Effect of Simulated Autogamy and Allogamy on the Success of Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) Fruit Set
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Sites
2.3. Experiment Design
2.4. Meteorological Conditions
2.5. Statistical Analysis
3. Results
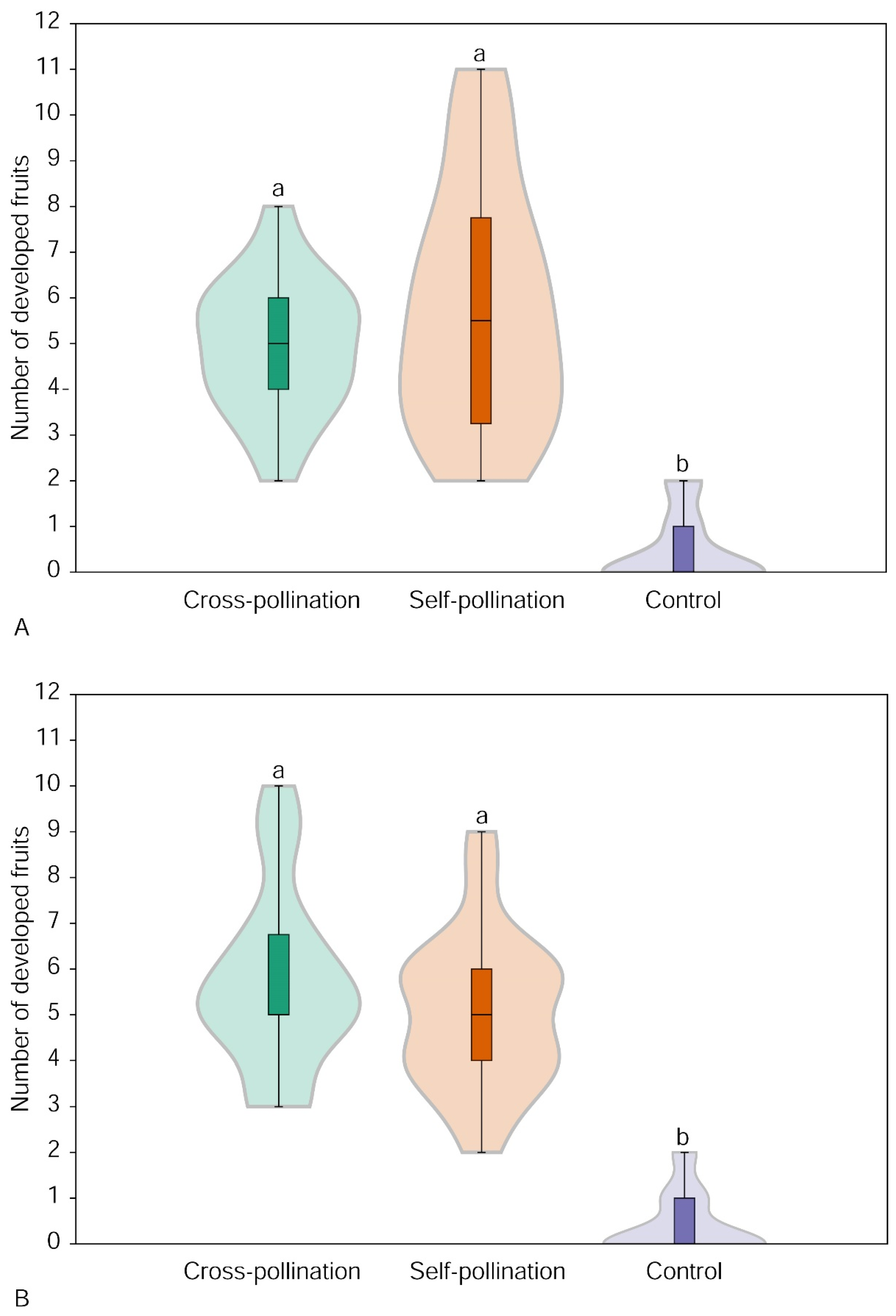
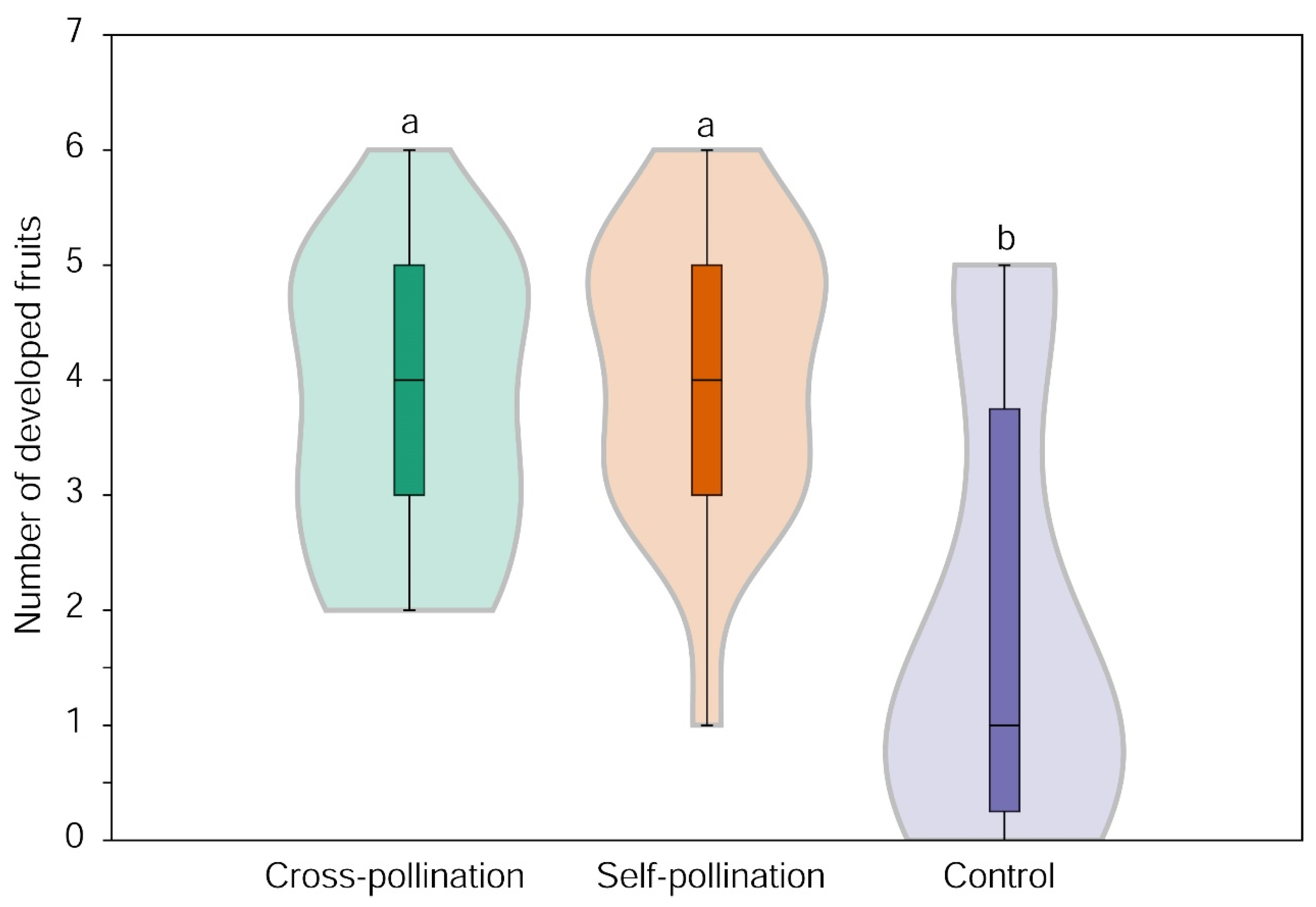
3.1. Effect of Simulated Cross-Pollination and Self-Pollination on Fruit Set
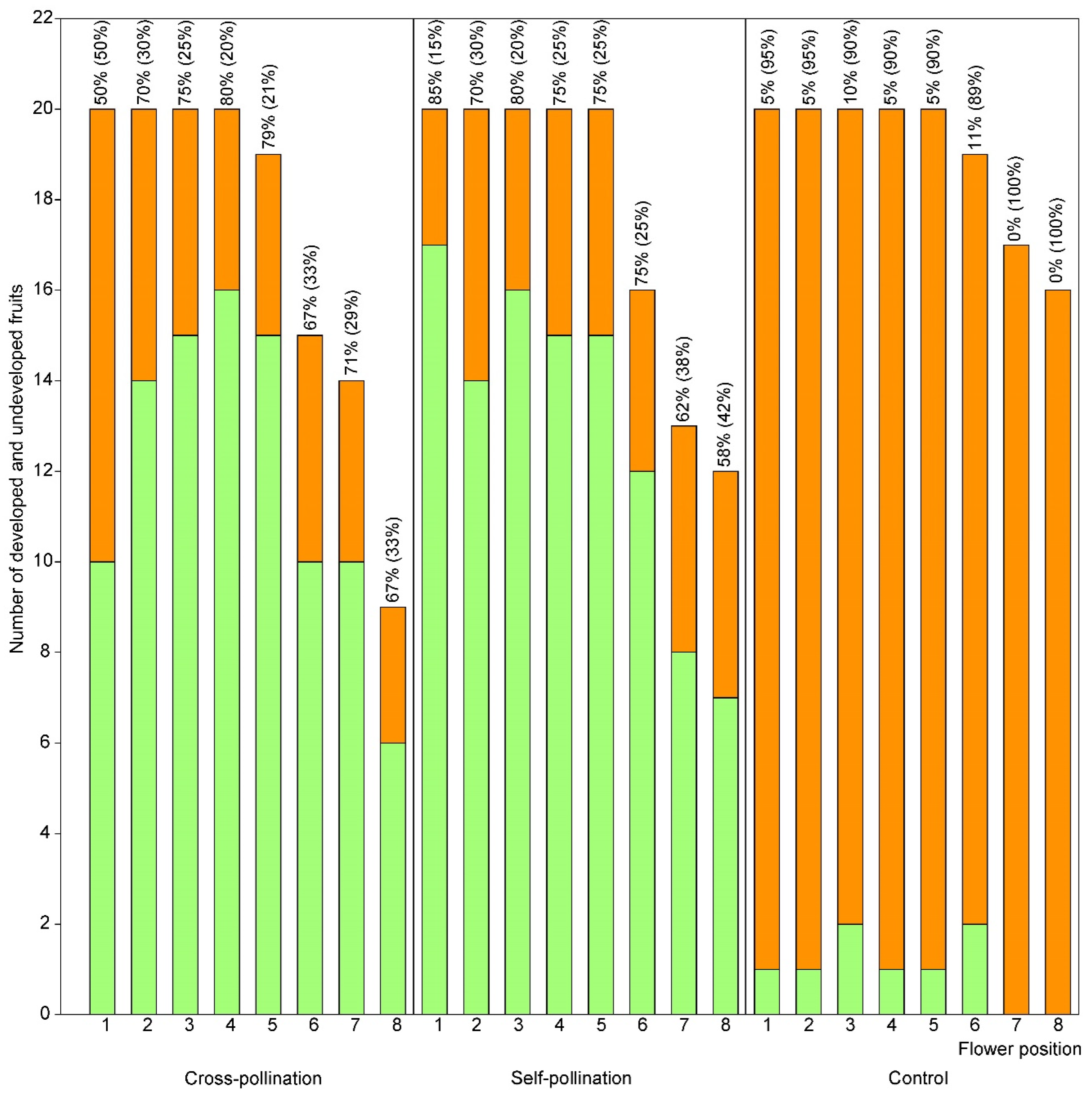
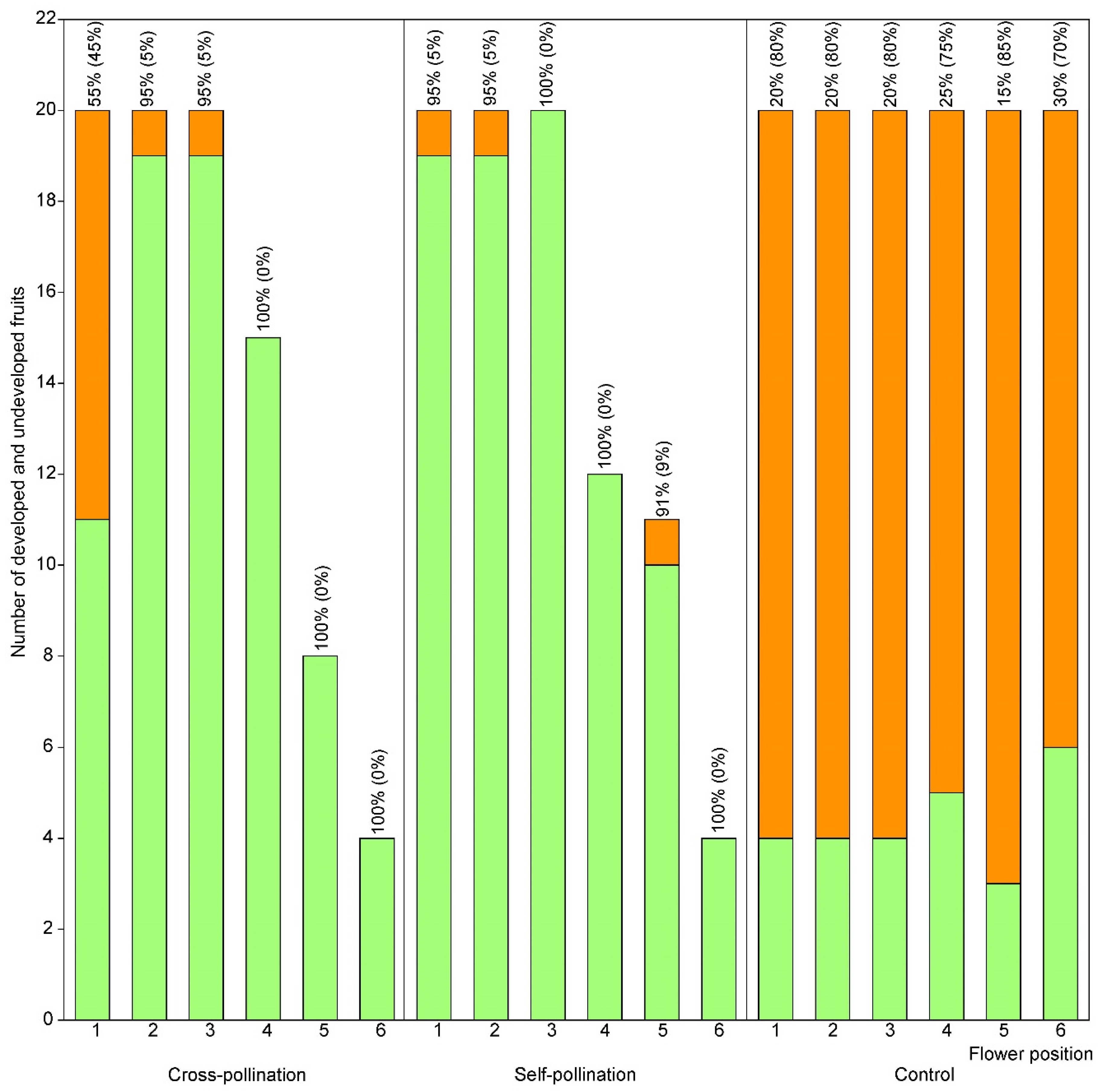
3.2. Effect of Flower Position on Fruit Set
3.3. Relationships Between Plant Traits and Fruit Set
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barman, D.; Devadas, R. Climate change on orchid population and conservation strategies: A review. J. Crop Weed 2013, 9, 1–12. [Google Scholar]
- Oliver, T.H.; Morecroft, M.D. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. Wiley Interdiscip. Rev. Clim. Change 2014, 5, 317–335. [Google Scholar] [CrossRef]
- Willmer, P. Climate change: Bees and orchids lose touch. Curr. Biol. 2014, 24, R1133–R1135. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, M.S.; Din, B.H.; Tan, S.H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar] [CrossRef]
- Prakash, S.; Verma, A.K. Anthropogenic activities and Biodiversity threats. Int. J. Biol. Innov. 2022, 4, 94–103. [Google Scholar] [CrossRef]
