Abstract
The Balsas Basin (BB) is a biogeographic province in south-central Mexico that straddles the Mexican Transition Zone and the Neotropical region. We provide a list of the amphibian and reptile species of the BB based on a detailed review and update of recent species lists of its constituent states. The BB is home to 51 native amphibian and 155 native reptile species, which represent 14.7% of the herpetofauna of Mexico. No amphibian and six reptile species are endemic to the BB. Six species of amphibians and seven of reptiles are categorized as being of conservation concern status (vulnerable, endangered, or critically endangered) on the International Union for Conservation of Nature’s (IUCN) Red List. The main threat these species face is habitat loss due to urbanization, agriculture, and pollution. The herpetofauna of the BB shows significant overlap with neighboring provinces. The composition of amphibian species in the BB is closest to that of the Pacific Lowlands, whereas the composition of reptile species is closest to the Sierra Madre del Sur and the Transvolcanic Belt. These findings suggest that while the BB supports significant amphibian and reptile diversity, its conservation importance may be limited due to the low levels of endemism and the relatively small proportion of species at risk.
1. Introduction
The Balsas Basin (BB) is a biogeographic province in south-central Mexico, located between the Transvolcanic Belt and the Sierra Madre del Sur (see [1,2,3]). It has usually been included in the Mexican Transition Zone between the Nearctic and Neotropical regions [4,5,6]; however, [7] placed the BB in the Neotropical region. Regardless of its classification in the Transition Zone or in the Neotropical region, the BB shows affinities with both the temperate Nearctic region and the tropical Neotropical region, possessing both physiographic regions in its borders [8]. Its location places it at a key position along important biogeographic tracks that are driven by historical climatic and geological events and occurs at important biogeographic nodes where species from neighboring biogeographic regions overlap, resulting in high biodiversity [9,10,11].
Because of its location between mountain ranges, the BB is geographically isolated from other biogeographic provinces, resulting in it being an area of high endemism for some taxa (e.g., plants [12,13,14], fireflies [15], birds [16]). In addition, species richness in the BB appears to be high for some taxa (e.g., fireflies [15], bats [17], amphibians [18]). Unfortunately, several threats to biodiversity impact the BB, including fragmentation of tropical deciduous forest [19], illegal poaching of reptiles, birds, and mammals for meat and the pet trade [20], and the introduction of non-native species [21,22]. Climate change may also cause shifts in the distributions of species, resulting in competition between species whose changed distributions now overlap [23]. Additionally, mortality in amphibians and reptiles is exacerbated by several factors, including disease outbreaks, such as chytridiomycosis in amphibians, and the transmission of pathogens from aquaculture, which may negatively affect native populations [21,24]. These factors, combined with environmental changes, pose significant risks to the persistence of native species.
One of the challenges to addressing conservation concerns in the BB is the lack of information on different taxonomic groups that occur in the area. For example, [25] provided a survey of richness, endemism, and endangered species in the BB as part of an examination of diversity hotspots for the herpetofauna of the Pacific Lowlands and surrounding areas. However, since then no study has integrated information on the species of amphibians and reptiles that inhabit this important biogeographic province, nor has their conservation status and their relationship with neighboring provinces been examined. In this work, we fill this gap by making this information accessible to people studying the diversity and conservation of the BB. We hope that this contribution lays the foundation for future studies on the herpetofaunal diversity of the BB and contributes to its long-term conservation.
2. Methods
2.1. Physiography
The BB is located in south-central Mexico, between the Transvolcanic Belt and the Sierra Madre del Sur between 16.2695° and 19.6905° latitude and −96.3003° and −103.0915° longitude, with an elevational range of 200–2800 m. It includes parts of the states of Jalisco, Michoacán, Guerrero, Mexico, Morelos, Oaxaca, and Puebla, with most of Morelos being included (Figure 1). To the north, the BB is bordered by the Transvolcanic Belt; to the south, east, and west by the Sierra Madre del Sur; and on a small portion of its western edge by the Pacific Lowlands [3].
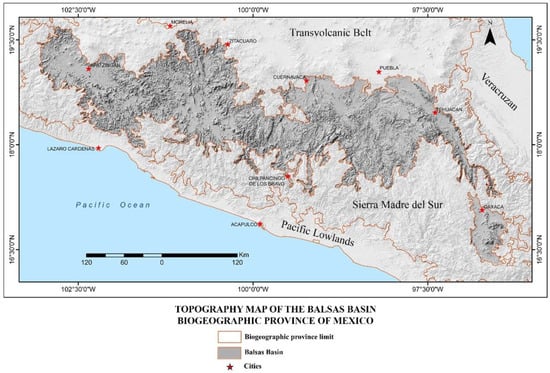
Figure 1.
Map showing the topography of the Balsas Basin and its neighboring biogeographical provinces of Mexico [26].
The climate is mainly warm and semi-warm, with significant areas of arid and semi-arid climate in the eastern, southern, and western extremes of the province and a small area of temperate climate scattered at higher elevations, mainly in the extreme northeast (Figure 2; [8,27]). Its vegetation ranges from xeric scrub, tropical forests, and oak and pine forests to grasslands; however, the main vegetation type is tropical deciduous forest, with oak and pine forests occurring at higher elevations [28]. These areas also support grasslands and are interspersed with extensive agricultural regions (Figure 3; [29]).
