribution. Indo-Pacific. East Africa (Somalia, Kenya, Tanzania, Madagascar, Seychelles, and Mauritius), Red Sea and Gulf of Suez, Persian Gulf and Gulf of Oman; Southeast Asia and Pacific (Thailand, Indonesia, Taiwan, Japan, and Pacific Islands), Australia, Hawaii, Socotra, and Caroline Islands and French Polynesia.
Remarks. For identification and distribution, see also [18,19,21,23,52].
| Genus Pachygrapsus Randall, 1840 |
| Pachygrapsus marmoratus (Fabricius, 1787) |
| Figure 9, Figure 10 and Figure 11 |

Figure 9.
Pachygrapsus marmoratus from Morocco, MNCN 20.04/10232. Scale bar: 1 cm.

Figure 10.
Pachygrapsus marmoratus, (A–F) specimens MNCN 20.04/01045; (G–K) specimens MNCN 20.04/10213. All from Morocco. Scale bars: 1 cm.

Figure 11.
Pachygrapsus marmoratus, (A–C) specimens MNCN 20.04/01068 (before MNCN 20.04/01155); (D–F) specimens MNCN 20.04/01062. All from Canary Islands (Spain). Scale bars: 1 cm.
Material examined. MOROCCO—Larache • Four ♂♂ (24.1 × 21.3, 19.8 × 17.7, 24.4 × 21.5, 16.5 × 14.8), 2 ♀♀ (18 × 16.1, 19.8 × 18.1); 1955; MNCN 20.04/01045. —Villa Alhucemas • One ♀ (41.6 × 36.4); MNCN 20.04/04026. —Tazougarte • One ♀ (19.4 × 16.6), one ♂ (21.2 × 19); 5 April 2001; I. Doadrio leg.; MNCN 20.04/10213. • Two ♀♀ (18.8 × 16.6, 17.1 × 15.1); 5 April 2001; I. Doadrio leg.; MNCN 20.04/10228. —Cabo Juby • Five ♂♂ (30.8 × 27.4, 31.3 × 28, 27 × 23.4, 29.1 × 26.1, 28.9 × 26.8), three ♀♀ (42.5 × 38.4, 33.2 × 30.4, 40.8 × 33.7); July 1933; MNCN 20.04/10232 and MNCN 20.04/10232-1 (DNA voucher ♂ 44.2 × 38.9).
SPAIN—Canary Islands, Gran Canarias, Las Palmas • Two ♀♀ ov. (29.8 × 26.5, 26.8 × 25.3); Playa San Cristóbal; MNCN 20.04/01062. —Canary Islands, Tenerife • Four ♀♀ (37.1 × 34.1, 22.2 × 18.5, 15.2 × 13.7, 18.2 × 16.3), five ♂♂ (17.6 × 15.7, 22.9 × 20.9, 19.2 × 17.1, 15.1 × 13.7, 25.5 × 23.2); A. Cabrera leg.; MNCN 20.04/01068.
Distribution. Eastern Atlantic, from the Gulf of Biscay to Morocco, including the Canary Islands, Azores, and Madeira. Mediterranean, as far as Palestine and the Black Sea.
Remarks. The specimens MNCN 20.04/01045, MNCN 20.04/04026, MNCN 20.04/01062, and MNCN 20.04/01068 were identified at family level, Grapsidae.
For identification and distribution, see [25].
DNA barcodes. Only a sequence of 438 bp of 16S (PX418379) was obtained for the specimen MNCN 20.04/10232-1 from Morocco, preserved 92 years ago. The sequence has a 100% similarity with four sequences (FR871307, FR871311, KM510117, and DQ079728) of specimens from Balearic Islands, Chile (probably erroneous locality and erroneously identified as Pachygrapsus pubescens), Greece, and a not-identified locality. It also has a similarity of about 99.30–99.77% with the other five sequences of 16S of this species deposited in GenBank.
| Pachygrapsus maurus (Lucas, 1846) |
| Figure 12 |
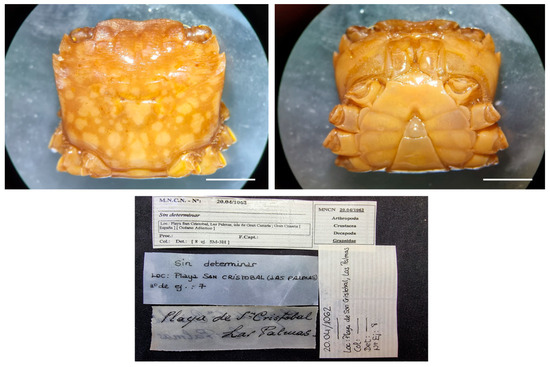
Figure 12.
Pachygrapsus maurus from Canary Islands (Spain), MNCN 20.04/20938. Scale bars: 0.5 cm.
Material examined. SPAIN—Canary Islands, Gran Canaria, Las Palmas • One ♂ (17.8 × 16.2); Playa San Cristóbal; MNCN 20.04/20938.
Distribution. Eastern Atlantic, from Azores to Cape Verde; Mediterranean Sea, with records in Spain, France, Italy, Greece, and Turkey.
Remarks. The specimen was mixed with P. marmoratus as “Sin determinar” (undetermined).
For identification and distribution, see also [25].
| Pachygrapsus transversus (Gibbes, 1850) |
| Figure 13 |
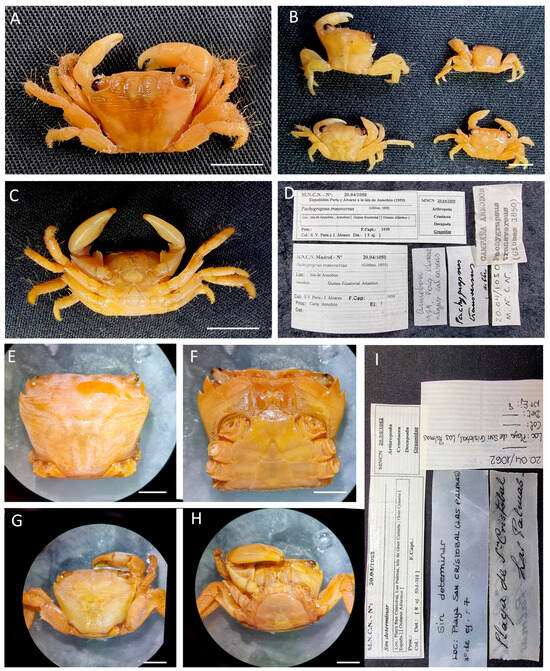
Figure 13.
Pachygrapsus transversus, (A–D) specimens from Equatorial Guinea, MNCN 20.04/01050; (E–I) specimens from Spain, MNCN 20.04/20939. Scale bars: 0.5 cm.
Material examined. EQUATORIAL GUINEA—Annobón Island • One ♂ (10 × 7.6), four juveniles; 1959; S. V. Peris & J. Álvarez leg.; MNCN 20.04/01050.
SPAIN—Canary Islands, Gran Canaria, Las Palmas • MNCN 20.04/20939; Two ♂ (14.2 × 10.9, 22.7 × 18.4), one ♀ (18.7 × 14.1); Playa San Cristóbal; A. Cabrera leg.; MNCN 20.04/01068b.
Distribution. Western Atlantic, from the Bermuda Islands, Bahamas, and Florida Keys to Uruguay; Eastern Atlantic, Spain, Western coasts of Africa, Madeira, Cape Verde, and the Canary Islands; Mediterranean, with records in France, Turkey, Israel, and Egypt; Pacific, from California to Peru, including the Galápagos Islands.
Remarks. The specimen MNCN 20.04/01050 was collected on calcareous algae. The specimens MNCN 20.04/20939 and MNCN 20.04/01068b were previously identified at family level, Grapsidae.
For identification and distribution see also [25].
| Family Percnidae Števčić, 2005 |
| Genus Percnon Gistel, 1848 |
| Percnon gibbesi (H. Milne Edwards, 1853) |
| Figure 14 and Figure 15 |
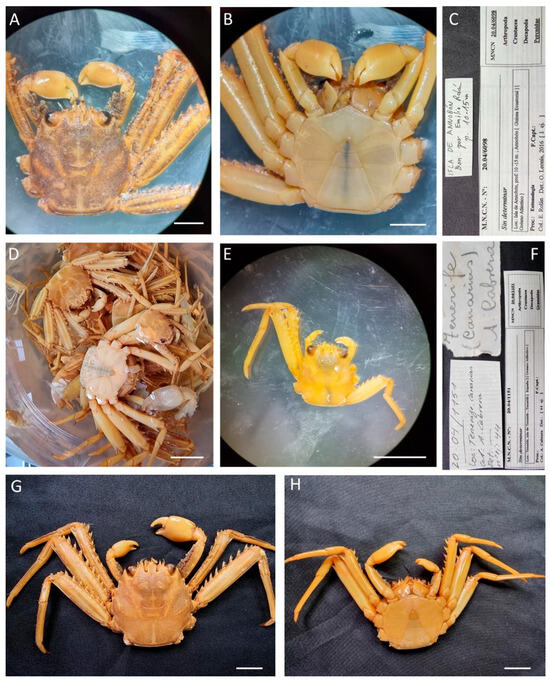
Figure 14.