- Downey, P.O.; Richardson, D.M. Alien plant invasions and native plant extinctions: A six-threshold framework. AoB Plants 2016, 8, plw047. [Google Scholar] [CrossRef] [PubMed]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The Effects of Invasive Species on the Decline in Species Richness: A Global Meta-Analysis. In Advances in Ecological Research; Bohan, D.A., Dumbrell, A.J., Massol, F., Eds.; Academic Press: London, UK, 2017; Volume 56, pp. 61–83. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.T.; Song, B.H. Plant adaptation to climate change—Where are we? J. Syst. Evol. 2020, 58, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014, 7, 123–139. [Google Scholar] [CrossRef]
- Poulsen, J.R.; Osenberg, C.W.; Clark, C.J.; Levey, D.J.; Bolker, B.M. Plants as reef fish: Fitting the functional form of seedling recruitment. Am. Nat. 2007, 170, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Hutchings, M.J. Biological flora of the British Isles: Spiranthes spiralis (L.) Chevall. J. Ecol. 2010, 98, 1253–1267. [Google Scholar] [CrossRef]
- Oostermeijer, J.G.B.; Hartman, Y. Inferring population and metapopulation dynamics of Liparis loeselii from single-census and inventory data. Acta Oecol. 2014, 60, 30–39. [Google Scholar] [CrossRef]
- Volis, S.; Deng, T. Importance of a single population demographic census as a first step of threatened species conservation planning. Biodivers. Conserv. 2020, 29, 527–543. [Google Scholar] [CrossRef]