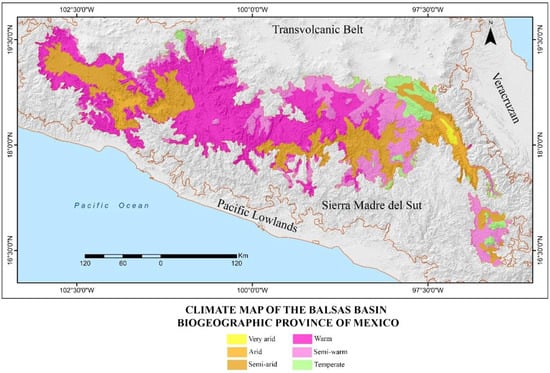
Figure 2.
Map showing the climatic regions found in the Balsas Basin biogeographic province of Mexico [27].
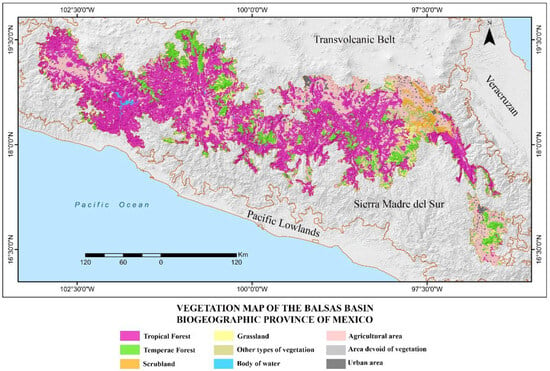
Figure 3.
Map showing the vegetation types found in the Balsas Basin biogeographic province of Mexico [29].
2.2. Methodology
We compiled a list of the species of amphibians and reptiles of the BB using the species lists of the Mexican states (Puebla, Oaxaca, Guerrero, Morelos, Mexico, Michoacán, and Jalisco) that contribute to the BB provided by [30] and updated with [31]. We defined the BB based on the delimitation given by [3,5,6,7]. We used this definition and the species lists for the Mexican states to derive our lists for the herpetofaunas of the BB and its neighboring provinces. For amphibian names we used [32,33], and [34] for reptile names. For each species in our list that has been evaluated, we determined its IUCN Red List conservation status and population trend [24], its listing by the Mexican government (SEMARNAT) [35], and its Environmental Vulnerability Score (EVS; [36,37]).
We used hierarchical clustering analyses using the Ward method for the BB and the neighboring biogeographic provinces (Sierra Madre del Sur, Transvolcanic Belt, and Pacific Lowlands) for amphibians and reptiles separately. To quantify the similarity in species composition between the provinces, we used Jaccard’s similarity coefficient for binary data. We calculated the length of shared borders between the biogeographic provinces using the Polygon Neighbors Tool in ArcGIS 10.8.1 (Environmental Systems Research Institute, Inc, Redlands, CA) and the straight-line distance between the centroids of the biogeographic provinces using the Feature to Point Tool and Point Distance (Table 1). We used non-parametric Spearman’s ρ tests to test for correlations between the Jaccard distance estimates and (1) the length of shared borders and (2) the distance between the centroids of the biogeographic provinces for amphibians and reptiles separately. We used JMP 17 Pro 17.2.0 (JMP Statistical Discovery LLC, Cary, NC) for all statistical analyses.

Table 1.
Geographic characteristics of the Balsas Basin (BB) and its neighboring provinces. Surface = surface area; length = length of the border with the BB; latitude = latitude of the geographic centroid of the province; distance = distance from the geographic centroid of the BB to the geographic centroid of the neighboring province.
3. Results
3.1. Species Richness and Endemism
The herpetofauna of the BB consists of 206 native species, 51 amphibians and 155 reptiles, that include 38 families, 12 amphibians (ten anurans and two salamanders) and 26 reptiles (one crocodile, thirteen lizards, eight snakes, and four turtles), and 82 genera (21 amphibians and 61 reptiles) (Table 2 and Table 3; Supplementary Table S1). Mexico has 1,399 species of native amphibians and reptiles (435 amphibians and 964 reptiles) in 55 families (16 amphibians and 39 reptiles) and 210 genera (55 amphibians and 155 reptiles) [31]; see also [38]. The BB thus houses 69.1% of the families (75.0% of amphibian families, 66.7% of reptile families), 52.9% of the genera (38.2% of amphibian genera, 56.8% of reptile genera), and 14.7% of the species (11.7% of amphibian species; 16.1% of reptile species) of amphibians and reptiles found in Mexico.

Table 2.
The number of native species shared by the Balsas Basin (BB) and the provinces that border it (Sierra Madre del Sur [SMS], Transvolcanic Belt [TVB], Pacific Lowlands [PL]). Total refers to the number of species found in all four provinces (i.e., regional species pool). Percentages are given in parentheses. — means no species in the taxon is shared between provinces.

Table 3.
The number of native species in different taxa in the Balsas Basin that are included in each IUCN Red List category: data deficient (DD), least concern (LC), near threatened (NT), vulnerable (VU), endangered (EN), critically endangered (CR). Mean EVS is the mean Environmental Vulnerability Score for a taxon (an EVS ≥ 14 is high vulnerability [36,37]). The SEMARNAT column provides the number of species in a particular taxon that are not listed (NL), subject to special protection (Pr), threatened (A), and in danger of extinction (P) according to [35].
Thirty-four of the fifty-one amphibian species are endemic to Mexico. The BB also harbors 110 endemic reptile species. Among these, 54 species of lizards and 54 species of snakes are endemic to Mexico. Six of the two hundred and six native species, all of them reptiles, are endemic to the BB biogeographic province: Kropotkin’s Leaf-toed Gecko (Phyllodactylus kropotkini), Papenfuss’s Leaf-toed Gecko (Phyllodactylus papenfussi), Río Márquez Valley Gecko (Phyllodactylus paucituberculatus), Clean Skink (Plestiodon lotus), Blackhead Stripeless Snake (Coniophanes melanocephalus), and Pueblan Coralsnake (Micrurus pachecogili). In addition, three reptile species have been introduced to the BB: the Common House Gecko (Hemidactylus frenatus); the Brahminy Blindsnake (Indotyphlops braminus); and the Spiny Softshell (Apalone spinifera).