Percnon gibbesi, (A–C) specimens from Equatorial Guinea, MNCN 20.04/01151; (D–H) specimen from Canary Islands (Spain), MNCN 20.04/06091. Scale bars: (A–C). 0.5 cm, (D–H). 1 cm.

Figure 15.
Percnon gibbesi, (A–E) MNCN 20.04/01144; (F–K) specimens MNCN 20.04/01145. All from Canary Islands (Spain). Scale bars: 1 cm.
Material examined. EQUATORIAL GUINEA—San Juan Cape • One ♂ (10.2 × 11.4); Muni River; 17 August 1901; M. Martínez de la Escalera leg.; MNCN 20.04/04409. —Annobón Island • One ♂ (14.1 × 14.2); between 10 and 15 m; E. Rolán leg.; MNCN 20.04/06098. • One ♂ (9.2 × 8.3); between 10 and 15 m; E. Rolán leg.; 20.04/06088. • Four ♀♀ (5.9 × 6.3, 7.5 × 8.3, 6.2 × 7, 6.6 × 7.1), two ♂♂ (6.3 × 7, 5.6 × 5.8); MNCN 20.04/06094.
SPAIN—Canary Islands, Santa Cruz de Tenerife • 44 unsexed (min. 8.6 × 9.1, max. 32.1 × 32.3); A. Cabrera leg.; MNCN 20.04/01151 • Two ♂♂ (27.1 × 28.7, 29.7 × 22.3); Doreste leg.; MNCN 20.04/01144. —Canary Islands, Gran Canaria, Las Palmas • Three ♀♀ (21.2 × 20.5, 21.9 × 23.3, 27.6 × 25.5), five ♂♂ (25.5 × 27.3, 23.9 × 24.3, 26.4 × 26.8, 22.4 × 23.6, 23.7 × 24.7); A. Cabrera leg.; MNCN 20.04/01145.
Distribution. Its natural distribution is along the Pacific coasts from Chile to California; Western Atlantic, from Brazil to Florida; Eastern Atlantic, from the Gulf of Guinea to Madeira.
Nowadays, the species is considered one of the most widely spread non-native marine species in the Mediterranean waters [53,54].
Remarks. All specimens identified were previously misidentified or identified as order/family level: MNCN 20.04/04409 as Decapoda, MNCN 20.04/01151 as Grapsidae, MNCN 20.04/01144 and MNCN 20.04/01145 as Homolidae, MNCN 20.04/06098 as Percnidae and MNCN 20.04/06088 as Plagusiidae.
For identification and distribution, see also [19,26,55].
| Family Plagusiidae Dana, 1851 |
| Genus Plagusia Latreille, 1804 |
| Plagusia squamosa (Herbst, 1790) |
| Figure 16 |
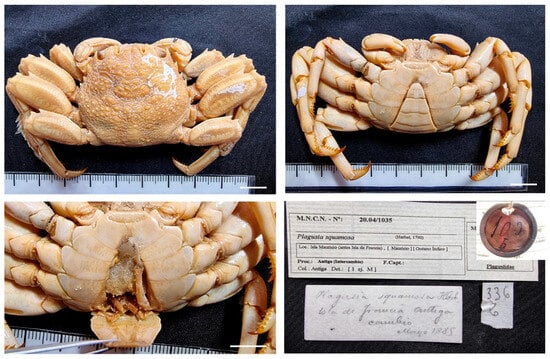
Figure 16.
Plagusia squamosa, from Mauritius, MNCN 20.04/01035. Scale bars: 1 cm.
Material examined. MAURITIUS • One ♂ (35.3 × 31.3); 1885? (exchange); MNCN 20.04/01035.
Distribution. Widely distributed across the Indo-Pacific region, from East Africa and the Red Sea to Japan, Australia, and Hawaii; extends into the eastern Pacific from Baja California to Chile, including Galápagos. Recently introduced into the Mediterranean Sea. Found in intertidal and shallow subtidal zones, often on rocks, driftwood, and floating debris.
Remarks. This specimen is stuffed with cotton, and it has been exposed in a dry state. Although the collection data is not available, due to the old round label, the comment on one of the labels about an exchange with Antiga suggests that this specimen was likely included in the MNCN collection in 1850 and collected years before (1824?).
For identification and distribution, see also [29,56,57].
| Family Sesarmidae Dana, 1851 |
| Genus Armases Abele, 1992 |
| Armases elegans (Herklots, 1851) |
| Figure 17 |
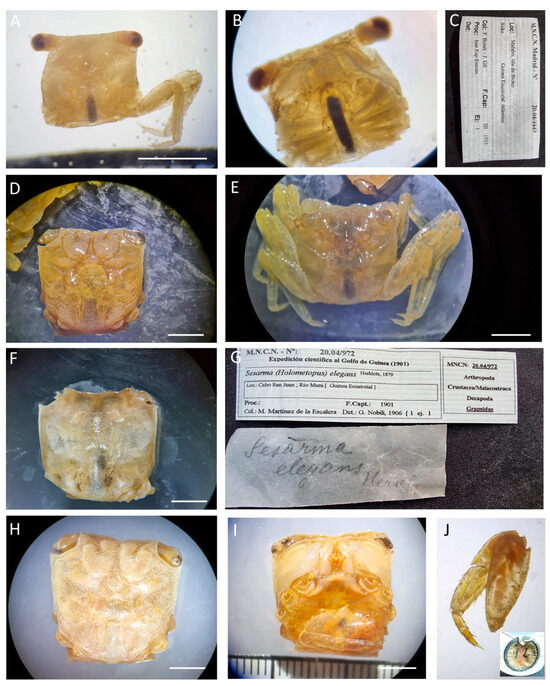
Figure 17.
Armases elegans, (A–C) specimen MNCN 20.04/04449; (D–G) specimens MNCN 20.04/00972; (H,I) specimen MNCN 20.04/01025. All from Equatorial Guinea. Scale bars: (A) 0.25 cm; (D–J) 0.5 cm.
Material examined. EQUATORIAL GUINEA—Malabo, Bioko • One ♂ (7.4 × 7.1); March 1933; F. Bonet & J. Gil Collado leg.; comes from the Instituto Español de Entomología; MNCN 20.04/04449. − San Juan Cape • One ♀ ov. (15.2 × 14); 17-08-1901; M. Martínez de la Escalera leg.; MNCN 20/04/01025. • One ♂ (17.4 × 17.7); unsexed; 17-08-1901; M. Martínez de la Escalera leg.; MNCN 20.04/00972.
Distribution. West Africa, from Guinea to Angola, including Cameroon, Nigeria, Gabon, and Sierra Leone.
Remarks. All specimens, at some point during their preservation, were kept dry, later rehydrated in trisodium phosphate, and subsequently placed in ethanol (comments in the MNCN database). Juvenile specimen MNCN 20.04/04449 was identified as Decapoda.
For identification and distribution, see also [19,24,26,58,59].
| Genus Guinearma Shahdadi & Schubart, 2017 |
| Guinearma huzardi (Desmarest, 1825) |
| Figure 18 |
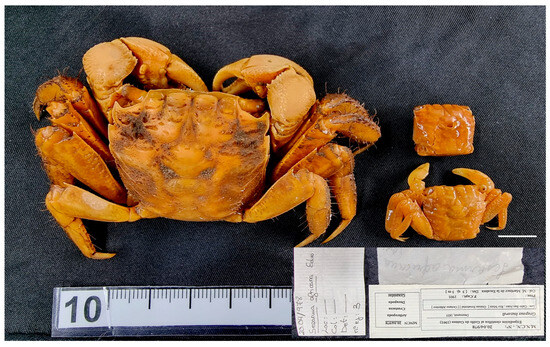
Figure 18.
Guinearma huzard from Equatorial Guinea MNCN 20.04/00978. Scale bar: 1 cm.
Material examined. EQUATORIAL GUINEA—San Juan Cape • One ♂ (34 × 30.4), two unsexed (15.3 × 13.4, 15.7 × 13.3); 1901; M. Martínez de la Escalera leg.; MNCN 20.04/00978.
Distribution. West Africa from the Senegal River to Angola.
Remarks. For identification and distribution, see also [26,60,61].
| Genus Neosarmatium Serène & Soh, 1970 |
| Neosarmatium meinerti (De Man, 1887) |
| Figure 19 |
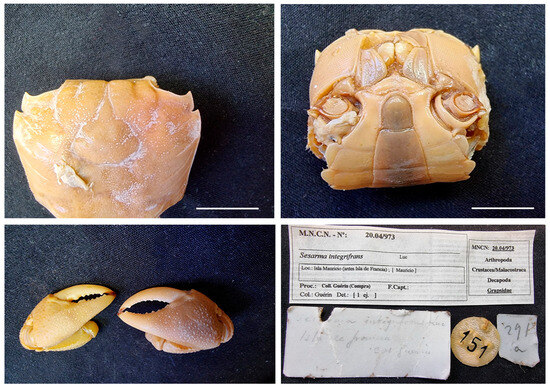
Figure 19.
Neosarmatium meinerti from Mauritius, MNCN 20.04/00973. Scale bars: 1 cm.
