Early Metabarcoding Detection of Eukaryotic Putative Pathogens Nearby Wastewater Effluents of Ría de Vigo (NW Spain)
Abstract
1. Introduction
2. Materials and Methods
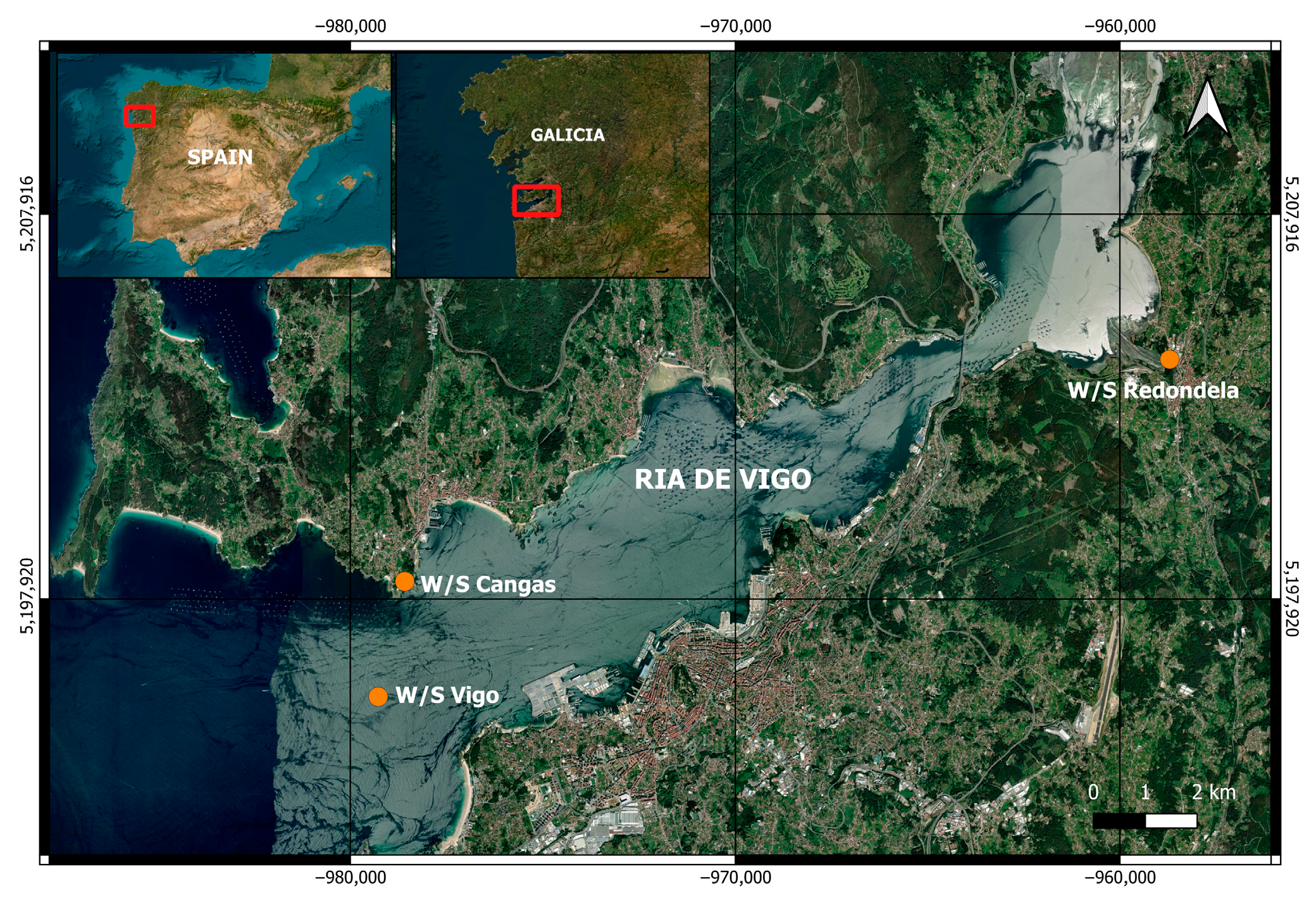
2.1. Sampling
2.2. DNA Isolation, Amplification and Sequencing
2.3. Bioinformatic Analisis
2.4. Diversity and Community Composition
3. Results
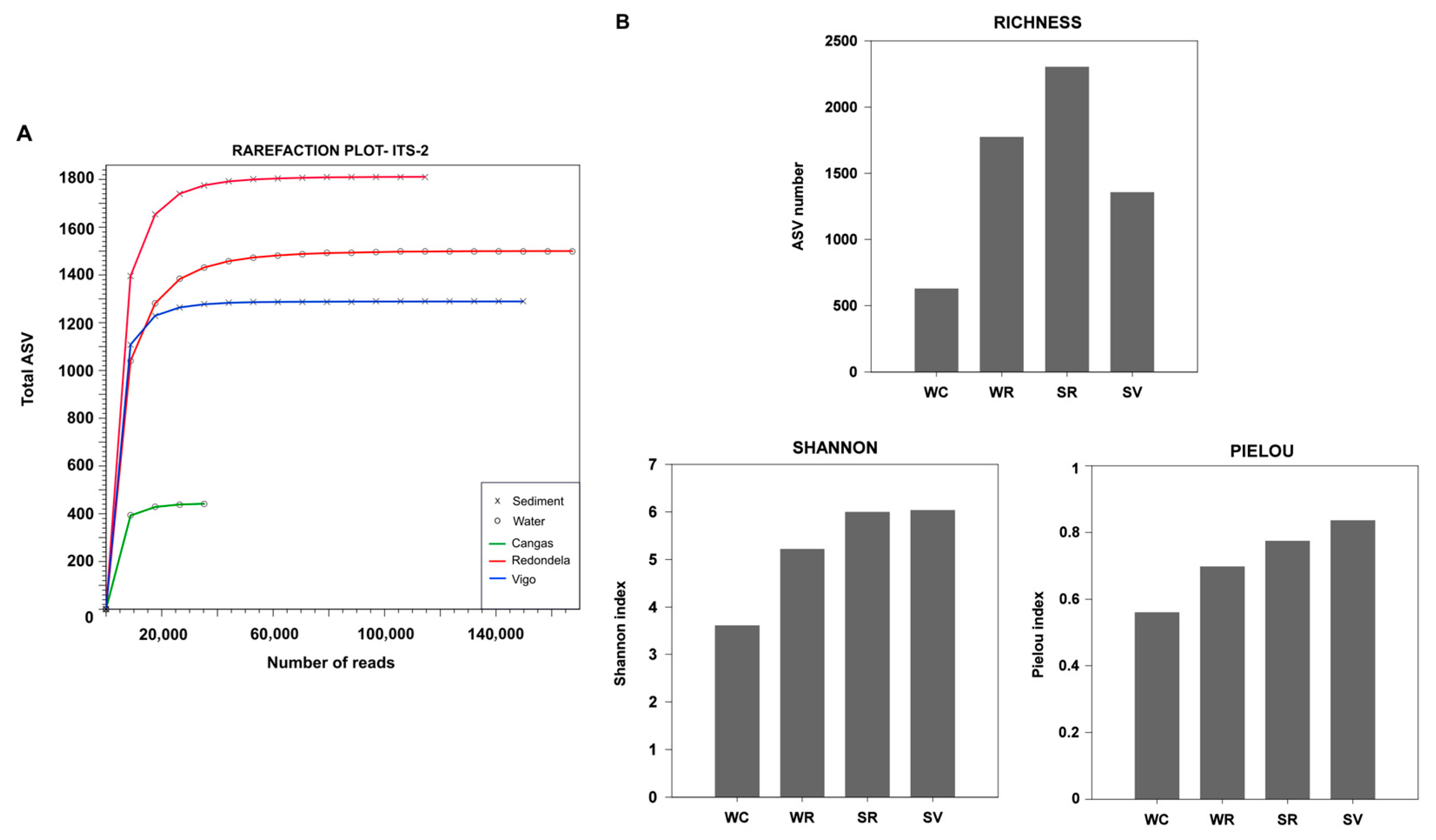
3.1. Overall Sequencing Data
3.2. Microeukaryote Diversity
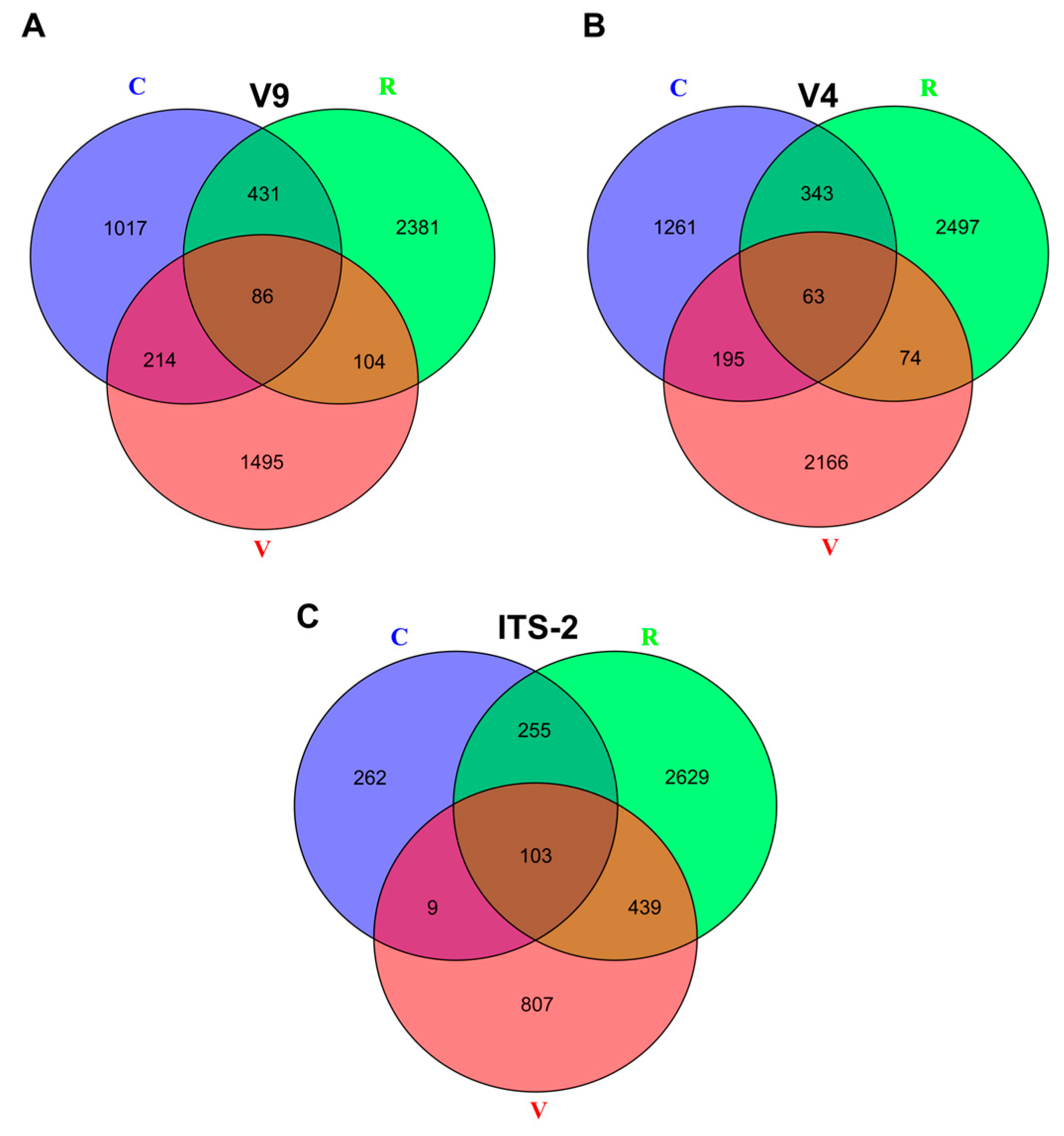
3.3. Exclusive/Common ASVs
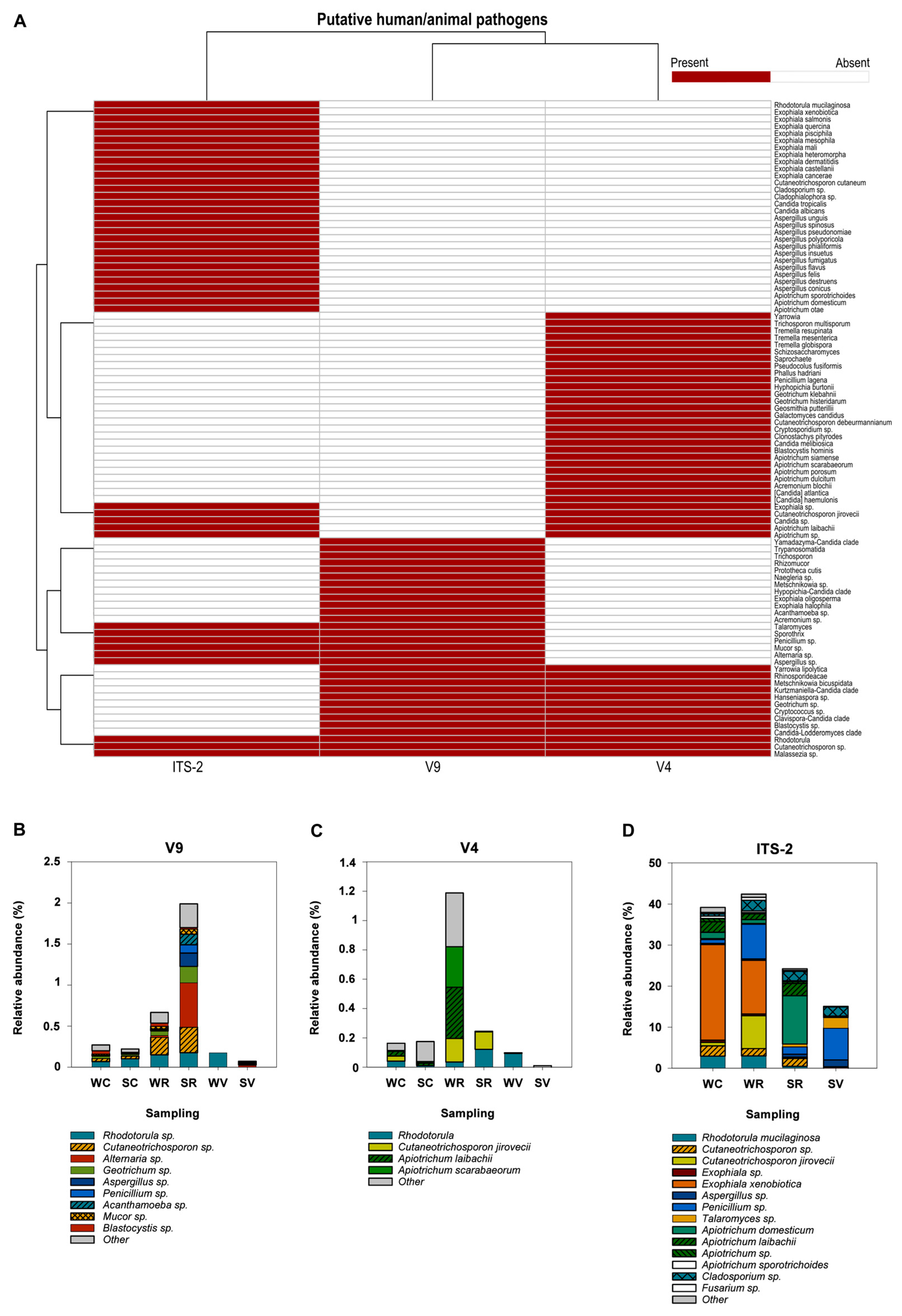
3.4. Putative Biohazards
3.4.1. Exclusive/Common Putative Biohazards
3.4.2. Biohazards of Human/Zoonotic Origin
3.4.3. Pathogens of Aquatic Organisms
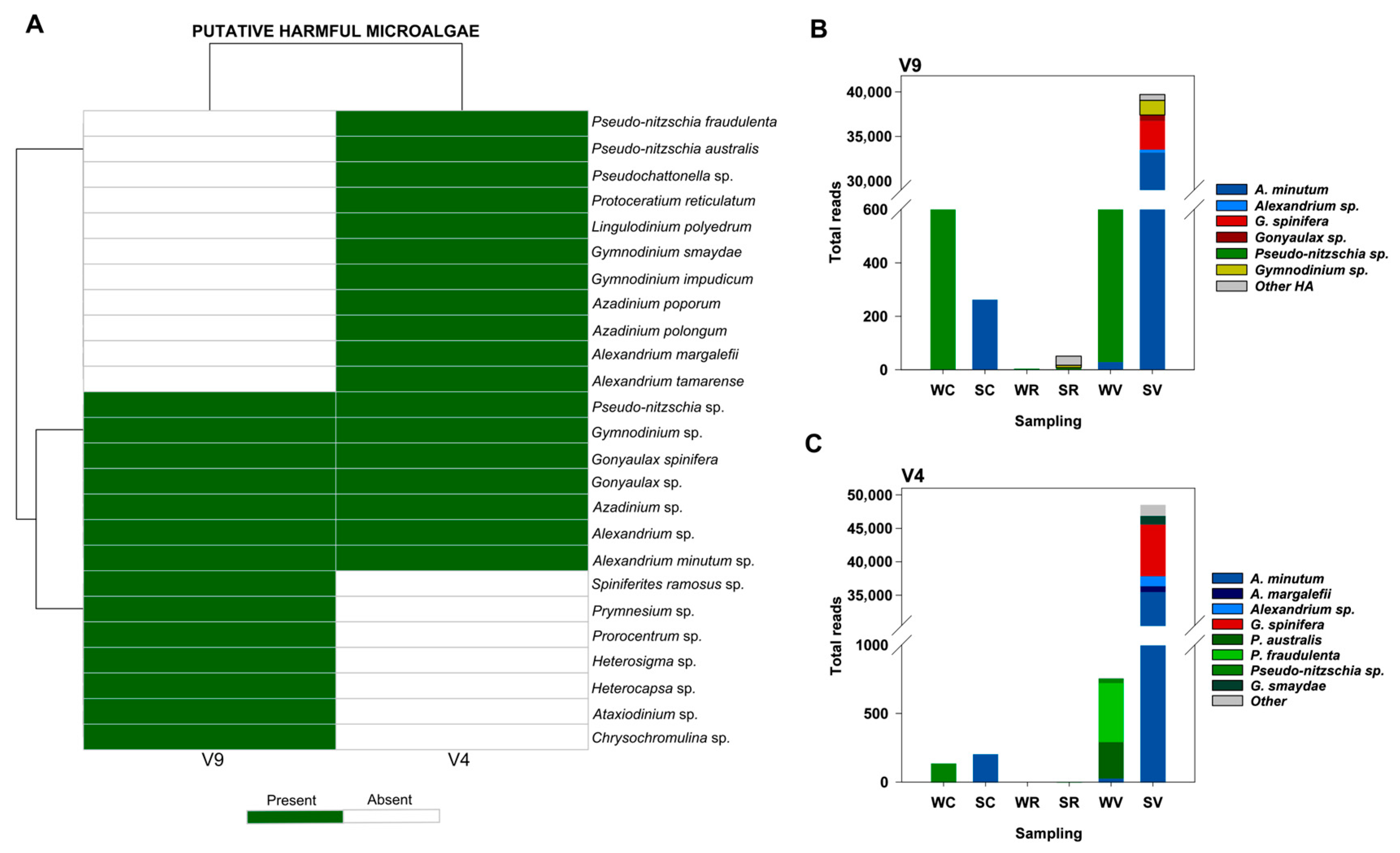
3.4.4. Harmful Algae (HA)
4. Discussion
4.1. Microeukaryotic Diversity
4.2. Putative Pathogen Diversity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human health and ocean pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef]
- Vidal-Dorsch, D.E.; Bay, S.M.; Maruya, K.; Snyder, S.A.; Trenholm, R.A.; Vanderford, B.J. Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ. Toxicol. Chem. 2012, 31, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Silliman, B.R. Climate change, human impacts, and coastal ecosystems in the Anthropocene. Curr. Biol. 2019, 29, R1021–R1035. [Google Scholar] [CrossRef]
- Kang, M.; Yang, J.; Kim, S.; Park, J.; Kim, M.; Park, W. Occurrence of antibiotic resistance genes and multidrug-resistant bacteria during wastewater treatment processes. Sci. Total Environ. 2022, 811, 152331. [Google Scholar] [CrossRef]
- Ariyadasa, S.; Taylor, W.; Weaver, L.; McGill, E.; Billington, C.; Pattis, I. Nonbacterial microflora in wastewater treatment plants: An underappreciated potential source of pathogens. Microbiol. Spectr. 2023, 11, e00481-23. [Google Scholar] [CrossRef]
- Freudenthal, J.; Ju, F.; Bürgmann, H.; Dumack, K. Microeukaryotic gut parasites in wastewater treatment plants: Diversity, activity, and removal. Microbiome 2022, 10, 27. [Google Scholar] [CrossRef]
- Cai, L.; Ju, F.; Zhang, T. Tracking human sewage microbiome in a municipal wastewater treatment plant. Appl. Microbiol. Biotechnol. 2014, 98, 3317–3326. [Google Scholar] [CrossRef]
- Sánchez, O.; Garrido, L.; Forn, I.; Massana, R.; Maldonado, M.I.; Mas, J. Molecular characterization of activated sludge from a seawater-processing wastewater treatment plant. Microb. Biotechnol. 2011, 4, 628–642. [Google Scholar] [CrossRef]