- Taura, L.; Kamaitytė-Bukelskienė, L.; Sinkevičienė, Z.; Gudžinskas, Z. Study on the Rare Semiaquatic Plant Elatine hydropiper (Elatinaceae) in Lithuania: Population Density, Seed Bank and Conservation Challenges. Front. Biosci. 2022, 27, 162. [Google Scholar] [CrossRef] [PubMed]
- Taura, L.; Gudžinskas, Z. What Factors Determine the Natural Fruit Set of Cephalanthera longifolia and Cephalanthera rubra? Diversity 2024, 16, 333. [Google Scholar] [CrossRef]
- Taura, L.; Gudžinskas, Z. Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) in Lithuania. Analysis of distribution, population dynamics and conservation issues. Botanica 2024, 30, 127–149. [Google Scholar] [CrossRef]
- Gudžinskas, Z.; Šablevičius, B.; Valiuška, Ž. Contribution to the native flora of Lithuania. First report. Botanica 2024, 30, 156–172. [Google Scholar] [CrossRef]
- Nigel, D.S.; Kingsley, W.D. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Jacquemyn, H.; Kull, T.; Hutchings, M.J. The demography of terrestrial orchids: Life history, population dynamics and conservation. Bot. J. Linn. Soc. 2020, 192, 315–332. [Google Scholar] [CrossRef]
- Lussu, M.; Ancillotto, L.; Labadessa, R.; Di Musciano, M.; Zannini, P.; Testolin, R.; Santi, F.; Dolci, D.; Conti, M.; Marignani, M.; et al. Prioritizing conservation of terrestrial orchids: A gap analysis for Italy. Biol. Conserv. 2024, 289, 110385. [Google Scholar] [CrossRef]