Material examined. MAURITIUS • One ♂ (29.5 × 25); 1824?; “La Coquille” corvette; Guérin-Méneville leg.; MNCN 20.04/00973.
Distribution. West Indian Ocean, Mayotte?, Seychelles, Mauritius, and Rodrigues.
Remarks. The specimen was previously identified as Sesarma integrifrans (Grapsidae), a species name which has not been possible to determine in historical records. The label inside the jar states that it comes from a purchase made by Guérin-Méneville.
Ragionieri et al. [62] studied the N. meinerti species complex, describing three new species and narrowing the distribution of this species to a more restricted region.
For identification and distribution, see also [21,62,63].
| Genus Platychirarma Schubart & Ng, 2020 |
| Platychirarma buettikoferi (De Man, 1883) |
| Figure 20 |
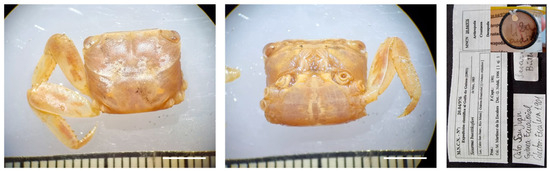
Figure 20.
Platychirarma buettikoferi from Equatorial Guinea, MNCN 20.04/00976. Scale bars: 0.5 cm.
Material examined. EQUATORIAL GUINEA—San Juan Cape • One ♂ (11.5 × 9.6); Muni River; 1901; M. Martínez de la Escalera leg. (G. Nobili, 1906); MNCN 20.04/00976.
Distribution. West Africa, from Liberia to Angola.
Remarks. For identification, see also [19,24,26,59].
| Family Varunidae H. Milne Edwards, 1853 |
| Subfamily Cyclograpsinae H. Milne Edwards, 1853 |
| Genus Pseudohelice Sakai, Türkay & Yang, 2006 |
| Pseudohelice sp. |
| Figure 21 |
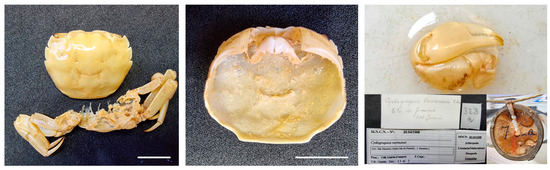
Figure 21.
Pseudohelice sp. from Mauritius MNCN 20.04/01008.
Material examined. MAURITIUS • One ♀ (27 × 24.4); 1824?; “La Coquille” corvette; Guérin-Méneville leg.; MNCN 20.04/01008.
Remarks. The specimen is in a ruined state, so its identification is not possible. It was identified as Cydograpsos verreavei (Grapsidae), where errors in the typography of the genus can be appreciated, since it is correctly written on the label; however, it was misspelt when digitised. It has not been possible to know which species this specimen was originally assigned.
For identification, see also [28,64,65,66].
| Subfamily Varuninae H. Milne Edwards, 1853 |
| Genus Brachynotus De Haan, 1833 |
| Brachynotus atlanticus Forest, 1957 |
| Figure 22 |
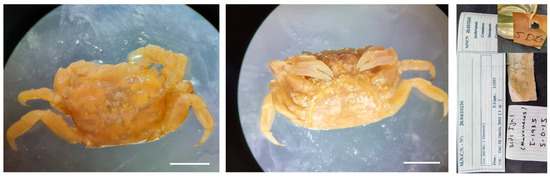
Figure 22.
Brachynotus atlanticus from Morocco, MNCN 20.04/10236.
Material examined. MOROCCO—Sidi Ifni • 1 ♀ (12.1 × 8.8) (moult); January 1935; MNCN 20.04/10236.
Distribution. Eastern Atlantic, from Portugal and Spain to Mauritania, and the Mediterranean Sea.
Remarks. The specimen was catalogued as “Sin determinar” (undetermined), assigned to Grapsidae. The specimen is not very well preserved, and it is highly likely that it was dry for some time, which is why fungi are observed on its surface and inside the abdomen.
For identification and distribution, see also [25,26,67,68].
| Superfamily Ocypodoidea Rafinesque, 1815 |
| Family Camptandriidae Stimpson, 1858 |
| Genus Calabarium Manning & Holthuis, 1981 |
| Calabarium crinodytes Manning & Holthuis, 1981 |
| Figure 23 |
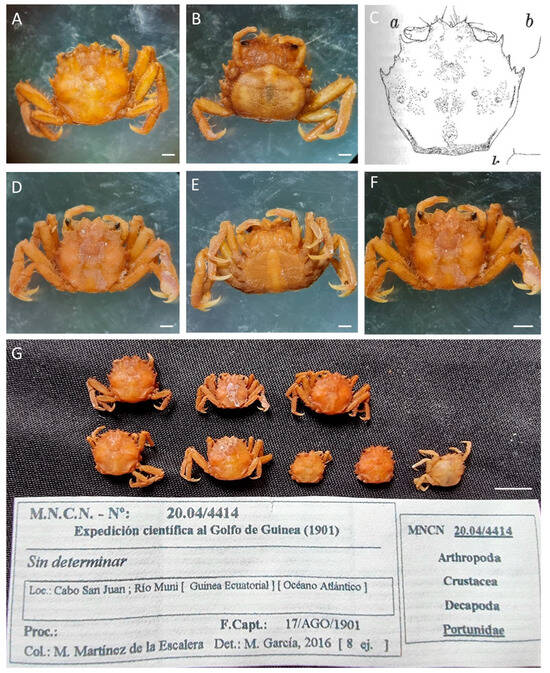
Figure 23.
(A,B,D–G). Calabarium crinodytes, MNCN 20.04/04414 and MNCN 20.04/04414-1; (C) illustration by Manning & Holthuis [26]. Scale bars: (A–F) 1 mm; (G) 0.5 cm.
Material examined. EQUATORIAL GUINEA—San Juan Cape • Five ♀♀ (7.8 × 7.3, 7.3 × 6.4, 6.2 × 6.5, 7.7 × 7.6, 7.3 × 7.5), three ♂♂ (6.3 × 5.7, 6 × 5.7, 6.7 × 6.3); “Expedición Científica al Golfo de Guinea”; 17 August 1901; M. Martínez de la Escalera leg.; MNCN 20.04/04414 and MNCN 20.04/04414-1 (DNA voucher ♂ 6.7 × 6.3).
Distribution. From the type locality in Nigeria.
Remarks. The specimens were labelled as “No identificado” (unidentified), assigned to Portunidae.
This is a species described by Manning & Holthuis in 1981 [26], based on 24 specimens collected in the Niger Delta (Nigeria) in 1978. Since then, the species had not been recorded. This is a new record of this species, although based on eight specimens collected more than 70 years earlier. This is the first record in the waters of Equatorial Guinea, clarifying that the distribution of Calabarium crynodites is not restricted to Nigeria (topotypical distribution), but rather it is a species very difficult to observe due to its small size and perhaps its habitat. Manning & Holthuis [26] described that the specimens were collected from submerged leaves of the aquatic lily Crinum natans Baker in the zone of mixed Rhizophora and Pandanus in the New Calabar River. The specimens deposited in the MNCN were located in the Muni River near San Juan Cape, an estuarine ecosystem, a similar habitat to that of the Calabar River in Nigeria.
For identification and distribution, see also [26].
DNA barcodes. Two sequences, one of 16S (438 bp) (PX418380) and another of COI (345 bp) (PX418257), were obtained for the specimen MNCN 20.04/04414-1, preserved 124 years ago. There are no molecular data for this species in any DNA database; therefore, these are the first DNA sequences known for this species. The 16S sequence has the highest match with the sequence ON379453 of Ilyogynnis microcheirum, but only with 87.73% of similarity, an expected intergeneric distance at intrafamilial level (Camptandriidae). However, the next matches, between 86.22 and 86.94%, are with the sequence of a Glyptograpsidae (Glyptograpsus jamaicensis) and several sesarmids (Neosarmatium spp., and Chiromantes spp.), and the next, a camptandriid (Nasima dotilliformis) match of <86.13%. In the case of the COI sequence, the highest matches (86.09–86.94% similarity) are with two sesarmids (Pseudosesarma glabrum and Karstarma boholano), and no matches appear with camptandriids in the first 250 sequences blasted, although there are COI sequences for several genera, as Cleistostoma, Danielella, Deiratonotus, Manningis, Nasima, and Opusia. However, when comparing these sequences with the 345 bp COI sequence of Calabarium crinodytes, all are below 83% of similarity. With only these first data, it is early to conclude about phylogenetic relationships of this genus and species.
| Family Macrophthalmidae Dana, 1851 |
| Genus Macrophthalmus Desmarest, 1823 |
| Macrophthalmus aff. depressus |
| Figure 24 |

Figure 24.
Macrophthalmus aff. depressus from Equatorial Guinea, MNCN 20.04/00926 and MNCN 20.04/00926-1. Scale bars: 1 cm.
Material examined. EQUATORIAL GUINEA—Bioko Island • Two ♂♂ (24.8 × 17.7, 25 × 17.9); (the surroundings of Malabo, Casa Juana); 21 April 1961; S. V. Peris leg.; MNCN 20.04/00926 and MNCN 20.04/00926-1 (DNA voucher ♂ 25 × 17.9).