3.2. Similarities with Neighboring Provinces
The BB and the Sierra Madre del Sur share the most amphibian species (92.2%), followed by BB and the Transvolcanic Belt (80.4%) and BB and the Pacific Lowlands (60.8%) (Table 2). The cluster analysis conducted on amphibian species across the four neighboring biogeographic provinces revealed that the BB and Pacific Lowlands are paired, then the Sierra Madre del Sur is added, and finally the Transvolcanic Belt joins (Figure 4A).
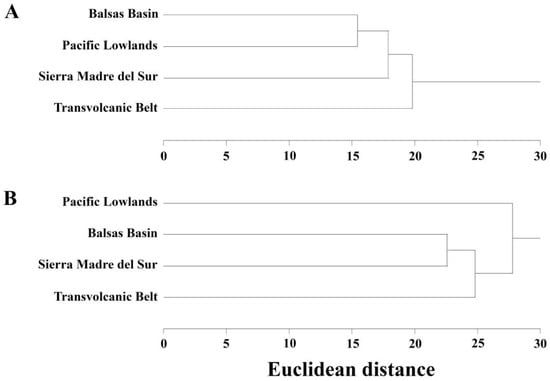
Figure 4.
Dendrograms for (A) amphibians and (B) reptiles visualizing the similarity in the herpetofaunas of the Balsas Basin and its three neighboring biogeographic provinces.
The BB shares 85.8% of its reptile species with the Sierra Madre del Sur; 71% of its species with the Transvolcanic Belt; and 56.1% with the Pacific Lowlands (Table 2). In the cluster analysis for reptiles, the Sierra Madre del Sur and the BB are paired, with this pairing joined next by the Transvolcanic Belt and then the Pacific Lowlands (Figure 4B).
Jaccard distances between pairs of provinces for amphibians were not correlated with the length of the shared border (n = 6, Spearman’s ρ = −0.20, p = 0.70) nor with the distance between their geographic centroids (n = 6, Spearman’s ρ = 0.43, p = 0.40). Similarly, Jaccard distances between pairs of provinces for reptiles were not correlated with the length of the shared border (n = 6, Spearman’s ρ = 0.26, p = 0.62); however, they were negatively correlated with the distance between their geographic centroids (n = 6, Spearman’s ρ = −0.94, p = 0.0048).
3.3. Conservation Status
Of the 206 native species of amphibians and reptiles found in the BB, 6.3% are categorized as vulnerable, endangered, or critically endangered by the IUCN Red List, 13.1% are listed as threatened (A) or in danger of extinction (P) by SEMARNAT, and 37.4% are considered to have a high-risk Environmental Vulnerability Score (Table 3). Three amphibian species are listed as vulnerable, two as endangered, and one as critically endangered, and all the listed amphibian species are Mexican endemics [24]. These species all face habitat loss due to conversion of natural lands to urban and agricultural use, and two species (Sarcohyla crassa and Sarcohyla pentheter) are also threatened by the chytrid fungus, Batrachochytrium dendrobatidis [24]. One amphibian species, the Transverse Volcanic Leopard Frog (Rana neovolcanica) (2.0% = 1/51), is categorized as threatened (A) by SEMARNAT [34], and nine species are considered of high risk by EVS (18.4% = 9/49) [36] (Table 3).
Of all the reptile species in the BB, 4.5% are categorized as vulnerable (five species) or endangered (two species) by the IUCN Red List [24], 16.8% are considered threatened (A) or in danger of extinction (P) by the Mexican government [35], and 43.9% are at high risk according to the Environmental Vulnerability Score [37] (Table 3). Six of the species listed by the IUCN Red List are endemic to Mexico. Habitat loss or degradation is the most important threat for all of these species, which is often aggravated by their restricted ranges [24]. In addition, fifteen species are data deficient (DD), and another 21 have not been evaluated by the IUCN Red List [24].
4. Discussion
The native herpetofauna of the BB consists of 51 amphibian and 155 reptile species. Compared to other biogeographic provinces in Mexico, the BB contains relatively few species endemic to the province [31], with only six reptile species endemic to the BB and no amphibians. However, a very high proportion of the amphibian and reptile species found in the BB are endemic to Mexico, likely due to BB’s centralized position within Mexico and the large geographic extent of the country. In addition, specific types of habitats and environments found within parts of the BB are very important sites of amphibian and reptile diversity and endemism in Mexico [18]. Two-thirds of the amphibian species found in the BB are Mexican endemics, showing a high level of unique evolutionary history within the country [39] and emphasizing its role as a center for endemism in Mexico [40]. Similarly, of the 68 native lizard species that inhabit the BB, 54 are endemic to Mexico. For example, four lizard species are endemic to the BB, highlighting the unique ecological niches and evolutionary adaptations within this province, reflecting localized patterns of distribution and ecological affinity [39,41,42]. Fifty-four of the eighty-one species of snakes in the BB are endemic to Mexico. In addition, the high percentage of the families and genera of amphibians and reptiles found in Mexico that are found in the BB indicates it may be a particularly important biogeographic province for conservation efforts aimed at preserving Mexico’s overall herpetofaunal biodiversity.