- Reiter, N.; Bohman, B.; Flemmati, G.R.; Phillips, R.D. Pollination by nectar foraging thynnine wasps: Evidence of a new specialized pollination strategy for Australian orchids. Bot. J. Linn. Soc. 2018, 188, 327–337. [Google Scholar] [CrossRef]
- Wraith, J.; Pickering, C. A continental scale analysis of threats to orchids. Biol. Conserv. 2019, 234, 7–17. [Google Scholar] [CrossRef]
- Phillips, R.D.; Reiter, N.; Peakall, R. Orchid conservation: From theory to practice. Ann. Bot. 2020, 126, 345–362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ackerman, D.J.; Phillips, R.D.; Tremblay, R.L.; Karremans, A.; Reiter, N.; Peter, C.I.; Bogarín, D.; Pérez-Escobar, O.A.; Liu, H. Beyond the various contrivances by which orchids are pollinated: Global patterns in orchid pollination biology. Bot. J. Linn. Soc. 2023, 202, 295–324. [Google Scholar] [CrossRef]
- Moore, D.M. Cephalanthera L. C. M. Richard. In Flora Europaea, Volume 5: Alismataceae to Orchidaceae (Monocotyledones); Tutin, E.D., Heywood, V.H., Burges, N.A., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1980; pp. 328–329. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.C.; Rasmussen, F.N. Epidendroideae (Part One). In Genera Orchidacearum; Oxford University Press: New York, NY, USA; Oxford, UK, 2006; Volume 4, pp. 1–672. [Google Scholar]
- Rankou, H. Cephalanthera longifolia (Europe Assessment). The IUCN Red List of Threatened Species. 2011. Available online: https://www.iucnredlist.org/species/176001/7167753 (accessed on 10 September 2024).