Remarks. The specimens were identified as “Sin determinar” (undetermined), assigned to Goneplacidae.
These two specimens, assigned to the genus Macrophthalmus, share some characteristics with M. (Mareotis) depressus Rüppell, 1830, which is the closer species following the keys of Barnes [69,70]. However, it is not this species; differences include carapace dimensions, and different antero-lateral teeth and chelae, among others. The two males show some morphological similarities with the monotypic genus Hemiplax Heller, 1865 from Australia, with only one species, H. hirtipes. Throughout West Africa, there are no records, at least to date, of species from the family Macrophthalmidae, making this record of two male specimens of Macrophthalmus sp. on the coasts of Malabo (Equatorial Guinea) a significant finding. New morphological and molecular studies are underway to determine whether this is a known species with a wide distribution range, or, on the contrary, a new species, and possibly a new subgenus.
For identification and distribution, see also [19,28,52,69,70,71].
| Family Ocypodidae Rafinesque, 1815 |
| Subfamily Gelasiminae Miers, 1886 |
| Genus Gelasimus Latreille, 1817 |
| Gelasimus tetragonon (Herbst, 1790) |
| Figure 25 |
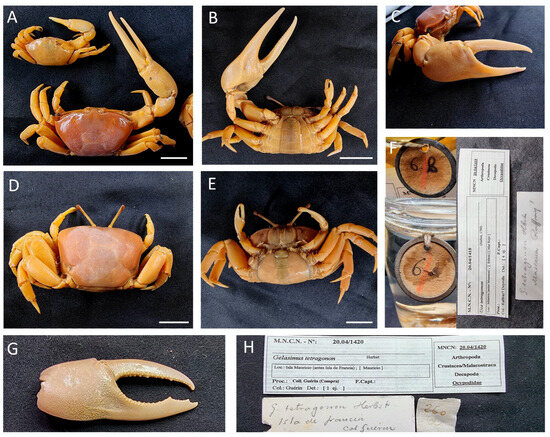
Figure 25.
Gelasimus tetragonon, (A–F) specimens from Eritrea, MNCN 20.04/01418; (G,H) major cheliped fragment of from Maurice specimen, MNCN 20.04/01420. Scale bars: 1 cm.
Material examined. ERITREA—Massaua (Mitsiwa) • Two ♂♂ (21.5 × 13.3, 29.5 × 20.8, 16.6 × 11.3), one ♀ ov. (29.7 × 22.1); 1825?; Hemprich & Ehrenberg Expedition (1824–1825)?; Raffray? Deyrolle leg.; MNCN 20.04/01418.
MAURITIUS • Unsexed (cheliped fragment); 1850; Guérin-Méneville leg.; MNCN 20.04/01420.
Distribution. Widely distributed in the Indo-Pacific and Indian Ocean regions, including: the Red Sea and East Africa (Egypt, Eritrea, Somalia, Kenya, Tanzania, and Madagascar); Indian Ocean (India, Maldives, Sri Lanka, Oman, Seychelles, Mauritius, Réunion, and Mayotte); Southeast Asia (Thailand, Malaysia, Indonesia, Philippines, Vietnam, and Taiwan); Pacific Islands (Australia, Papua New Guinea, New Caledonia, Fiji, Vanuatu, Samoa, Tonga, Cook Islands, French Polynesia, and Hawaiian Islands).
Remarks. For identification and distribution, see also [19,20,28,72].
| Subfamily Ocypodinae Rafinesque, 1815 |
| Genus Afruca Crane, 1975 |
| Afruca tangeri (Eydoux, 1835) |
| Figure 26, Figure 27 and Figure 28 |
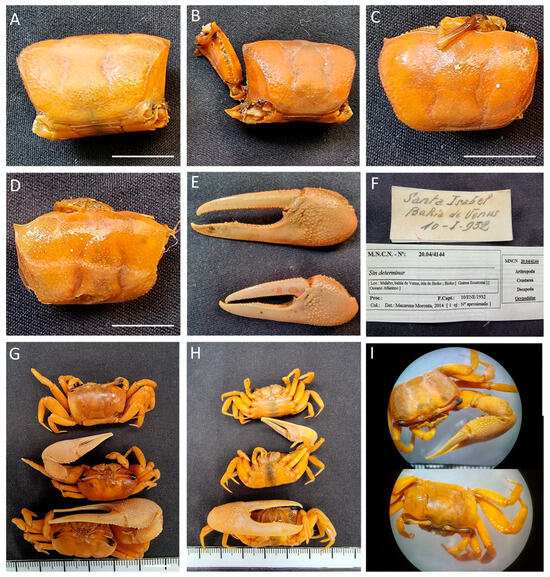
Figure 26.
Afruca tangeri, (A–F) specimens MNCN 20.04/04144; (G–I) specimens MNCN 20.04/01429. All from Equatorial Guinea. Scale bars: 1 cm.

Figure 27.
Afruca tangeri, (A–E) specimens MNCN 20.04/01422; (F–J) specimens MNCN 20.04/01467. All from Morocco. Scale bars: 1 cm.

Figure 28.
Afruca tangeri, (A–F) specimens MNCN 20.04/03979; (G–L) specimens MNCN 20.04/03981. All from Morocco. Scale bars: 1 cm.
Material examined. EQUATORIAL GUINEA—Bioko Island, Malabo • Two ♂♂ (22.6 × 15.3, 21.6 × 15.4), one ♀ (8.6 × 15.4); (Venus Bay); 10-01-1932; MNCN 20.04/04144. • one ♀ (12.2 × 17.3), two ♂♂ (13.8 × 18.3, 13.8 × 20.3); MNCN 20.04/01429. —Gulf of Guinea • One unsexed (cheliped fragment); uncertain locality in Equatorial Guinea; July 1891; J. Valero leg.; MNCN 20.04/01411.
MOROCCO—Oro River • Three ♂♂ (35.9 × 26.8, 36.5 × 27.5, 35 × 25.9); Quiroga leg.; MNCN 20.04/01422. —Larache • Two ♂♂ (31.2 × 22.7, 27.7 × 18.1); 1955; MNCN 20.04/01467.
WESTERN SAHARA—Dakhla • Four ♂♂ (38.3 × 28.1, 40.4 × 29.8, 26.2 × 18.9, 36.1 × 26.5); August 1933; MNCN 20.04/03979. • Three ♀♀ (30.1 × 22.5, 24.4 × 18.3, 23.6 × 18.9), one ♂ (41.2 × 30.6); August 1933; MNCN 20.04/03981.
Distribution. Atlantic coasts from Morocco and Portugal to Angola, including the Cape Verde Islands, Gulf of Guinea, and parts of West Africa (Senegal, Guinea, Sierra Leone, Liberia, and Nigeria). It is also reported from the Canary Islands and the West Indies (inaccurate).
Remarks. The records with codes MNCN 20.04/04144 and MNCN 20.04/01467 came in as “Sin determinar” (undetermined), assigned to Ocypodidae; MNCN 20.04/01411 was identified as Gelasimus platydactilus, actually as Uca major (Herbst, 1782) and with East American distribution, and the rest of the specimens were labelled with the old name, Uca tangeri. The specimens included in record MNCN 20.04/01429 are known to have been collected in the Gulf of Guinea, most likely in Equatorial Guinea, but the current record itself is marked with a question mark.
For identification and distribution, see also [19,20,28,73].
| Genus Ocypode Weber, 1795 |
| Ocypode africana De Man, 1881 |
| Figure 29 |
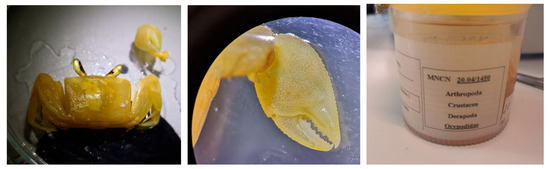
Figure 29.
Ocypode africana, one of the specimens of Equatorial Guinea, MNCN 20.04/01450.
Material examined. EQUATORIAL GUINEA—San Juan Cape • Five unsexed (nor sized, nor sexed); 17 August 1901; M. Martínez de la Escalera leg.; MNCN 20.04/01450. − Annobón Island • Seven ♂♂ (18.6 × 15.2, 20.4 × 15.9, 20.2 × 15.5, 18.7 × 15.6, 22.3 × 17.1, 23.6 × 18.2, 22.7 × 17); 1959; S.V. Peris and J. Álvarez leg.; MNCN 20.04/01475 (not photografied).
Distribution. Tropical West and Central Africa, from southern Mauritania to northern Namibia, including the Gulf of Guinea. It is found in countries like Senegal, Guinea, Sierra Leone, Liberia, Ghana, Côte d’Ivoire, Nigeria, Cameroon, Angola, and Cape Verde Islands.
Remarks. The specimens were identified at family level (Ocypodidae) and appeared to have been dry for some time and have subsequently been rehydrated.