- Geisen, S.; Vaulot, D.; Mahé, F.; Lara, E.; de Vargas, C.; Bass, D. A user guide to environmental protistology: Primers, metabarcoding, sequencing, and analyses. bioRxiv 2019. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Cai, Z.; Bartlam, M.; Wang, Y. Comparison of ITS and 18S rDNA for estimating fungal diversity using PCR-DGGE. World J. Microbiol. Biotechnol. 2015, 31, 1387–1395. [Google Scholar] [CrossRef]
- Guerra, A.; Lens, S.; Rocha, F. Impacto del hombre sobre el ecosistema de la Ría de Vigo: Hacia una gestión integrada. In La Ría de Vigo: Una Aproximación Integral al Ecosistema Marino de la Ría de Vigo; González-Garcés Santiso, A., Vilas-Martín, F., Álvarez-Salgado, X.A., Eds.; Instituto de Estudios Vigueses: Vigo, Spain, 2002; pp. 51–84. [Google Scholar]
- Balseiro, P.; Montes, J.; Fernández Conchas, R.; Novoa, B.; Figueras, A. Comparison of diagnostic techniques to detect the clam pathogen Perkinsus olseni. Dis. Aquat. Organ. 2010, 90, 143–151. [Google Scholar] [CrossRef]
- Broullón, E.; López-Mozos, M.; Reguera, B.; Chouciño, P.; Doval, M.D.; Fernández-Castro, B.; Gilcoto, M.; Nogueira, E.; Souto, C.; Mouriño-Carballido, B. Thin layers of phytoplankton and harmful algae events in a coastal upwelling system. Prog. Oceanogr. 2020, 189, 102449. [Google Scholar] [CrossRef]
- Bellisario, B.; Fais, M.; Duarte, S.; Vieira, P.E.; Canchaya, C.; Costa, F.O. The network structure of intertidal meiofaunal communities from environmental DNA metabarcoding surveys in Northwest Iberia. Aquat. Sci. 2021, 83, 71. [Google Scholar] [CrossRef]
- Ríos-Castro, R.; Costas-Selas, C.; Pallavicini, A.; Vezzulli, L.; Novoa, B.; Teira, E.; Figueras, A. Co-occurrence and diversity patterns of benthonic and planktonic communities in a shallow marine ecosystem. Front. Mar. Sci. 2022, 9, 934976. [Google Scholar] [CrossRef]
- Costas-Selas, C.; Martínez-García, S.; Logares, R.; Hernández-Ruiz, M.; Teira, E. Role of bacterial community composition as a driver of the small-sized phytoplankton community structure in a productive coastal system. Microb. Ecol. 2023, 86, 777–794. [Google Scholar] [CrossRef]
- Ríos-Castro, R.; Cabo, A.; Teira, E.; Cameselle, C.; Gouveia, S.; Payo, P.; Novoa, B.; Figueras, A. High-throughput sequencing as a tool for monitoring prokaryote communities in a wastewater treatment plant. Sci. Total Environ. 2023, 861, 160531. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.; Breiner, H.W.; Richards, T.A. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 2010, 19, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Op De Beeck, M.; Lievens, B.; Busschaert, P.; Declerck, S.; Vangronsveld, J.; Colpaert, J.V. Comparison and validation of some ITS primer pairs useful for fungal metabarcoding studies. PLoS ONE 2014, 9, e97629. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 12 August 2025).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: Sequences, taxa, and classifications reconsidered. Nucleic Acids Res. 2023, 52, D791–D797. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O′HAra, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecology Package, R package version 2.8-0. 2025. Available online: https://vegandevs.github.io/vegan/ (accessed on 3 April 2025).
- European Parliament and Council of the European Union. Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work. Off. J. Eur. Communities 2000, L262, 21–45. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0054 (accessed on 20 December 2024).
- Real Decreto 664/1997, de 12 de mayo, sobre la protección de los trabajadores contra los riesgos relacionados con la exposición a agentes biológicos durante el trabajo. Boletín Oficial del Estado 1997, 124, 15114–15119. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-1997-11144 (accessed on 2 February 2025).
- IOC-UNESCO. Taxonomic Reference List of Harmful Microalgae. Available online: https://www.marinespecies.org/hab/index.php (accessed on 5 March 2025).
- Velavan, T.P.; Schulenburg, H.; Michiels, N.K. Detection of multiple infections by Monocystis strains in a single earthworm host using ribosomal internal transcribed spacer sequence variation. Parasitology 2010, 137, 45–51. [Google Scholar] [CrossRef]
- Gomes, G.B.; Miller, T.L.; Vaughan, D.B.; Jerry, D.R.; McCowan, C.; Bradley, T.L.; Hutson, K.S. Evidence of multiple species of Chilodonella (Protozoa, Ciliophora) infecting Australian farmed freshwater fishes. Vet. Parasitol. 2017, 237, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Paramá, A.; Alvarez, M.F.; Leiro, J.; Fernández, J.; Sanmartín, M.L. Philasterides dicentrarchi (Ciliophora, Scuticociliatida) as the causative agent of scuticociliatosis in farmed turbot Scophthalmus maximus in Galicia (NW Spain). Dis. Aquat. Organ. 2001, 46, 47–55. [Google Scholar] [CrossRef]