For amphibians, the Pacific Lowlands and Balsas Basin (BB) form the initial cluster, followed by the Sierra Madre del Sur and then the Transvolcanic Belt. This clustering pattern suggests a gradient of species similarity among these provinces, possibly reflecting shared evolutionary histories or ecological similarities within these provinces (e.g., [31,43,44]). The clustering for reptiles shows a different pattern, with the BB initially clustering with the Sierra Madre del Sur, followed by the Transvolcanic Belt, and the Pacific Lowlands as the last to join the tree (e.g., [11,31]). Much of the Balsas Basin (BB) and the Pacific Lowlands are separated by the Sierra Madre del Sur. However, for amphibians, these two provinces clustered together. This clustering pattern suggests that the similarity in amphibian fauna between the BB and the Pacific Lowlands may be influenced by shared environmental conditions that could potentially override the geographic barrier posed by the Sierra Madre del Sur. Although further studies are needed to substantiate this hypothesis, it is possible that factors such as moisture availability and proximity to water bodies could play a significant role in shaping the distribution of amphibians in these regions, as amphibians are often more limited by moisture and water availability than by geographic barriers [45,46]. The lack of correlations between the Jaccard distances for amphibian species composition between the provinces and the distances between their geographic centroids and the length of their shared borders suggests that geography is less important than other factors in driving the similarity of amphibian faunas among these provinces. In contrast, for reptiles, the BB clusters with the Transvolcanic Belt and the Sierra Madre del Sur. This clustering suggests that proximity may play a more significant role for reptiles, as well as the possibility that reptiles are capable of occupying a broader range of environmental conditions across the provinces. Indeed, the negative correlation between Jaccard distances and the distance between geographic centroids we found for reptiles supports this conclusion (i.e., provinces that are closer together have greater similarities in the composition of their reptile species). Reptiles are more limited by the availability of sunny habitats, as they often use a complex array of physiological and behavioral mechanisms for maintaining body temperatures. Indeed, the richness of reptiles is strongly correlated with the amount of sunshine an area receives [47], and ambient temperature is considered the most important climatic factor affecting their distribution [48]. According to [49], the distribution of reptiles is therefore influenced by areas that provide enough sunlight for thermoregulation, which may explain their broader clustering across diverse environmental conditions.
Few (7%) of the amphibian and reptile species in the BB are considered to be Vulnerable, Endangered, or Critically Endangered by the IUCN Red List relative to its neighboring provinces, especially the Sierra Madre del Sur (25.9%) [31] and the Transvolcanic Belt (21.9%) [50]. Of the thirteen species with conservation concern status in the BB, twelve are shared with one or both of the provinces mentioned above. The only species with conservation concern status in the BB, but not in the Sierra Madre del Sur or the Transvolcanic Belt, is Crocodylus acutus. The fact that the BB shares most of its species of conservation concern with these neighboring provinces suggests that some environmental threats, such as habitat loss, may be widespread across these regions. However, the relatively low percentage of imperiled species in the BB compared to the other provinces may indicate that different or less acute environmental threats are affecting the BB, potentially offering a different ecological context for conservation efforts. For amphibians, additional threats such as emerging diseases, like chytridiomycosis, may also play a role in species decline [24]. However, other threats, such as climate change, the pet trade, and the limited distribution of some species, increase the risk of decline or even extirpation of amphibian and reptile populations in the BB [20,24,51,52,53]. In addition, some habitat types, such as tropical dry forest, in the BB are becoming increasingly fragmented [19].
For reptiles in particular, the lack of knowledge about the conservation status of many species (15 with data deficient status and 21 not evaluated) represents a challenge for their conservation in the BB [54,55,56]. It is essential to promote studies aimed at determining the conservation status of these 36 species, for which sufficient information is not available [57]. In addition, it is crucial to establish a greater number of protected areas in the province to guarantee the conservation of amphibian and reptile populations in their natural habitats [58,59,60,61,62]. Environmental education programs must also be implemented that involve and educate the population, creating awareness and promoting the active protection of biodiversity in general [63,64]. Through these actions, the protection of the BB’s biodiversity can be strengthened and its long-term survival in a changing environment ensured [65]. Interdisciplinary collaboration between scientists, natural resource managers, and local communities will be essential to implement effective conservation and monitoring strategies [66]. By strengthening biodiversity protection in the BB, not only are individual species preserved, but also ecosystem services vital to human well-being [65].
In addition, there are seven amphibian species and six reptile species in the LC category with a decreasing population trend (Table 4). All of these thirteen species face habitat loss as their primary threat to survival [24]. Sarcohyla bistincta may also be threatened by amphibian chytrid fungus, Batrachochytrium dendrobatidis, while Rana montezumae faces predation from species introduced through aquaculture, such as fish that prey upon tadpoles, although the specific species involved are not yet clear. Human consumption is a threat to some populations of Rana spectabilis [24]. The Balsas River system in the state of Morelos, which is home to 22 fish species, has over half (64%–14/22) of these species introduced, primarily for fisheries or ornamental aquaculture purposes [21]. Additional specific threats for reptiles include human persecution for Heloderma horridum, human consumption for Ctenosaura acanthura and Ctenosaura pectinata, and the introduction of Kinosternon integrum in parts of the distribution of Kinosternon hirtipes, which could be contributing to a decline in its range [24]. These thirteen species should be reassessed to determine the appropriate protection category they warrant on the IUCN Red List. In particular, Heloderma horridum, Ctenosaura pectinata, and Thamnophis godmani are also classified as threatened (A) by the Mexican government [35]. Two of these species, C. pectinata and T. godmani, have a high Environmental Vulnerability Score (EVS), and one more, Sceloporus asper, also has a high EVS but is not currently listed by the Mexican government.

Table 4.