For identification and distribution, see also [22].
| Ocypode cursor (Linnaeus, 1758) |
| Figure 30 |
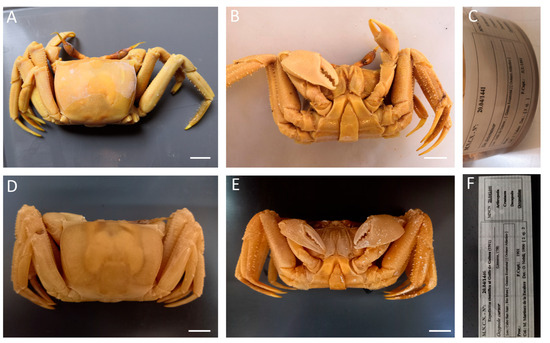
Figure 30.
Ocypode cursor, (A–C) specimen from Equatorial Guinea, MNCN 20.04/01441; (D–F) specimen from Equatorial Guinea, MNCN 20.04/01446. Scale bars: 1 cm.
Material examined. EQUATORIAL GUINEA • Three ♂♂ (42.8 × 34, 31.8 × 25.8, 42.7 × 38.1); July 1891; J. Valero leg.; MNCN 20.04/01441. One ♂ (unmesured); Martínez de la Escalera leg.; MNCN 20.04/01446.
Distribution. Along the East Atlantic from southern Mauritania to Angola, including the Gulf of Guinea and Cape Verde Islands; Mediterranean Sea, from Egypt and Israel to Turkey and Greece. It inhabits sandy beaches in intertidal zones.
Remarks. Specimens from Equatorial Guinea were previously identified as “No identificado”, (unidentified), assigned to Ocypodidae. This record provides the first one in Eritrea waters.
For identification and distribution, see also [22].
| Ocypode pallidula Hombron & Jacquinot, 1846 |
| Figure 31 |

Figure 31.
Ocypode pallidula from Eritrea, MNCN 20.04/01444b. Scale bars: 1 cm.
Material examined. ERITREA • Massaua; one ♂ (14.4 × 10.9); 1825?; Hemprich & Ehrenberg Expedition (1824–1825)?; Raffray? Deyrolle leg.; MNCN 20.04/01444b.
Distribution. Indo-Pacific, Hawaii (type locality), French Polynesia, Australia (Great Barrier Reef), New Zealand (Kermadec Islands), Madagascar, Mauritius, and Indian Ocean islands (Réunion, Juan de Nova, Europa). Common on sandy beaches, often in burrows, from the supratidal zone to intertidal areas.
Remarks. The specimen was identified at family level (Ocypodidae).
For identification and distribution, see also [22].
| Ocypode saratan (Forskål, 1775) |
| Figure 32 |
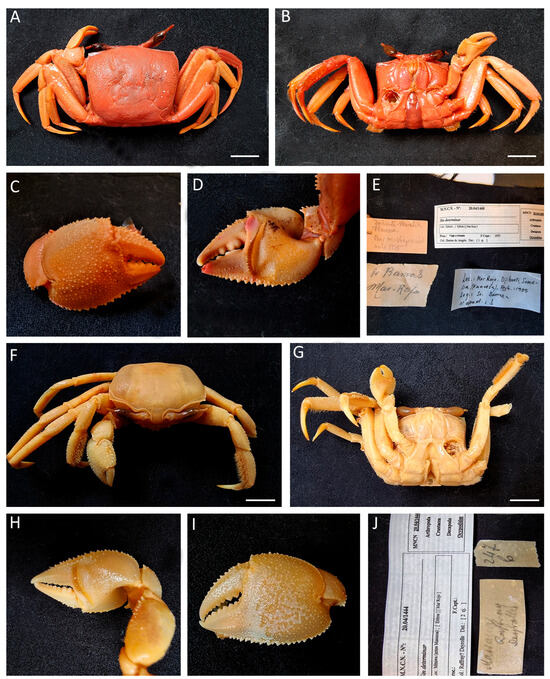
Figure 32.
Ocypode saratan (A–E) specimen from Djibouti MNCN 20.04/01468; (F–J) specimen from Eritrea MNCN 20.04/01444a. Scale bars: 1 cm.
Material examined. DJIBOUTI • One ♂ (32.5 × 29.6); 1935; Barras de Aragón leg.; MNCN 20.04/01468.
ERITREA − Massaua • One ♂ (31.6 × 28.6); 1825?; Hemprich & Ehrenberg Expedition (1824–1825)?; Raffray? Deyrolle leg.; MNCN 20.04/01444a.
Distribution. Red Sea, Gulf of Suez, Gulf of Aqaba, and along the coasts of Egypt, Eritrea, Sudan, Saudi Arabia, and Yemen; Gulf of Aden, Socotra, and parts of the Arabian Peninsula, including Oman and Somalia, primarily in sandy intertidal zones and mangrove areas.
Remarks. The specimen was previously identified as “No identificado” (unidentified), assigned to Ocypodidae.
Although some authors have described this species as having a “curved, prolonged eyestalk,” not all specimens exhibit such an elongated eyestalk. It is common to find specimens with shorter, non-curved eyestalks. In fact, in Sakai’s key, the length of the eyestalk is not used as an identifying feature; instead, the stridulating ridge composed of 67–87 fine striae, is the key characteristic.
For identification see also [19,21,22,23].
DNA barcodes. For the specimen from Djibouti, preserved 90 years ago, we have obtained two sequences, one of 16S (432 bp) (PX418382) and another of COI (308 bp) (PX418259). In both cases, the sequences match 98.84–97.22% (5–12 mut.) and 95.72% (13 mut.) with sequences of 16S (NC_087790, PP389282, LC150369, MF509788–MF509792, and MF673804–MF673812) and COI (NC_087790, PP389282, and LC150424), respectively, of Ocypode rotundata from Pakistan and Persian Gulf (Iran). For the specimen from Eitrea, preserved 200 years ago, we have only obtained a sequence of 16S (432 bp) (PX418383) that differ in two mutations from the sequence of the specimen from Djibouti.
There is no molecular data of this species in any DNA database; therefore, these are the first sequences known for this species and reflex a clear relationship with O. rotundata, a species with a partial overlapping distribution.
3.2. Additional Specimens
| Leptuca pugilator (Bosc, 1801) |
| Figure 33 |

Figure 33.
Leptuca pugilator, MNCN 20.04/01432 and MNCN 20.04/01432-1 from unknown country. Scale bars: 0.5 cm.
Material examined. UNKNOWN COUNTRY• One ♀ (14.2 × 9.9), one ♂ (16.7 × 11.2); 1824?; “La Coquille” corvette; Guérin-Méneville leg.; MNCN 20.04/01432 and MNCN 20.04/01432-1 (DNA voucher ♂ 16.7 × 11.2).
Distribution. Subtropical and temperate Western Atlantic, including Caribbean and the Gulf of Mexico.
Remarks. It was identified as Uca chlorophthalmus (Paraleptuca chlorophthalmus (H. Milne Edwards, 1837)) and labelled as being from Mauritius. However, its correct identification is Leptuca pugilator, a species common in the Western Atlantic. These two specimens were purchased from Guérin-Méneville and come from the La Coquille expedition. However, we consider it very likely that the specimen does not originate from the waters of Mauritius, but rather from somewhere in the Atlantic or Caribbean American waters, which were also visited during the expedition. This could be another case of mislabelling in the recorded locality of old specimens deposited in scientific collections, as the species Pugettia producta (Randall, 1840), previously reported in Muñoz et al. [3].
As it is not a species native to African waters, it should not be included in this catalogue, but we have chosen to cite it in the “additional specimens” section because mislabelling in ancient collections is not uncommon and because, in doing so, we highlight the challenges that can arise when studying ancient collections.
For identification and distribution, see also [20,29,72].
DNA barcodes. For the male of these specimens, preserved 200 years ago, we have obtained two sequences, one of 16S (338 bp) (PX418381) and another of COI (318 bp) (PX418256). In both cases, the sequences match 100% with sequences of 16S (AB813662) and COI (FJ969880, FJ969885, and EU329173) of Leptuca pugilator from South Carolina and Florida (USA).
| Neohelice granulata (Dana, 1851) |
| Figure 34 |
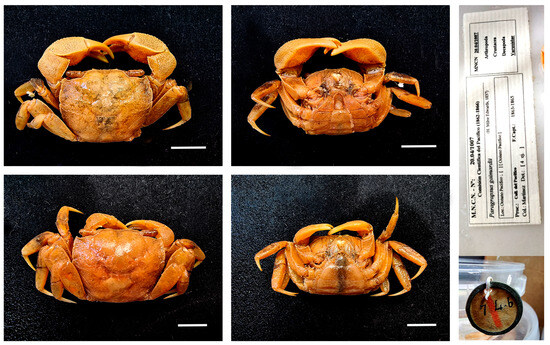
Figure 34.
Neohelice granulata, MNCN 20.04/01007 and MNCN 20.04/01007-1 from unknown location on the Atlantic coast of South America. Scale bars: 0.5 cm.
Material examined. UNKNOWN COUNTRY • Two ♀♀ (23.7 × 20, 27 × 23), two ♂♂ (26 × 22.1, 27 × 23.4); 1863–1865; Martínez y Sáez leg.; MNCN 20.04/01007 and MNCN 20.04/01007-1 (DNA voucher ♂ 27 × 23.4).
Distribution. Along the Atlantic coast of South America, primarily in salt marshes, mudflats, and estuaries from northern Patagonia (Argentina) to Rio de Janeiro (Brazil).