- Rankou, H. Cephalanthera rubra (Europe Assessment). The IUCN Red List of Threatened Species. 2011. Available online: https://www.iucnredlist.org/species/176009/7170277 (accessed on 10 September 2024).
- Grulich, V. Red List of Vascular Plants of the Czech Republic: 3rd Edition. Preslia 2012, 84, 631–645. [Google Scholar]
- Jakubska-Busse, A.; Pielech, R.; Szczesniak, E. The extinction of terrestrial orchids in Europe: Does disappearance of Cephalanthera Rich., 1817 (Orchidaceae, Neottieae) species show pattern consistent with the elevation gradient. Life Sci. J. 2014, 11, 140–144. [Google Scholar]
- Podgórska, M. Locality of Cephalanthera damasonium (Orchidaceae) on the former iron-ore mining remnants. Fragm. Florist. Geobot. Pol. 2015, 22, 106–109. [Google Scholar]
- Véla, E. Cephalanthera cucullata. The IUCN Red List of Threatened Species 2018: E.T161912A123982623. Available online: https://www.iucnredlist.org/species/161912/123982623 (accessed on 28 September 2024).
- Véla, E. Cephalanthera epipactoides. The IUCN Red List of Threatened Species 2019: E.T176038A21336941. Available online: https://www.iucnredlist.org/species/176038/21336941 (accessed on 28 September 2024).
- Rašomavičius, V. (Ed.) The Red Data Book of Lithuania. Animals, Plants, Fungi; Ministry or Environment: Vilnius, Lithuania, 2021.
- Gilián, L.D.; Endrédi, A.; Zsinka, B.; Neményi, A.; Nagy, J.G. Morphological and reproductive trait-variability of a food deceptive orchid, Cephalanthera rubra along different altitudes. Appl. Ecol. Environ. Res. 2019, 17, 5619–5639. [Google Scholar] [CrossRef]
- Summerhayes, V.S. Wild Orchids of Britain; Collins: London, UK, 1985; 384p. [Google Scholar]
- Scacchi, R.; De Angelis, G.; Corbo, R.M. Effect of the breeding system on the genetic structure in three Cephalanthera spp. (Orchidaceae). Plant Syst. Evol. 1991, 176, 53–62. [Google Scholar] [CrossRef]
- Chung, M.Y.; Lu, N.T.; López-Pujol, J.; Herrando-Moraira, S.; Chung, J.M.; Tian, H.Z.; Suetsugu, K.; Kawahara, T.; Yukawa, T.; Maki, M.; et al. Effect of historical factors on genetic variation in three terrestrial Cephalanthera species (Orchidaceae) with different breeding system on the Korean Peninsula. Nord. J. Bot. 2018, 36, e01862. [Google Scholar] [CrossRef]
- Füller, F. Epipactis und Cephalanthera; Orchideen Mitteleuropas, 5. Teil; A. Ziemsen Verlag: Leipzig, Germany, 1974; p. 96. [Google Scholar]
- Claessens, J.; Beentjes, K.K.; Heijerman, T.; Miller, J.; Graven-Deel, B. Beobachtungen von Miarus campanulae als Bestäuber von Cephalanthera rubra. J. Eur. Orchid. 2015, 47, 77–87. [Google Scholar]