- Kim, S.M.; Cho, J.B.; Lee, E.H.; Kwon, S.R.; Kim, S.K.; Nam, Y.K.; Kim, K.H. Pseudocohnilembus persalinus (Ciliophora: Scuticociliatida) is an additional species causing scuticociliatosis in olive flounder Paralichthys olivaceus. Dis. Aquat. Organ. 2004, 62, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.R.; Prosperi-Porta, G.; LaPatra, S.E. First isolation of Pseudocohnilembus persalinus (Ciliophora: Scuticociliatida) from freshwater-reared rainbow trout, Oncorhynchus mykiss. J. Parasitol. 2010, 96, 1014–1016. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.; Xu, S.; Song, S.; Li, C. Variation of Amoebophrya community during bloom of Prorocentrum donghaiense Lu in coastal waters of the East China Sea. Estuar. Coast. Shelf Sci. 2020, 243, 106887. [Google Scholar] [CrossRef]
- Gleason, F.H.; Carney, L.T.; Lilje, O.; Glockling, S.L. Ecological potentials of species of Rozella (Cryptomycota). Fungal Ecol. 2012, 5, 651–656. [Google Scholar] [CrossRef]
- Baker, G.C.; Beebee, T.J.C.; Ragan, M.A. Prototheca richardsi, a pathogen of anuran larvae, is related to a clade of protistan parasites near the animal-fungal divergence. Microbiology 1999, 145, 1777–1784. [Google Scholar] [CrossRef]
- Azevedo, C.; Conchas, R.F.; Montes, J. Description of Haplosporidium edule n. sp. (Phylum Haplosporidia), a parasite of Cerastoderma edule (Mollusca, Bivalvia) with complex spore ornamentation. Eur. J. Protistol. 2003, 39, 161–165. [Google Scholar] [CrossRef]
- Dyková, I.; Kostka, M.; Wortberg, F.; Nardy, E.; Pecková, H. New data on aetiology of nodular gill disease in rainbow trout, Oncorhynchus mykiss. Folia Parasitol. 2010, 57, 157–163. [Google Scholar] [CrossRef]
- FioRito, R.; Leander, C.; Leander, B. Characterization of three novel species of Labyrinthulomycota isolated from ochre sea stars (Pisaster ochraceus). Mar. Biol. 2016, 163, 8. [Google Scholar] [CrossRef]
- Mendoza, L.; Taylor, J.W.; Walker, E.D.; Vilela, R. Description of three novel Lagenidium (Oomycota) species causing infection in mammals. Rev. Iberoam. Micol. 2016, 33, 83–91. [Google Scholar] [CrossRef]
- Svoboda, J.; Mrugała, A.; Kozubíková-Balcarová, E.; Petrusek, A. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. J. Fish Dis. 2017, 40, 127–140. [Google Scholar] [CrossRef]
- Maritz, J.M.; Rogers, K.H.; Rock, T.M.; Liu, N.; Joseph, S.; Land, K.M.; Carlton, J.M. An 18S rRNA workflow for characterizing protists in sewage, with a focus on zoonotic trichomonads. Microb. Ecol. 2017, 74, 923–936. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.S. Comparative analyses of the V4 and V9 regions of 18S rDNA for the extant eukaryotic community using the Illumina platform. Sci. Rep. 2020, 10, 6519. [Google Scholar] [CrossRef] [PubMed]