Remarks. These specimens were identified as Paragrapsus gaimardii (H. Milne Edwards, 1837), and the locality of these specimens is the Pacific Ocean (as stated on the label), as they were collected during the Scientific Commission of the Pacific expedition conducted between 1863 and 1865. However, the DNA shows that they belong to Neohelice granulata, a species with a restricted distribution on the Atlantic coasts of South America. Initially, they were included in this study for comparisons with other African varunid, but after the DNA results, we have included them in this additional section, because we consider important to show the validity of DNA barcoding to correct another mistake in species identification and in the locality assigned to a sample, as well as to the success of its sequencing 160 years after it was collected.
The expedition passed through the Atlantic coasts in its way to the Pacific, so surely these samples were collected in this zone.
For identification and distribution, see also [29,64].
DNA barcodes. For one of the males of these specimens, preserved 160 years ago, we have obtained one sequence of 16S (196 bp) (PX418384). The sequences have a 98.96–98.44% similarity (2–3 mutations) with the 16S sequences AJ250640, KT279702, KU313178 and Z79652, the only 16S sequences in GenBank of N. granulata.
4. Discussion
As mentioned in the Introduction Section, many zoological collections require a thorough taxonomic review by experts in different taxa. This is the situation of the decapod crustacean from African waters that are deposited at the Crustacean collection of the MNCN.
Following the review conducted for this work, an annotated catalogue of Thoracotremata marine crabs has been compiled. This catalogue is based on the revision of 197 specimens of African marine crabs, representing 25 species grouped into eight families (Camptandriidae, Gecarcinidae, Grapsidae, Ocypodidae, Percnidae, Plagusiidae, Sesarmidae, and Varunidae) and 2 additional species from other waters, Leptuca pugilator and Neohelice granulate. There are specimens requiring further studies (Pseudohelice sp. and Macrhopthalmus aff. depressus), which could increase the number of species (up to 27 and nine families, including Macrophthalmidae. Families listed in this catalogue represent a high percentage compared with the 13 families of Thoracotremata present in African waters out of the 17 known for this subsection [28], according to the information provided by the African Register of Marine Species platform [74].
Of the total specimens reviewed, 22.9% were previously correctly identified taxonomically, including synonyms (less than 29% when considering records), while the remaining 77.2% were either unidentified (or only identified at family level) or misidentified; more than 70% considering records. The fact that 77.2% of the specimens were not correctly identified is remarkable, and even more so if we consider that this percentage is very similar to that of Part I of this work focused on Heterotremata [3], where this value was close to 80%. This means that not only for many years did a large number of specimens remain at the MNCN facilities without proper identification, as in other scientific collections, but also that we missed the opportunity to learn more about the collecting naturalists or the Spanish expeditions to African waters in past centuries. This study represents the first step toward a better understanding of African crabs through the MNCN collections, a starting point for conducting molecular studies on Thoracotremata specimens deposited in the museum, some of which are up to 200 years old, necessary to clarify the correct taxonomic status of some specimens without accurate identification due to different causes.
How it happened in the study of the Heterotremata [3] was that there were species described in 1981 [26] which already had specimens housed in the MNCN since 1901, such as Calabarium crinodytes, in this case. In fact, after the description of this species by Manning & Holthuis [26], this is the second record worldwide, also expanding its distribution range, which was previously considered restricted to the Niger Delta.
This type of collaboration between specialists and museums provides the necessary resources for revising and updating the classifications of families, genera, and species, which is crucial for keeping taxonomic knowledge updated. Additionally, the use of new molecular techniques, such as DNA barcoding, allow us to conduct more detailed studies on specific taxa. This is the first application of the extraction protocol developed by Ruane & Austin [32] in Decapods, because previously it was used mainly in Vertebrata, especially Reptiles and Fishes [75,76]. The successful results with crab specimens preserved until 200 years ago are promising and open an interesting opportunity for molecular studies of decapod specimens deposited in museums and collections that previously where are not attempted because they were preserved many years ago.
Author Contributions
Conceptualisation, I.M., J.A.C., and J.E.G.-R.; methodology, I.M., J.A.C., and J.E.G.-R.; software, J.A.C.; validation, I.M., J.A.C., J.E.G.-R., and B.S.; investigation, I.M., J.A.C., and J.E.G.-R.; data curation, I.M., J.A.C., J.E.G.-R., and B.S.; writing—original draft preparation, I.M.; writing—review and editing, I.M., J.A.C., and J.E.G.-R.; supervision, I.M., J.A.C., J.E.G.-R., and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Information on the specimens examined can be obtained by contacting the curator at https://www.mncn.csic.es/en/colecciones/cientificas/arthropods (accessed on 20 August 2025).
Acknowledgments
We wish to thank the MNCN for allowing us to access and study the specimens deposited in its collections, Alejandro Cano (ICMAN-CSIC) for laboratory work, and Esmeralda Muñoz for her help with taking some photographs. We are grateful to two anonymous reviewers for their constructive comments, suggestions, and corrections that help to improve the current work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IWP | Indo-West Pacific Ocean |
| leg. | (legit, legerunt) people who donated or sold specimens to the MNCN |
| ov. | ovigerous |
| MNCN | Museo Nacional de Ciencias Naturales |
References
- Bakker, F.T.; Antonelli, A.; Clarke, J.A.; Cook, J.A.; Edwards, S.V.; Ericson, P.G.P.; Faurby, S.; Ferrand, N.; Gelang, M.; Gillespie, R.G.; et al. The Global Museum: Natural history collections and the future of evolutionary science and public education. PeerJ 2020, 8, e8225. [Google Scholar] [CrossRef]
- Parrinha, D.; Soares, L.B.; Cartaxana, A.; Alves, M.J. The zoological collections of Portuguese oceanographic campaigns in former colonial territories. Nat. Hist. Collect. Museomics 2024, 1, 1–34. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Raso, J.E.; Sánchez Chillón, B.; Cuesta, J.A. Marine crabs from African waters deposited in the MNCN. An opportunity for biogeographic and systematic studies. Part I. Heterotremata. Eur. J. Taxon. 2025, 996, 1–94. [Google Scholar] [CrossRef]
- ACN0284/005. Expediente por el que la Comisión del Muni solicita y obtiene del Mº de Estado la financiación necesaria para publicar un tomo de las Memorias con los resultados de la expedición a la Guinea española. Ignacio Bolívar et al., 1902–1907; 24p. Available online: https://csic-primo.hosted.exlibrisgroup.com/primo-explore/search?query=any,contains,ACN0284~2F005&tab=default_tab&search_scope=default_scope&vid=34CSIC_VU1&lang=es_ES&offset=0 (accessed on 20 August 2025).
- ACN0347/007. Proyecto de expedición científica a Guinea Ecuatorial Española, a la Isla de Annobón y Fernando Poo. Museo Nacional de Ciencias Naturales, Madrid, 1930–1932; 8pp. Available online: https://csic-primo.hosted.exlibrisgroup.com/primo-explore/search?query=any,contains,ACN0347~2F007&tab=default_tab&search_scope=default_scope&vid=34CSIC_VU1&lang=es_ES&offset=0 (accessed on 20 August 2025).
- ACN0281/035. Proyecto de expediciones científicas a los territorios españoles del Golfo de Guinea, Sahara español e Ifni, remitido mediante oficio del Director del Museo de Ciencias al Director Gral. de Marruecos y Colonias. Museo Nacional de Ciencias Naturales, Madrid, 1939; 5pp. Available online: https://csic-primo.hosted.exlibrisgroup.com/primo-explore/search?query=any,contains,ACN0347~2F007&tab=default_tab&search_scope=default_scope&vid=34CSIC_VU1&lang=es_ES&offset=0 (accessed on 20 August 2025).
- ACN0261/002. Expediente relativo a la entrega al Museo de Ciencias de las colecciones de historia natural, principalmente etnológicas, recogidas en África y posesiones españolas del Golfo de Guinea por el Oficial de Marina Luis Sorela. Luis Sorela. Museo de Ciencias Naturales de Madrid, Junta de Profesores, 1888; 14pp. Available online: https://csic-primo.hosted.exlibrisgroup.com/primo-explore/search?query=any,contains,ACN0347~2F007&tab=default_tab&search_scope=default_scope&vid=34CSIC_VU1&lang=es_ES&offset=0 (accessed on 20 August 2025).
- RJB01/0143/0006. Libro diario de viajes de Francisco de las Barras y de Aragón: “Diario de un viaje al Extremo Oriente en 1935”. Real Jardín Botánico 1935. Available online: https://bibdigital.rjb.csic.es/records/?navigation=&perpage=&page=1&sort=_score&search=&fulltext=0&bookmarks=0&child=0&search_hide[Categorie%20Real%20Jard%C3%ADn%20Bot%C3%A1nico%20][]=(Categories.title:Real%20Jard%C3%ADn%20Bot%C3%A1nico%20) (accessed on 20 August 2025).