- Suetsugu, K.; Naito, R.S.; Fukushima, S.; Kawakita, A.; Kato, M. Pollination system and the effect of inflorescence size on fruit set in the deceptive orchid Cephalanthera falcata. J. Plant Res. 2015, 128, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, A.; Kikuchi, S.; Yonekura, R.; Maruyama, A.; Yanagisawa, N.; Kagami, M.; Nakagawa, M.; Mahoro, S.; Kohmatsu, Y.; Hatada, A.; et al. The influence of floral symmetry and pollination systems on flower size variation. Nord. J. Bot. 2004, 24, 593–598. [Google Scholar] [CrossRef]
- Claessens, J.; Kleynen, J. The pollination of European orchids: Part 2: Cypripedium and Cephalanthera. J. Hardy Orchid Soc. 2013, 10, 114–120. [Google Scholar]
- Dafni, A.; Ivri, Y. The flower biology of Cephalanthera longifolia (Orchidaceae) pollen limitation and facultative floral mimicry. PI Syst. Evol. 1981, 137, 229–240. [Google Scholar] [CrossRef]
- Püttsepp, Ü.; Kull, T. Cephalanthera longifolia and Cephalanthera rubra in Estonia. Bot. Lith. 1997, 1, 133–135. [Google Scholar]
- Newman, R.D.; Showler, A.J.; Harvey, M.C.; Showler, D.A. Hand pollination to increase seedset of red helleborine Cephalanthera rubra in the Chiltern Hills, Buckinghamshire, England. Conserv. Evid. 2007, 4, 88–93. [Google Scholar]
- Shefferson, R.P.; Roy, M.; Püttsepp, Ü.; Selosse, M.A. Demographic shifts related to mycoheterotrophy and their fitness impacts in two Cephalanthera species. Ecology 2016, 97, 1452–1462. [Google Scholar] [CrossRef]
- Tałałaj, I.; Ostrowiecka, B.; Włostowska, E.; Rutkowska, A.; Brzosko, E. The ability of spontaneous autogamy in four orchid species: Cephalanthera rubra, Neottia ovata, Gymnadenia conopsea, and Platanthera bifolia. Acta Biol. Cracov. Ser. Bot. 2017, 59, 51–61. [Google Scholar] [CrossRef]
- Batty, P. Long-term study of the Sword-leaved Helleborine Cephalanthera longifolia (Orchidaceae) in Knapdale, Argyll. Br. Ir. Bot. 2022, 4, 106–124. [Google Scholar] [CrossRef]
- Ito, A.; Shoji, A.; Akasaki, H.; Matsumae, M.; Yamazaki, J.; Yukawa, T. The effect of hand-pollination and bagging treatment in Cephalanthera longifolia and Cephalanthera falcata in planted forests of reclaimed land. J. Jpn. Soc. Reveget. Technol. 2016, 42, 271–274. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Hermy, M.; Willems, J.H. Does nectar reward affect rarity and extinction probabilities of orchid species? An assessment using historical records from the Netherlands and Belgium. Biol. Cons. 2005, 121, 257–263. [Google Scholar] [CrossRef]
- Claessens, J.; Kleynen, J. The Flower of the European Orchid: Form and Function; Claessens & Kleynen: Voerendaal, The Netherlands, 2011; 440p. [Google Scholar]
- Kazlauskas, M.; Taura, L.; Gudžinskas, Z. Current state of critically endangered Neotinea ustulata (Orchidaceae) in Lithuania and report on a new record of the species. Botanica 2022, 28, 91–101. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Gudžinskas, Z.; Ryla, M. Orchids (Orchidaceae) of Lithuania. Lietuvos gegužraibiniai (Orchidaceae); Botanikos Instituto Leidykla: Vilnius, Lithuania, 2006; 104p. [Google Scholar]