Amphibians and reptiles of the Balsas Basin biogeographic province of Mexico with a conservation status (IUCN Status) of least concern and a decreasing population trend according to the IUCN Red List [24]. Environmental Vulnerability Score: (EVS) [36,37]; Mx indicates category of risk in Mexico according to [35]: (P = in danger of extinction, A = threatened, Pr = subject to special protection, NL—not listed); and main threats reported by [24]: 1 = habitat loss; 2 = human consumption in part of its distribution; 3 = human persecution; 4 = introduction of non-native predators through aquaculture; 5 = introduction of non-native predators [cats and dogs]; 6 = introduction of non-native competitors; 7* = its populations might be affected by chytridiomycosis.
The BB is now known to house at least three non-native species of reptiles: Hemidactylus frenatus, Indotyphlops braminus, and Apalone spinifera. Direct evidence for these species having impacts on the native herpetofauna in the BB, or Mexico in general, is very limited. However, evidence from elsewhere suggests that these three species may have a variety of effects on native species. Studies from other invasions by H. frenatus suggest it could compete with and potentially exclude native geckos [67,68,69], prey on smaller native lizards [70,71], or spread parasites [72,73]. In addition, in Mexico, H. frenatus may be better able to perform physiologically compared to native species, suggesting they may successfully invade and negatively impact native species of lizards [74]. The Brahminy Blindsnake appears likely to have relatively limited interactions with native species; however, its presence could impact local invertebrate populations and potentially impact native snake species sharing the same ecological niche [75,76]. As for A. spinifera, its ecological effects remain unclear but could involve competition or predation on native freshwater species, including frogs [22,77], and they are believed to be a threat for range expansion and establishment outside their native range [78]. Further research is needed to evaluate the full impact of these introduced species on native biodiversity in the BB.
5. Conclusions
The biogeographic province of the Balsas Basin (BB) stands out as an important center of biodiversity for amphibians and reptiles within Mexico, characterized by its notable species richness (206 native species), the presence of six species of reptiles endemic to the BB, and a high percentage of its species endemic to Mexico. The six reptile species endemic to the BB further highlight the importance of conservation efforts aimed at safeguarding unique evolutionary lineages. However, the different clustering patterns of amphibians and reptiles suggest the factors driving reptile and amphibian distributions across the BB and its neighboring provinces differ.
Despite its biological richness, the BB faces significant conservation challenges; pressures such as habitat loss, emerging diseases, climate change, and other anthropogenic threats threaten the long-term survival of these populations. Efforts to mitigate these threats should include expanding protected areas, improving research on species with uncertain conservation status (data deficient) or lacking studies (not evaluated), and implementing robust environmental education initiatives to engage local communities in the conservation of biodiversity. Understanding the BB’s biodiversity patterns and conservation needs is necessary to developing effective strategies that ensure the persistence of its rich amphibian and reptile communities. By encouraging interdisciplinary collaboration and proactive conservation measures, we can strive to preserve not only individual species but also the vital ecosystem services they provide, ensuring the biodiversity of the BB for future generations amid a changing environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17010044/s1, Table S1 (Supplementary Table S1): Amphibians and reptiles of the Balsas Basin (BB) biogeographic province of Mexico.
Author Contributions
Both authors contributed to the study conception and design; material preparation, data collection and analysis were performed by J.A.L.-E. and G.R.S.; the first draft of the manuscript was written by J.A.L.-E. and G.R.S. and both authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Support for this study was provided by Dirección General de Asuntos del Personal Académico, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (DGAPA-PAPIIT), through the Project IN200225.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All of the data that support the findings of this study are available in the main text.
Acknowledgments
We thank Alejandra Núñez Merchand from the National Commission for the Understanding and Use of Biodiversity (CONABIO) for making the maps and obtaining the geographic variables, and two anonymous reviewers for very helpful comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Morrone, J.J. Toward a cladistic model for the Caribbean subregion: Delimitation of areas of endemism. Caldasia 2001, 23, 43–76. [Google Scholar]
- Morrone, J.J.; Espinosa Organista, D.; Llorente Bousquets, J. Mexican biogeographic provinces: Preliminary scheme, general characteristics and synonymies. Acta Zool. Mex. (N.S.) 2002, 85, 83–108. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G. Mexican biogeographic provinces: Map and shapefiles. Zootaxa 2017, 4277, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rodríguez, G.; Morrone, J.J. The diversification of Nearctic mammals in the Mexican transition zone. Biol. J. Linn. Soc. 2004, 83, 327–339. [Google Scholar] [CrossRef]
- Morrone, J.J. Hacia una síntesis biogeográfica de México. Rev. Mex. Biodivers. 2005, 76, 207–252. [Google Scholar] [CrossRef]