- Duperrey, L.-I.; Bertrand, A.; Bory de Saint-Vincent, M.; Brongniart, A.; Dumont d’Urville, J.-S.-C.; Garnot, P.; Guérin-Méneville, F.-É.; Lesson, R.-P.; Firmin-Didot. Voyage Autour du Monde: Exécuté par Ordre du roi, sur la Corvette de Sa Majesté, la Coquille, Pendant les Années 1822, 1823, 1824, et 1825; Arthus Bertrand: Paris, France, 1838; Volume 2, 162p, Available online: https://www.biodiversitylibrary.org/item/340077 (accessed on 10 July 2025).
- Guérin-Méneville, F.E. Crustacés et Arachnides. In Duperrey, L.I., Capitaine de Frégate, Chevalier de Saint-Louis et Membre de la Légion D’Honneur, Commandant de l’Expédition, Voyage Autour du Monde, exécuté par Ordre du Roi, sur la Corvette de Sa Majesté, La Coquille, pendant les années 1822, 1823, 1824 et 1825, sous le ministère et conformément aux instructions de S.E.M. Le Marquis de Clermont-Tonnerre, Ministre, de la Marine; et publié sous les auspices de son Excellence Mgr le Cte de Chabrol, Ministre de la Marine et des Colonies; Zoologie par R.P. Lesson; Arthus Bertrand: Paris, France, 1838; Volume 2, pp. i–xii+9–56. [Google Scholar]
- Ehrenberg, C.G. Naturgeschichtliche reisen durch Nord-Afrika und West-Asien in den jahren 1820 bis 1825 von Dr. W.F. Hemprich und Dr. C.G. Ehrenberg. In Historischer Theil; Ernst Siegfried Mittler: Berlin, Germany, 1828; 162p. [Google Scholar]
- Raxworthy, C.J.; Smith, B.T. Mining museums for historical DNA: Advances and challenges in museomics. Trends Ecol. Evol. 2021, 36, 1049–1060. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Isarch, E.; Cuesta, J.A. Annotated and updated checklist of marine crabs (Decapoda: Brachyura) of Mozambique supported by morphological and molecular data from shelf and slope species of the “MOZAMBIQUE” surveys. Zootaxa 2021, 5056, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; García-Raso, J.E.; Gónzalez, J.A.; Lopes, E.P.; Dos Santos, A.M.; Cuesta, J.A. Taxonomic revision and molecular phylogeny of Pisa (Decapoda: Majoidea: Epialtidae), including the description of a new genus of Pisinae. Sci. Mar. 2023, 87, e076. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Raso, J.E.; Abello, P.; Cuesta, J.A. Marine crabs of Guinea-Bissau, with emphasis on the deep fauna, supported by an integrative taxonomy. Diversity 2024, 16, 93. [Google Scholar] [CrossRef]
- Muñoz, I.; García-Raso, J.E.; Cuesta, J.A. A new Varunid subfamily (Decapoda, Brachyura, Grapsoidea, Varunidae) for crabs from European and West African waters, with the description of two new genera and two new species. Ecol. Evol. 2025, 15, e71712. [Google Scholar] [CrossRef] [PubMed]
- Barnard, K.H. Descriptive Catalogue of South African decapod crustacea (Crabs and Shrimps). Ann. S. African Mus. 1950, 38, 1–837. [Google Scholar]
- Banerjee, S.K. Biological results of the Snellius expedition. XVIII. The genera Grapsus, Geograpsus, and Metopograpsus (Crustacea Brachyura). Temminckia 1960, 10, 132–199. [Google Scholar]
- Crosnier, A. Crustacés décapodes. Grapsidae et Ocypodidae. Faune Madag. 1965, 18, 1–143. [Google Scholar]
- Crane, J. Fiddler Crabs of the World (Ocypodidae: Genus Uca); Princeton University Press: Princeton, NJ, USA, 1975; pp. 1–736. [Google Scholar]
- Bouchard, J.-M.; Poupin, J.; Cleva, R.; Dumas, J.; Dinhut, V. Land, Mangrove and Freshwater Decapod Crustaceans of Mayotte Region (Crustacea Decapoda); Atoll Research Bulletin; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2013; Volume 592, pp. 1–60. [Google Scholar] [CrossRef]
- Sakai, K.; Türkay, M. Revision of the genus Ocypode with the description of a new genus, Hoplocypode (Crustacea: Decapoda: Brachyura). Mem. Queensl. Mus. 2013, 56, 665–793. [Google Scholar] [CrossRef]
- Al-Hindi, A.N. Brachyuran Crabs of the Yemeni Coastal Waters (Red Sea, Gulf of Aden, Arabian Sea and Socotra Isands). Ph.D. Dissertation, Universität Rostock, Rostock, Germany, 2019. [Google Scholar]
- Monod, T. Hippidea et Brachyura ouest-africains. In Mémoires de l’Institut Français d’Afrique Noire (IFAN); IFAN: Dakar, Senegal, 1956; Volume 45, pp. 1–674. [Google Scholar]
- Zariquiey Álvarez, R. Crustáceos Decápodos Ibéricos. Investig. Pesq. 1968, 32, xv + 510 pp. [Google Scholar]
- Manning, R.B.; Holthuis, L.B. West African Brachyuran crabs. Smithson. Contrib. Zool. 1981, 306, 1–379. [Google Scholar] [CrossRef]
- Manning, R.B.; Chace, F.A. Decapod and Stomatopod Crustacea from Ascension Island, South Atlantic Ocean. Smithson. Contrib. Zool. 1990, 503, 91. [Google Scholar]
- Poore, G.; Ahyong, S. Marine Decapod Crustacea. A Guide to Families and Genera of the World; CSIRO Publishing: Clayton, Australia, 2023; pp. 1–928. [Google Scholar]
- Sasaki, J. The Species List of Decapoda, Euphausiacea, and Stomatopoda, all of the World. Version. 07-8.12-Worldwide species list of Decapoda, Euphausiacea and Stomatopoda. 2023. Available online: https://www.researchgate.net/publication/376519923_The_Species_List_of_Decapoda_Euphausiacea_and_Stomatopoda_all_of_the_World_Version_07-812-Worldwide_species_list_of_Decapoda_Euphausiacea_and_Stomatopoda-?channel=doi&linkId=657bcf906610947889ccc159&showFulltext=true (accessed on 20 July 2025).
- DecaNet. (World List of Decapoda). Available online: https://www.decanet.info (accessed on 20 August 2025).
- Ng, P.K.L.; Guinot, D.; Davie, P. Systema Brachyurorum: Part I. An annotated checklist of extant Brachyuran crabs of the world. Raffles Bull. Zool. 2008, 17, 1–296. [Google Scholar]
- Ruane, S.; Austin, C.C. Phylogenomics using formalin-fixed and 100+ year-old intractable natural history specimens. Mol. Ecol. Resour. 2017, 17, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (NCBI) [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 1988. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 20 August 2025).