- Vilas Martín, F.; Rey García, D.; Rubio Armesto, B.; Bernabeu Tello, A.; Méndez Martínez, G.; Duran Gallego, R.; Mohamed Falcón, K. Los fondos de la Ría de Vigo: Composición, distribución y origen del sedimento. In La Ría de Vigo: Una Aproximación Integral al Ecosistema Marino de la Ría de Vigo; González-Garcés Santiso, A., Vilas Martín, F., Álvarez-Salgado, X.A., Eds.; Instituto de Estudios Vigueses: Vigo, Spain, 2002; pp. 17–50. [Google Scholar]
- Ramos, A.; Cabero, M.; Orden, B.; Ángel-Moreno, A.; Forés, R. Fungemia por Rhodotorula mucilaginosa. Presentación de dos casos. Rev. Esp. Quimioter. 2012, 25, 76–78. [Google Scholar]
- Yoo, I.Y.; Heo, W.; Kwon, J.A.; Lee, M.; Park, Y.J. Identification of the rare yeast Cutaneotrichosporon (Trichosporon) debeurmannianum from diabetic foot infection. J. Clin. Lab. Anal. 2022, 36, e24785. [Google Scholar] [CrossRef]
- Morio, F.; Berre, J.Y.; Garcia-Hermoso, D.; Najafzadeh, M.J.; de Hoog, S.; Benard, L.; Michau, C. Phaeohyphomycosis due to Exophiala xenobiotica as a cause of fungal arthritis in an HIV-infected patient. Med. Mycol. 2012, 50, 513–517. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, W.; Wu, P.; Zhang, T.; Xiang, P.; Wu, Q.; Zou, L.; Gui, M. The first two mitochondrial genomes from Apiotrichum reveal mitochondrial evolution and different taxonomic assignment of Trichosporonales. IMA Fungus 2023, 14, 7. [Google Scholar] [CrossRef]
- Connelly, L.; Anijeet, D.; Tole, D.; Alexander, C.L. Acanthamoeba keratitis: Molecular typing of Acanthamoeba species directly from ocular tissue. Curr. Res. Parasitol. Vector Borne Dis. 2023, 4, 100141. [Google Scholar] [CrossRef]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Hublin, J.S.Y.; Maloney, J.G.; Santin, M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021, 135, 260–282. [Google Scholar] [CrossRef]
- Ríos-Castro, R.; Romero, A.; Aranguren, R.; Pallavicini, A.; Banchi, E.; Novoa, B.; Figueras, A. High-throughput sequencing of environmental DNA as a tool for monitoring eukaryotic communities and potential pathogens in a coastal upwelling ecosystem. Front. Vet. Sci. 2021, 8, 765606. [Google Scholar] [CrossRef]
- Pohl, N.; Solbach, M.D.; Dumack, K. The wastewater protist Rhogostoma minus (Thecofilosea, Rhizaria) is abundant, widespread, and hosts Legionellales. Water Res. 2021, 203, 117566. [Google Scholar] [CrossRef] [PubMed]
- Delafont, V.; Rodier, M.H.; Maisonneuve, E.; Cateau, E. Vermamoeba vermiformis: A free-living amoeba of interest. Microb. Ecol. 2018, 76, 991–1001. [Google Scholar] [CrossRef]