- Morrone, J.J. Biogeographic areas and transition zones of Latin America and the Caribbean Islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu. Rev. Entomol. 2006, 51, 467–494. [Google Scholar] [CrossRef]
- Morrone, J.J. Regionalización biogeográfica y evolución biótica de México: Encrucijada de la biodiversidad del Nuevo Mundo. Rev. Mex. Biodivers. 2019, 90, e902980. [Google Scholar] [CrossRef]
- Flores-Tolentino, M.; Ramírez-Rodríguez, J.R.; Morales-Linares, J.; Ibarra-Manríquez, G.; Dorado, Ó.; Villaseñor, J.L. Delimitación geográfica y florística de la provincia fisiográfica de la Depresión del Balsas, México, con énfasis en el bosque tropical estacionalmente seco. Rev. Mex. Biodivers. 2023, 94, 1–22. [Google Scholar] [CrossRef]
- García-Marmolejo, G.; Escalante, T.; Morrone, J.J. Establecimiento de prioridades para la conservación de mamíferos terrestres Neotropicales de México. Mastozool. Neotrop. 2008, 15, 41–65. [Google Scholar]
- Morrone, J.J.; Márquez, J. Biodiversity of Mexican terrestrial arthropods (Arachnida and Hexapoda): A biogeographical puzzle. Acta Zool. Mex. (N.S.) 2008, 24, 15–41. [Google Scholar] [CrossRef]
- Hernández-Salinas, U.; Ramírez-Bautista, A.; Montiel-Canales, G.; Cruz-Elizalde, R. Historical and ecological biogeography of the genus Crotalus in Mexico. Herpetol. J. 2017, 26, 99–108. [Google Scholar]
- Montaño-Arias, G.; Luna-Vega, I.; Morrone, J.J.; Espinosa, D. Biogeographical identity of the Mesoamerican dominion with emphasis on seasonally dry tropical forests. Phytotaxa 2016, 376, 277–290. [Google Scholar] [CrossRef]
- Estrada-Márquez, A.S.; Morrone, J.J.; Villaseñor, J.L. Areas of endemism of two biogeographic provinces in central Mexico based on their endemic Asteraceae: A conservation proposal. Rev. Mex. Biodivers. 2021, 92, 1–17. [Google Scholar] [CrossRef]
- Arenas-Navarro, M.; Escalante, T.; Miguel-Talonia, C.; Silva-Galicia, A.; Téllez-Valdés, O. Areas of endemism and environmental heterogeneity: A case study in Mexican legumes. Aust. Syst. Bot. 2023, 36, 21–37. [Google Scholar] [CrossRef]
- Pérez-Hernández, C.X.; Zaragoza-Caballero, S.; Romo-Galicia, A. Updated checklist of the fireflies (Coleoptera: Lampyridae) of Mexico. Zootaxa 2022, 5092, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Llanes-Quevedo, A.; Sánchez-Ramos, L.E.; Navarro-Sigüenza, A.G. Patrones históricos y actuales de diversidad y relaciones biogeográficas de la avifauna residente de los bosques tropicales de México. Rev. Mex. Biodivers. 2024, 95, e955341. [Google Scholar] [CrossRef]
- Arita, H.T.; Santos-del-Prado, K. Conservation biology of nectar-feeding bats in Mexico. J. Mammal. 1999, 80, 31–41. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Ochoa-Ochoa, L.M.; Flores-Villela, O.A.; Velasco, J.A. Taxonomic distinctiveness and phylogenetic variability of amphibians and reptiles in the cloud forest of Mexico. Community Ecol. 2022, 23, 87–102. [Google Scholar] [CrossRef]
- Galindo-Cruz, A.; Sahagún-Sánchez, F.J.; López-Barrera, F.; Rojas-Soto, O. Recent changes in tropical-dry-forest connectivity within the Balsas Basin Biogeographic Province: Potential effects on endemic-bird distributions. Nat. Conserv. 2024, 55, 177–199. [Google Scholar] [CrossRef]
- Guzmán, J.C.C.; Saldaña, M.E.S.; Grosselet, M.; Gámez, J.S. The Illegal Parrot Trade in Mexico: A Comprehensive Assessment; Defenders of Wildlife: Washington, DC, USA, 2007. [Google Scholar]
- Contreras-MacBeath, T.; Mejia, M.H.; Carrillo, W.R. Negative impact on the aquatic ecosystems of the state of Morelos, Mexico, from introduced aquarium and other commercial fish. Aquar. Sci. Conserv. 1998, 2, 67–78. [Google Scholar] [CrossRef]
- Lemos-Espinal, J.A.; Webb, R.G.; Smith, H.M. Emory’s softshell turtle, Apalone spinifera emoryi, in México. Bull. Md. Herpetol. Soc. 1999, 35, 40–42. [Google Scholar]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature’s (IUCN). The IUCN Red List of Threatened Species Version 2024-1. Available online: https://www.iucnredlist.org/ (accessed on 21 June 2024).
- García, A. Using ecological niche modelling to identify diversity hotspots for the herpetofauna of Pacific Lowlands and adjacent interior valleys of Mexico. Biol. Conserv. 2006, 130, 25–46. [Google Scholar] [CrossRef]
- Aster, G. Advanced Spaceborne Thermal Emission and Reflection Radiometer Global Digital Elevation Model Version 2 (ASTER GDEM2)—Modelo Digital de Elevación Global ASTER Versión 2. 1:50,000. 2010. Available online: https://asterweb.jpl.nasa.gov/gdem.asp (accessed on 27 November 2024).
- García, E. “Climas (Clasificación de Köppen, Modificado por García).” Escala 1:1 000 000; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO): Mexico City, Mexico, 1998. [Google Scholar]
- Fernández-Nava, R.; Rodríguez-Jiménez, C.; Arreguín-Sánchez, M.; Rodríguez-Jiménez, A. Listado florístico de la cuenca del Río Balsas, México. Polibotánica 1998, 9, 1–151. [Google Scholar]
- Instituto Nacional de Estadística y Geografía [INEGI]. Conjunto de Datos Vectoriales de Uso de Suelo y Vegetación. Escala 1:250 000. Serie VI (Capa Unión), Escala: 1:250 000, 1st ed.; Instituto Nacional de Estadística y Geografía: Aguascalientes, México, 2016. [Google Scholar]
- Lemos-Espinal, J.A.; Smith, G.R. An analysis of the inter-state similarity of the herpetofaunas of Mexican states. Nat. Conserv. 2023, 53, 223–256. [Google Scholar] [CrossRef]
- Lemos-Espinal, J.A.; Smith, G.R. The distribution, diversity and conservation of the Mexican herpetofauna among its biogeographic provinces. J. Nat. Conserv. 2024, 82, 126714. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference; Version 6.2; American Museum of Natural History: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- AmphibiaWeb. University of California, Berkeley, CA, USA. Available online: https://amphibiaweb.org (accessed on 20 July 2024).