- Meyer, C.P.; Paulay, G. DNA Barcoding: Error Rates Based on Comprehensive Sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Palumbi, S.R.; Martin, A.; Romano, S.; Mcmillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR–A Collection of PCR Protocols, 2nd ed.; University of Hawai: Honolulu, HI, USA, 1991. [Google Scholar]
- Crandall, K.A.; Fitzpatrick, J.F.J. Crayfish molecular systematics: Using a combination of procedures to estimate phylogeny. Syst. Biol. 1996, 45, 1–26. [Google Scholar] [CrossRef]
- Wangensteen, O.S.; Palacin, C.; Guardiola, M.; Turon, X. DNA Metabarcoding of littoral hardbottom communities: High diversity and database gaps revealed by two molecular markers. PeerJ 2018, 5, e4705. [Google Scholar] [CrossRef]
- Schubart, C.D.; Huber, M.G.J. Genetic comparisons of German populations of the stone crayfish, Austropotamobius torrentium (Crustacea: Astacidae). Bull. Fr. Pêche Piscic. 2006, 380–381, 1019–1028. [Google Scholar] [CrossRef]
- Astrin, J.; Stuben, P. Phylogeny in cryptic weevils: Molecules, morphology and new genera of Western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebr. Syst. 2008, 22, 503–522. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Ng, P.Y.C. Land crabs (Crustacea: Brachyura: Gecarcinidae) of Singapore. Nat. Singap. 2021, 14, e2021001. [Google Scholar] [CrossRef]
- Cuesta, J.A.; Anger, K. Larval morphology and salinity tolerance of a land crab from West Africa, Cardisoma Armatum (Brachyura: Grapsoidea: Gecarcinidae). J. Crust. Biol. 2005, 25, 640–654. [Google Scholar] [CrossRef]
- Rathbun, M.J. The brachyuran crabs collected by the American Museum Congo Expedition, 1909–1915. In Scientific Results of The American Museum Congo Expedition. General Invertebrate Zoology; American Museum of Natural History: New York, NY, USA, 1921. [Google Scholar]
- Kingsley, J.S. Carcinological notes, no. IV. –Synopsis of the Grapsidae. Proc. Acad. Nat. Sci. Phila. 1880, 32, 187–224. [Google Scholar]
- De Man, J.G. Bericht über die von Herrn Schiffscapitän Storm zu Atjeh, an den westlichen Küsten von Malakka, Borneo und Celebes sowie in der Java-See gesammelten Decapoden und Stomatopoden. Zool. Jahrb. Abt. Syst. Geog. Biol. Tiere 1898, 10, 75–218. [Google Scholar]
- Fujita, Y. Terrestrial and semi-terrestrial decapod crustaceans from Fude-iwa Island, Miyako Group, the Ryukyu Islands, Japan [In Japanese with English abstract]. Bull. Miyakojima City Mus. 2016, 20, 37–52. [Google Scholar]
- Schubart, C.D. Reconstruction of phylogenetic relationships within Grapsidae (Crustacea: Brachyura) and comparison of trans-isthmian versus amphi-atlantic gene flow based on mtDNA. Zool. Anz. 2011, 250, 472–478. [Google Scholar] [CrossRef]
- Ip, B.H.Y.; Schubart, C.D.; Tsang, L.M.; Chu, K.H. Phylogeny of the shore crab family Grapsidae (Decapoda: Brachyura: Thoracotremata) based on a multilocus approach. Zool. J. Linn. Soc 2015, 174, 217–227. [Google Scholar] [CrossRef]
- Capart, A. Crustacés Décapodes Brachyures. In: Expédition océanographique Belge dans les eaux côtières africaines de l’Atlantique Sud (1948–1949). Résultats Sci. Inst. R. Sci. Nat. Belg. 1951, 3, 11–205. [Google Scholar]
- Forest, J.; Guinot, D. Crustacés Décapodes: Brachyoures. In Campagne de la Calypso dans le Golfe de Guinée et aux îles Principe, São Tomé, et Annobon (1956). Résultats scientifiques des Campagnes de la ‘Calypso’. Fascicule VII. 16. Annales de l’Institut Océanographique; Masson: Paris, France, 1966; Volume 44, pp. 23–124. [Google Scholar]
- Chace, F.A.; Hobbs, H.H. The freshwater and terrestrial decapod crustaceans of the West Indies with special reference to Dominica. Bull. U.S. Nat. Mus. 1969, 292, 1–258. [Google Scholar] [CrossRef]
- Ng, P.K.L. Crabs. In FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific; Carpenter, K.E., Niem, V.H., Eds.; Cephalopods, Crustaceans, Holothurians and Sharks; FAO: Rome, Italy, 1998; Volume 2, pp. 687–1396. [Google Scholar]
- Naderloo, R. Atlas of Crabs of the Persian Gulf; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–444. [Google Scholar] [CrossRef]
- Katsanevakis, S. Unpublished Mediterranean records of marine alien and cryptogenic species. BioInvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Iveša, N.; Brajković, A.; Piria, M.; Buršić, M. The northernmost record of Percnon gibbesi (H. Milne Edwards, 1853) in the Mediterranean Sea. BioInvasions Rec. 2024, 13, 767–776. [Google Scholar] [CrossRef]
- Schmitt, W.L. Decapod and other Crustacea collected on the presidential cruise of 1938. Smithson. Misc. Collect. 1939, 98, 1–29. [Google Scholar] [CrossRef]
- Garth, J.S. The Brachyuran Crabs of Easter Island; Proceedings of the California Academy of Sciences, 4th Series; California Academy of Sciences: San Francisco, CA, USA, 1973; Volume 39, pp. 331–336. [Google Scholar]
- Schubart, C.D.; Ng, P.K.L. On the identities of the rafting crabs Cancer depressus Fabricius, 1775, Cancer squamosus Herbst, 1790, Plagusia immaculata Lamarck, 1818 (Crustacea: Decapoda: Brachyura: Plagusiidae). Raffles Bull. Zool. 2000, 48, 327–3356. [Google Scholar]
- Abele, L.G. A review of the Grapsid crab genus Sesarma (Crustacea: Decapoda: Grapsidae) in America, with the description of a new genus. Smithson. Contrib. Zool. 1992, 527, 1–60. [Google Scholar] [CrossRef]
- Schubart, C.D.; Ng, P.K.L. Revision of the intertidal and semiterrestrial crab genera Chiromantes Gistel, 1848, and Pseudosesarma Serène & Soh, 1970 (Crustacea: Brachyura: Sesarmidae), using morphology and molecular phylogenetics, with the establishment of nine new genera and two new species. Raffles Bull. Zool. 2020, 68, 891–994. [Google Scholar] [CrossRef]
- Shahdadi, A.; Schubart, C.D. Taxonomic review of Perisesarma (Decapoda: Brachyura: Sesarmidae) and closely related genera based on morphology and molecular phylogenetics: New classification, two new genera and the questionable phylogenetic value of the epibranchial tooth. Zool. J. Linn. Soc. 2017, 182, 517–548. [Google Scholar] [CrossRef]
- Shahdadi, A.; Ndongo, P.A.M.; Suess, T.; Schubart, C.D. Reappraisal and redescription of the three species of the recently defined genus Guinearma Shahdadi & Schubart, 2017, with a key to the West African Sesarmidae (Decapoda, Brachyura). Crustaceana 2019, 92, 307–334. [Google Scholar] [CrossRef]
- Davie, P. Revision of Neosarmatium Serène and Soh (Crustacea: Brachyura: Sesarminae) with descriptions of two new species. Mem. Qld. Mus. 1994, 35, 35–74. [Google Scholar]
- Ragionieri, L.; Fratini, S.; Schubart, C.D. Revision of the Neosarmatium meinerti species complex (Decapoda: Brachyura: Sesarmidae), with descriptions of three pseudocryptic Indo-West Pacific species. Raffles Bull. Zool. 2012, 60, 71–87. [Google Scholar]
- Sakai, K.; Türkay, M.; Yang, S.-L. Revision of the Helice/Chasmagnathus complex. (Crustacea: Decapoda: Brachyura). Abh. Senckb. Naturforsch. Ges. 2006, 565, 1–76. [Google Scholar]
- Prema, M.; Hsu, J.; Shih, H.; Ravichandran, S. First record of the genus Pseudohelice Sakai, Türkay & Yang, 2006 from India and description of a new pseudocryptic species (Crustacea: Brachyura: Varunidae). Zool. Stud. 2022, 61, e56. [Google Scholar] [CrossRef]
- Hsu, J.-W.; Shih, H.-T.; Innocenti, G. A review of the mud crab genus Pseudohelice Sakai, Türkay & Yang, 2006 (Crustacea: Brachyura: Varunidae), with redescription of Cyclograpsus latreillii H. Milne Edwards, 1837, from the western Indian Ocean. Raffles Bull. Zool. 2022, 70, 94107. [Google Scholar] [CrossRef]
- Forest, J. Mise au point sur les Brachynotus de Méditerranée et d’Afrique occidentale: Brachynotus sexdentatus Risso et Brachynotus atlanticus nov. sp. Bull. Inst. Franç. Afrique Noire A 1957, 19, 501–510. [Google Scholar]
- García-Raso, J.E. Brachyura of the coast of Southern Spain. Spixiana 1984, 7, 105–113. [Google Scholar]
- Barnes, R.S.K. Biological results of the snellius expedition XXIII. The genus Macrophthalmus (Crustacea, Brachyura). Zool. Verh. 1971, 115, 3–40. [Google Scholar]
- Barnes, R.S.K. A review of the sentinel and allied crabs (Crustacea: Brachyura: Macrophthalmidae), with particular reference to the genus Macrophthalmus. Raffles Bull. Zool. 2010, 58, 31–49. [Google Scholar] [CrossRef]
- Wada, K.; Sakai, K. A new species of Macrophthalmus closely related to M. japonicus (de Haan) (Crustacea: Decapoda: Ocypodidae). Senckenb. Marit. 1989, 20, 131–146. [Google Scholar]
- Shih, H.T.; Ng, P.K.L.; Christy, J. Uca (Petruca), a new subgenus for the rock fiddler crab Uca panamensis (Stimpson, 1859) from Central America, with comments on some species of the American broad-fronted subgenera. Zootaxa 2015, 4034, 471–494. [Google Scholar] [CrossRef]
- Shih, H.-T.; Ng, P.; Davie, P.; Schubart, C.; Türkay, M.; Naderloo, R.; Jones, D.; Liu, M.-Y. Systematics of the family Ocypodidae Rafinesque, 1815 (Crustacea: Brachyura), based on phylogenetic relationships, with a reorganization of subfamily rankings and a review of the taxonomic status of Uca Leach, 1814, sensu lato and its subgenera. Raffles Bull. Zool. 2016, 64, 139–175. [Google Scholar]
- Odido, M.; Appeltans, W.; BelHassen, M.; Mussai, P.; Nsiangango, S.E.; Vandepitte, L.; Wambiji, N.; Zamouri, N.; Jiddou, A.M. (Eds.) African Register of Marine Species. Available online: https://www.marinespecies.org/afremas (accessed on 20 August 2025).
- Burroughs, R.W.; Parham, J.F.; Stuart, B.L.; Smits, P.D.; Angielczyk, K.D. Morphological species delimitation in the Western pond turtle (Actinemys): Can machine learning methods aid in cryptic species identification? Integr. Org. Biol. 2024, 6, obae010. [Google Scholar] [CrossRef]
- Meng, H.; Wang, Y.; Qiao, G.-X.; Chen, J. Mitochondrial genome data provide insights into the phylogenetic relationships within Triplophysa dalaica (Kessler, 1876) (Cypriniformes, Nemacheilidae). ZooKeys 2024, 1197, 43–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).