- John, A.W.G.; Reid, P.C. Possible resting cysts of Dissodinium pseudolunula Swift ex Elbrächter et Drebes in the Northeast Atlantic and the North Sea. Br. Phycol. J. 1983, 18, 61–67. [Google Scholar] [CrossRef]
- Desportes, I.; Schrével, J. Treatise on Zoology—Anatomy, Taxonomy, Biology: The Gregarines; Brill: Leiden, The Netherlands, 2013. [Google Scholar]
- Rodríguez, F.; Escalera, L.; Reguera, B.; Nogueira, E.; Bode, A.; Ruiz-Villarreal, M.; Rossignoli, A.E.; Ben-Gigirey, B.; Rey, V.; Fraga, S. Red tides in the Galician rías: Historical overview, ecological impact, and future monitoring strategies. Environ. Sci. Process. Impacts 2024, 26, 16–34. [Google Scholar] [CrossRef]
- Nogueira, E.; Bravo, I.; Montero, P.; Díaz-Tapia, P.; Calvo, S.; Ben-Gigirey, B.; Figueiras, R.I.; Garrido, J.L.; Ramilo, I.; Lluch, N.; et al. HABs in coastal upwelling systems: Insights from an exceptional red tide of the toxigenic dinoflagellate Alexandrium minutum. Ecol. Indic. 2022, 137, 108790. [Google Scholar] [CrossRef]
- Trainer, V.; Pitcher, G.; Reguera, B.; Smayda, T. The distribution and impacts of harmful algal bloom species in eastern boundary upwelling systems. Prog. Oceanogr. 2010, 85, 33–52. [Google Scholar] [CrossRef]
- Barton, E.D.; Largier, J.L.; Torres, R.; Sheridan, M.; Trasviña, A.; Souza, A.; Pazos, Y.; Valle-Levinson, A. Coastal upwelling and downwelling forcing of circulation in a semi-enclosed bay: Ría de Vigo. Prog. Oceanogr. 2015, 134, 173–189. [Google Scholar] [CrossRef]



| Target | Name | Reads BT | Avg. Length BT | Reads AT | Retained Reads (%) | Avg. Length AT | Reads in ASVs | Unique Sequences | ASV with Taxonomy | Reads in ASV with Taxonomy | Total ASV with Taxonomy | Total Reads ASV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V9 | WC | 724,544 | 250 | 720,142 | 99.39 | 133 | 657,268 | 10,304 | 2447 | 328,634 | 6039 | 1,646,724 |
| SC | 762,576 | 250 | 757,774 | 99.37 | 136 | 711,936 | 7043 | 1031 | 355,968 | |||
| WR | 1,302,636 | 250 | 1,289,876 | 99.02 | 132 | 1,152,146 | 12,908 | 3095 | 576,073 | |||
| SR | 681,264 | 250 | 672,756 | 98.75 | 133 | 616,402 | 14,073 | 4342 | 308,201 | |||
| WV | 620,302 | 250 | 616,648 | 99.41 | 135 | 550,780 | 9281 | 1597 | 275,390 | |||
| SV | 615,656 | 250 | 612,126 | 99.43 | 134 | 548,908 | 8834 | 2002 | 274,454 | |||
| C-neg-W | 40 | 250 | 34 | 85.00 | 137 | 28 | 5 | 3 | 14 | NA | ||
| C-neg-S | 178 | 250 | 140 | 78.65 | 138 | 108 | 12 | 10 | 54 | |||
| Total | 4,707,196 | NA | 4,669,496 | NA | NA | 4,237,576 | 62,460 | NA | 2,118,788 | |||
| V4 | WC | 1,068,608 | 250 | 1,067,386 | 99.89 | 231 | 714,460 | 26,643 | 2388 | 357,230 | 6679 | 1,865,868 |