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Kudera, J.; Hošek, J. (Eds.) The Reptile Database. Available online: http://www.reptile-database.org (accessed on 20 July 2024).
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Modificación al Anexo Normativo III, Lista de Especies en Riesgo de la Norma Oficial Mexicana NOM-059-Ecol-(2010) Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2010. [Google Scholar]
- Wilson, L.D.; Johnson, J.D.; Mata-Silva, V. A conservation reassessment of the amphibians of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 97–127. [Google Scholar]
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 1–47. [Google Scholar]
- Ramírez-Bautista, A.; Torres-Hernández, L.A.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernández-Salinas, U.; Wilson, L.D.; Johnson, J.D.; Porras, L.W.; Balderas-Valdivia, C.J.; González-Hernández, A.J.; et al. An updated list of the Mexican herpetofauna: With a summary of historical and contemporary studies. ZooKeys 2023, 1166, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Rosauer, D.; Laffan, S.W.; Crisp, M.D.; Donnellan, S.C.; Cook, L.G. Phylogenetic endemism: A new approach for identifying geographical concentrations of evolutionary history. Mol. Ecol. 2009, 18, 4061–4072. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Wilson, L.D.; Mata-Silva, V.; García-Padilla, E.; DeSantis, D.L. The endemic herpetofauna of Mexico: Organisms of global significance in severe peril. Mesoamer. Herpetol. 2017, 4, 543–620. [Google Scholar]
- Rivero, J.A. Distribution, species-richness, endemism, and conservation of Venezuelan amphibians and reptiles. Amphib. Reptile Conserv. 2000, 2, 42–70. [Google Scholar]
- Kafash, A.; Ashrafi, S.; Yousefi, M.; Rastegar-Pouyani, E.; Rajabizadeh, M.; Ahmadzadeh, F.; Grüning, M.; Pellissier, L. Reptile species richness associated to ecological and historical variables in Iran. Sci. Rep. 2020, 10, 18167. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.M.; Berlanga-Robles, C.A.; Ruiz-Luna, A. High amphibian diversity related to unexpected environmental values in a biogeographic transitional area in north-western Mexico. Contrib. Zool. 2014, 83, 151–166. [Google Scholar] [CrossRef]
- Rivas, G.A.; Lasso-Alcalá, O.M.; Rodríguez-Olarte, D.; De Freitas, M.; Murphy, J.C.; Pizzigalli, C.; Weber, J.C.; de Verteuil, L.; Jowers, M.J. Biogeographical patterns of amphibians and reptiles in the northernmost coastal montane complex of South America. PLoS ONE 2021, 16, e0246829. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.B.; Jetz, W. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. Lond. B Biol. Sci. 2007, 274, 1167–1173. [Google Scholar] [CrossRef]
- Çömden, A.E.; Yenmiş, M.; Çakır, B. The complex bridge between aquatic and terrestrial life: Skin changes during development of amphibians. J. Dev. Biol. 2023, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Pianka, E.R. Ecology and Natural History of Desert Lizards; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Heatwole, H. Ecology of Reptiles; University of Queensland Press: St. Lucia, Australia, 1976. [Google Scholar]
- Wang, Y.; Lin, X.; Zheng, P.; Hou, Y.; Wang, G.; Gong, Y.; Shu, G.; Jiang, J.; Ran, J.; Xie, F. Species richness, composition, distribution and conservation status of herpetofauna in a global hotspot: The mountains of southwest China. Glob. Ecol. Conserv. 2024, 54, e03122. [Google Scholar] [CrossRef]
- Lemos-Espinal, J.A.; Smith, G.R. Amphibians and reptiles of the Transvolcanic Belt biogeographic province of Mexico: Diversity, similarities, and conservation. Nat. Conserv. 2024, 56, 37–76. [Google Scholar] [CrossRef]
- Ochoa-Ochoa, L.M.; Rodríguez, P.; Mora, F.; Flores-Villela, O.; Whittaker, R.J. Climate change and amphibian diversity patterns in Mexico. Biol. Conserv. 2012, 150, 94–102. [Google Scholar] [CrossRef]
- Masés-García, C.A.; Briones-Salas, M.; Sosa-Escalante, J.E. Assessment of wildlife crime in a high biodiversity region of Mexico. J. Nat. Conserv. 2021, 59, 125932. [Google Scholar] [CrossRef]
- Salas-Picazo, R.I.; Ramírez-Bravo, O.E.; Meza-Padilla, I.; Camargo-Rivera, E.E. The role of socal media groups in illegal wildlife trade in four Mexican states: A year-long assessment. Glob. Ecol. Conserv. 2023, 45, e02539. [Google Scholar]
- Bland, L.M.; Collen, B.E.N.; Orme, C.D.L.; Bielby, J.O.N. Predicting the conservation status of data-deficient species. Conserv. Biol. 2015, 29, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Bland, L.M.; Bielby, J.; Kearney, S.; Orme, C.D.L.; Watson, J.E.; Collen, B. Toward reassessing data-deficient species. Conserv. Biol. 2017, 31, 531–539. [Google Scholar] [CrossRef]
- Roberts, D.L.; Taylor, L.; Joppa, L.N. Threatened or data deficient: Assessing the conservation status of poorly known species. Divers. Distrib. 2016, 22, 558–565. [Google Scholar] [CrossRef]