- van Doorn, W.G. Effects of pollination on floral attraction and longevity. J. Exp. Bot. 1997, 48, 1615–1622. [Google Scholar] [CrossRef]
- Abdala-Roberts, L.; Parra-Tabla, V.; Navarro, J. Is floral longevity influenced by reproductive costs and pollination success in Cohniella ascendens (Orchidaceae)? Ann. Bot. 2007, 100, 1367–1371. [Google Scholar] [CrossRef]
- Fung, H.F.; Thomson, J. Does lack of pollination extend flower life? J. Pollinat. Ecol. 2017, 21, 86–91. [Google Scholar] [CrossRef]
- Stpiczyńska, M. Stigma receptivity during the life span of Platanthera chlorantha Custer (Rchb.) flowers. Acta Biol. Cracov. Ser. Bot. 2003, 45, 37–41. [Google Scholar]
- Cozzolino, S.; Widmer, A. Orchid diversity: An evolutionary consequence of deception? Trends Ecol. Evol. 2005, 20, 487–494. [Google Scholar] [CrossRef]
- Phillips, R.D.; Peakall, R.; Hutchinson, M.F.; Linde, C.C.; Xu, T.; Dixon, K.W.; Hopper, S.D. Specialized ecological interactions and plant species diversification in the Cape flora. Ann. Rev. Ecol. Evol. Syst. 2014, 45, 621–645. [Google Scholar] [CrossRef]
- Tremblay, R.L.; Ackerman, J.D.; Zimmerman, J.K.; Calvo, R.N. Variation in sexual reproduction in orchids and its evolutionary consequences: A spasmodic journey to diversification. Biol. J. Lin. Soc. 2005, 84, 1–54. [Google Scholar] [CrossRef]
- Peakall, R.; Beattie, A.J. Ecological and genetic consequences of pollination by sexual deception in the orchid Caladenia tentaculata. Evolution 1996, 50, 2207–2220. [Google Scholar] [CrossRef]
- Dressler, R.L. Phylogeny and Classification of the Orchid Family; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Brzosko, E.; Wróblewska, A. Genetic variation and clonal diversity in island Cephalanthera rubra populations from the Biebrza National Park. Bot. J. Lin. Soc. 2003, 143, 99–108. [Google Scholar] [CrossRef]
- Brzosko, E.; Wróblewska, A. Genetic diversity of nectar-rewarding Platanthera chlorantha and nectarless Cephalanthera rubra. Bot. J. Lin. Soc. 2013, 171, 751–763. [Google Scholar] [CrossRef]
- Micheneau, C.; Duffy, K.J.; Smith, R.J.; Stevens, L.J.; Stout, J.C.; Civeyrel, L.; Cowan, R.S.; Fay, M.F. Plastid microsatellites for the study of genetic variability in the widespread Cephalanthera longifolia, C. damasonium and C. rubra (Neottieae, Orchidaceae), and cross-amplification in other Cephalanthera species. Bot. J. Linn. Soc. 2010, 163, 181–193. [Google Scholar] [CrossRef]
- Ito, A.; Shoji, A.; Matsumoto, T.; Akasaki, H.; Kaido, T.; Matsuzawa, H.; Yamazaki, S.; Yukawa, T. Attempt at propagation of golden orchid (Cephalanthera falcata (Thunb.) Blume.) by field sowing test method in plantation forest on reclaimed land. J. Jpn. Soc. Reveget. Eng. 2015, 41, 279–282. [Google Scholar]
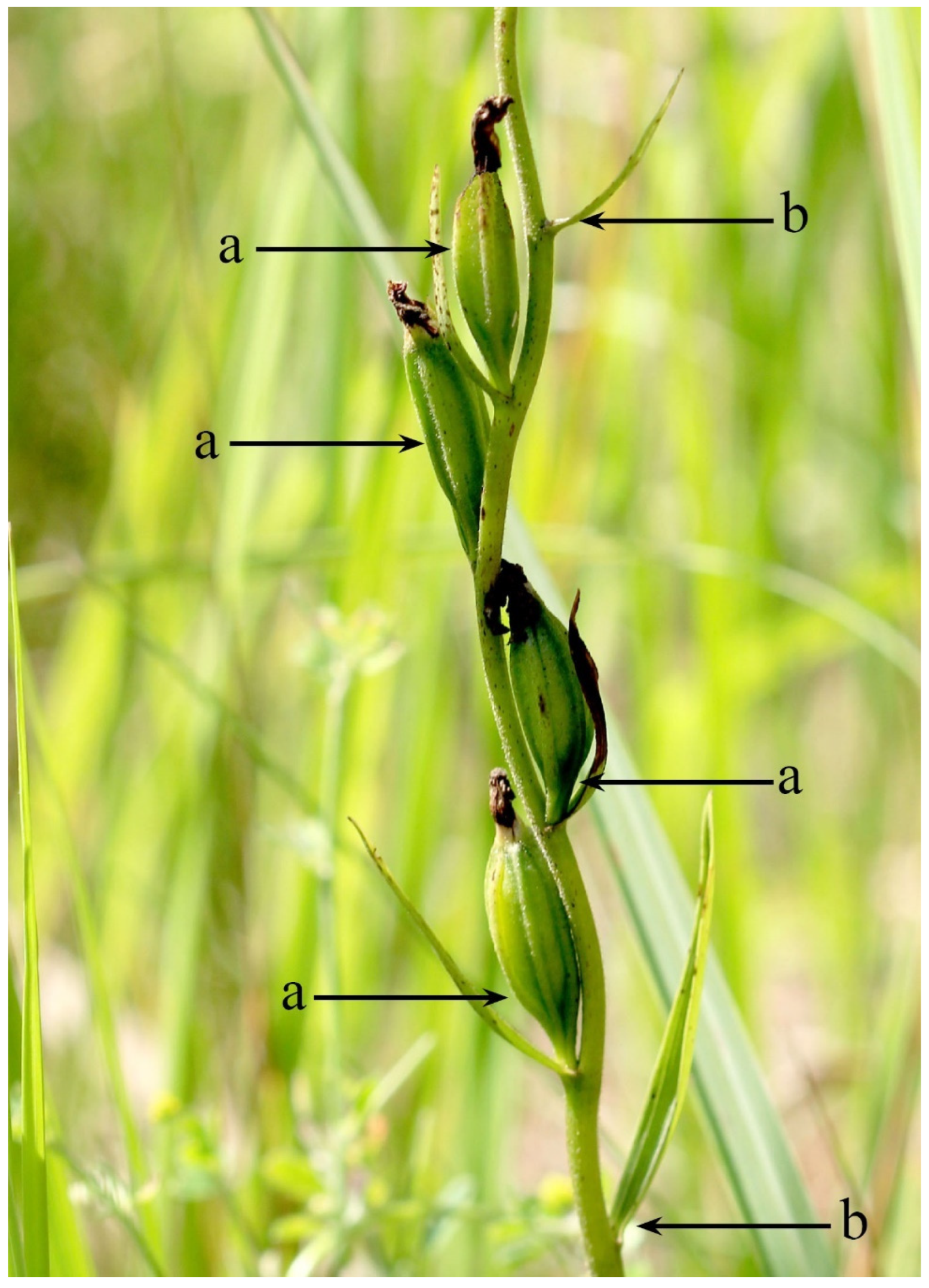

| Species and Site Name | District | Latitude (°N) | Longitude (°E) | Cover (%) | ||
|---|---|---|---|---|---|---|