| SC | 885,248 | 250 | 884,204 | 99.88 | 231 | 698,978 | 16,031 | 614 | 349,489 | |||
| WR | 1,779,298 | 250 | 1,778,564 | 99.96 | 231 | 1,469,394 | 34,231 | 2363 | 734,697 | |||
| SR | 968,022 | 250 | 966,652 | 99.86 | 231 | 754,382 | 28,880 | 3162 | 377,191 | |||
| WV | 961,382 | 250 | 960,270 | 99.88 | 231 | 685,738 | 29,211 | 2090 | 342,869 | |||
| SV | 865,048 | 250 | 864,228 | 99.91 | 231 | 621,956 | 25,644 | 1945 | 310,978 | |||
| C-neg-W | 68 | 250 | 56 | 82.35 | 237 | 36 | 4 | 3 | 18 | NA | ||
| C-neg-S | 254 | 250 | 244 | 96.06 | 247 | 118 | 9 | 8 | 59 | |||
| Total | 6,527,928 | NA | 6,521,604 | NA | NA | 4,945,062 | 160,653 | NA | 2,472,531 | |||
| ITS-2 | WC | 481,222 | 250 | 480,380 | 99.83 | 227.79 | 368,624 | 9877 | 630 | 40,124 | 4885 | 40,124 |
| SC | 618,584 | 250 | 616,790 | 99.85 | 229.08 | 496,138 | 8667 | 165 | 3325 | NA | ||
| WR | 2,153,728 | 250 | 2,147,658 | 99.86 | 228.39 | 1,766,638 | 25,375 | 1915 | 169,673 | 40,124 | ||
| SR | 658,958 | 250 | 657,288 | 99.87 | 227.34 | 509,194 | 16,849 | 2462 | 123,820 | |||
| WV | 502,398 | 250 | 501,206 | 99.88 | 229.27 | 330,594 | 7404 | 120 | 6173 | |||
| SV | 594,614 | 250 | 593,596 | 99.83 | 228.46 | 461,186 | 14,223 | 1401 | 156,731 | NA | ||
| C-neg-W | 306 | 250 | 306 | 100 | 229.48 | 214 | 19 | 13 | 107 | NA | ||
| C-neg-S | 68 | 250 | 68 | 100 | 230.04 | 62 | 3 | 3 | 31 | |||
| Total | 5,009,878 | NA | 4,000,320 | NA | NA | 3,932,650 | 82,417 | 6709 | 499,846 |
| Group | Phylum/ Order | Genus/ Specie | V9 Number of Reads (%) | V4 Number of Reads (%) | Host | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WC | SC | WR | SR | WV | SV | WC | SC | WR | SR | WV | SV | |||||
| Alveolata | Gregarinasina | Monocystis sp. | NA | 350 (0.24) | 67 (0.05) | 1369 (0.96) | NA | NA | 1880 (0.70) | NA | 636 (0.24) | 68 (0.03) | 1176 (0.44) | NA | Earthworm | [31] |
| Chlamydodontida | Chilodonella uncinata | 54 (0.04) | NA | 147 (0.10) | NA | NA | NA | 155 (0.05) | NA | 66 (0.02) | NA | NA | NA | Farmed freshwater fishes | [32] | |
| Philasterida | Miamiensis avidus | 16 (0.01) | NA | NA | NA | NA | NA | 7 (0.002) | NA | NA | NA | NA | NA | Farmed fishes | [33,34,35] | |
| Pseudocohnilembus hargisi | NA | NA | 266 (0.18) | 12 (0.01) | NA | NA | NA | NA | 422 (0.16) | 6 (0.002) | NA | NA | ||||
| Pseudocohnilembus persalinus | NA | NA | NA | NA | NA | NA | 79 (0.03) | NA | NA | NA | NA | |||||
| Scuticociliatia sp. | 476 (0.33) | NA | 45 (0.03) | NA | NA | NA | 613 (0.23) | NA | 49 (0.02) | NA | NA | NA | ||||