- Bland, L.M.; Böhm, M. Overcoming data deficiency in reptiles. Biol. Conserv. 2016, 204, 16–22. [Google Scholar] [CrossRef]
- Escalona Segura, G.; Navarro Sigüenza, A.G. Protected Areas of Guerrero. In Protected Areas of Western Mexico: Status, Management, and Needs; Aid, C.S., Carter, M.F., Peterson, A.T., Eds.; Colorado Bird Observatory: Brighton, UK, 1997; pp. 90–108. [Google Scholar]
- Peterson, A.T.; Salazar, R.M. Protected Areas of Oaxaca. In Protected Areas of Western Mexico: Status, Management, and Needs; Aid, C.S., Carter, M.F., Peterson, A.T., Eds.; Colorado Bird Observatory: Brighton, UK, 1997; pp. 109–125. [Google Scholar]
- Villaseñor Gómez, J.F.; Villaseñor Gómez, L.E.; Villaseñor Gómez, A.E.; Sosa Gutiérrez, N.; Guzmán Pérez, A.M. Protected Areas of Michoacán. In Protected Areas of Western Mexico: Status, Management, and Needs; Aid, C.S., Carter, M.F., Peterson, A.T., Eds.; Colorado Bird Observatory: Brighton, UK, 1997; pp. 61–80. [Google Scholar]
- Urbina Torres, F.; Argote Cortés, A.; Jiménez Piedragil, D. Protected Areas of Morelos. In Protected Areas of Western Mexico: Status, Management, and Needs; Aid, C.S., Carter, M.F., Peterson, A.T., Eds.; Colorado Bird Observatory: Brighton, UK, 1997; pp. 81–89. [Google Scholar]
- Calderón-Patrón, J.M.; Peña-Joya, K.E.; Téllez-López, J.; Canales-Gómez, P.E. Dissimilarity among species and higher taxa of amphibians in a hotspot of biodiversity and endemism in the Neotropics. Diversity 2024, 16, 224. [Google Scholar] [CrossRef]
- Sousa, E.; Quintino, V.; Palhas, J.; Rodrigues, A.M.; Teixeira, J. Can environmental education actions change public attitudes? An example using the pond habitat and associated biodiversity. PLoS ONE 2016, 11, e0154440. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.H.; Pilliod, D.S. Elevating human dimensions of amphibian and reptile conservation, a USA perspective. Conserv. Sci. Pract. 2022, 4, e12685. [Google Scholar] [CrossRef]
- Corlett, R.T. Safeguarding our future by protecting biodiversity. Plant Divers. 2020, 42, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ewel, K.C. Natural resource management: The need for interdisciplinary collaboration. Ecosystems 2001, 4, 716–722. [Google Scholar] [CrossRef]
- Case, T.J.; Bolger, D.T.; Petren, K. Invasions and competitive displacement among house geckos in the tropical Pacific. Ecology 1994, 75, 464–477. [Google Scholar] [CrossRef]
- Dame, E.A.; Petren, K. Behavioural mechanisms of invasion and displacement in Pacific island geckos (Hemidactylus). Anim. Behav. 2006, 71, 1165–1173. [Google Scholar] [CrossRef]
- Cole, N.C.; Harris, S. Environmentally-induced shifts in behavior intensify indirect competition by an invasive gecko in Mauritius. Biol. Invas. 2011, 13, 2063–2075. [Google Scholar] [CrossRef]
- Gardner, C.; Jasper, L. Paroedura picta in southern Madagascar: Diet and predation by the introduced Hemidactylus frenatus. Herpetol. Notes 2012, 5, 457–458. [Google Scholar]
- Nordberg, E.J. Potential impacts of intraguild predation by invasive Asian house geckos. Austr. Ecol. 2019, 44, 1487–1489. [Google Scholar] [CrossRef]
- Barnett, L.K.; Phillips, B.L.; Heath, A.C.G.; Coates, A.; Hoskin, C.J. The impact of parasites during range expansion of an invasive gecko. Parasitology 2018, 145, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.A.; Torres, R.A.; Paternina, L.E.; Santana, D.J.; Miranda, R.J. Traveling with an invader: Ectoparasitic mites of Hemidactylus frenatus (Squamata: Gekkonidae) in Colombia. Cuad. Herpetol. 2020, 34, 79–82. [Google Scholar] [CrossRef]
- Romero-Báez, O.; Santos-Bibiano, R.; Domínguez-Godoy, A.M.; Miles, D.B.; Muñoz-Nolasco, F.J. Thermal ecophysiology of a native and an invasive gecko species in a tropical dry forest of Mexico. J. Therm. Biol. 2020, 90, 102607. [Google Scholar] [CrossRef]
- Rorabaugh, J.R. Brahminy Blindsnake (Indotyphlops braminus). Tucson Herpetological Society. Available online: https://tucsonherpsociety.org/amphibians-reptiles/snakes/brahminy-blindsnake-2/ (accessed on 27 December 2024).
- DeVos, T.B.; Giery, S.T. Establishment of the introduced Brahminy Blindsnakes (Indotyphlos braminus) on Abaco Island, The Bahamas, with notes on potential niche overlap with the native Cuban Brown Blindsnake (Typhlops lumbricalis). IRCF Amphib. Rept. 2021, 28, 555–557. [Google Scholar] [CrossRef]
- Rorabaugh, J.C.; Hossack, B.R.; Muths, E.; Sigafus, B.H.; Lemos-Espinal, J.A. Status of the threatened Chiricahua leopard frog and conservation challenges in Sonora, Mexico, with notes on other Ranid frogs and non-native predators. Herpetol. Conserv. Biol. 2018, 13, 17–32. [Google Scholar]
- Kopecký, O.; Kalous, L.; Patoka, J. Establishment risk from pet-trade freshwater turtles in the European Union. Knowl. Manag. Aquatic. Ecosyst. 2013, 410, 17–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).