| Tree | Shrub | Herb | ||||
| Cephalanthera longifolia | ||||||
| Raisteliai | Vilnius | 54.6180 | 25.2101 | 40 | 30 | 30 |
| Paneriai | Vilnius | 54.6218 | 25.2065 | 40 | 40 | 60 |
| Cephalanthera rubra | ||||||
| Kapiniškiai | Varėna | 54.0340 | 24.2915 | 0 | 0 | 70 |
| Species and Site | Experimental Group | Number of Used Flowers | Number of Produced Fruits | Fruit Set Rate (%) |
|---|---|---|---|---|
| Cephalanthera longifolia | ||||
| Raisteliai | Cross-pollination | 145 | 99 | 68.3 |
| Self-pollination | 159 | 115 | 72.3 | |
| Control | 179 | 9 | 5.0 | |
| Paneriai | Cross-pollination | 164 | 114 | 69.5 |
| Self-pollination | 148 | 101 | 68.2 | |
| Control | 157 | 8 | 5.1 | |
| Pooled | Cross-pollination | 309 | 213 | 68.9 |
| Self-pollination | 307 | 216 | 70.4 | |
| Control | 336 | 17 | 5.1 | |
| Cephalanthera rubra | ||||
| Kapiniškiai | Cross-pollination | 85 | 75 | 88.2 |
| Self-pollination | 85 | 82 | 96.5 | |
| Control | 214 | 38 | 17.8 |
| Species, Site and Experimental Group | Plant Height (cm) | Number of Leaves | Inflorescence Length (cm) | Number of Flowers |
|---|---|---|---|---|
| Cephalanthera longifolia | ||||
| Raisteliai | ||||
| Cross-pollination | 40.2 ± 7.1 a | 7.5 ± 0.6 a | 8.9 ± 2.6 a | 9.1 ± 2.4 a |
| Self-pollination | 38.0 ± 8.9 a | 7.1 ± 0.6 b | 9.3 ± 2.9 a | 8.8 ± 3.3 a |
| Control | 40.6 ± 6.0 a | 7.8 ± 0.8 a | 9.3 ± 2.8 a | 9.0 ± 2.1 a |
| Paneriai | ||||
| Cross-pollination | 41.8 ± 6.7 a | 7.8 ± 0.7 a | 10.3 ± 3.1 a | 8.5 ± 2.6 a |
| Self-pollination | 36.2 ± 7.7 b | 8.0 ± 1.4 a | 9.2 ± 2.7 a | 8.1 ± 2.1 a |
| Control | 39.6 ± 7.2 ab | 8.1 ± 0.9 a | 9.1 ± 3.3 a | 7.9 ± 2.5 a |
| Cephalanthera rubra | ||||
| Kapiniškiai | ||||
| Cross-pollination | 41.8 ± 5.6 a | 5.2 ± 0.7 a | 9.4 ± 2.7 a | 10.1 ± 2.8 a |
| Self-pollination | 41.4 ± 10.8 a | 4.8 ± 0.9 a | 11.1 ± 4.4 a | 10.0 ± 4.0 a |
| Control | 40.1 ± 5.1 a | 5.0 ± 0.8 a | 8.3 ± 4.1 b | 10.7 ± 3.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taura, L.; Gudžinskas, Z. Effect of Simulated Autogamy and Allogamy on the Success of Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) Fruit Set. Diversity 2025, 17, 73. https://doi.org/10.3390/d17010073
Taura L, Gudžinskas Z. Effect of Simulated Autogamy and Allogamy on the Success of Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) Fruit Set. Diversity. 2025; 17(1):73. https://doi.org/10.3390/d17010073
Chicago/Turabian StyleTaura, Laurynas, and Zigmantas Gudžinskas. 2025. "Effect of Simulated Autogamy and Allogamy on the Success of Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) Fruit Set" Diversity 17, no. 1: 73. https://doi.org/10.3390/d17010073
APA StyleTaura, L., & Gudžinskas, Z. (2025). Effect of Simulated Autogamy and Allogamy on the Success of Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) Fruit Set. Diversity, 17(1), 73. https://doi.org/10.3390/d17010073