| Syndiniales | Amoebophrya sp. | 37 (0.03) | NA | NA | NA | 692 (0.48) | 12 (0.01) | 2454 (0.91) | 59 (0.02) | NA | NA | NA | 2330 (0.87) | Dinophyceae | [36] | |
| Opisthokonta | Fungi | Cryptomycota sp. | 12 (0.01) | 16 (0.01) | 33 (0.0232) | 1053 (0.73) | NA | 18 (0.01) | NA | NA | NA | NA | NA | NA | Phytoplankton | [37] |
| Ichthyophonida | Anurofeca sp. | 897 (0.63) | 367 (0.26) | 202 (0.14) | 10,685 (7.5) | NA | 20 (0.01) | 97 (0.04) | 570 (0.21) | 265 (0.1) | 10,005 (3.73) | NA | 78 (0.03) | Anuran larvae | [38] | |
| Rhizaria | Haplosporida | Haplosporidium edule | NA | NA | 5 (0.003) | 172 (0.12) | NA | 2 (0.01) | NA | NA | NA | NA | NA | NA | Bivalves (C. edule) | [39] |
| Thecofilosea | Rhogostoma sp. | 900 (0.63) | 8 (0.006) | 48,675 (34.18) | 1319 (0.93) | NA | NA | 39 (0.01) | NA | 444 (0.16) | 33 (0.01) | NA | NA | Fish. NGD | [40] | |
| Rhogostoma minus | NA | NA | NA | NA | NA | NA | 99 (0.037) | NA | 6589 (2.46) | 200 (0.07) | NA | NA | ||||
| Stramenopiles | Labirinthulomycetes | Aplanochytrium sp. | 31 (0.02) | 4 (0.003) | 16 (0.01) | 58 (0.04) | 273 (0.19) | 171 (0.12) | 918 (0.340) | 49 (0.02) | 36 (0.01) | 17 (0.01) | 72 (0.02) | 543 (0.2) | Seastar | [41] |
| Lagenidiales | Lagenidium sp. | NA | NA | 676 (0.47) | 6 (0.004) | NA | NA | NA | NA | NA | NA | NA | NA | Mammals | [42] | |
| Oomycetes | Aphanomyces sp. | NA | NA | 6 (0.004) | 6 (0.004) | NA | NA | 234 (0.09) | 124 (0.05) | NA | 78 (0.03) | 32 (0.01) | NA | Crustaceans | [43] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Castro, R.; Ramilo, A.; Pascual, S.; Abollo, E. Early Metabarcoding Detection of Eukaryotic Putative Pathogens Nearby Wastewater Effluents of Ría de Vigo (NW Spain). Diversity 2025, 17, 671. https://doi.org/10.3390/d17100671
Ríos-Castro R, Ramilo A, Pascual S, Abollo E. Early Metabarcoding Detection of Eukaryotic Putative Pathogens Nearby Wastewater Effluents of Ría de Vigo (NW Spain). Diversity. 2025; 17(10):671. https://doi.org/10.3390/d17100671
Chicago/Turabian StyleRíos-Castro, Raquel, Andrea Ramilo, Santiago Pascual, and Elvira Abollo. 2025. "Early Metabarcoding Detection of Eukaryotic Putative Pathogens Nearby Wastewater Effluents of Ría de Vigo (NW Spain)" Diversity 17, no. 10: 671. https://doi.org/10.3390/d17100671
APA StyleRíos-Castro, R., Ramilo, A., Pascual, S., & Abollo, E. (2025). Early Metabarcoding Detection of Eukaryotic Putative Pathogens Nearby Wastewater Effluents of Ría de Vigo (NW Spain). Diversity, 17(10), 671. https://doi.org/10.3390/d17100671









